Abstract
Sarcopenia, the loss of skeletal muscle mass and function with aging, is a multifactorial condition that slowly develops over decades and becomes a significant contributor to disability in the older population. Malnutrition and alterations in the muscle anabolic response to nutritional stimuli have been identified as potentially preventable factors that may significantly contribute to sarcopenia. Thus, nutritional interventions may be useful for the prevention and treatment of sarcopenia.
Keywords: aging, skeletal muscle, dietary protein, metabolism, anabolism
Introduction
The current length of the human life span averages 75 to 78 years and may increase to 85 years within the next two decades [1]. The projected increase in the number of older adults will most likely result in an increase in the number of persons with sarcopenia. Sarcopenia is the involuntary decline in lean muscle mass, strength and function that occurs with aging [2–4]. Sarcopenia increases the risk of disability and loss of functional capacity in the elderly [5], which is not necessarily the result of disease but is very prevalent in aging adults, including master athletes [6–9].
Skeletal muscle mass and strength generally peak between 20 and 35 years of age [10]. Thereafter, 3 to 8% of that muscle mass may be lost per decade, and this loss rate accelerates after the age of 60 [11–14]. Accompanying the muscle loss is a reduction in voluntary strength between (about 30% between 50 and 70 years of age) [15]. The majority of strength loss may be attributed to a preferential loss of Type II muscle fibers [16,17], which are capable of producing four times the power of Type I fibers [18], and may explain the reduced muscle power production of older adults [19–22]. Muscle power has been suggested to be a better predictor of physical function than muscle strength in older adults [21]. Moreover, aging is associated with declines in muscle quality [12,23]. As sarcopenia progresses, activities of daily living and mobility are further impaired, which may result in osteoporosis, falls, fractures, thrombophlebitis, pulmonary embolism, isolation, depression, and other adverse consequences. It is estimated that 14% of persons between the age of 65 to 75 require assistance with activities of daily living (ADL), and this figure increases to 45% for persons over 85 years of age [1].
Although the etiology of sarcopenia remains to be determined, several mechanisms have been proposed including:
Declines in the number and conduction velocity of motor neurons, specifically the largest diameter and fasted conducting type II motor units [24,25],
Muscle fiber alterations such as fiber type conversions favoring slow oxidative fibers or permanent denervation resulting in a loss of contact between nerve and muscle fiber [17],
Diminished excitation-contraction coupling [24] which may be attributed to the decline in the number of dihydropyridine receptors [26],
Mitochondrial DNA deletion mutations subsequent to oxidative damage [27],
Changes in satellite cell activation/proliferation [28],
Altered endocrine function (e.g. changes in insulin, growth factor, and/or cytokine release) and/or impaired tissue responsiveness to the hormonal stimuli [31–35],
Changes in tissue response to nutrients and/or malnutrition [36–38],
Diminished physical activity [39].
Sarcopenia, likely results from a compilation of each of the above factors, albeit in varying proportions depending on the individual. It remains that skeletal muscle fiber atrophy, regardless of the underlying mechanisms, results from the imbalance between muscle protein synthesis and breakdown.
Over time, the age-associated bias towards skeletal muscle catabolism leads to a net loss of muscle proteins and the associated decline in muscle mass and force production. Therefore, the study of skeletal muscle protein metabolism is a very useful tool to assess the mechanism(s) that are involved in the development of sarcopenia. This review focuses on the potential role nutrition plays in the pathophysiology and treatment of sarcopenia as it relates to protein synthesis and breakdown.
