Abstract
Experimentally increasing metabolic flux in a pathway in which an essential step is catalyzed by a vitamin-dependent enzyme (a challenge test) has been used in assessing functional vitamin status and elucidating common and alternate metabolic pathways. Conversion of 3-methylcrotonyl CoA to 3-methylglutaconyl CoA in the leucine catabolic pathway is catalyzed by the biotin-dependent enzyme methylcrotonyl-CoA carboxylase (MCC). Marginal biotin deficiency reduces MCC activity and increases urinary excretion of 3-hydroxyisovaleric acid (3HIA) and 3-hydroxyisovaleryl carnitine (3HIA-carnitine) measured in 24-h urine collections. We assessed urinary excretion of 3HIA and 3HIA-carnitine in response to a leucine challenge in humans made progressively biotin deficient by egg white consumption. In 2 cohorts of healthy adults (Study 1: n = 5; Study 2: n = 7) rendered biotin deficient over 28 d, urinary excretion of 3HIA and 3HIA-carnitine in response to a leucine challenge was quantitated weekly for 3 or 4 wk, respectively. In both studies, mean urinary excretion of both 3HIA and 3HIA-carnitine increased >2-fold by d 14 (P < 0.002 for both indicators for both studies). Diagnostically, both indicators were highly sensitive, but diagnostic sensitivities were not superior to those of 24-h excretion of 3HIA and 3HIA-carnitine. These studies provide evidence that urinary excretions of 3HIA and 3HIA-carnitine in response to an oral leucine challenge are early and sensitive indicators of marginal biotin deficiency in humans. The variability of the proportion of leucine catabolites excreted as 3HIA suggests substantial population heterogeneity in the metabolic capacity of the 3HIA-carnitine detoxification pathway.
Introduction
There is increasing evidence that marginal biotin deficiency develops in a substantial proportion of normal human pregnancies in the first trimester (1, 2). Marginal biotin deficiency is teratogenic in a variety of mammalian and nonmammalian species (3–13); thus, there is legitimate concern that biotin deficiency may be a significant human teratogen (14). Such concerns increase the importance of developing robust, valid indicators of marginal biotin deficiency and of understanding the metabolic consequences of biotin deficiency (15).
Challenge tests have been useful in assessing functional vitamin status and elucidating common and alternate metabolic pathways. Established examples include: 1) increased urinary excretion of xanthurenic acid in response to a tryptophan challenge because of reduced activity of the pyridoxine-dependent enzyme, aromatic L-amino acid decarboxylase, in pyridoxine deficiency (16–18); 2) increased urinary excretion of formiminoglutamic acid in response to a histidine challenge because of reduced status of the cosubstrate tetrahydrofolic acid or reduced activity of the coenzyme formiminoglutamic acid transferase in folic acid deficiency (19); and 3) increased plasma concentration of homocysteine due to reduced activity of pyridoxine-dependent enzyme cystathionine-β-synthase in response to a methionine challenge (20, 21).
Conversion of 3-methylcrotonyl CoA to 3-methylglutaconyl CoA in the leucine catabolic pathway is catalyzed in the mitochondria by the biotin-dependent enzyme MCC10. Marginal biotin deficiency reduces MCC activity (12) and leads to a buildup of its substrate, 3-methylcrotonyl CoA, which is converted to 3-hydroxyisovaleryl CoA by the enzyme enoyl-CoA hydratase (22, 23). Because acyl-CoA compounds are compartmentalized in the mitochondria, increased concentrations of 3-methylcrotonyl CoA and 3-hydroxyisovaleryl CoA lead to a disruption of the esterified CoA:free CoA ratio and ultimately to mitochondrial toxicity (24, 25). Detoxification of these metabolic end products and defense of CoA ratios are thought to occur by the transfer of the 3-hydroxyisovaleryl moiety to carnitine forming 3HIA-carnitine (22), transfer of the 3HIA-carnitine across the inner mitochondrial membrane, and release the 3HIA moiety as the free acid. The details and kinetics of this process are not well characterized.
Studies have shown that marginal biotin deficiency causes an increased fasting plasma concentration of 3HIA-carnitine (26) and increased urinary excretion of both 3HIA (27, 28) and 3HIA-carnitine (29) measured in 24-h urinary collections. We hypothesized that the urinary excretion rate of 3HIA and 3HIA-carnitine will increase relative to the 24-h rate in response to a leucine challenge administered to healthy adults rendered marginally biotin deficient. Here, we report confirmation of that hypothesis in 2 cohorts of healthy adults experimentally rendered marginally biotin deficient.
