Abstract
Objective
The study investigated the relative degree and timing of cortical activation associated with phonological decoding in poor readers.
Method
Regional brain activity was assessed during performance of a pseudoword reading task and a less demanding, letter-sound naming task by three groups of students: children who experienced reading difficulties without attention problems (N = 50, RD) and nonreading impaired (NI) readers either with (N = 20) or without attention-deficit/hyperactivity disorder (ADHD; N = 50). Recordings were obtained with a whole-head neuromagnetometer, and activation profiles were computed through a minimum norm algorithm.
Results
Children with RD showed decreased amplitude of neurophysiological activity in the superior temporal gyrus, bilaterally, and in the left supramarginal and angular gyri during late stages of decoding, compared to typical readers. These effects were restricted to the more demanding pseudoword reading task. No differences were found in degree of activity between NI and ADHD students. Regression analyses provided further support for the crucial role of left hemisphere temporoparietal cortices and the fusiform gyrus for basic reading skills.
Conclusions
Results were in agreement with fMRI findings and replicate previous MEG findings with a larger sample, a higher density neuromagnetometer, an overt pseudoword reading task, and a distributed current source-modeling method.
Keywords: phonological decoding, dyslexia, attention-deficit/hyperactivity disorder, magnetoencephalography, functional brain imaging
The ability to recognize printed words depends on several cognitive and linguistic skills, some of which are unique to reading, such as visual discrimination of graphemic features (Eden, VanMeter, Rumsey, & Zeffiro, 1996) and phonological awareness (Blachman, 1997; Liberman, 1998; Lukatela & Turvey, 1998; Snow, Burns, & Griffin, 1998). On the basis of the study of reading deficits caused by brain lesions (e.g., Damasio & Damasio, 1983; Henderson, 1986) and from functional brain imaging studies (reviewed in Jobard, Crivello, & Jurio-Mazoyer, 2003; Joseph, Noble, & Eden, 2001; Pugh, Mencl, Jenner, et al., 2000), several left hemisphere regions appear to be involved, more or less critically, in the brain mechanism that supports skilled reading. Some of these areas have also been implicated in receptive (superior temporal, middle temporal, supramarginal, and angular gyri) and expressive language functions (inferior frontal gyrus), whereas others (lateral occipitotemporal region and fusiform gyrus) are part of the visual association cortex.
Neural Circuits That Underlie Reading Difficulties
Multiple functional imaging modalities demonstrate that children who experience difficulties in acquiring basic reading skills (decoding and word recognition) exhibit reduced neurophysiological and hemodynamic activity in posterior temporal and inferior parietal regions in the left hemisphere (e.g., B. A. Shaywitz et al., 2002; Temple et al., 2001). Magnetoencephalography (MEG) studies have further shown that failure to engage these regions (Simos et al., 2000, 2002; Simos, Fletcher, et al., 2007) characterizes relatively late stages in the processing of print (approximately between 250 ms and 700 ms after stimulus onset).
However, evidence for a disruption of reading-related engagement of occipitotemporal regions, the angular gyrus, and inferior frontal cortex in children who experience reading difficulties (RD) was less consistently noted across studies. With respect to the left inferior frontal gyrus, some studies have reported decreased activity in children with RD (e.g., Cao, Bitan, Chou, Burman, & Booth, 2006; B. A. Shaywitz et al., 2002), whereas others reported increased activity (e.g., Hoeft et al., 2007). More recent MEG studies (Simos, Fletcher, et al., 2007, Simos, Papanicolaou, et al., 2007) that use neuromagnetometers that are more sensitive to neurophysiological activity produced by the frontal lobes than earlier generation machines indicated that frontal hyperactivation in RD is associated with an aberrant temporal progression of regional activity. Similarly, some studies have reported underactivation of the left occipitotemporal cortex (most notably the fusiform gyrus) in children with RD, even for tasks that require phonological decoding (Cao et al., 2006; Hoeft et al., 2007; McCrory, Mechelli, Frith, & Price, 2005; van der Mark et al., 2009). Moreover, a positive association between degree of activity in left occipitotemporal cortices and phonological decoding has also been reported in children spanning a wide range of reading skill (B. A. Shaywitz et al., 2002). Moreover, the hypothesis of a functional disruption of the angular gyrus in RD is based on relatively sparse lesion data that establishes a selective link between damage to or electrical interference with this region and acquired alexia (Greenblatt, 1976; Henderson, 1986; Philipose et al., 2007; Roux et al., 2004). Direct evidence for angular gyrus hypoactivation in children with developmental RD is relatively sparse (S. E. Shaywitz et al., 1998; Temple et al., 2001; Simos et al., 2007).
Comorbidity of RD and Attention-Deficit/Hyperactivity Disorder
Children with RD often have associated disorders including math disability and attention-deficit/hyperactivity disorder (ADHD; e.g., Willcutt, Pennington, Olson, & DeFries, 2007). It has been estimated that RD and ADHD show comorbidity ranging between 30% and 70% of children with RD in the school-age population (Pennington et al., 2009). Although the cognitive correlates of RD and ADHD vary, the latter involving difficulties with executive functions and sustained attention (Bonafina, Newcorn, McKay, Koda, & Halperin, 2000; Laasonen, Lehtinen, Leppämäki, Tani, & Hokkanen, 2009; Willcutt, Pennington, Olson, & DeFries, 2007), there is genetic overlap in the heredity of the two disorders (Pennington, McGrath, Rosenberg, et al., 2009). However, the impact of comorbidity on brain activation patterns from neuroimaging studies of RD is not well understood. Accordingly, the impact of ADHD symptoms on brain activation profiles associated with phonological decoding is explored systematically in this MEG study.
MEG Studies of RD
Earlier MEG studies on RD suffered from a number of potential limitations. Spatiotemporal activation profiles were obtained by iterative application of the single equivalent dipole model (e.g., Sarvas, 1987). Although this method has been routinely applied to estimate the location of sensory- and language-evoked magnetic fields, and subjected to external validation against invasive diagnostic techniques (Kamada et al., 2007; Papanicolaou et al., 2004; Szymanski et al., 2001), a potential drawback of this approach is susceptibility to spatial undersampling of scalp field distributions. An approach proposed for addressing this potential problem is the application of distributed source-modeling techniques, such as minimum-norm estimates (MNE; Hämäläinen & Ilmoniemi, 1994; Moran & Tepley, 2000). The present study uses MNE in an effort to replicate previous MEG studies on children with RD that used a single dipole method for detecting active sources.
Another potential limitation of previous MEG studies (as well as of some PET and fMRI studies) is their reliance on convenience sampling methods that inadvertently result in overrepresentation of RD children from middle to high SES families and above average general cognitive abilities. Both potential limitations of earlier studies are addressed in this study.
Rationale for This Study
This study had several goals. First, we sought to extend previous MEG findings in regard to differences in regional degree of activation between nonreading impaired (NI) and RD children, by using larger more representative samples of children with and without RD. Special care was taken in this study to recruit children from the general school population, ensuring a better representation of socioeconomic (SES), ethnic backgrounds, achievement, and general cognitive ability scores. The second goal of the study was to obtain brain-activation profiles from children with ADHD and no RD to ensure that our previous results were not confounded by the high rate of comorbidity between RD and ADHD. Third, in addition to regional degree of activity we examined the relative timing of regional activation based on the peak latency of estimated activity to replicate previous MEG reports in regard to distinct timing patterns for RD and NI children, and to ascertain that such differences are indeed related to reading ability (and do not reflect more general disruptions in functional brain organization common to both RD and ADHD). The fourth goal of the study was to determine whether group differences in degree, relative timing, or both, of regional activity depend on task demands for phonological decoding, by assessing spatiotemporal activation profiles obtained during performance of a pseudoword decoding task and during an easier letter-sound naming task. Finally, by taking advantage of the size of the present sample, combined with the statistical properties of the activation data provided by the MNE procedure (near-Gaussian distribution of individual activation values), we applied linear regression models to identify brain regions where the degree of activity predicted individual scores on reading achievement tests.
Method
Participants
The current sample included 50 children with reading difficulties (RD group) as indicated by scores below the 25th percentile (standard score of 90) on the Basic Reading composite (average of Word Attack and Letter-Word Identification subtest scores of the Woodcock-Johnson Tests of Achievement-III [W-J III]; Woodcock, McGrew, & Mather, 2001). A second group of 20 children carried a clinical diagnosis of ADHD and a negative history of reading difficulties (they also scored above the 30th percentile, corresponding to a standard score of 92, on the W-J III Basic Reading composite). A third group of 50 children who had never experienced difficulties in reading (NI group) served as controls, having standard scores 92 on the Basic Reading composite index and no indication of ADHD as described below. Table 1 displays demographic and psychoeducational information for each of the three groups of participants, which were comparable on age, ethnicity, handedness, and performance IQ. As expected, the RD group scored significantly lower than the other two groups on measures of reading, spelling, and verbal IQ. Moreover, the RD group contained a higher proportion of boys than the ADHD and NI groups (80% vs. 60% and 54%, respectively).
Table 1.