Amino Acid Administration and Muscle Protein Anabolism in Aging
The common classification of amino acids into the essential amino acid (EAA) and non-essential amino acid (NEAA) categories, according the body's ability to synthesize them (NEAA) or not (EAA), is important not only to define the amino acid synthesis site (endogenous or exogenous), but also to characterize their effect on protein metabolism. Observations made in younger subjects point to the EAA as being responsible for the amino acid induced stimulation of muscle protein synthesis [40,41], primarily the branched-chain amino acids [42,43], leucine in particular [44–46]. Whereas, NEAA failed to elicit an anabolic response even at very high doses [40]. The absence of EAA from the intracellular pool down-regulates protein synthetic rates by inhibiting steps in translation [47] while their presence is stimulatory, and may involve increased formation of the eukaryotic initiation factor 4E complex, activation of ribosomal protein S6, and other mechanisms that need yet to be elucidated [45,46].
Introduction of EAA in young men stimulates muscle protein synthesis by increasing the amino acid availability for the muscle cytosol [48,49], although more recent data suggest that the extracellular availability may be more important than the intracellular concentration for the amino acid stimulation of muscle protein synthesis [50,51]. We have shown previously in older adults that the intravenous (I.V.) infusion of a mixture of amino acids directly into the blood stream increased the transport of those amino acids into skeletal muscle [52]. Moreover, the increased availability of the infused amino acids increased the muscle protein synthesis rate while leaving protein breakdown unchanged [52].
Parenteral administration (infusion) of amino acids is, however, un-physiologic as this route circumvents both intestinal absorption dynamics and first pass splanchnic processing. This is important because it appears that orally administered protein digestion rates differentially effect the anabolic response in young and older adults such that slowly digested protein favors anabolism in the young whereas proteins digested at a faster rate favor anabolism in older individuals [53,54]. In addition, the splanchnic tissues may use dietary amino acids for their own metabolism [55]. Thus, a proportion of the ingested amino acids will not be made available to muscle due to the splanchnic bed utilization (oxidation and protein synthesis and breakdown) and this first pass extraction may increase with age [56,57]. Consequently, it appears that older adults extract more amino acids from oral protein administration at the level of the liver than do younger individuals.
We recently tested whether that greater amino acid extraction in older individuals may play a role in the age-associated chronic imbalance in protein anabolism and catabolism. Using stable isotope methodologies we measured the response of splanchnic and muscle protein metabolism to the oral administration of mixed amino acids in young and older subjects. We found that despite a higher first pass splanchnic phenylalanine extraction in the elderly, the delivery of phenylalanine to the leg and incorporation into final muscle protein, increased to the same extent in the old as it did in the young [58]. In a follow-up study we found that an amino acid load could stimulate muscle protein anabolism in older subjects regardless of the administration route (oral or intravenous), confirming that the splanchnic tissues are not a limiting factor for the amino acid stimulation of muscle protein synthesis in older people [59]. Collectively, these studies demonstrate that despite a greater first pass extraction by the gut and liver of orally administered amino acids, the amino acid-induced muscle protein anabolism in older subjects is not different from that of younger individuals.
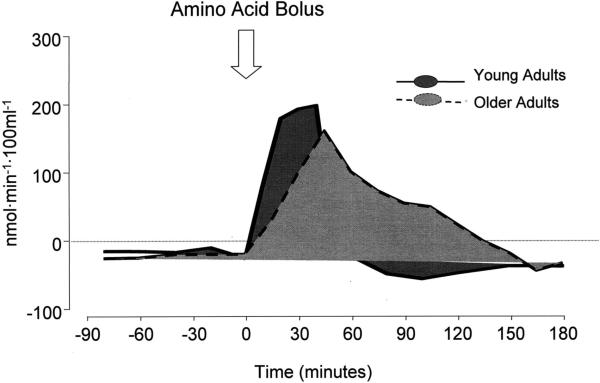
In a recent report, Paddon-Jones and colleagues have shown that the muscle protein synthetic rates of young and older men and women were similarly stimulated by the bolus oral administration of 15 g of EAA [60], which is consistent with our previous results using the continuous oral or intravenous administration [52,58]. Nonetheless, although the muscle protein synthetic rate increased significantly and similarly in both young and older individuals 4 hours after the EAA meal [60], the peak of net phenylalanine uptake by the leg, an index of net muscle protein anabolism, was delayed and blunted in the older subjects as compared to the young [60] (Fig. 1). Yet, the integrated anabolic response over the entire experimental period was slightly better in the older subjects. In both young and older subjects, the net phenylalanine uptake by the leg closely followed the response of the phenylalanine arterial concentration to the amino acid bolus, which was sharp and rapid in the young, blunted and slower in the older subjects. This was possibly due to either a delay in gastric emptying, which is often observed in older adults [61], and/or greater uptake at first pass by the splanchnic tissues with subsequent slower release of the alimentary amino acids [56,58].