Materials and Methods
Study participants.
The Institutional Review Board of the University of Arkansas approved human research protocols for both studies for medical sciences. Written consent was obtained from each participant at enrollment and consent was assessed intermittently throughout the study as part of the informed consent process.
Selection of leucine dose and urine collection interval.
A dose of leucine suitable for the leucine challenge was established empirically (30). Briefly, the biotin status of 3 healthy adults was altered as follows: 1) marginally biotin deficient (induced by avidin consumption); 2) normal biotin status (mixed general diet); or 3) augmented biotin status (produced by supplementation with 300 μg biotin/d). These marginally biotin-deficient participants consumed a self-selected mixed general diet and, before each meal, drank an avidin solution containing sufficient avidin to bind ~14-fold dietary biotin as determined from 3-d diet records (30). After providing a baseline void, participants consumed 1 of 2 leucine doses suspended in water: either 35 mg/kg, which is about one-half of the estimated daily dietary intake, or 70 mg/kg, which is about equal to the daily dietary intake. To establish the urine collection interval, urine voids subsequent to ingesting the challenge dose were collected at ~2-h intervals for 6 h on d 0 and 14 of avidin consumption. The increase in urinary excretion of 3HIA over baseline lasted for <4 h. Thus, the collection interval was set to 5 h. Maximal increase in 3HIA excretion occurred at 70 mg/kg leucine; this dose was chosen for all subsequent challenges.
Design of Study 1.
Details of this study design were previously published (30). Briefly, 11 participants (8 women) completed the overall study designed to experimentally induce marginal biotin deficiency. Of these, the final 5 participants (2 women) agreed to participate in the leucine challenge.
To ensure biotin sufficiency prior to biotin depletion, we incorporated a biotin loading and washout regimen consisting of 7 d of supplementation with a 300-μg biotin supplement [10-fold greater than the RDA for healthy adults (31)] followed by 7 d of supplementation with a vitamin that contained no biotin (washout). For each individual, biotin sufficiency on d 0 was judged by 4 established indicators of biotin status (26–29, 32). The multivitamin supplement with no biotin was given for the duration of the depletion phase of the study (d 0 through 28).
On d −1, participants were admitted to the University of Arkansas for Medical Sciences CRC. A research diet consisting of dietitian-planned, low-biotin menu cycles plus an egg-white beverage consumed with each meal commenced on d 0 and continued for 28 d. Participants spent evenings and nights in the CRC for meal provision, monitoring of health status, and assurance of protocol compliance.
Leucine challenge participants were given 70 mg/kg body weight (534 μmol/kg) leucine (dissolved in orange juice) orally at ~0800 h on d 0, 7, 14, and 21. All postchallenge voids were collected for ~5-h time intervals after administration; mean collection time ± 1 SD = 5.3 ± 0.6 h and range = 3.8–6.6 h for the 5 participants.
Design of Study 2.
Details of loading, washout, and induction of biotin deficiency for this study were previously published (33). Briefly, 7 participants (3 women) completed the overall study successfully. All 7 participants took part in the leucine challenge portion of the study design.
As a result of our concern that the combination of the large loading dose of biotin and the relatively short duration of washout used in Study 1 may have resulted in mean biotin status that was somewhat greater than sufficient, a smaller loading dose and longer washout period was used for Study 2. Participants consumed a 30-μg biotin supplement daily for 7 d (loading phase). After 7 d, biotin supplementation was discontinued and participants were supplemented with a multivitamin containing no biotin for 14 d (washout phase). For each individual, biotin sufficiency on d 0 was measured by 4 established indicators of biotin status (26–29, 32). The multivitamin supplement with no biotin was given for the duration of the depletion phase of the study.
Participants were admitted on d −1 to the CRC. The biotin depletion regimen was identical to Study 1 described above. Health status was monitored daily. For the leucine challenges, participants were given 70 mg/kg body weight (534 μmol/kg) leucine orally at ~0800 on d 0, 7, 14, 21, and 28. All postchallenge voids were collected for ~5-h time intervals after administration; mean collection time ± 1 SD = 5.2 ± 0.4 h and range = 4.3–6.6 h for the 7 participants.
Urine collection and storage.