Demographic Data, Educational History, and Performance on Standardized Tests for Each Group of Participants (Mean and SD in Parentheses)
| Variable | NI (n = 50) | RD (n = 50) | Additional poor readers (n = 20) | ADHD (n = 20) |
|---|---|---|---|---|
| Gender (boys/girls)a | 27/23 | 40/10 | 9/11 | 12/8 |
| Age (months, η2 = .05) | 84–156 (127 ± 25) | 92–190 (134 ± 22) | 102–165 (142 ± 18) | 86–156 (135 ± 24) |
| Ethnicity (C, H, A-AMb)c | 21/10/19 | 29/8/13 | 6/5/9 | 13/2/5 |
| No. retainedd | 0 | 6 | 1 | 0 |
| Reading remediation (group–individualized) | 0 | 40/2 | 9/5 | 0 |
| Handedness (L/R)e | 45/5 | 43/7 | 16/4 | 19/1 |
| VIQ (η2 = .13) | 85–144 (111 ± 16)† | 80–128 (100 ± 14)†* | 80–114 (96.5 ± 10) | 90–147 (110 ± 15)* |
| PIQ (η2 = .03) | 83–118 (100 ± 8) | 77–117 (97 ± 11) | 80–132 (103 ± 15) | 80–114 (99 ± 10) |
| FSIQ (η2 = .10) | 85–130 (106 ± 11)@ | 82–118 (98 ± 11)@ | 81–126 (101 ± 13) | 87–130 (104 ± 11) |
| W-J-IIIf Reading Composite (η2 = .75) | 93–126 (106 ± 10)† | 54–85 (76 ± 7)†§ | 81–92 (86 ± 4) | 92–123 (105 ± 9)§ |
| W-J-III Word Attack η2 = .71) | 92–131 (105 ± 10)† | 49–85 (79 ± 6)†§ | 84–94 (89 ± 3) | 94–124 (106 ± 9)§ |
| W-J-III Letter-Word Identification (η2 = .69) | 89–130 (106 ± 11)† | 35–86 (72 ± 12)†§ | 75–96 (85 ± 5) | 88–126 (104 ± 11)§ |
| W-J-III Spelling (η2 = .72) | 88–136 (110 ± 12)† | 20–88 (71 ± 14)†§ | 78–100 (89 ± 8) | 94–136 (107 ± 8)§ |
Note. Participants in the supplementary Poor reader group scored significantly lower than nonreading impaired (NI) and attention-deficit/hyperactivity disorder (ADHD) participants on VIQ, and each of the W-J-III subtests. VIQ = Verbal IQ; PIQ = Performance IQ; FSIQ = Full Scale IQ.
Phi = .274, p < .01.
C = Caucasian, H = Hispanic, A-AM = African American.
Phi = .26, p = .1
Number of children retained in the same grade.
Phi = .09, p > .75.
W-J-III = Woodcock-Johnson Tests of Achievement-III.
p < .05.
p < .01.
p < 0001.
p < 0001.
For the presence or absence of ADHD, participants were selected for inclusion in the RD or NI groups only if they had T scores < 55 on the Attention Problems scale of the parent form of the Child Behavior Checklist (CBCL; Achenbach, 1991; n = 15) or a mean score lower than 1.67 on the Inattention and Hyperactivity-Impulsivity scales of the parent completed Swanson, Nolan, Achenbach, Pelham questionnaire (SNAP-IV; Swanson, 1992), indicating low risk for ADHD (Chen, Faraone, Biederman, & Tsuang, 1994). Given the high rates of comorbidity between RD and ADHD, approximately 140 children had to be screened to form the NI and RD groups, matching the above criteria. In addition to having scores on the CBCL or SNAP-IV in the clinically significant range, all children in the group with ADHD had a clinical diagnosis of ADHD and were treated with stimulant medication. Prior to the MEG session, an overnight washout was used. The child skipped the morning and second dose (if there was one) to do the study and then went back to their standard regimen. Though some residual receptor excitability remains, this is minimal. If the family was reluctant to stop for a day or two, a Monday assessment was offered so that the child could be off at home rather than at school. Children on medications other than stimulants were not included. A licensed psychologist reviewed all data pertaining to ADHD and also completed a semi-structured interview with the parents to verify the presence or absence of ADHD (using the Diagnostic Interview Schedule for Children-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). All participants in the ADHD group scored above the 85th percentile on the Hyperactivity/Impulsivity or Inattention subscales of the SNAP-IV (or on both). Average percentile rankings for this group were 79 ± 15 and 85 ± 13 on each subscale, respectively.
To ensure sufficient range and variability in reading achievement scores among poor readers when conducting correlational analyses, the group of poor readers was enriched by including 20 students with Word Attack scores in the low average range (see Table 1) and at least one of their Letter-Word Identification or Spelling scores below 85 points, indicating difficulties in word identification or spelling with relatively preserved decoding skills. All 140 participants had FSIQ scores > 80 on the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
Procedures
Tasks
Each participant was tested on two naming tasks that varied on decoding difficulty. For the letter-sound task, stimuli were four randomized sequences of 24 single, lowercase letters subtending 0.6° of visual angle. For the pseudoword reading task, stimuli were three-letter pronounceable nonwords (e.g., lan), subtending 2.0° of visual angle, which were adapted versions of those used previously by our group in studies of reading Simos, Fletcher, et al. (2007). For each task, 100 stimuli were presented randomly arranged in four blocks of 25 items each. Stimuli were presented for 1 s, one at a time (with a randomly varied interstimulus interval of 3–4 s), through a Sony LCD projector (Model VPL-PX21) on a back-projection screen located approximately 60 cm in front of the participant. Task order was counterbalanced across participants, who were instructed to name (on the Letter-Sound task) and read aloud (on the Pseudoword task) each stimulus immediately after it had disappeared from the screen. Prior to each scan children were asked to practice this response strategy while magnetic activity was monitored online to ensure that movement artifacts associated with articulation systematically occurred only after the end of the recording epoch. Epochs containing such movement artifacts (when, occasionally, verbal responses were produced earlier than instructed) were not included in further data analyses.
Imaging procedures
MEG recordings were obtained with a whole-head neuromagnetometer array (4-D Neuroimaging, Magnes WH3600), that consisted of 248 first-order axial gradiometer coils, housed in a magnetically shielded chamber and arranged to cover the entire head surface. The magnetic flux measurements were digitized at 250 Hz, filtered with a bandpass filter between 0.1 and 20 Hz and subjected to baseline adjustment (using the 150 ms prestimulus recording) and to a noise reduction algorithm that is part of the 4D-Neuroimaging software. The single-trial event-related field segments (ERFs) in response to 60–80 stimulus presentations, were averaged after excluding those containing eye movement or other myogenic or mechanical artifacts.
To identify the intracranial origin of ERFs, the magnetic flux distribution recorded simultaneously over the entire head surface at successive points (4 ms apart) was analyzed using a minimum norm model to obtain estimates of the time-varying strength of intracranial currents (MNE Software, Version 2.5; Hämäläinen, (2006). This method affords greater spatial resolution and allows detection of simultaneous magnetic sources distributed along the entire cortical surface. The model assumes a continuous distribution of current along the cortical surface, which has some minimum norm (Hämäläinen & Ilmoniemi, 1994). Estimated current sources were anatomically constrained by an MRI-derived surface model of each participant's brain (T1-weighted: TR 13.6 ms; TE 4.8 ms; recording matrix 256 × 256 pixels, 1 excitation, 240 mm field of view, and 1.4 mm slice thickness), obtained on a Philips 3 T scanner with SENSE (sensitivity encoding) technology.
This surface model was generated by a fully automated cortical surface reconstruction procedure using FreeSurfer software (Dale, Fischl, & Sereno, 1999) for producing a detailed geometric description (regular tessellation of the cortical surface consisting of equilateral triangles known as vertices) of the gray-white matter boundary of the neocortical mantle and the mesial temporal lobe. Each hemisphere consisted of approximately 150,000 vertices (depending on each subject's cortical surface area). For estimating current sources, the MNE software requires the Freesurfer-derived cortical surface reconstruction for defining the boundaries of a solution source space. A grid-spacing of 7 mm was used to construct icosahedrons to decimate the number of vertices from 150,000 to approximately 3,000 per hemisphere. Additionally, the MNE software was used to construct a single-compartment boundary element model, using triangular tessellations to model each vertex as a potential current dipole perpendicular to the cortical surface during the forward calculations. The inverse solution was subsequently reduced to obtaining an estimate of the scalar distribution of dipole strength across current sources within orientation-specific cortical patches of vertices (Dale, Fischl, & Sereno, 1999). Coregistration of each MEG dataset with its corresponding MRI dataset was performed by using an automated coregistration routine within MNE, which aligns digitization points in the MEG headshape file with the fiducial points demarcated on the outer skin surface reconstruction of the MRI.