Fig. 1.
Effect of an oral essential amino acid bolus on net muscle phenylalanine balance, an index of net muscle protein anabolism.
Overall these data indicate that despite some changes in the splanchnic handling of the alimentary amino acids, the general response of muscle proteins to amino acids is not significantly impaired with aging.
Mixed Nutrient Feeding and Muscle Protein Anabolism in Aging
Despite these findings, prior attempts to attenuate muscle loss in older adults utilizing nutritional supplements have proven unsuccessful in humans [62–64] and in a rat model [37]. In a study of frail adults, resistance exercise resulted in increased muscle strength and mass whereas nutritional supplementation alone failed to increase either muscle mass or strength [63]. However, in that experiment the nutritional supplementation was counterbalanced by reducing energy intake so that subjects in the supplement group did not increase their caloric intake [63]. Moreover, the addition of nutritional supplementation to resistance exercise did not increase muscle mass, strength, and/or muscle protein synthesis above the level achieved with resistive exercise alone [62–64]. Nonetheless, in a follow up study, it was found that increasing energy intake was associated with positive changes in strength and type II fiber size [65].
These reports may appear in conflict with our data indicating that amino acids supplementation can acutely stimulate muscle protein anabolism in both young and older subjects [58,60]. However, our positive data were obtained using a mixture of amino acids alone, while the studies demonstrating no benefit [62–64] or some benefit [65] from nutritional supplements in older adults utilized a balanced meal consisting of protein, fat, and carbohydrate [62–64]. Thus, the differences in the makeup of the nutritional supplements could have been responsible for the disparity between studies.
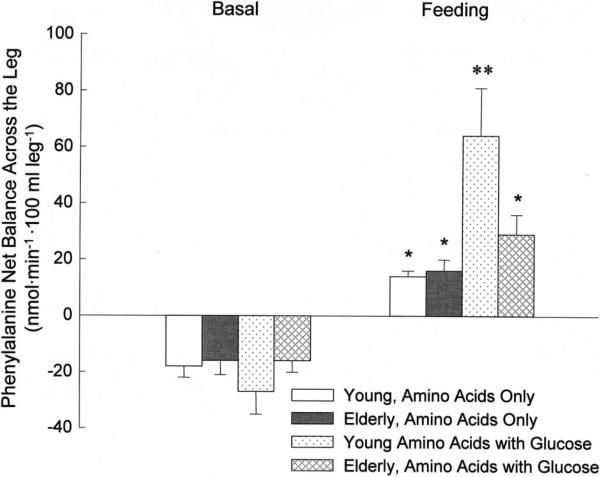
Consequently, we hypothesized that the protein synthetic response to feeding may be impaired in aging under certain circumstances, namely the composition of the nutritional stimulus. Therefore, we have recently completed a set of experiments designed to assess the response of muscle protein synthesis, breakdown and net balance to the ingestion of a mixture of amino acids combined with glucose in healthy older subjects as compared to younger controls. Our results demonstrate that phenylalanine net balance became positive in both age groups, which indicates that phenylalanine was being retained in the muscle tissue for protein synthesis, because it is not oxidized in the muscle. However, the increase was significantly blunted in the elderly [38] (Fig. 2). Moreover, in the older group protein synthesis was completely unresponsive to this mixed meal while protein breakdown was reduced, resulting in a net positive phenylalanine balance. However, since the increase in net balance was due almost entirely to a decrease in protein breakdown with no change in protein synthesis, amino acid and glucose intake overall decreased muscle protein turnover in the older subjects. Thus, despite its overall anabolic effect, this combination of nutrients does not appear to be as beneficial for muscle as amino acids alone because it may reduce muscle tissue remodeling. These results have been recently confirmed by another group [66] mimicking the postprandial state using the hyperinsulinemic, hyperaminoacidemic clamp technique and point to an impaired activation of the 70 kDa ribosomal protein S6 kinase (S6K1) as a potential mechanism for the attenuated protein synthetic response in the elderly.
