Abstract
Some previous studies have suggested that consuming dairy products, particularly the low-fat variety, lowers the incidence of type 2 diabetes. However, no study to our knowledge has focused on an ethnically diverse group of postmenopausal women, a population with a high risk of this disease. We conducted a prospective cohort study of 82,076 postmenopausal women enrolled in the Women’s Health Initiative Observational Study who did not report diabetes at enrollment. Total, low-fat, and high-fat dairy product and yogurt intakes were estimated from FFQ at baseline and 3 y of follow-up. Treated diabetes incidence was ascertained from annual follow-up questionnaires. During 8 y of follow-up, 3946 cases of incident treated diabetes were reported (annual incidence, 0.73%; cumulative incidence, 4.8%). After multivariable adjustment, low-fat dairy product consumption was inversely associated with the risk of type 2 diabetes. RR was roughly 0.5–0.6 in the upper quintiles compared with the lowest quintile (median servings/d, 2.8 in the 5th quintile and 1.5 in the 4th quintile vs. 0.05 in the first quintile; P-trend < 0.001). The inverse relationship was more pronounced in women with a higher BMI. High yogurt consumption was associated with a significant decrease in diabetes risk, whereas there was no relationship between high-fat dairy product consumption and diabetes risk. A diet high in low-fat dairy products is associated with lower diabetes risk in postmenopausal women, particularly those who are obese.
Introduction
It is estimated that 24 million people in the United States have diabetes and 1 million more receive a new diagnosis of diabetes each year (1). Thus, there is an urgent need for effective preventive strategies, including dietary approaches. Recent epidemiological studies have suggested beneficial effects of dairy product intake on risk factors for diabetes, including body weight (2, 3), body composition (4), and insulin resistance (5–7). Low-fat dairy foods were one of the main components of the diets shown to be effective in 2 randomized trials for type 2 diabetes prevention (8, 9).
Several epidemiologic studies have found an association of low-fat dairy product intake with lower risk of type 2 diabetes in men (10) and women (11, 12). For the most part, these associations were independent of dietary calcium, vitamin D, glycemic load, fat, fiber, and magnesium intake. In middle-aged and elderly men, each serving per day increase in total dairy food intake was associated with a 9% lower risk for type 2 diabetes, with the effect confined to low-fat dairy foods (10). In women aged 45 y or older, the association was slightly weaker, with each serving per day increase in dairy food intake showing a 4% nonsignificantly lower risk for type 2 diabetes (11). As in men, the association appeared strongest for low-fat dairy products and there was also an 18% lower risk of diabetes for women who consumed more than 2 servings of yogurt/wk compared with women who consumed <1 serving/mo. In a study of black women aged 21–69 y, daily consumption of low-fat dairy products was associated with a 13% lower risk of type 2 diabetes compared with consumption less than once per week (12).
In this paper, we examined the association of dairy product intake, particularly low-fat dairy products and yogurt, with diabetes incidence in postmenopausal women enrolled in the Women’s Health Initiative Observational Study (WHI-OS).
Methods
Study population.
As described elsewhere, the Women’s Health Initiative (WHI) has clinical trial and observational study components (13, 14). The WHI-OS is an ongoing prospective cohort study of postmenopausal women designed to examine the association of clinical, socioeconomic, behavioral, and dietary risk factors and subsequent health outcomes. Between September 1, 1994, and December 31, 1998, the WHI-OS enrolled 93,676 women aged 50–79 y at 40 clinical centers throughout the United States.
Participants were recruited from areas surrounding the clinical centers in 24 states and the District of Columbia (15). Women were eligible to participate in the WHI-OS if they were postmenopausal; unlikely to change residence or die within 3 y; did not have complicating conditions such as alcoholism, drug dependency, or dementia; and were not enrolled in any clinical trial, including the WHI clinical trials. The baseline characteristics of the WHI-OS cohort have been described in detail (16). All participants provided informed consent using materials approved by institutional review boards at each center.
Data collection.
Participants underwent initial screening visits during which personal information, medical history, family history, and health-related habits were assessed using standardized questionnaires. Medication and supplement use were assessed by a medication inventory. Certified staff performed physical measurements at the baseline clinic visit and again at y 3.