For both Studies 1 and 2, complete urine collections were obtained for the 24-h period ending just before the leucine challenge (24-h urine samples) as well as for the 5-h postadministration of leucine (leucine challenge). As previously described (30), urine samples were subdivided into aliquots and centrifuged at 3000 × g for 10 min. The supernatant was removed and frozen at −70°C until assayed. As previously described (27–29), urinary excretion rates are expressed per mass of urinary creatinine. Urinary creatinine was determined by the picric acid method as previously described (34).
Measurement of urinary 3HIA.
Urinary 3HIA was quantitated without prior extraction using a novel, high-throughput UPLC-MS/MS method recently described (35). The mean CV for this assay method was ~6% for samples whose 3HIA concentration was normal to modestly increased.
Measurement of urinary 3HIA-carnitine
Urinary 3HIA-carnitine was quantitated without prior extraction by LC-MS/MS as previously described (36). The mean CV for this assay method was ~7% for samples whose 3HIA-carnitine concentration was normal to modestly increased.
Measurement of other biotin status indicators.
PCC activities in peripheral blood lymphocytes were measured by 14CO2 incorporation as previously described (29, 32). Activities were determined in triplicate for each participant at each time point and were normalized by lymphocyte protein content. Plasma 3HIA-carnitine concentrations were measured as previously described (37).
Statistical analysis.
To assess whether the different loading and washout protocols used for Studies 1 and 2 produced different biotin status on d 0, the significance of differences in means of established biotin status indicators (PCC activities in peripheral blood lymphocytes, plasma 3HIA-carnitine, urinary 3HIA-carnitine, and urinary 3HIA) were tested by using the Mann-Whitney U test. Differences over time consuming the egg-white diet in the group mean urinary excretion rates of 3HIA and of 3HIA-carnitine in response to the leucine challenge and in the 24-h urine samples were tested for significance by 1-way ANOVA with repeated measures and post hoc testing by Dunnett’s relative to d −1/0. All statistical analyses were performed using α = 0.05 and KaleidaGraph (version 4.1.1; Synergy Software).
Normal ranges.
The “normal range” denotes urine excretion rates of 3HIA and 3HIA-carnitine from biotin-sufficient, normal participants in response to the standard leucine challenge; biotin sufficiency was established by loading and washout as described above (26). Because no prior data were available for this index of biotin status, the normal ranges for the leucine challenge for urinary excretion of 3HIA and 3HIA-carnitine was chosen as the full range of values measured at d 0 before beginning the egg-white diet when the participants were biotin sufficient. Using the described analytical methods, normal ranges for 24-h urine excretion rates of 3HIA and 3HIA-carnitine were established from 10th and 90th percentiles from a group of 82 individuals who were either free-living or had received the loading and washout protocols described above; the mean excretion rates for the subgroups of the free-living participants and the participants who had received the loading and washout protocols were not significantly different.
Results
Biotin status on d 0.
Based on a comparison of mean and individual values for 4 established indicators of biotin status to the normal range, loading and washout protocols did produce biotin sufficiency in all participants in both studies on d 0 (Table 1). With regard to whether the loading and washout protocols produced equivalent initial biotin status, mean lymphocyte PCC activity, urinary excretion of 3HIA, and urinary excretion of 3HIA-carnitine, mean values did not differ at d 0 between participants in Study 1 and Study 2. These findings indicate equivalent biotin status. However, group mean plasma 3HIA-carnitine was lower in Study 1 than Study 2 (P = 0.008). Whether this finding indicates a slightly higher biotin status in the Study 1 group at d 0 or reflects individual variation in this indicator is not clear.
TABLE 1.
Biotin status on d 0 of participants in Studies 1 and 21
| Indicator of biotin status | Normal range1 | Study 12 | Normal at d 0, n | Study 22 | Normal at d 0, n | P value3 |
| Lymphocyte PCC activity, pmol ·min−1 · mg protein−1 | 320–760 | 411 ± 104 | 4 | 478 ± 107 | 6 | >0.05 |
| Plasma concentration of 3HIA-carnitine, μmol/L | 0.005–0.018 | 0.009 ± 0.003 | 5 | 0.014 ± 0.004 | 6 | 0.008 |
| Urinary excretion of 3HIA-carnitine, mmol/mol creatinine | 0.06–0.16 | 0.12 ± 0.03 | 5 | 0.13 ± 0.04 | 6 | >0.05 |
| Urinary excretion of 3HIA, mmol/mol creatinine | 3.3–12.0 | 6.2 ± 2.5 | 5 | 13.5 ± 11.0 | 6 | >0.05 |
10th and 90th percentiles used as range limits for each indicator. PCC: calculated from 18 individuals; plasma 3HIA-carnitine: calculated from 51 individuals; urinary 3HIA-carnitine: calculated from 80 individuals; urinary 3HIA: calculated from 80 individuals. 3HIA, 3-hydroxyisovaleric acid; 3HIA-carnitine, 3-hydroxyisovaleryl carnitine; PCC, propionyl-CoA carboxylase.