Fig. 2.
Effects of continuous oral administration of balanced amino acids alone or combined with glucose in young and older adults. *p < 0.01 vs. basal. **p < 0.05 vs. others during feeding.
In the young, on the other hand, the protein synthetic rate increased significantly while breakdown was unchanged. Since our prior work indicated that young and older individuals responded equally to amino acids and that this response was due to increased protein synthesis [52,58], these results suggest that the addition of glucose may abolish the anabolic effects of amino acids in older adults, probably due to the glucose stimulation of insulin secretion [38]. It is in fact unlikely that glucose per se impaired muscle protein synthesis, since protein synthesis is an energy consuming process [67], which may be limited by lack of energy. Because glucose is the main source of energy for the muscle during meals and exercise [68], an increase in glucose availability, as we achieved in our study, provides additional energy for the muscle cells, thus facilitating, rather than impeding, the anabolic processes including protein synthesis.
Protein Requirements in Aging
To compound the muscle metabolic changes with aging mentioned above, food and energy intake appear to be reduced in older adults [69], possibly due to a combination of changes in the taste sensation, alterations in dentition, social isolation and depression [36]. In addition to the less than optimal food intake, older individuals appear to substitute protein in preference to fat and carbohydrate rich meals, which may again reflect changes in taste [36].
A recent report from the Institute of Medicine has extended the protein dietary reference intake (DRI) for adults 55 years and younger (0.8 g/kg/day) [70] to individuals older than 55 years. However, this recommendation may fall short of the protein needs of older adults. For example, it has recently been proposed that protein requirements for older individuals may be higher than the most recent DRI, and potentially greater than 1.0 g/kg/day [71,72]. Campbell and colleagues (2001) placed 10 healthy older men and women (55–77 years of age) on a 14-week eucaloric diet that contained 0.8 g/kg/day of protein under controlled conditions. They observed no change over time in protein turnover, oxidation, incorporation into muscle proteins (i.e., protein synthesis), or muscle protein breakdown [71]. However, nitrogen excretion declined, suggesting adaptation to a lower intake, and the mid-thigh muscle cross-sectional area decreased significantly as determined by computerized tomography [71]. These findings are consistent with our observation of a blunting in the acute response of muscle protein anabolism to a mixed meal, a defect that when repeated over time at each meal may result in a net muscle loss. These data from Campbell and colleagues thus suggest that the DRI for protein in older men and women may be set too low to afford preservation of muscle protein mass in these individuals. Nonetheless, further studies are necessary to exactly define the protein DRI for older subjects, possibly focusing on various older age ranges (young-old, middle age-old, and old-old), as well as on health status, as it is likely that the protein DRI changes as people grow older and with the development of chronic illnesses, most prevalent in the oldest segments of the population.