Dietary intake was measured by a semiquantitative FFQ designed for the WHI that inquired about the average use of 122 line items covering >300 foods and beverages in the previous 3 mo. Measurement characteristics of the WHI FFQ have been published (17). In the WHI-OS, the FFQ was administered at baseline and repeated at y 3. The WHI FFQ was based on instruments previously used in large-scale dietary intervention trials (18, 19). WHI scientists modified the questionnaire to include additional questions on fat-related food preparation methods and reduced-fat foods to increase its sensitivity to changes in fat intake as well as to reflect regional and ethnic eating patterns throughout the United States (17).
We calculated dairy product consumption based on responses to questions about intake of milk as a beverage, on cereal, or in coffee or tea as well as the use of other dairy products. Low-fat milk was considered to be nonfat milk, skim milk, or 1% milk; all other types of milk were considered high fat. Other dairy products included on the FFQ were cheese (including cheese in entrees and mixed dishes and cottage cheese and ricotta cheese), cream soups, yogurt, ice cream, pudding, custard, and flan. Specific questions covered low-fat cottage cheese, part-skim or reduced-fat cheese, nonfat yogurt, and low-fat or nonfat frozen desserts; these items were included in the calculations for consumption of low-fat dairy foods. A serving of milk was considered to be an 8 oz glass (250 g) (20).
Follow-up and ascertainment of cases.
The WHI-OS follow-up was conducted by annual, mailed, self-administered questionnaires. Response rates for y 1–8 medical history updates in the WHI-OS ranged from 93 and 96%, with only 1.9% of the participants lost to follow-up. An additional 2.2% discontinued their participation in the study and 6.1% died during the follow-up period.
Participants who reported physician-diagnosed diabetes at enrollment were excluded from this analysis. At each annual contact, participants were asked to report whether they had been newly treated with insulin or oral medication for diabetes. At the baseline and y 3 visits, participants were asked to bring all medications for a medication inventory. The product name or generic name of each medication was entered into the study database (Master Drug Data Base, Medi-Span). In the WHI-OS, self-reports of diabetes treatment were matched by the medication inventory in 77% at baseline and in 72% at the y 3 follow-up visit (21). A validation study found that 82% of new-onset self-reported diabetes was confirmed by review of medical records obtained from WHI participants (K.L. Margolis, unpublished data).
Statistical analysis.
Of 93,676 participants enrolled in the WHI-OS, we excluded 4692 women who had diabetes at enrollment, 5321 women with missing data on key variables on the FFQ or covariates (blood pressure, weight, and height), 950 women who reported implausibly low total energy intake (≤500 kcal/d), and 637 women who reported implausibly high total energy intake (≥3500 kcal/d). The final analytic sample size was 82,076. We computed person-time of follow-up from the beginning of the study to the date of diagnosis of type 2 diabetes, loss to follow-up, death from any cause, or the end of the study period (September 12, 2005), whichever came first. The median duration of follow-up was 7.9 y. We examined intakes of total dairy, low-fat dairy, high-fat dairy, and yogurt in separate analyses. To reduce within-person variation and best represent long-term diet, we used data from both FFQ in a method that employs repeated measures of diet during follow-up (22). If new-onset diabetes was reported between enrollment and the y 3 FFQ, we used only the baseline FFQ to estimate dietary intake.
We created hierarchical Cox proportional hazards models to estimate the HR, or estimates of RR, and 95% CI for developing incident type 2 diabetes for each category of intake compared with the lowest category. We tested the proportional hazard assumption using interactions between the predictors and type 2 diabetes-free survival time. Where the proportional hazards assumption was not met, an interaction term with time and the main exposure was incorporated in the multivariable models (23).
Trend tests were performed by assigning the median value for each quintile category and modeling this as a continuous variable. We also examined dairy food and yogurt intake modeled as continuous variables, with the RR expressed as change in risk with an increment of 1 serving/d of the medium portion size. The initial models were adjusted for age, race/ethnicity, and total energy intake. In subsequent multivariable models, we additionally adjusted for income, education, smoking, alcohol consumption, use of postmenopausal hormone therapy, physical activity, family history of diabetes, BMI, and blood pressure. In additional models, we adjusted for some key dietary factors linked to type 2 diabetes (glycemic load, total fat, dietary fiber, and magnesium.) Food group and nutrient intakes were adjusted for total energy intake using the residual method (24).