Preliminary equivalent current dipole modeling of magnetic activity, using procedures described in detail elsewhere (Simos, Fletcher, et al., 2007), indicated that the vast majority (94%) of activity sources were found in the following areas in both hemispheres: superior (STG; BA 22) and middle temporal gyri (MTG; BA 21), excluding cortex along the banks of the superior temporal sulcus (STS); the cortical surface within STS; supramarginal gyrus (SMG; BA 40); angular gyrus (ANG; BA 39); pars opercularis of the inferior frontal gyrus (IFG; BA 44); rostral inferior (BA 47), and rostral middle frontal cortices (BA 46/9); motor cortex (BA 4); fusiform gyrus (BA 37); and lateral occipitotemporal cortex (BA 19). The program outputs a current estimate value for each voxel and each 4-ms time point. This value was then used to compute the two dependent measures used in the analyses outlined in the following sections. First, the average current across all voxels defining each of the Regions of Interest (ROIs) listed above and across all of the 4-ms time points comprising 14 successive 50-ms time bins (100–150 ms, 150–200 ms, etc., up to 800 ms). Second, the latency (in milliseconds after stimulus onset) when averaged current in a given ROI reached peak amplitude within the entire recording epoch. This approach was designed to address the following questions: (a) determine whether group differences in the degree of regional activity were time and hemisphere dependent (i.e., more systematic for particular time windows, restricted to one hemisphere, or both); (b) determine group differences in peak latency; (c) establish the temporal progression of regional activity in each group of participants; and (d) identify ROIs and time bins where activity correlated with standardized reading achievement measures.
Analytic approach
To address the first, second, and fourth main goals of the study, the average current for each 50-ms time bin and each ROI were initially submitted to an analysis of variance (ANOVA) with task (2), area (11), hemisphere (2), and time bin (14) as the within-subjects variables and group (3) as the between-subjects variable. Significant five-way interactions involving group were further evaluated by examining four-way (e.g., Time × Area × Hemisphere × Group for each task) or three-way interactions (e.g., Time × Area × Group for each task) which, if significant, were explored by testing two-way interactions (Time × Group or Time × Hemisphere). These lower order ANOVAs were performed separately for NI versus RD and NI versus ADHD participants. Significant hemisphere asymmetries in the degree of regional activity will be mentioned, even in the absence of group-specific hemisphere effects, to facilitate comparisons between the present and findings from previous studies using similar tasks. All ANOVA results were evaluated using the Huynh-Feldt method as a precaution against inhomogeneity of variance problems.
The second set of analyses (addressing the third main goal of the study) established the outline of the spatiotemporal profiles of activity associated with the performance of each task by each group of participants— essentially the temporal progression of regional activity for each group. The dependent variable in these analyses was the median time point of the 50-ms time bin when average MNE current reached peak value. ROIs from both hemispheres were ranked according to peak latency, and a series of dependent-sample t tests were computed between ROIs (earliest peaking ROI with each subsequent ROI, second earliest ROI with each subsequent ROI, etc.), to test the hypothesis that regional onset latency differences were statistically significant. There were eight potential reading-related areas resulting in 15 ROIs (in both hemispheres—LOC was not examined separately in each hemisphere). The nominal alpha value to control for family-wise Type I error (for each task and each group of participants) for multiple comparisons, would be .0004. However, the ultimate goal was to identify sets of ROIs showing similar peak latencies and also sets of ROIs that peaked consistently earlier than activity in the majority of ROIs in the set of subsequently active regions. To achieve both goals, we set the alpha level to .001, a criterion that has been proven to be neither overly conservative nor too lax, producing replicable results across studies (Simos et al., 2005, Simos, Fletcher, et al., 2007, Simos, Papanicolaou, et al., 2007).
The final set of analyses consisted of partial correlations between reading measures and peak latency–average current in ROIs (and time bins) where significant group differences emerged from previous analyses. Notably, the metric of the degree of regional activation provided by MNE is void of the deviation-from-normality problems to which equivalent current count data is particularly susceptible, permitting the application of linear regression algorithms.
Results
In-Scanner Task Performance
Significant group main effects (controlling for age) were found for performance on the pseudoword reading task, F(2, 119) = 118.17, p < .0001. As shown in Table 2, performance was significantly higher for the NI and ADHD groups as compared to the RD group, whereas the two former groups did not differ from each other. The subgroup of 20 additional poor readers used in the correlational analyses had a significantly lower correct pseudoword reading rate than children in the NI group (p < .0001), although they performed significantly higher than the RD group (p < .002). The four groups (three main groups plus the supplementary group of poor readers) performed comparably on the letter-sound naming task (p < .8).
Table 2.
In Scanner Performance (Mean ± SD of Percentage of Correct Responses in Each Task) for the Four Groups of Participants
| Task | NI | RD | Additional poor readers | ADHD |
|---|---|---|---|---|
| Pseudoword Reading | 85.9 ± 11†# | 49.5 ± 16†§$ | 65.1 ± 17#,$ | 89.5 ± 9§ |
| Letter Sound Naming | 93.1 ± 5 | 93.2 ± 5 | 92.6 ± 6 | 94.4 ± 4 |
Note. NI = nonreading impaired; RD = reading difficulties; ADHD = attention-deficit/hyperactivity disorder. *p < .05.
p < .01.
p < .0001.
MEG Data
The omnibus ANOVA revealed a significant Task × Hemisphere × Time × Area × Group interaction, F(260, 15210) = 2.59, p < .01. Follow-up four-way ANOVAs indicated that the Hemisphere × Time × Area × Group interaction was restricted to the pseudoword task, F(260, 15210) = 2.35, p < .01. These effects were further explored by performing three-way ANOVAs (Time × Hemisphere × Group) separately for each ROI. The initial focus was on differences between the RD and NI groups.
RD Versus NI Groups: Pseudoword Reading Task
Degree of activity
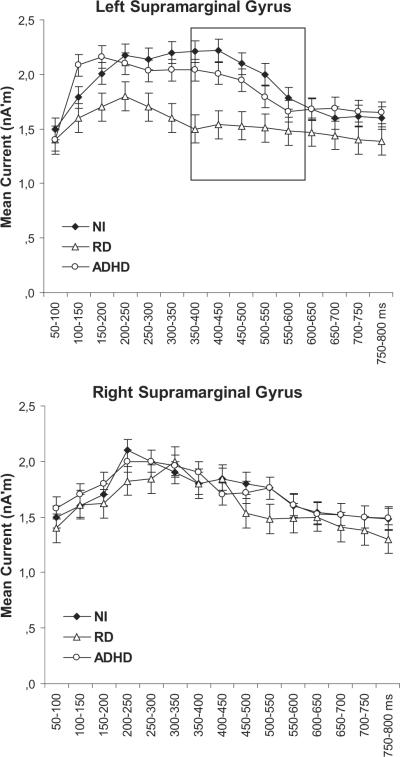
The key finding was that RD participants as a group showed reduced degree of activity in the left SMG as compared with RD students (see Table 3 for details). Follow-up two-way ANOVAs (Hemisphere × Group) indicated that his effect was restricted to late activity (between 350 and 650 ms after stimulus onset; see Figure 1, top row). The two groups showed comparable degree of activity during this latency window in the right hemisphere. Greater degree of late activity in STS for the NI as compared with the RD group was also found, bilaterally. A trend in the same direction, which failed to reach the stringent criterion (α= .01), was found for late activity in STG, bilaterally. Further, the degree of hemisphere asymmetry differentiated the two groups in the SMG and ANG, where significant leftward asymmetries were restricted to the NI group.
Table 3.
Significant Analysis of Variance Results: Group Differences in the Degree and Latency of Regional Activity
| Degree of activity |
||
|---|---|---|
| Effect | ROI | Latency window |
| Hem × Time × Group | SMG, F(13, 1274) = 3.50, p < .0001 | 350–650 ms |
| NI > RD | (LH), F(1, 98) = 5.86, p < .01 | |
| Time × Group | STS, F(13, 1274) = 1.99, p < .01 | 450–650 ms |
| NI > RD | (LH/RH) (p < .04) | |
| Time × Group | STG, F(13, 1274) = 2.04, p < .01 | 350–550 ms |
| NI > RD | (LH/RH) (p < .04) | |
| Peak latency | ||
| NI > RD | IFG (LH), F(l, 98) = 5.39, p < .02 | |
| NI > RD | STG (LH), F(l, 98) = 5.89, p < .02 | |
| NI > ADHD | IFG (RH), F(1, 68) = 5.83, p < .01 | |
| NI > ADHD | Fusiform (RH), F(1, 68) = 5.16, p < .02 | |
| NI < ADHD | LOC (LH), F(1, 68) = 7.02, p < .01 |
Note. Results were restricted to the pseudoword task. ROI = Region of Interest; SMG = supramarginal gyrus; NI = nonreading impaired; RD = reading difficulties; STS = superior temporal sulcus; STG = superior tempored gyrus; IFG = inferior frontal gyrus; ADHD = attention-deficit/hyperactivity disorder; LOC = ; LH = left hemisphere (Hem); RH = right hemisphere.
Figure 1.
Time course of estimated neurophysiological activity (nano-Amperes) associated with pseudoword reading in the supramarginal and angular gyri for the nonreading impaired (NI; n = 50), reading difficulties (RD; n = 50), and attention-deficit/hyperactivity disorder (ADHD) participants (n = 20). Stimulus onset is at 0 ms. Time windows of significant group differences are marked by squares. Vertical bars represent standard deviation.