Conclusions
The ability of amino acids to stimulate skeletal muscle protein anabolism appears to be preserved in older subjects, despite an increased splanchnic amino acid turnover. Nonetheless, the combination of carbohydrate with amino acids, which is more anabolic in younger people, may potentially abrogate the desired anabolic effects known to occur with amino acids alone in older adults. This differential response of young and older muscle to amino acids and carbohydrate combinations may be due to an age-associated disregulation in the response of muscle proteins to insulin. Undernutrition and a tendency for protein to be underrepresented in the daily diet of older individuals complicate the underlying dynamics of aging muscle. It appears that eliminating carbohydrate from protein meals for older adults might be useful in order to maximize the anabolic effects of the protein is a necessary step to combat sarcopenia. However, before recommending such a drastic dietary intervention, long term studies are necessary to determine its safety and efficacy. Further, additional studies are needed to assess the protein DRIs for the various age and health cohorts of older subjects, the effects of the incorporation of exercise (endurance and resistive) to nutritional interventions and the nutritional timing relative to the exercise bout, the combination of hormonal and nutritional interventions, and the optimal interactions therein, in order to better understand and positively impact muscle mass and strength in older adults.
Key teaching points.
The ability of older muscle to mount a protein anabolic response following amino acid feeding.
The effects of mixed nutrient feeding on muscle protein metabolism in aging.
Protein requirements in aging.
Acknowledgements
This work was supported in part by NIH grants # R01 AG18311, R01 AR049877, and by NIDRR grant # H133P040003.
Footnotes
Presented in part at the American College of Nutrition 45th meeting, at Long Beach, CA, September 30, 2004.
REFERENCES
- 1.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 2.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50:5–8. doi: 10.1093/gerona/50a.special_issue.5. Spec No. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 4.Roubenoff R, Castaneda C. Sarcopenia-understanding the dynamics of aging muscle. JAMA. 2001;286:1230–1231. doi: 10.1001/jama.286.10.1230. [DOI] [PubMed] [Google Scholar]
- 5.Vandervoot AA, Symons TB. Functional and metabolic consequences of sarcopenia. Can J Appl Physiol. 2001;26:90–101. doi: 10.1139/h01-007. [DOI] [PubMed] [Google Scholar]
- 6.Dreyer HC, Hawkins SA, Schroeder ET, Wiswell RA. Muscle quality in master athletes drops significantly after age 65. Med Sci Sports Exerc. 2002;34:S98. [Google Scholar]
- 7.Hawkins SA, Wiswell RA, Marcell TJ. Exercise and the master athlete—a model of successful aging? J Gerontol A Biol Sci Med Sci. 2003;58:M1009–1011. doi: 10.1093/gerona/58.11.m1009. [DOI] [PubMed] [Google Scholar]
- 8.Pollock ML, Mengelkoch LJ, Graves JE, Lowenthal DT, Limacher MC, Foster C, Wilmore JH. Twenty-year follow-up of aerobic power and body composition of older track athletes. J Appl Physiol. 1997;82:1508–1516. doi: 10.1152/jappl.1997.82.5.1508. [DOI] [PubMed] [Google Scholar]
- 9.Wiswell RA, Hawkins SA, Jaque SV, Hyslop D, Constantino N, Tarpenning K, Marcell T, Schroeder ET. Relationship between physiological loss, performance decrement, and age in master athletes. J Gerontol A Biol Sci Med Sci. 2001;56:M618–26. doi: 10.1093/gerona/56.10.m618. [DOI] [PubMed] [Google Scholar]
- 10.Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci. 1999;54:B207–218. doi: 10.1093/gerona/54.5.b207. [DOI] [PubMed] [Google Scholar]
- 11.Holloszy JO. The biology of aging. Mayo Clin Proc. 2000;75(Suppl):S3–8. discussion S8–9. [PubMed] [Google Scholar]
- 12.Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, Fleg JL, Hurley BF. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999;86:188–194. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 13.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83:1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ, 3rd, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- 15.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 16.Hameed M, Harridge SD, Goldspink G. Sarcopenia and hypertrophy: a role for insulin-like growth factor-1 in aged muscle? Exerc Sport Sci Rev. 2002;30:15–19. doi: 10.1097/00003677-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–6. doi: 10.1093/gerona/50a.special_issue.11. Spec No. [DOI] [PubMed] [Google Scholar]