Values are mean ± SD, = 5 (Study 1) or 7 (Study 2).
Determined by U test.
Successful induction of biotin deficiency.
As previously reported (26, 29, 33, 38), marginal biotin deficiency was successfully induced in both studies by the low-biotin plus egg-white diet. This assessment is based on appropriate changes in each of 4 established indices of biotin status: lymphocyte PCC activity, plasma concentration of 3HIA-carnitine, urinary 3HIA-carnitine, and 3HIA excretion.
Characterizing the response to a leucine challenge in biotin-sufficient individuals: urinary 3HIA and 3HIA-carnitine on d 0.
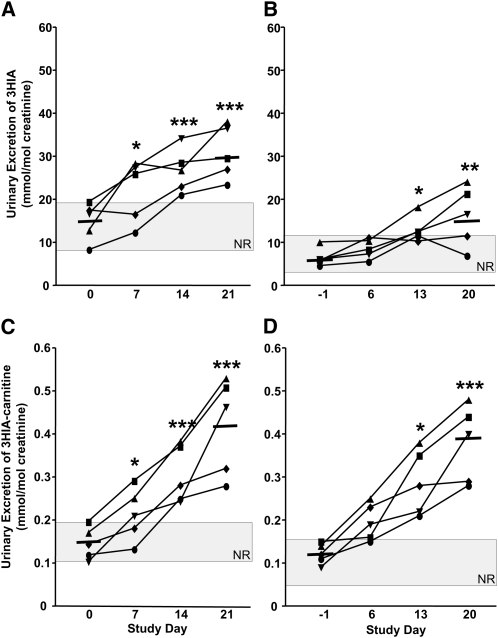
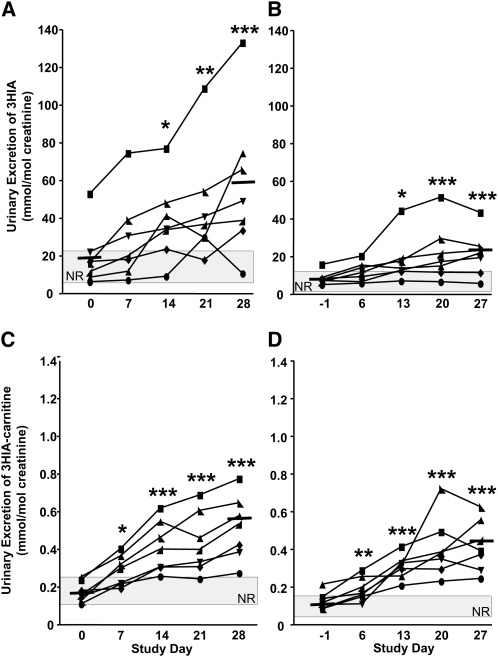
Even on d 0 when the participants were biotin sufficient, the mean urinary excretion of 3HIA in response to the leucine challenge increased >2-fold over the 24-h urine sample in both Studies 1 and 2 (Figs. 1 and 2A,B). Likewise, 3HIA-carnitine excretion increased in response to the leucine challenge on d 0 (Figs. 1 and 2C,D). However, the mean increase was only ~30% over the baseline. On d 0, the absolute excretion rate of 3HIA in response to the leucine challenge was ~50-fold greater than that of 3HIA-carnitine. Together, these observations provide evidence that MCC can be rate-limiting under a leucine load even in biotin-sufficient individuals and that 3HIA is the primary metabolic fate of leucine catabolites when MCC is limiting.
FIGURE 1.
Urinary 3HIA and 3HIA-carnitine responses to leucine challenge (A,C) and in the preceding 24-h urine samples (B,D) increase with biotin depletion in 5 healthy adults (Study 1). The gray rectangle denotes the NR. Solid bars denote mean excretion at d 0 and 28. Asterisks indicate that the mean on that day differs from d 0: *P < 0.02, **P < 0.002, ***P < 0.0005. 3HIA, e-hydroxyisovaleric acid; 3HIA-carnitine, 3-hydroxyisovaleryl carnitine; NR, normal range.
FIGURE 2.