Laterality index
Given that hemisphere and group effects were found only in the SMG and ANG, average late activity in these ROIs was used to calculate a laterality index (LI) for each participant based on the formula: (LH activity − RH activity)/(LH activity + RH activity), which was then used to group participants into left dominant (LI > .03), and nonleft dominant (LI < .03). The RD and NI groups differed in the relative proportions of the two LI subgroups, χ2(3, N = 90) = 8.32, p < .004. There was a significantly higher proportion of left-dominant than nonleft-dominant participants in the NI group (76% vs. 24%), χ2(1, N = 46) = 13.52, p < .0001, although the corresponding relative proportions did not differ significantly among children in the RD group (48% vs. 52%, p > .7). The two subgroups of RD children did not differ on age, gender, ethnicity, handedness, or on any of the psychoeducational measures obtained in the study (see Table 1).
Inferior frontal region
In view of previous reports of differential (left) inferior frontal activity in RD students (e.g., Cao et al., 2006; Hoeft et al., 2007; B. A. Shaywitz et al., 2002), we explored further the apparent lack of such effects in our sample. Specifically, we searched for factors associated with enhanced left-IFG activity in RD children by grouping them into those (n = 22) who showed activity greater than the median normative left-IFG activity (based on the distribution of scores within the NI group) and those who showed less than median activity (n = 24). Although the two subgroups showed nearly identical scores on reading and spelling tests (including inscanner pseudoword reading performance, p > .6 in all cases), enhanced left-IFG activity was significantly associated with age, with younger RD students (M = 126.7 ± 19 months), showing more than median activity than older students (M = 141.7 ± 20 months), F(1, 45) = 8.22, p < .006). This finding was further corroborated by the observation of a moderate negative correlation between age and left-IFG activity in the RD group (r = −.42, p < .003)—revealing a decreasing trend with age—whereas a much smaller, nonsignificant age trend was found for the NI group (r = −.16, p > .3).
Peak latency
On average, activity peaked earlier in the RD as compared to the NI group in the left IFG (362 ± 135 ms vs. 440 ± 150 ms) and left STG, (289 ± 91 ms vs. 341 ± 120 ms) as shown in Table 3. There were no other differences in peak latency between the two groups.
Controls Versus RD: Letter-Sound Naming Task
There were no significant main effects or interactions involving group on degree or latency of activity in any ROI. Both groups demonstrated significant leftward asymmetry in the degree of activity when performing this task in the SMG and fusiform gyrus.
Controls Versus ADHD: Pseudoword Reading Task
There were no significant main effects or interactions involving group in any ROI. Both groups showed significant leftward asymmetries in the degree of activity in STS, SMG, ANG, and the fusiform gyrus. Neurophysiological activity peaked earlier in the group of ADHD participants as compared to the NI group in the right IFG (285 ± 110 ms vs. 391 ± 168 ms) and right fusiform gyrus (240 ± 67 ms vs. 290 ± 90 ms), and later in the left lateral occipitotemporal cortex (272 ± 95 ms vs. 215 ± 74 ms).
Controls Versus ADHD: Letter-Sound Naming Task
There were no significant main effects or interactions involving group, and both groups demonstrated significant leftward asymmetry in the degree of activity when performing this task in SMG and the fusiform gyrus. No significant main effects of group were noted for peak latency.
Temporal Progression and Profiles of Regional Activity: Pseudoword Reading Task
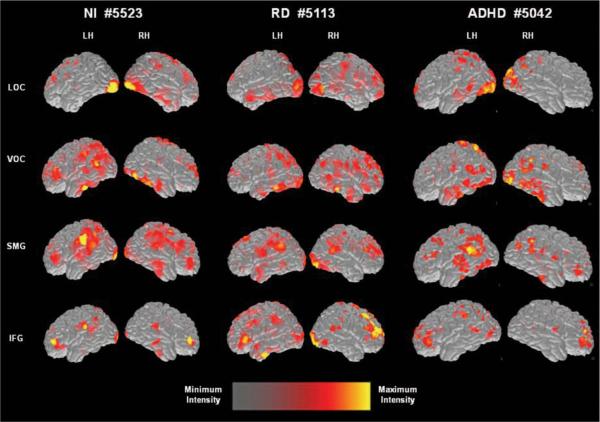
NI group
The left-hand pair of columns in Figure 2 displays active voxels projected on the brain surface of a representative NI participant at different time points. The average spatiotemporal brain activation profile in this group involved early activity in lateral occipitotemporal regions, bilaterally. This was followed after a significant delay by near-simultaneous peaks of activity in the fusiform gyri, ANG, and MTG. Subsequent activity peaks were noted in the STG, STS, SMG, and in the right IFG. Activity in the left IFG peaked last at a significant temporal delay following activity peaks in all other ROIs. Table 5 ranks peak latency values in increasing order for each group.
Figure 2.
Brain activation map snapshots for the pseudoword reading task from three representative participants: a typically developing reader (left-hand pair of columns), a student with reading difficulties (RD; middle pair of columns), and a student with attention-deficit/hyperactivity disorder without RD (right-hand pair of columns). The relative intensity of activated voxels is shown at the bottom of the figure. Each set of images presents activity at a different latency after stimulus onset (at 125, 200, 350, and 500 ms from top to bottom, respectively), in the left hemisphere (LH) and right hemisphere (RH). LOC = lateral occipitotemporal cortex; VOC = ventral occipitotemporal; SMG = supramarginal gyrus; and IFG = inferior frontal gyrus.
Table 5.
Temporal Progression of Regional Magnetic Activity
| NI |
RD |
ADHD |
|||
|---|---|---|---|---|---|
| ROI | Peak latency (ms) | ROI | Peak latency (ms) | ROI | Peak latency (ms) |
| LOC (L) | 215 ± 74 | LOC (L) | 234 ± 100 | LOC (R) | 230 ± 88 |
| (R) | 224 ± 73 | (R) | 246 ± 94* | Fusiform (R) | 240 ± 67 |
| Fusiform (L) | 289 ± 110 | Fusiform (L) | 247 ± 90 | ANG (R) | 237 ± 66 |
| (R) | 290 ± 90 | MTG (L) | 273 ± 96 | MTG (R) | 247 ± 54 |
| ANG (L) | 300 ± 94 | (R) | 262 ± 80 | STS (R) | 260 ± 76 |
| (R) | 275 ± 105 | STS (R) | 277 ± 102 | MTG (L) | 260 ± 65 |
| MTG (L) | 303 ± 108 | STS (L) | 280 ± 96 | SMG (R) | 262 ± 111 |
| (R) | 290 ± 98 | Fusiform (R) | 282 ± 106 | LOC (L) | 272 ± 95 |
| STG (L) | 341 ± 110 | STG (L) | 289 ± 91* | Fusiform (L) | 282 ± 137 |
| (R) | 345 ± 133 | STG (R) | 296 ± 111 | STS (L) | 280 ± 95 |
| STS (L) | 318 ± 111 | ANG (L) | 302 ± 92 | ANG (L) | 282 ± 96 |
| (R) | 322 ± 119 | ANG (R) | 313 ± 98 | STG (R) | 285 ± 91 |
| SMG (L) | 342 ± 108 | SMG (L) | 346 ± 132 | IFG (R) | 347 ± 131 |
| (R) | 312 ± 145 | SMG (R) | 350 ± 127 | SMG (L) | 352 ± 131 |
| IFG (R) | 375 ± 119 | IFG (L) | 359 ± 148 | STG (L) | 370 ± 130 |
| (L) | 440 ± 160 | IFG (R) | 362 ± 167 | IFG (L) | 417 ± 135 |
Note. Horizontal lines separate ROIs that differed significantly in peak latency from earlier peaking ones. Latencies in bracketed ROIs differed significantly from corresponding latencies in each of the ROIs below the horizontal line. RD = reading difficulties; ADHD = attention-deficit/hyperactivity disorder; L = left; R = right; LOC = lateral occipitotemporal cortex; ANG = angular gyrus; STG = superior temporal gyrus; MTG = middle temporal gyrus; STS = superior temporal sulcus; SMG = supramarginal gyrus; IFG = inferior frontal gyrus.
RD group
In this group magnetic activity peaked first in lateral occipitotemporal cortices, the left fusiform gyrus, MTG, right STS, and the right fusiform gyrus. At a significant delay following activity peaks in lateral occipitotemporal cortices and the left fusiform gyrus, activity peaked in the STG and left ANG. Activity in the latter group of ROIs peaked significantly earlier than activity in the left SMG and IFG. Notably there were no significant differences in peak latency between bilateral SMG, left STS, right ANG, and bilateral IFG.
ADHD group
The earliest activity peak was noted in the right lateral occipitotemporal region. Next, activity peaks were noted near simultaneously in a group of diverse right hemisphere areas including the fusiform gyrus, STS, ANG, MTG, SMG, and STG. Left-hemisphere regions in this group of active ROIs included the lateral occipitotemporal region, fusiform gyrus, and STS. After a significant temporal delay, activity peaks were found in the IFG, left STG, and left SMG.
The majority of NI participants demonstrated earlier activity peaks in the left STG and SMG than in IFG (66%), whereas the majority of RD students (60%) showed the opposite trend. The RD and NI groups differed significantly in the relative proportions of participants with early and late prefrontal peaks (ϕ = .25, p < .024). The RD and ADHD groups did not differ on the relative proportions of participants with early and late frontal peaks (ϕ = .29, p > .3; the proportion of late prefrontal cases in the ADHD group was 50%). It should be noted, however, that this trend may have been mediated by gender, given that the proportion of children with early frontal peaks was larger among boys regardless of reading ability status: 53% among NI boys versus 17% among NI girls, χ2(1, N = 50) = 5.25, p < .02, and 68% for RD boys versus. 30% for RD girls, χ2(1, N = 50) = 4.53, p < .04.