- 18.Faulkner JA. Power output of fast and slow fibers form skeletal muscles. In: Jones NL, McCartney N, McComas AJ, editors. “Human Muscle Power.”. Human Kinetics; Champaign, IL: 1986. pp. 61–94. [Google Scholar]
- 19.Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 20.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA, Singh MA. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 21.Evans WJ. Exercise strategies should be designed to increase muscle power. J Gerontol A Biol Sci Med Sci. 2000;55:M309–310. doi: 10.1093/gerona/55.6.m309. [DOI] [PubMed] [Google Scholar]
- 22.Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand. 2003;177:69–78. doi: 10.1046/j.1365-201X.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- 23.Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol. 2000;279:C611–618. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- 24.Payne AM, Delbono O. Neurogenesis of excitation-contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev. 2004;32:36–40. doi: 10.1097/00003677-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 26.Renganathan M, Messi ML, Delbono O. Overexpression of IGF-1 exclusively in skeletal muscle prevents age-related decline in the number of dihydropyridine receptors. J Biol Chem. 1998;273:28845–28851. doi: 10.1074/jbc.273.44.28845. [DOI] [PubMed] [Google Scholar]
- 27.Cortopassi GA, Shibata D, Soong NW, Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci USA. 1992;89:7370–4. doi: 10.1073/pnas.89.16.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 29.Welle S, Brooks A, Thornton CA. Senescence-related changes in gene expression in muscle: similarities and differences between mice and men. Physiol Genomics. 2001;5:67–73. doi: 10.1152/physiolgenomics.2001.5.2.67. [DOI] [PubMed] [Google Scholar]
- 30.Welle S, Bhatt K, Thornton CA. High-abundance mRNAs in human muscle: comparison between young and old. J Appl Physiol. 2000;89:297–304. doi: 10.1152/jappl.2000.89.1.297. [DOI] [PubMed] [Google Scholar]
- 31.Kelijman M. Age-related alterations of the growth hormone/insulin-like-growth-factor I axis. J Am Geriatr Soc. 1991;39:295–307. doi: 10.1111/j.1532-5415.1991.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 32.Tenover JS, Matsumoto AM, Clifton DK, Bremner WJ. Age-related alterations in the circadian rhythms of pulsatile luteinizing hormone and testosterone secretion in healthy men. J Gerontol. 1988;43:M163–169. doi: 10.1093/geronj/43.6.m163. [DOI] [PubMed] [Google Scholar]
- 33.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75:1092–1098. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 34.Roubenoff R. Hormones, cytokines and body composition: can lessons from illness be applied to aging? J Nutr. 1993;123:469–473. doi: 10.1093/jn/123.suppl_2.469. [DOI] [PubMed] [Google Scholar]
- 35.Roubenoff R, Rall LC, Veldhuis JD, Kehayias JJ, Rosen C, Nicolson M, Lundgren N, Reichlin S. The relationship between growth hormone kinetics and sarcopenia in postmenopausal women: the role of fat mass and leptin. J Clin Endocrinol Metab. 1998;83:1502–1506. doi: 10.1210/jcem.83.5.4809. [DOI] [PubMed] [Google Scholar]
- 36.Morley JE. Anorexia of aging: physiologic and pathologic. Am J Clin Nutr. 1997;66:760–773. doi: 10.1093/ajcn/66.4.760. [DOI] [PubMed] [Google Scholar]
- 37.Mosoni L, Valluy MC, Serrurier B, Prugnaud J, Obled C, Guezennec CY, Mirand PP. Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol. 1995;268:E328–335. doi: 10.1152/ajpendo.1995.268.2.E328. [DOI] [PubMed] [Google Scholar]
- 38.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiatarone MA, Evans WJ. The etiology and reversibility of muscle dysfunction in the aged. J Gerontol. 1993;48:77–83. doi: 10.1093/geronj/48.special_issue.77. Spec No. [DOI] [PubMed] [Google Scholar]