To assess possible effect modification, we conducted tests for interaction for variables that are strongly associated with incident diabetes: BMI (continuous variable in kg/m2), physical activity (continuous variable in metabolic equivalent task/week), and family history of diabetes (yes/no). Because lactose intolerance is more common in certain ethnic groups (African Americans, Asians) with higher risk of diabetes, we also tested for interactions with race/ethnicity. We tested the significance of the interactions using the likelihood ratio test by comparing a model with the main effects of dietary intake and interaction term with a reduced model with only the main effects. In addition, we examined the association between dairy product intake (low fat, high fat, and yogurt) and weight gain in the follow-up period to determine if any association of dairy food consumption with risk of diabetes was moderated by changes in weight. All statistical analyses were conducted using SAS (version 9.0, SAS Institute).
Results
The median intakes were 1.5 servings/d for all dairy products, 0.8 servings/d for low-fat dairy products, and 0.4 servings/ d for high-fat dairy products. Yogurt consumption was low in this group of older women (median intake was about one-half serving/w, and 38% reported rarely or never consuming yogurt). The baseline characteristics of the cohort comparing the lowest and highest quintiles of intake of low-fat dairy, high-fat dairy, and total dairy products are shown in Table 1.
TABLE 1.
Baseline characteristics of the study population according to dairy food intake1
| Low-fat dairy food intake3 |
High-fat dairy food intake4 |
All dairy food intake5 |
||||
| Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | |
| Servings/d, range (median)2 | 0–0.2 (0.05) | 1.9–13.5 (2.8) | 0–0.1 (0.06) | 0.9–11.9 (1.3) | 0–0.7 (0.5) | 2.6–15.7 (3.4) |
| Age, % | ||||||
| 50–59 y | 31.6 | 30.4 | 29.8 | 30.9 | 33.3 | 30.8 |
| 60–69 y | 42.9 | 44.8 | 45.4 | 43.0 | 43.0 | 44.0 |
| 70–79 y | 25.5 | 24.8 | 24.8 | 26.1 | 23.7 | 25.3 |
| Race, % | ||||||
| American Indian or Alaskan Native | 0.6 | 0.2 | 0.4 | 0.4 | 0.5 | 0.2 |
| Asian or Pacific Islander | 5.5 | 0.9 | 4.1 | 1.5 | 6.4 | 0.9 |
| Black or African-American | 13.7 | 1.9 | 8.5 | 5.4 | 14.1 | 2.5 |
| Hispanic/Latino | 5.2 | 1.6 | 3.2 | 4.2 | 4.7 | 2.1 |
| White (not of Hispanic origin) | 73.2 | 94.4 | 82.4 | 87.3 | 72.5 | 93.1 |
| Other/unknown | 1.7 | 1.1 | 1.5 | 1.3 | 1.8 | 1.2 |
| Income, % | ||||||
| <$34,999 | 46.8 | 33.1 | 35.5 | 44.6 | 40.9 | 36.6 |
| $35,000–74,999 | 37.8 | 44.1 | 41.1 | 39.3 | 39.5 | 42.7 |
| ≥$75,000 | 15.5 | 22.9 | 23.5 | 16.2 | 19.6 | 20.8 |
| Education, % | ||||||
| High school diploma or less | 28.1 | 14.1 | 19.7 | 22.5 | 24.8 | 15.4 |
| School after high school | 38.2 | 32.6 | 35.4 | 37.7 | 38.4 | 33.8 |
| College degree or higher | 33.0 | 52.6 | 44.9 | 39.9 | 36.8 | 50.7 |
| Smoking, % | ||||||
| Never smoked | 52.0 | 52.1 | 49.5 | 51.8 | 51.0 | 53.1 |