Letter-Sound Naming
The relative timing of activity during performance of the letter-sound naming task was generally similar to that observed during the pseudoword reading task. Notably, in the NI and ADHD groups activity in the SMG and STG peaked significantly earlier (302–391 ms and 275–303 ms, respectively, on average) than activity in inferior frontal regions (390–468 ms and 309–384 ms, respectively). In contrast, in the RD group, activity in prefrontal areas (352–420 ms) peaked earlier than activity in the left SMG (430 ms).
Correlations Between Neurophysiological Activity and Reading Ability
Although the three main groups (RD, NI, ADHD) were matched on age, moderate yet significant negative correlations were found between age and achievement scores, as well as between age and degree of activity in the majority of ROIs of interest (both within and across groups). Accordingly, the relationship between degree of activity and achievement scores was explored by controlling for the effects of age on both sets of variables. Initially, and in order to preserve the temporal information that is inherent in the MNE time series data, partial correlation coefficients were computed between estimates of the magnitude of regional neurophysiological activity during the pseudoword reading task for each time window in each of the 11 ROIs and W-J-III Word Attack scores (i.e., the test that most closely resembled the main activation task in this study). These analyses were conducted on participants without ADHD, separately for NI students and for a more inclusive group of poor readers, consisting of all of the students from the RD group plus 20 students with low-average scores described in the Participants section (n = 70). The highest significant (at p < .005, to control for family-wise Type I error) correlation coefficients for each ROI are reported in Table 6.
Table 6.
Significant Pearson Correlation Coefficients Between Degree or Peak Latency of Activity and Woodcock-Johnson Tests of Achievement-III Word Attack Standard Scores
| Degree of activity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Latency window (ms) | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 | 550 |
| NI | |||||||||
| ROI (LH) | |||||||||
| SMG | .44 | ||||||||
| STG | .40 | ||||||||
| ANG | .39 | ||||||||
| IFG | .44 | ||||||||
| Fusiform | .50 | ||||||||
| Peak latency | |||||||||
| Fusiform | −.50 | ||||||||
|
| |||||||||
| RD | |||||||||
| ROI (RH) | |||||||||
| SMG | −.30 | ||||||||
| STG | −.35 | ||||||||
| Fusiform | −.30 | −.38 | |||||||
| MTG | −.31 | ||||||||
| IFG | −.30 | ||||||||
| Peak latency | |||||||||
| IFG | −.37 | ||||||||
Note. ROI = Region of Interest; LH = left hemisphere; SMG = supramarginal gyrus; RH = right hemisphere; STG = superior temporal gyrus; ANG = angular gyrus; IFG = inferior frontal gyrus; MTG = middle temporal gyrus.
Results of correlational analyses were generally consistent with the conclusions of the findings on group differences in the degree of activity. For NI readers, significant positive correlations were obtained between Word Attack scores and degree of activity in the left SMG, STG, ANG, IFG, and fusiform gyrus. A significant negative correlation was also found between peak latency in the fusiform gyrus and Word Attack scores.
For the group of poor readers, only negative correlations were found between Word Attack scores and five right hemisphere regions: SMG, STG, MTG, IFG, and fusiform gyrus. Among peak latency measures, the only significant correlate of Word Attack scores was peak IFG latency in the right hemisphere. Incidentally, coefficients between MEG measures and Letter-Word Identification scores were generally slightly smaller in absolute value than those with Word Attack scores for both groups, whereas none of the correlations with Verbal IQ or Performance IQ scores were significant.
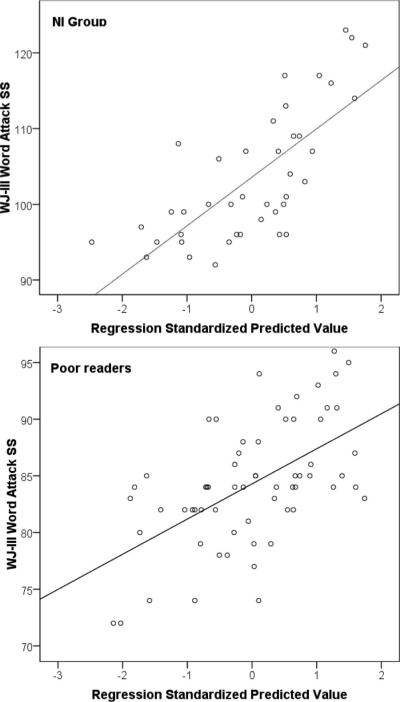
Finally, each of the MNE measures associated with significant partial correlations listed above for each group were entered into hierarchical linear multiple regression analyses as predictors of W-J-III Word Attack scores, after controlling for age. For NI readers, degree of activity measures accounted for 40% of the variance in reading scores (adjusted R2 = .40), F(5, 44) = 3.65, p < .005. When added in the third step (after age and degree of activity measures), peak latency in the fusiform gyrus accounted for an additional 12% of total variance in Word Attack scores, which represented a significant improvement of the model, F(1, 43) = 6.05, p < .009. The regression plot in Figure 3 shows a rather linear relationship between the combined ERF measures and Word Attack scores. It is interesting to note that none of the degree of activity measures was associated with significant regression coefficients in this group, probably due to considerable shared variance among them. In contrast, the regression coefficient of peak latency in the fusiform gyrus was significant, β = −.40, t(39) = −2.84, partial r = −.35.
Figure 3.
Regression plots of Word Attack scores over a linear combination of MEG variables for typically achieving (upper panel) and poor readers (lower panel). For students with average to above average reading achievement scores (N = 50) significant positive predictors of decoding ability included degree of activity in left hemisphere regions (SMG, STG, ANG, IFG, and fusiform gyri) and peak latency in the left fusiform gyrus. Conversely, increased activity in right hemisphere regions (SMG, MTG, IFG, and fusiform gyri) was associated with lower Word Attack scores among poor readers (N = 70).
For the group of poor readers, degree of activity measures accounted for 32% of the variance in Word Attack, adjusted R2 = .44, F(6, 62) = 6.85, p < .0001. There were only two significant independent (negative) predictors of reading scores: degree of late activity in the right SMG, β = −.32, t(56) = −2.80, p < .007, partial r = −.30, and degree of late activity in the right fusiform gyrus, β = −.28, t(56) = −2.14, p < .036, partial r = −.23. Adding IFG peak latency did not improve the regression model.
Discussion
This study examined reading skill-related variability in patterns of neurophysiological activity on a large sample of children while controlling for age and performance IQ. The study had four main goals, which were addressed as follows. First, our data concur with earlier smaller-scale MEG studies in demonstrating that the most notable differences between students who experience difficulties in learning to read—not accountable by ADHD—and typical readers occur in temporoparietal regions. Specifically, greater degree of neurophysiological activity was found in the left SMG and ANG for the NI group and greater degree of activity in the corresponding right hemisphere regions for the RD group. Reduced activity for children with RD was also found in the STG, extending into the banks of the STS, bilaterally. Moreover, and in agreement with previous MEG studies, using phonological decoding tasks (Simos et al., 2000; Simos, Fletcher, et al., 2007), the hemispheric laterality profile for activity in these regions was clearly different between groups: Whereas NI children demonstrated strong leftward asymmetry, RD children generally showed bilaterally symmetric degree of activity in the SMG and ANG. These effects were noted as early as 350 ms after stimulus onset and persisted for up to approximately 650 ms.
In terms of the second goal of the study, hypoactivation in posterior temporal and inferior parietal regions was found to be independent of the presence of ADHD, given that children in the NI and ADHD groups (who scored in the average to above-average range on word-level reading tests), generally presented with similar activation profiles.
The activation profiles associated with the RD and NI groups also differed in their timing features (thus addressing the third goal of the study). For children in the NI group, there was a regular progression of activity from lateral occipitotemporal cortex to the fusiform gyri, in both hemispheres. This activity was temporally distinct from (subsequent) activity peaks in temporal and inferior parietal regions. Activity in the left IFG peaked significantly later than activity in temporal and inferior parietal cortices. In contrast, the pattern of temporal progression in regional activity was not consistently present among children with RD: We did not observe a clear temporal differentiation between activity peaks in the left fusiform gyrus and other temporal and inferior parietal areas or between the left SMG and IFG. The lack of temporal differentiation between left fusiform and inferior frontal regions was also observed among students with ADHD, and therefore does not seem to be characteristic of developmental difficulties in reading acquisition (or related to increased subjective difficulty in performing the task given that NI and ADHD students performed comparably on the pseudoword reading task). An interesting finding that may account for previous reports (e.g., Simos et al., 2005) is that the apparent aberrant temporal profile of regional activation was more prominent among boys regardless of reading ability.