Urinary 3HIA and 3HIA-carnitine responses to leucine challenge (A,C) and the preceding 24-h urine samples (B,D) increase with biotin depletion in Study 2 (7 participants). The gray rectangle denotes NR. Solid bars denote mean excretion at d 0 and 28. Significance of changes in group mean data were tested by 1-way ANOVA with repeated measures and Dunnett’s post hoc test relative to d 0; *P ≤ 0.02, **P < 0.002, ***P ≤ 0.0009. 3HIA, e-hydroxyisovaleric acid; 3HIA-carnitine, 3-hydroxyisovaleryl carnitine; NR, normal range.
Response of urinary 3HIA and 3HIA-carnitine excretion to a leucine challenge during biotin depletion.
In Study 1 (Fig. 1), urinary excretion rates of 3HIA in response to the leucine challenge (Fig. 1A) and in 24-h urine samples (Fig. 1B) increased during progressive biotin depletion. Similarly, 3HIA-carnitine in response to the leucine challenge (Fig. 1C) and in 24-h urine samples (Fig. 1D) increased during progressive biotin depletion. For both 3HIA and 3HIA-carnitine, the mean increases in response to the leucine challenge were ~2-fold by d 14 (P < 0.02) and were >2-fold by d 21 (P < 0.0005). By d 14, individual urinary excretions of 3HIA and 3HIA-carnitine in response to the leucine challenge were both increased above the upper limit of normal in all 5 participants. Thus, the diagnostic sensitivities at d 21 were high and similar to 24-h urine 3HIA (80%) and 3HIA-carnitine (100%).
In Study 2 (Fig. 2), urinary excretion rates of 3HIA and 3HIA-carnitine in response to the leucine challenge (Fig. 2A,C) and in 24-h urine samples (Fig. 2B,D) increased during progressive biotin depletion. Mean increases in 3HIA and 3HIA-carnitine excretion in response to the leucine challenge were ~2-fold by d 14 (P < 0.02) and ~3-fold by d 28 (P < 0.0001). By d 14, 6 of the 7 participants excreted urinary 3HIA beyond the upper limit of normal and 3HIA-carnitine excretion rates were above the upper limit of normal in all participants. The diagnostic sensitivities by d 28 were again high and similar to 24-h urine 3HIA (86%) and 3HIA-carnitine (100%).
This study was notable for one participant (denoted by the square symbols) who exhibited increased urinary excretion of 3HIA and 3HIA-carnitine in 24-h urine samples and after leucine challenge. These indicators were elevated on d 0 (despite biotin loading and washout and despite normal lymphocyte PCC activity) and remained elevated throughout the study.
Discussion
Here, we evaluated the urinary excretion rates of both 3HIA and 3HIA-carnitine in response to a leucine challenge administered to 2 cohorts of healthy adults during experimentally induced marginal biotin deficiency. We found that the excretion rates of both 3HIA and 3HIA-carnitine substantially increase with progressive (but still asymptomatic) biotin deficiency. The diagnostic sensitivities were similar but not importantly superior to those of PCC activity measured in peripheral blood lymphocyte and to the urinary excretion rates of 3HIA and 3HIA-carnitine determined in 24-h urine collections that preceded the 5-h urine collections for the leucine challenge.
Given the diagnostic sensitivities of these 2 novel indicators of marginal biotin status, we infer that the utility of urinary 3HIA and 3HIA-carnitine excretion in response to a leucine challenge is potentially technically superior to that of lymphocyte PCC activity. As previously discussed (26), lymphocyte isolation, sample storage, and assay characteristics render assaying lymphocyte PCC time consuming, problematic, and costly for population studies. However, the utility of urinary 3HIA and 3HIA-carnitine responses to a leucine challenge is likely inferior to that of 3HIA and 3HIA-carnitine in 24-h urine collections because of the difficulty in conducting the leucine challenge. The leucine challenge requires fasting overnight and during the challenge as well as the collection of a urine sample pooled over the 5 h after the challenge.
Whether from timed (or untimed) baseline urine samples or from leucine challenge urine samples, measurement of urinary 3HIA and 3HIA-carnitine excretion shares the following advantages: 1) only small volumes (maximum of 50 μL) of urine are required; 2) collection and storage at −70°C are technically straightforward; 3) 3HIA and 3HIA-carnitine stored frozen in urine are likely stable for years; and 4) quantitation by UPLC-MS/MS and LC-MS/MS is both precise and accurate (36). These advantages likely render urinary 3HIA and 3HIA-carnitine more suitable for application to population studies than lymphocyte PCC activity. However, the broader applicability of these findings remains to be established. Twelve participants is not a sufficiently large group from which to confidently generalize.