| Past smoker | 37.2 | 44.6 | 45.8 | 40.2 | 40.5 | 42.7 |
| Current smoker | 10.8 | 3.3 | 4.7 | 8.1 | 8.5 | 4.3 |
| Alcohol,6% | ||||||
| Nondrinker | 14.9 | 8.6 | 11.7 | 10.5 | 13.9 | 9.3 |
| Past drinker | 21.4 | 16.5 | 20.0 | 18.5 | 20.3 | 17.1 |
| <7 drinks/wk | 52.1 | 63.1 | 56.8 | 57.9 | 53.1 | 62.0 |
| ≥7 drinks/wk | 11.6 | 11.8 | 11.4 | 13.1 | 12.7 | 11.6 |
| Family history of diabetes, % | 30.1 | 28.6 | 29.8 | 28.9 | 31.0 | 28.6 |
| Hormone use, % | ||||||
| Never used hormones | 34.7 | 26.7 | 29.7 | 32.1 | 32.3 | 28.1 |
| Past hormone user | 22.3 | 20.6 | 20.7 | 21.9 | 21.3 | 21.0 |
| Current hormone user | 43.0 | 52.7 | 49.7 | 46.1 | 46.4 | 51.0 |
| Systolic blood pressure, mm Hg | 127 ± 16 | 125 ± 16 | 126 ± 16 | 127 ± 16 | 127 ± 16 | 126 ± 16 |
| Diastolic blood pressure, mm Hg | 75 ± 9 | 74 ± 8 | 74 ± 8 | 74 ± 8 | 75 ± 9 | 74 ± 8 |
| BMI, kg/m2 | 27.2 ± 5.8 | 26.9 ± 5.3 | 26.1 ± 5.0 | 27.9 ± 6.1 | 26.8 ± 5.5 | 27.3 ± 5.6 |
| Total metabolic equivalent task/wk | 10.9 ± 12.4 | 16.3 ± 13.8 | 16.4 ± 14.7 | 11.6 ± 12.1 | 12.8 ± 13.4 | 15.2 ± 13.5 |
| Dietary energy, kcal/d | 1340 ± 540 | 1770 ± 480 | 1210 ± 420 | 1870 ± 550 | 1150 ± 420 | 1890 ± 510 |
| Dietary glycemic load/d | 79 ± 34 | 111 ± 33 | 80 ± 32 | 107 ± 36 | 71 ± 29 | 115 ± 33 |
| Dietary total fat, g/d | 53.5 ± 28.2 | 53.6 ± 23.3 | 34.4 ± 15.9 | 74.9 ± 28.4 | 41.0 ± 20.5 | 62.3 ± 27.9 |
| Dietary fiber, g/d | 12.7 ± 5.8 | 19.4 ± 6.4 | 15.5 ± 6.9 | 16.7 ± 6.2 | 12.7 ± 5.9 | 19.3 ± 6.5 |
| Supplemental + dietary magnesium, mg/d | 260 ± 150 | 410 ± 150 | 320 ± 180 | 350 ± 150 | 260 ± 170 | 410 ± 150 |
Values are mean ± SD or percentage.
One serving of milk = 1 cup (250 g).
Low-fat dairy products: first quintile 0–0.2 (0.05); second quintile 0.2–0.6 (0.4); 3rd quintile 0.6–1.1 (0.8); 4th quintile 1.1–1.9 (1.5); 5th quintile 1.9–13.5 (2.8).
High-fat dairy products: first quintile 0–0.1 (0.06); second quintile 0.1–0.3 (0.2); 3rd quintile 0.3–0.5 (0.4); 4th quintile 0.5–0.9 (0.7); 5th quintile 0.9 – 11.9 (1.3).
Total dairy products: first quintile 0–0.7 (0.5); second quintile 0.7–1.2 (1.0); 3rd quintile 1.2–1.8 (1.5); 4th quintile 1.8–2.6 (2.1); 5th quintile 2.6 – 15.7 (3.4).
One serving of alcohol = 14 g ethanol.
Compared with women with the lowest consumption of low-fat dairy foods, women with the highest consumption were more likely to be white, have higher income and education, be a nonsmoker, have no a family history of diabetes, use hormones, have a slightly lower BMI, and be more physically active. Similar patterns were observed among women with the highest intake of total dairy products and yogurt, except that the women with the highest total dairy food intake had higher BMI and women with the highest yogurt intake were younger (data not shown). Compared with women with the lowest intake of high-fat dairy products, women with the highest intake were more likely to be white, have lower income and education, be a smoker, not use hormones, have a higher BMI, and be less physically active.