With respect to the fourth main goal of the study, differences between the groups of NI and RD children in the degree of regional activity were restricted to the more demanding pseudoword reading task and not found during performance of an easier task that required conversion of single letters into phonemes. Both tasks had identical response requirements (oral pronunciation of responses), although the pseudoword reading task required production of more complex utterances than the (single) letter-sound naming task. If reduced demands for phonological–articulatory complexity of the response underlay the lack of group differences in regional activation during the letter-sound naming task, then one may have expected to find group differences in frontal (motor, premotor, and inferior frontal–opercular regions), during the more complex pseudoword reading task, but this was clearly not the case in the present study. Instead, differences between the RD and NI groups were found mostly in temporal and temporoparietal regions, a finding consistent with the notion that functional disruptions in the brain mechanism for reading in RD become apparent only under increased demands for phonological processing (Cao et al., 2006; S. E. Shaywitz et al., 1998).
With respect to the fifth goal of the study, multiple regression analyses indicated that, after controlling for age, the degree of neurophysiological activity in several left-hemisphere regions jointly account for individual differences in phonological decoding scores among typical readers. Estimates of the degree of late activity (between 250 and 450 ms after the onset of the pseudoword stimuli) in the left SMG, ANG, STG, and IFG, and early activity in the left fusiform gyrus (between 150 and 200 ms) along with peak latency of left fusiform activity accounted for 50% of the variance in standard pseudoword decoding scores. The pattern of regression results for the group of poor readers was dramatically different, revealing significant negative associations between degree of activity in occipitotemporal, temporoparietal, and inferior frontal regions in the right hemisphere. The latter finding lends further support to the notion that relying on a right hemisphere temporoparietal network of brain regions for reading is a correlate of developmental reading difficulties. Failure to identify loci of brain activity that were positively associated with reading performance in RD children is also consistent with previous fMRI findings, and it may reflect underdeveloped functional organization of cortical networks to support reading (Hoeft et al., 2007; Pernet, Anderson, Paulesu, & Demonet, 2009).
To summarize, this study demonstrated signs of aberrant brain function in children with RD, essentially involving all of the key components of the putative brain circuit for word-level reading (e.g., Jobard et al., 2003; Joseph et al., 2001; Pugh, Mencl, Jenner, et al., 2000). Whereas direct evidence of reduced neurophysiological signaling during decoding was found for predominantly left temporoparietal areas, there was also indirect (correlational) evidence of aberrant contribution of the left fusiform gyrus to reading skill in children with RD. In addition, our findings suggest that aberrant brain activation patterns are largely dependent on demands for phonological decoding and independent from commonly encountered comorbidities between RD and ADHD.
The Left Temporoparietal Cortex
The most robust finding that emerged from the current data set was reduced late activity in left hemisphere posterior temporal and inferior parietal sites (STG, SMG, and ANG) for children with RD compared to NI readers. These regions have long been postulated to serve as crucial components of the brain mechanism for reading and the prevailing notion among researchers is that they host neurophysiological processes, which are in some manner involved in phonological processing of print.
In adult skilled readers there is direct evidence implicating both the ANG and SMG, in the left hemisphere, as indispensable components of the reading circuit, including recent large-scale lesion (Philipose et al., 2007) and electrocortical stimulation studies (Roux et al., 2004). Functional neuroimaging studies corroborate the importance of inferior parietal cortices for reading. For instance, Xu et al. (2001) reported selective activations by pseudowords within the SMG, and Booth et al. (2003) found significant correlations between the magnitude of the hemodynamic response in the SMG and ANG with performance on visual rhyming tasks. Thus, different studies that use fMRI, MEG, and other imaging modalities show that inferior parietal cortices host neurophysiological processes involved in converting print to sound, rather than in accessing stored visual word forms.
Furthermore, there is ample evidence linking impairments in phonological processing of spoken and written language to acquired damage to the posterior portion of the left STG (Beauvois &Dérouesné, 1979; Caplan, Gow, & Makris, 1995). Results of hemodynamic brain-imaging studies are also consistent with a role of posterior BA 22 sites in subword level phonological processing and analysis (Jacquemot, Pallier, LeBihan, Dehaene, & Dupoux, 2003; Majerus et al., 2005; Scott, Blank, Rosen, & Wise, 2000; Specht et al., 2003). It should be noted, however, that competing views suggest a role for both the STG and ANG in lexical access, storage, or both (Binder, Westbury, McKiernan, Possing, & Medler, 2005; Hickok & Poeppel, 2000; Mechelli et al., 2007; Mustovic et al., 2003; Wise et al., 2001).
Although findings of reduced hemodynamic response in the left STG (extending into the banks of STS (B. A. Shaywitz et al., 2002) and ANG (Temple et al., 2001; see also Eden & Zeffiro, 1998) in children with RD are relatively sparse, several studies have reported underactivation of the left SMG (Cao et al., 2006; Hoeft et al., 2007; Maisog, Einbinder, Flowers, Turkeltaub, & Eden, 2008; van der Mark et al., 2009). Recent MEG studies have repeatedly reported reduced activation of the left STG, SMG, and ANG (or trends in that direction for certain ROIs) during pseudoword naming in kindergarten students who were at risk for developing reading problems (Simos et al., 2005) and in older students with documented reading difficulties (Simos, Fletcher, et al., 2007). As in the present study, these MEG studies reported bilaterally symmetric activity in superior temporal and inferior parietal regions for students with RD and for at-risk students. In the most recent MEG study, using a similar task and the same high-density neuromagnetometer system (Simos, Fletcher, et al., 2007), reduced activity in the STG was found bilaterally, as in this study, but in contrast to earlier MEG reports (Simos et al., 2000, 2002), where group differences were restricted to the left STG and RD students showed hyperactivation of the right STG. We did, however, find evidence in this study that reliance on temporoparietal regions in the right hemisphere by children with RD may have detrimental effects for reading ability, as indicated by significant negative activity-skill correlations. The opposite trend (positive relationship) was found between corresponding left-hemisphere areas and reading skill among NI. It is important to note that the degree of hemispheric asymmetry in the degree of temporoparietal activation, per se, was not a predictor of reading skill (and was not affected by demographic or other cognitive measures). Consistent with this conclusion is the finding that significant hemispheric asymmetries in temporoparietal cortex in both NI and ADHD groups were found regardless of task difficulty.
Failure to find enhanced right temporoparietal activity (especially in the STG) among children with RD may be due to differences in sample and task characteristics between the present and earlier MEG studies (Simos et al., 2000, 2002). One possibility, which is not supported by the present data, is that the RD sample included children who had significant ADHD. Thus, ADHD alone, in the absence of RD, was not associated with an activation profile associated with RD. Another possibility is that our initial MEG studies included students with more severe reading disabilities (defined by word-level reading achievement scores below the 13th percentile) whereas the majority of NI students were scoring in the high average to superior range. This account did not receive support from additional exploratory analyses performed on the current data set. Thus, using stricter selection criteria, closely matching those used in our earlier studies, did not affect the relative proportion of left- and nonleft-dominant (for temporoparietal activity) children with RD.
An alternative account for the reduced tendency of children with RD to rely on right hemisphere temporoparietal regions during pseudoword reading in this study implicates task differences with our initial MEG studies (Simos et al., 2000, 2002), where we had employed a pseudoword rhyming task. In these studies children were asked to silently decode a pseudoword and briefly maintain its phonological representation in short-term memory to be subsequently compared with either a rhyming (e.g., kume-noom) or a nonrhyming pseudoword (e.g., kume-wote). Evoked magnetic fields were recorded to the first pseudoword of each pair. In addition to increased phonological decoding difficulty (four-to-five letter pseudowords were used in the earlier studies as compared to three-letter pseudowords in this study), the rhyming task posed potentially significant demands for phonological short-term memory. These demands were negligible in this study. Assuming that this function depends on both temporoparietal and inferior frontal cortices (see Jonides et al., 1998; Maestu et al., 2005; Rypma & D'Esposito, 1999; Vallar, Di Betta, & Silveri, 1997), then increased task demands on the phonological loop may have been more taxing for the underlying brain mechanism and thus inflating group differences in regional activity. Such differences would appear to be more pronounced in temporoparietal areas given that the lower density neuromagnetometer and source-modeling technique used in these earlier studies were relatively insensitive to prefrontal activity. That differences between groups with RD and NI were task dependent in this study (i.e., observed only in the context of the pseudoword reading task and not the less demanding letter-sound naming task) supports the notion that differences between the present and earlier MEG studies from our group can be attributed, at least to some extent, to task differences between studies. It should also be noted that previous MEG studies, which relied on visual, anatomical characterization of dipolar sources, considered STG sources localized in the posterior third of the superior temporal gyrus, whereas reconstruction of the cortical surface inherent to the head modeling procedure used in this study by default treats the entire surface of the gyrus as one ROI.