Reduced activity of MCC impairs catalysis of an essential step in the mitochondrial catabolism of the BCAA leucine. Metabolic impairment diverts methylcrotonyl CoA to 3-hydroxyisovaleryl CoA in a reaction catalyzed by enoyl-CoA hydratase (22, 23). 3-Hydroxyisovaleryl CoA accumulation can inhibit cellular respiration either directly or via effects on the ratios of acyl CoA:free CoA if further metabolism and detoxification of 3-hydroxyisovaleryl CoA does not occur (22). The transfer to carnitine by 4 carnitine acyl-CoA transferases distributed in subcellular compartments likely serves as an important reservoir for acyl moieties (39–41). 3-Hydroxyisovaleryl CoA is likely detoxified by carnitine acetyltransferase producing 3HIA-carnitine, which is transported across the inner mitochondrial membrane (and hence effectively out of the mitochondria) via carnitine-acylcarnitine translocase (39). 3HIA-carnitine is thought to be either directly deacylated by a hydrolase to 3HIA or to undergo a second CoA exchange to again form 3-hydroxyisovaleryl CoA followed by release of 3HIA and free CoA by a thioesterase.
The observation that the urinary excretion rate of free 3HIA exceeds that of the precursor 3HIA-carnitine by >50-fold despite the increased flux in the pathway of about 3-fold caused by marginal deficiency of MCC provides evidence that the enzymes catalyzing the ultimate release of 3HIA from 3HIA-carnitine are generally not limiting in this alternative pathway. This seems to remain true despite the further increase in flux in the pathway caused by the leucine challenge.
A point of individual variability that deserves consideration is the participant whose 3HIA excretion in response to the leucine challenge on d 0 was already strikingly greater than that of any of the other 11 participants. We speculate that this individual could have heterozygous MCC deficiency. Support for this speculation comes from the work of Baumgartner et al. (42, 43), who reported asymptomatic and symptomatic individuals who have allele-specific, dominant-negative effects of heterozygous, isolated MCC deficiency, including increased urinary 3HIA. These investigators estimated that the incidence of heterozygous MCC deficiency is ~1 in 100. Thus, inadvertent selection of an individual who is heterozygous for MCC deficiency is statistically plausible. Genetic material is not available from that participant.
In conclusion, these studies provide evidence that urinary excretions of 3HIA and 3HIA-carnitine in response to an oral leucine challenge are early and sensitive indicators of marginal biotin deficiency in humans but are not superior to other indicators of biotin status. Substantial population heterogeneity in the metabolic capacity of the 3HIA-carnitine detoxification pathway was observed and deserves further investigation.
Acknowledgments
The authors thank Joel Bradley, Ron Trolard, and Rosemarie Bachand of Cambridge Isotope Laboratories for the generous gift of the authentic 3HIA-carnitine and deuterated-3HIA-carnitine used in the work described herein. D.M.M., T.D.H., G.B., and J.M. designed research; S.L.S., T.D.H., A.B., N.I.M., C.L.H., A.M.D., and S.N.O. conducted research; G.B. and J.M. provided essential equipment for research; D.M.M., S.L.S., and H.J.S. analyzed data; D.M.M. and S.L.S. wrote the paper; and D.M.M. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by NIH R37DDK36823-28, R37DDK36823-26S1 (D.M.M.); National Center for Research Resources UL1RR029884 (University of Arkansas for Medical Sciences Translational Research Institute); Arkansas Biosciences Institute, Arkansas Tobacco Settlement Proceeds Act of 2000 (D.M.M., G.B.); and CDC Agreement Contract 200-2007-21729 and Bioterrorism Cooperative agreement U90/CCU616974-07 (J.H.M.).
Abbreviations used: CRC, Clinical Research Center; 3HIA, 3-hydroxyisovaleric acid; 3HIA-carnitine, 3-hydroxyisovaleryl carnitine; LC-MS/MS, liquid chromatography-tandem MS; MCC, methylcrotonyl-CoA carboxylase; PCC, propionyl-CoA carboxylase; UPLC-MS/MS, ultra high-performance liquid chromatography- tandem MS.