During 8 y of follow-up, 3946 cases of incident treated diabetes were reported (mean annual incidence, 0.73%; cumulative incidence, 4.8%). After adjustment for age, race, and energy intake, low-fat dairy food consumption was inversely associated with the development of type 2 diabetes, with RR ~0.7 in the upper quintiles compared to the lowest quintile (P-trend < 0.0001) (Table 2). Further multivariable adjustment strengthened this association; although the lower RR in the 4th quintile (0.5) relative to the slightly higher risk in the 5th quintile (0.6) was more exaggerated, the trend was still significant (P-trend = 0.0006). Further adjustment for glycemic load, total fat, dietary fiber, and magnesium had little effect on the results, which remained significant. Additional adjustment for intake of calcium, vitamin D, fruit juice, and sweetened beverages also did not change the results, nor did including women with implausibly low or high total energy intake (data not shown). In the multivariable model unadjusted for dietary variables, the RR for incident diabetes with each serving/d increase in low-fat dairy food intake was significant, at 0.93 (95% CI = 0.87, 0.99; P = 0.03), although with additional adjustment for dietary fat, the association became nonsignificant (P = 0.12).
TABLE 2.
RR of incident diabetes in postmenopausal women by quintile of low-fat dairy food intake
| Quintiles of intake |
||||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend3 | |
| n | 18,037 | 15,915 | 16,024 | 16,050 | 16,047 | |
| Person years | 132,589 | 119,273 | 121,631 | 122,769 | 122,664 | |
| Diabetes cases, n (%) | 1,078 (6.0) | 842 (5.3) | 714 (4.4) | 644 (4.0) | 668 (4.2) | |
| Age, race, energy1 | 1.00 (reference) | 0.86 (0.78, 0.95) | 0.75 (0.68–0.83) | 0.68 (0.61–0.75) | 0.70 (0.64–0.77) | <0.0001 |
| Multivariable model2 | 1.00 (reference) | 0.97 (0.68–1.38) | 0.63 (0.43–0.93) | 0.48 (0.32–0.73) | 0.60 (0.41–0.89) | 0.0006 |
| Multivariable model2 + dietary glycemic load | 1.00 (reference) | 0.98 (0.68–1.40) | 0.64 (0.44–0.95) | 0.49 (0.32–0.74) | 0.62 (0.42–0.91) | 0.001 |
| Multivariable model2 + dietary total fat | 1.00 (reference) | 0.99 (0.69–1.41) | 0.66 (0.45–0.97) | 0.51 (0.33–0.77) | 0.66 (0.45–0.98) | 0.004 |
| Multivariable model2 + dietary fiber | 1.00 (reference) | 0.98 (0.68–1.40) | 0.64 (0.43–0.95) | 0.48 (0.32–0.74) | 0.62 (0.42–0.91) | 0.001 |
| Multivariable model2 + total magnesium | 1.00 (reference) | 0.97 (0.68–1.39) | 0.64 (0.43–0.94) | 0.49 (0.32–0.74) | 0.62 (0.42–0.91) | 0.001 |
| Multivariable model2 + dietary glycemic load + dietary total fat + dietary total fiber + total magnesium | 1.00 (reference) | 0.96 (0.69–1.41) | 0.65 (0.44–0.97) | 0.50 (0.33–0.76) | 0.65 (0.44–0.96) | 0.003 |
RR (95% CI) adjusted for age, race/ethnicity, total energy intake
RR (95% CI) adjusted for age, race/ethnicity, total energy intake, income, education, smoking, alcohol intake, family history of diabetes, use of postmenopausal hormone therapy, systolic blood pressure, diastolic blood pressure, BMI, physical activity, and an interaction term between quintiles of low-fat dairy food and BMI.
Linear trends were tested by using the median value of each category as an ordinal variable.
There was no significant association of high-fat dairy product intake with incident type 2 diabetes before or after adjustment for covariates (data not shown). There was a significant association of total dairy food intake with increased risk of type 2 diabetes in the model adjusted only for age, race, and energy intake, but the association was attenuated by further adjustment and was not significant in the fully adjusted model (Table 3). Yogurt consumption also was associated with a decreased risk of type 2 diabetes and the association persisted after additional multivariable adjustment (Table 4). Compared with women who ate yogurt less than once per month, more frequent yogurt intake was associated with a lower risk of diabetes [1–3 times/mo, RR = 0.61 (95% CI = 0.41–0.92); once per week, RR = 0.55 (95% CI = 0.37–0.82); more than twice per week, RR = 0.46 (95% CI = 0.31–0.68)]. There was no significant association of any type of dairy product intake with measured weight change between baseline and y 3 after adjustment for energy intake.
TABLE 3.