Ventral Occipitotemporal Cortex (Fusiform Gyrus)
Although direct evidence of generally reduced activity in the left fusiform gyrus in children with RD was not found in this study (e.g., Cao et al., 2006; Hoeft et al., 2007; van der Mark et al., 2009), there were clear indications that both the degree and latency (potentially an index of neural efficiency) of activity in this region are closely associated with decoding skill during normal reading development. One of the few large-scale scales on RD to date (S. E. Shaywitz et al., 2003) also reported a significant positive correlation between BOLD signal strength during pseudoword reading and Word Attack scores, without finding generally reduced activity in this region in the RD as compared to the NI group. Although the importance of occipitotemporal cortices (the left fusiform gyrus in particular) both in skilled and in reading development is well established, as discussed in more detail in the next paragraph, degree of engagement of this region in reading during development may be affected by general cognitive ability. This notion may explain the lack of reading group differences in our study and in S. E. Shaywitz et al. (2003), because both studies used groups comparable in PIQ versus Simos et al. (2007a), where children with RD had significantly lower PIQ and VIQ on average than NI participants. This hypothesis is also consistent with the finding that significantly reduced left occipitotemporal activation (as compared to adults who never experienced reading difficulties) was restricted to the group of persistent poor readers: adults who demonstrated persistent reading difficulties since childhood and overall lower FSIQ. Task difficulty and stimulus complexity may also affect degree of occipitotemporal engagement, given that the majority of fMRI studies that report underactivation of the left fusiform gyrus have used rhyming tasks.
There is strong evidence that the ventral occipitotemporal region plays a crucial role in the (mature) brain mechanism responsible for storing (or automatically retrieving) orthographic information (knowledge regarding frequently occurring graphemic patterns) (McCandliss, Cohen, & Dehaene, 2003). Neuroimaging studies and studies using intracranial recordings have reported stronger or more extensive activation in this region to words and word-like nonwords than to consonant letter strings or nonsense characters (Cohen et al., 2000, 2002; Tarkiainen, Helenius, Hansen, Cornelissen, & Salmelin, 1999). Perhaps the strongest evidence that the fusiform gyrus is an indispensable component of the mechanism for reading aloud words and pseudowords comes from lesion studies that document severe forms of alexia characterized by “letter-by-letter reading,” following damage to this region (Binder & Mohr, 1992; Cohen et al., 2000; Philipose et al., 2007). Moreover, MEG data obtained from skilled adult readers in the context of a lexical-decision task is consistent with the notion that ventral occipitotemporal cortices are involved in storing or gaining access to abstract orthographic representations (Simos et al., 2009). The latter may take the shape of “word forms” (Kronbichler et al., 2004) or simply familiar letter combinations regardless of lexicality (Binder, Medler, Westbury, Liebenthal, & Buchanan, 2006).
The Left Inferior Frontal Gyrus
In this data set, we did not find reliable group differences (RD vs. NI) in the degree of inferior frontal activity, although a trend toward reduced prefrontal activity (extending across inferior and middle frontal regions) for the RD group could be detected. Failure to find significant group differences in this region is not surprising given the inconsistency of previous reports with some studies showing increased frontal activation in RD children (Richards et al., 2002; Simos, Fletcher, et al., 2007; Simos, Papanicolaou, et al., 2007), while others showing effects in the opposite direction (Cao et al., 2006; B. A. Shaywitz et al., 2002) or lack of significant differences (Hoeft et al., 2007; Maisog et al., 2008; Temple et al., 2001). Closer examination of the current data suggest that this phenomenon may be mediated by age: Whereas, younger children with RD showed greater inferior frontal activity than older students with reading difficulties, no significant age effect was found among NI readers. This possibility is supported by the finding that increased inferior frontal activation had subsided by the end of Grade 1 in children who were at a high risk for developing reading difficulties (Simos et al., 2005). This age-related account is also consistent with studies that reported reduced left IFG activation in adults with persistent RD during tasks that pose heavy demands for phonological decoding (Georgiewa et al., 1999; Maisog et al., 2008). Given that tasks used in previous studies vary widely on several important aspects, it is important to examine children varying on reading skill on tasks that manipulate articulatory, memory demands, and overall difficulty level, to assess the relative contribution of these factors on the degree of task-related prefrontal activation during reading.
Conclusions and Implications for Future Studies
Anatomically, the network of left-hemisphere regions that support word-level reading uses corticocortical connections that can be imaged directly in vivo with MRI tractography methods (e.g., Lawes et al., 2008). Neuronal inputs from the occipital cortices travel along the inferior longitudinal fasciculus to the left fusiform gyrus, which is in turn connected to the SMG and ANG through the posterior-inferior branches of the arcuate fasciculus. Inferior parietal regions connect to posterior temporal cortices (mainly the posterior portion of the STG) through smaller bundles that are part of the arcuate fasciculus (Catani, Jones, & ffytche, 2005). Direct fiber tracts linking both posterior temporal and inferior parietal areas with inferior frontal regions have also been documented. It is interesting to note that tractography studies have demonstrated hemispheric asymmetries in the volume of the arcuate fasciculus in the majority (approximately 80%) of typically developing adults (Catani et al., 2007; Powell et al., 2006). The presence of a direct anatomical connection between ventral occipitotemporal and inferior frontal cortices has also been discovered, and predominant signaling along this pathway may in part account for the tendency of, at least some, children with RD to show early activation of frontal opercular cortices in this study. Future studies may take advantage of information in regard to the pattern of anatomical connections between brain regions believed to be critical for reading, and MEG data regarding the sequence of regional activity peaks in real time, to explore parallel patterns of functional interdependencies between occipitotemporal, temporoparietal, and inferior frontal regions that enable this function. Studies that simultaneously coregister structural and functional imaging modalities may be especially useful in elucidating these structure–function relations.
Table 4.
Significant Analysis of Variance Results: Hemisphere (Hem) Asymmetries for Degree of Activity
| Pseudoword reading | ||
|---|---|---|
| Effect | ROI | Latency window |
| Hem × Time × Group | LOC (NI & RD groups), F(1, 98) = 9.52, p < .003 | 350–650 ms |
| LH > RH | NI & ADHD groups, F(1, 49) = 28.55, p < .0001 | |
| Hem × Time × Group | ANG, F(13, 1274) = 2.03, p < .01 | 350–500 ms |
| LH > RH | NI & ADHD groups, F(1, 49) = 12.42, p < .001 | |
| LH > RH | STS (all groups), F(1, 98) = 13.14, p < .0001 | 450–650 ms |
| LH > RH | Fusiform (all groups), F(1, 98) = 4.59, p < .03 | 100–700 ms |
| LH > RH | LOC (NI & RD groups), F(1, 98) = 9.52, p < .003 | 100–700 ms |
| LH > RH | IFG (NI & RD groups), F(1, 98) = 7.54, p < .007 | 100–800 ms |
| Letter-Sound Naming | ||
| LH > RH | SMG (all groups; p < .002) | 100–800 ms |
| LH > RH | Fusiform (all groups; p < .006) | 100–800 ms |
Note. ROI = Region of Interest; LH = left hemisphere; RH = right hemisphere; SMG = supramarginal gyms; NI = nonreading impaired; ADHD = attention-deficit/hyperactivity disorder; ANG = angular gyms; STS = superior temporal sulcus; LOC = lateral occipitotemporal cortex; IFG = inferior
Acknowledgments
This research was supported in part by Grant P50 HD052117 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 & 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Beauvois MF, Dérouesné J. Phonological alexia: Three dissociations. Journal of Neurology, Neurosurgery and Psychiatry. 1979;42:1115–1124. doi: 10.1136/jnnp.42.12.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. NeuroImage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Mohr JP. The topography of callosal reading pathways. A case-control analysis. Brain. 1992;115:1807–1826. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience. 2005;17:905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Blachman BA. Early intervention and phonological awareness: A cautionary tale. In: Blachman BA, editor. Foundations of reading acquisition and dyslexia: Implications for early intervention. Erlbaum; Mahwah, NJ: 1997. pp. 409–430. [Google Scholar]
- Bonafina MA, Newcorn JH, McKay KE, Koda VH, Halperin JM. ADHD and reading disabilities: A cluster analytic approach for distinguishing subgroups. Journal of Learning Disabilities. 2000;33:297–307. doi: 10.1177/002221940003300307. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Relation between brain activation and lexical performance. Human Brain Mapping. 2003;19:155–169. doi: 10.1002/hbm.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Bitan T, Chou T-L, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Gow D, Makris N. Analysis of lesions by MRI in stroke patients with acoustic–phonetic processing deficits. Neurology. 1995;45:293–298. doi: 10.1212/wnl.45.2.293. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin M, Husain M, Pugliese L, Mesulam M, Murray R, Jones DK. Symmetries in human brain language pathways correlate with verbal recall. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Faraone SV, Biederman J, Tsuang MT. Diagnostic accuracy of the Child Behavior Checklist scales for attention-deficit hyperactivity disorder: A receiver-operating characteristic analysis. Journal of Consulting and Clinical Psychology. 1994;62:1017–1025. doi: 10.1037/0022-006X.62.5.1017. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H. The anatomic basis of pure alexia. Neurology. 1983;33:1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Anatomie des centres nerveux. Vol. 1. Rueff et Cie; Paris, France: 1895. [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Zeffiro TA. The visual deficit theory of developmental dyslexia. NeuroImage. 1996;4:108–117. doi: 10.1006/nimg.1996.0061. [DOI] [PubMed] [Google Scholar]