Literature Cited
- 1.Mock DM, Stadler DD. Conflicting indicators of biotin status from a cross-sectional study of normal pregnancy. J Am Coll Nutr. 1997;16:252–7 [DOI] [PubMed] [Google Scholar]
- 2.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr. 1997;127:710–6 [DOI] [PubMed] [Google Scholar]
- 3.Watanabe T, Endo A. Teratogenic effects of avidin-induced biotin deficiency in mice. Teratology. 1984;30:91–4 [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Endo A. Species and strain differences in teratogenic effects of biotin deficiency in rodents. J Nutr. 1989;119:255–61 [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T. Teratogenic effects of biotin deficiency in mice. J Nutr. 1983;113:574–81 [DOI] [PubMed] [Google Scholar]
- 6.Watanabe T, Endo A. Teratogenic effects of maternal biotin deficiency in mouse embryos examined at midgestation. Teratology. 1990;42:295–300 [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T. Dietary biotin deficiency affects reproductive function and prenatal development in hamsters. J Nutr. 1993;123:2101–8 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe T, Dakshinamurti K, Persaud TVN. Biotin influences palatal development of mouse embryos in organ culture. J Nutr. 1995;125:2114–21 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Endo A. Biotin deficiency per se is teratogenic in mice. J Nutr. 1991;121:101–4 [DOI] [PubMed] [Google Scholar]
- 10.Takechi R, Taniguchi A, Ebara S, Fukui T, Watanabe T. Biotin deficiency affects the proliferation of human embryonic palatal mesenchymal cells in culture. J Nutr. 2008;138:680–4 [DOI] [PubMed] [Google Scholar]
- 11.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr. 2003;133:2519–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sealey WM, Stratton SL, Hansen DK, Mock DM. Marginal maternal biotin deficiency in CD-1 mice reduces fetal mass of biotin-dependent carboxylases. J Nutr. 2005;135:973–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mock DM. Biotin. : Ziegler EE, Filer LJ, Jr, Present knowledge in nutrition. 7th ed p. 220–35 Washington, DC: International Life Sciences Institutes-Nutrition Foundation; 1996 [Google Scholar]
- 14.Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in the mouse. J Nutr. 2009;139:154–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Said HM. Biotin bioavailability and estimated average requirement: why bother? Am J Clin Nutr. 1999;69:352–3 [DOI] [PubMed] [Google Scholar]
- 16.Okayama A, Fun L, Yamatodani A, Ogawa Y, Wada H, Goto S. Effect of exposure to carbon disulfide on tryptophan metabolism and the tissue vitamin B6 contents of rats. Arch Toxicol. 1987;60:450–3 [DOI] [PubMed] [Google Scholar]
- 17.Coon WW, Nagler E. The tryptophan load as a test for pyridoxine deficiency in hospitalized patients. Ann N Y Acad Sci. 1969;166:30–43 [DOI] [PubMed] [Google Scholar]
- 18.Coon WW. Tryptophan load and pyridoxine deficiency. Am J Clin Pathol. 1966;46:345. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed SD, Roberts M. Relative importance of formiminoglutamic and urocanic acid excretion after a histidine load. J Clin Pathol. 1966;19:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ubbink JB, Van Der Merwe A, Delport R, Allen R, Stabler S, Riezler R, Vermaak W. The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J Clin Invest. 1996;98:177–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46 [DOI] [PubMed] [Google Scholar]
- 22.Sweetman L, Williams JC. Branched chain organic acidurias. : Scriver CR, Beaudet AL, Sly WS, Valle D, The metabolic and molecular bases of inherited disease. 7th ed New York: McGraw-Hill Inc; 1995. p. 1387–1422 [Google Scholar]
- 23.Maeda Y, Ito T, Ohmi H, Yokoi K, Nakajima Y, Ueta A, Kurono Y, Togari H, Sugiyama N. Determination of 3-hydroxyisovalerylcarnitine and other acylcarnitine levels using liquid chromatography-tandem mass spectrometry in serum and urine of a patient with multiple carboxylase deficiency. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;870:154–9 [DOI] [PubMed] [Google Scholar]
- 24.Röschinger W, Millington DS, Gage DA, Huang ZH, Iwamoto T, Yano S, Packman S, Johnston K, Berry SA, Sweetman L. 3-Hydroxyisovalerylcarnitine in patients with deficiency of 3-methylcrotonyl CoA carboxylase. Clin Chim Acta. 1995;240:35–51 [DOI] [PubMed] [Google Scholar]