- 40.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–78. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- 41.Smith K, Barua JW, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1–13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1–13C]valine. Am J Physiol. 1992;262:E372–376. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- 42.Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. 1988;254:579–584. doi: 10.1042/bj2540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta. 1978;544:351–359. doi: 10.1016/0304-4165(78)90103-4. [DOI] [PubMed] [Google Scholar]
- 44.Buse MG, Reid SS. Leucine: A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 46.Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, Kimball SR, Jefferson LS. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 2002;51:928–936. doi: 10.2337/diabetes.51.4.928. [DOI] [PubMed] [Google Scholar]
- 47.Kimball SR, Jefferson LS. Regulation of translation initiation in mammalian cells by amino acids. In: Sondergaard SR, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. pp. 561–579. [Google Scholar]
- 48.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 49.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 50.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi H, Borsheim E, Anthony TG, Traber DL, Badalamenti J, Kimball SR, Jefferson LS, Wolfe RR. Reduced amino acid availability inhibits muscle protein synthesis and decreases activity of initiation factor eIF2B. Am J Physiol Endocrinol Metab. 2003;284:E488–498. doi: 10.1152/ajpendo.00094.2002. [DOI] [PubMed] [Google Scholar]
- 52.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340–348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- 54.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol. 1993;264:E109–118. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- 56.Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997;65:489–495. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- 57.Young VR. Amino acids and proteins in relation to the nutrition of elderly people. Age Ageing. 1990;19:S10–24. doi: 10.1093/ageing/19.suppl_1.s10. [DOI] [PubMed] [Google Scholar]
- 58.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277:E513–20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 59.Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6:358–362. [PMC free article] [PubMed] [Google Scholar]
- 60.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 61.O'Mahony D, O'Leary P, Quigley EM. Aging and intestinal motility: a review of factors that affect intestinal motility in the aged. Drugs Aging. 2002;19:515–527. doi: 10.2165/00002512-200219070-00005. [DOI] [PubMed] [Google Scholar]
- 62.Campbell WW, Crim MC, Young VR, Joseph LJ, Evans WJ. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol. 1995;268:E1143–1153. doi: 10.1152/ajpendo.1995.268.6.E1143. [DOI] [PubMed] [Google Scholar]
- 63.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 64.Welle S, Thornton CA. High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62- to 75-yr-old men and women. Am J Physiol. 1998;274:E677–683. doi: 10.1152/ajpendo.1998.274.4.E677. [DOI] [PubMed] [Google Scholar]
- 65.Singh MA, Ding W, Manfredi TJ, Solares GS, O'Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol. 1999;277:E135–143. doi: 10.1152/ajpendo.1999.277.1.E135. [DOI] [PubMed] [Google Scholar]
- 66.Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. Faseb J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 67.Welle S, Nair KS. Relationship of resting metabolic rate to body composition and protein turnover. Am J Physiol. 1990;258:E990–998. doi: 10.1152/ajpendo.1990.258.6.E990. [DOI] [PubMed] [Google Scholar]
- 68.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 69.McDowell MA, Briefel RR, Alaimo K, Bischof AM, Caughman CR, Carroll MD, Loria CM, Johnson CL. Energy and macronutrient intakes of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988–91. Adv Data. 1994:1–24. [PubMed] [Google Scholar]
- 70.Series Protein and Amino Acids. The National Academies Press; Washington: 2002. Protein and Amino Acids. pp. 465–608. [PubMed] [Google Scholar]
- 71.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–380. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 72.Campbell WW, Crim MC, Dallal GE, Young VR, Evans WJ. Increased protein requirements in elderly people: new data and retrospective reassessments. Am J Clin Nutr. 1994;60:501–509. doi: 10.1093/ajcn/60.4.501. [DOI] [PubMed] [Google Scholar]