- Eden GF, Zeffiro TA. Neural systems affected in developmental dyslexia revealed by functional neuroimaging. Neuron. 1998;21:279–282. doi: 10.1016/s0896-6273(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Hopf JM, Knab R, Glauche V, Kaiser WA, Blanz B. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Greenblatt SH. Subangular alexia without agraphia or hemianopsia. Brain & Language. 1976;3:229–245. doi: 10.1016/0093-934x(76)90019-5. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M. MNE Software User's Guide. v. 2.5 Athinoula A. Martinos Center for Biomedical Imaging; Charlestown: Massachusetts: 2006. [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: Minimum norm estimates. Medical & Biological Engineering & Computing. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Henderson V. Anatomy of posterior pathways in reading: A reassessment. Brain & Language. 1986;29:119–133. doi: 10.1016/0093-934x(86)90037-4. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, Gabrieli JD. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Science of the United States of America. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemot C, Pallier C, LeBihan D, Dehaene S, Dupoux E. Phonological grammar shapes the auditory cortex: A functional magnetic resonance imaging study. Journal of Neuroscience. 2003;23:9541–9546. doi: 10.1523/JNEUROSCI.23-29-09541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Willis CR. The role of parietal cortex in verbal working memory. Journal of Neuroscience. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Noble K, Eden G. The neurobiological basis of reading. Journal of Learning Disabilities. 2001;34:566–579. doi: 10.1177/002221940103400609. [DOI] [PubMed] [Google Scholar]
- Kamada K, Sawamura Y, Takeuchi F, Kuriki S, Kawai K, Morita A, Todo T. Expressive and receptive language areas determined by a non-invasive reliable method using functional magnetic resonance imaging and magnetoencephalography. Neurosurgery. 2007;60:296–305. doi: 10.1227/01.NEU.0000249262.03451.0E. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: Evidence from a parametric fMRI study. NeuroImage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Laasonen M, Lehtinen M, Leppämäki S, Tani P, Hokkanen L. Project DyAdd: Phonological processing, reading, spelling, and arithmetic in adults with dyslexia or ADHD. Journal of Learning Disabilities. 2010;43:3–14. doi: 10.1177/0022219409335216. [DOI] [PubMed] [Google Scholar]
- Lawes IN, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, Clark CA. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. NeuroImage. 2008;39:62–79. doi: 10.1016/j.neuroimage.2007.06.041. [DOI] [PubMed] [Google Scholar]
- Liberman AM. Why is speech so much easier than reading? In: Hulme C, Malatesha RM, editors. Reading and spelling: Development and disorders. Erlbaum; Mahwah, NJ: 1998. [Google Scholar]
- Lukatela G, Turvey MT. Reading in two alphabets. American Psychologist. 1998;53:1057–1072. doi: 10.1037//0003-066x.53.9.1057. [DOI] [PubMed] [Google Scholar]
- Maestu F, Simos PG, Campo C, Paul N, Capilla A, Fernandez S, Ortiz T. Prefrontal brain magnetic activity: Effects of memory task demands. Neuropsychology. 2005;19:301–308. doi: 10.1037/0894-4105.19.3.301. [DOI] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Majerus S, Linden MV, Collette F, Laureys S, Poncelet M, Degueldre C, Salmon E. Modulation of brain activity during phonological familiarization. Brain & Language. 2005;92:320–331. doi: 10.1016/j.bandl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, Mechelli A, Frith U, Price CJ. More than words: A common neural basis for reading and naming deficits in developmental dyslexia? Brain. 2005;128:261–267. doi: 10.1093/brain/awh340. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Josephs O, Lambon Ralph MA, McClelland JL, Price CJ. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Human Brain Mapping. 2007;28:205–217. doi: 10.1002/hbm.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JE, Tepley N. Two dimensional inverse imaging (2DII) of current sources in magnetoencephalography. Brain Topography. 2000;12:201–217. doi: 10.1023/a:1023441924015. [DOI] [PubMed] [Google Scholar]
- Mustovic HK, Scheffler F, Di Salle F, Esposito JG, Neuhoff JG, Hennig J, Seifritz E. Temporal integration of sequential auditory events: Silent period in sound pattern activates human planum temporale. NeuroImage. 2003;20:429–434. doi: 10.1016/s1053-8119(03)00293-3. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Sarkari S, Pataraia E, Maggio WW. Magnetoencephalography: A non-invasive alternative to the Wada procedure. Journal of Neurosurgery. 2004;100:867–876. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- Pennington BF, McGrath LM, Rosenberg J, Barnard H, Smith SD, Willcutt EG, Olson RK. Gene × Environment interactions in reading disability and attention-deficit/hyperactivity disorder. Developmental Psychology. 2009;45:77–89. doi: 10.1037/a0014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet C, Anderson J, Paulesu E, Demonet JF. When all hypotheses are right: A multifocal account of dyslexia. Human Brain Mapping. 2009;30:2278–2292. doi: 10.1002/hbm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipose LE, Gottesman RF, Newhart M, Kleinman JT, Herskovits EH, Pawlak MA, Hillis AE. Neural regions essential for reading and spelling of words and pseudowords. Annals of Neurology. 2007;62:481–492. doi: 10.1002/ana.21182. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Duncan JS. Hemispheric asymmetries in language-related pathways: A combined functional MRI and tractography study. NeuroImage. 2006;32:388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, Gore JC. The angular gyrus in developmental dyslexia: Task-specific differences in functional connectivity within posterior cortex. Psychological Science. 2000;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Richards TL, Berninger VW, Aylward EH, Richards AL, Thomson JB, Nagy WE, Abbott RD. Reproducibility of proton MR spectroscopic imaging (PEPSI): Comparison of dyslexic and normal-reading children and effects of treatment on brain lactate levels during language tasks. American Journal of Neuroradiology. 2002;23:1678–1685. [PMC free article] [PubMed] [Google Scholar]
- Roux FE, Lubrano V, Lauwers-Cances V, Trémoulet M, Mascott CR, Démonet JF. Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients. Brain. 2004;127:1796–1810. doi: 10.1093/brain/awh204. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: Effects of memory load and individual differences. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvas J. Basic mathematical and electromagnetic concepts of the biomagnetic problem. Physics in Medicine and Biology. 1987;32:11–22. doi: 10.1088/0031-9155/32/1/004. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Gore JC. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biological Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, Papanicolaou AC. Dyslexia-specific brain activation profile become normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Denton CA, Papanicolaou AC. Altering the brain circuits for reading through intervention: A magnetic source imaging study. Neuropsychology. 2007;21:485–496. doi: 10.1037/0894-4105.21.4.485. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley-Marshall RL, Francis DJ, Castillo EM, Papanicolaou AC. Early development of neurophysiological processes involved in normal reading and reading disability. Neuropsychology. 2005;19:787–798. doi: 10.1037/0894-4105.19.6.787. [DOI] [PubMed] [Google Scholar]
- Simos PG, Papanicolaou AC, Breier JI, Fletcher JM, Foorman BR, Bergman E, Papanicolaou AC. Brain activation profiles in dyslexic children during nonword reading: A magnetic source imaging study. Neuroscience Letters. 2000;290:61–65. doi: 10.1016/s0304-3940(00)01322-7. [DOI] [PubMed] [Google Scholar]
- Simos PG, Papanicolaou AC, Fletcher JM, Sarkari S, Denton C. Intensive instruction affects brain magnetic activity associated with reading fluency in children with dyslexia. Journal of Learning Disabilities. 2007;40:37–48. doi: 10.1177/00222194070400010301. [DOI] [PubMed] [Google Scholar]
- Simos PG, Pugh K, Mencl E, Frost S, Fletcher JM, Sarkari S, Papanicolaou AC. Temporal course of word recognition in skilled readers: A magnetoencephalography study. Behavioral Brain Research. 2009;197:45–54. doi: 10.1016/j.bbr.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow CE, Burns MS, Griffin P. Preventing reading difficulties in young children. National Academy Press; Washington, DC: 1998. [Google Scholar]
- Specht K, Holtel C, Zahn R, Herzog H, Krause BJ, Motthagy FM, Huber W. Lexical decision of nonwords and pseudowords in humans: A positron emission tomography study. Neuroscience Letters. 2003;345:177–181. doi: 10.1016/s0304-3940(03)00494-4. [DOI] [PubMed] [Google Scholar]
- Swanson J. School-based assessments and interventions for ADD children. K. C. Publications; Irvine, CA: 1992. [Google Scholar]
- Szymanski MD, Perry DW, Gage NM, Rowley HA, Walker J, Berger MS, Roberts TP. Magnetic source imaging of late evoked field responses to vowels: Toward an assessment of hemispheric dominance for language. Journal of Neurosurgery. 2001;94:445–453. doi: 10.3171/jns.2001.94.3.0445. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122:2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JD. Disrupted neural responses to phonological and orthographic processing in dyslexic children: An fMRI study. Neuroreport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Vallar G, Di Betta AM, Silveri MC. The phonological short-term store-rehearsal system: Patterns of impairment and neural correlates. Neuropsychologia. 1997;35:795–812. doi: 10.1016/s0028-3932(96)00127-3. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmüller J, Brandeis D. Children with dyslexia lack multiple specializations along the visual word form (VWF) system. NeuroImage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, DeFries JC. Understanding comorbidity: A twin study of reading disability and attention-deficit/hyperactivity disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2007;144B:709–714. doi: 10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within “Wernicke”s area'. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Riverside; Itasca, IL: 2001. [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, Theodore W. Conjoint and extended neural networks for the computation of speech codes: The neural basis of selective impairment in reading words and pseudowords. Cerebral Cortex. 2001;11:267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]