- 25.Pasquali M, Monsen G, Richardson L, Alston M, Longo N. Biochemical findings in common inborn errors of metabolism. Am J Med Genet C Semin Med Genet. 2006;142C:64–76 [DOI] [PubMed] [Google Scholar]
- 26.Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Spencer HJ, Moran JH, Mock DM. Plasma concentration of 3-hydroxyisovaleryl carnitine is an early and sensitive indicator of marginal biotin deficiency in humans. Am J Clin Nutr. 2010;92:1399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased status in experimental biotin deficiency. Am J Clin Nutr. 1997;65:951–8 [DOI] [PubMed] [Google Scholar]
- 28.Mock DM, Jackson H, Lankford GL, Mock NI, Weintraub ST. Quantitation of urinary 3-hydroxyisovaleric acid using deuterated 3-hydroxyisovaleric acid as internal standard. Biomed Environ Mass Spectrom. 1989;18:652–6 [DOI] [PubMed] [Google Scholar]
- 29.Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Spencer HJ, Moran JH, Mock DM. Urinary excretion of 3-hydroxyisovaleryl carnitine is an early and sensitive indicator of marginal biotin deficiency in humans. J Nutr. 2011;141:353–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mock DM, Henrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validation of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am J Clin Nutr. 2002;76:1061–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NRC Dietary reference intakes tables–the complete set (pdf). : Food and Nutrition Board Institute of Medicine, editor Dietary reference intakes. Washington, DC: The National Academies; 2005 [Google Scholar]
- 32.Mock D, Henrich CL, Carnell N, Mock NI, Swift L. Lymphocyte propionyl-CoA carboxylase and accumulation of odd-chain fatty acid in plasma and erythrocytes are useful indicators of marginal biotin deficiency. J Nutr Biochem. 2002;13:462–70 [DOI] [PubMed] [Google Scholar]
- 33.Vlasova TI, Stratton SL, Wells AM, Mock NI, Mock DM. Biotin deficiency reduces expression of SLC19A3, a potential biotin transporter, in leukocytes from human blood. J Nutr. 2005;135:42–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mock DM. Rayon balls and disposable-diaper material selectively adsorb creatinine. Am J Clin Nutr. 1992;55:326–30 [DOI] [PubMed] [Google Scholar]
- 35.Horvath TD, Matthews NI, Stratton SL, Mock DM, Boysen G. Measurement of 3-hydroxyisovaleric acid in urine from marginally biotin deficient humans by UPLC-MS/MS. Anal Bioanal Chem. In press 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horvath TD, Stratton SL, Bogusiewicz A, Owen SO, Mock DM, Moran JH. Quantitative measurement of urinary excretion of 3-hydroxyisovaleryl carnitine by LC-MS/MS as an indicator of biotin status in humans. Anal Chem. 2010;82:9543–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horvath TD, Stratton SL, Bogusiewicz A, Pack L, Moran J, Mock DM. Quantitative measurement of plasma 3-hydroxyisovaleryl carnitine by LC-MS/MS as a novel biomarker of biotin status in humans. Anal Chem. 2010;82:4140–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stratton SL, Bogusiewicz A, Mock MM, Mock NI, Wells AM, Mock DM. Lymphocyte propionyl-CoA carboxylase and its activation by biotin are sensitive indicators of marginal biotin deficiency in humans. Am J Clin Nutr. 2006;84:384–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zammit VA, Ramsay RR, Bonomini M, Arduini A. Carnitine, mitochondrial function and therapy. Adv Drug Deliv Rev. 2009;61:1353–62 [DOI] [PubMed] [Google Scholar]
- 40.Westin MA. Hunt MC, Alexson SE. Short- and medium-chain carnitine acyltransferases and acyl-CoA thioesterase in mouse provide complementary systems for transport of beta-oxidation products out of peroxisomes. Cell Mol Life Sci. 2008;65:982–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsay RR. The carnitine acyltransferases: modulators of acyl-CoA-dependent reactions. Biochem Soc Trans. 2000;28:182–6 [DOI] [PubMed] [Google Scholar]
- 42.Baumgartner M. Molecular mechanism of dominant expression in 3-methylcrotonyl-CoA carboxylase deficiency. J Inherit Metab Dis. 2005;28:301–9 [DOI] [PubMed] [Google Scholar]
- 43.Baumgartner MR, Dantas MF, Suormala T, Almashanu S, Giunta C, Friebel D, Gebhardt B, Fowler B, Hoffmann GF, Baumgartner ER, Valle D. Isolated 3-methylcrotonyl-CoA carboxylase deficiency: evidence for an allele-specific dominant negative effect and responsiveness to biotin therapy. Am J Hum Genet. 2004;75:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]