RR of incident diabetes in postmenopausal women by quintile of total dairy product intake
| Quintiles of intake |
||||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend3 | |
| n | 17,894 | 16,032 | 16,051 | 16,047 | 16,049 | |
| Person years | 133,432 | 120,668 | 121,239 | 121,926 | 121,662 | |
| Diabetes cases, n (%) | 968 (5.4) | 807 (5.0) | 724 (4.5) | 701 (4.4) | 746 (4.7) | |
| Age, race, energy1 | 1.00 (reference) | 0.87 (0.79–0.96) | 0.80 (0.73–0.89) | 0.76 (0.69–0.84) | 0.79 (0.72–0.87) | <0.0001 |
| Multivariable model2 | 1.00 (reference) | 0.91 (0.83–1.00) | 0.85 (0.77–0.94) | 0.83 (0.75–0.92) | 0.90 (0.82–1.00) | 0.03 |
| Multivariable model2 + dietary glycemic load | 1.00 (reference) | 0.91 (0.83–1.00) | 0.85 (0.77–0.94) | 0.83 (0.75–0.93) | 0.91 (0.82–1.00) | 0.03 |
| Multivariable model2 + dietary total fat | 1.00 (reference) | 0.92 (0.84–1.02) | 0.87 (0.78–0.96) | 0.86 (0.78–0.96) | 0.96 (0.87–1.07) | 0.39 |
| Multivariable model2 + dietary fiber | 1.00 (reference) | 0.92 (0.83–1.01) | 0.85 (0.77–0.94) | 0.84 (0.75–0.93) | 0.90 (0.82–1.00) | 0.03 |
| Multivariable model2 + total magnesium | 1.00 (reference) | 0.92 (0.83–1.01) | 0.86 (0.77–0.95) | 0.84 (0.76–0.94) | 0.92 (0.83–1.02) | 0.09 |
| Multivariable model2 + dietary glycemic load + dietary total fat + dietary total fiber + total magnesium | 1.00 (reference) | 0.92 (0.83–1.01) | 0.86 (0.78–0.95) | 0.85 (0.76–0.95) | 0.93 (0.83–1.04) | 0.15 |
RR (95% CI) adjusted for age, race/ethnicity, total energy intake.
RR (95% CI) adjusted for age, race/ethnicity, total energy intake, income, education, smoking, alcohol intake, family history of diabetes, use of postmenopausal hormone therapy, systolic blood pressure, diastolic blood pressure, BMI, and physical activity.
Linear trends were tested by using the median value of each category as an ordinal variable.
TABLE 4.
RR of incident diabetes in postmenopausal women by frequency of yogurt intake
| Frequency of yogurt intake |
|||||
| <1/mo | 1/mo to≤3/mo | >3/mo to <2/wk | ≥2/wk | P-trend3 | |
| n | 31,198 | 14,202 | 18,687 | 17,986 | |
| Person years | 231,201 | 107,565 | 142,322 | 137,839 | |
| Diabetes cases, n (%) | 1816 (5.8) | 674 (4.8) | 740 (4.0) | 716 (4.0) | |
| Age, race, energy1 | 1.00 (reference) | 0.72 (0.58, 0.88) | 0.55 (0.45,0.68) | 0.52 (0.42, 0.64) | <0.0001 |
| Multivariable model#x2020 | 1.00 (reference) | 0.61 (0.40, 0.92) | 0.54 (0.37, 0.80) | 0.44 (0.30, 0.66) | 0.002 |
| Multivariable model2 + dietary glycemic load | 1.00 (reference) | 0.61 (0.40, 0.91) | 0.54 (0.37, 0.80) | 0.45 (0.30, 0.67) | 0.003 |
| Multivariable model2 + dietary total fat | 1.00 (reference) | 0.61 (0.41, 0.92) | 0.55 (0.37, 0.82) | 0.46 (0.31, 0.69) | 0.005 |
| Multivariable model2 + dietary fiber | 1.00 (reference) | 0.61 (0.41, 0.92) | 0.55 (0.37, 0.81) | 0.45 (0.30, 0.67) | 0.003 |
| Multivariable model2 + total magnesium | 1.00 (reference) | 0.61 (0.41, 0.93) | 0.55 (0.37, 0.81) | 0.45 (0.30, 0.67) | 0.003 |
| Multivariable model2 + dietary glycemic load + dietary total fat + dietary total fiber + total magnesium | 1.00 (reference) | 0.61 (0.41, 0.92) | 0.55 (0.37, 0.82) | 0.46 (0.31, 0.68) | 0.004 |
RR (95% CI) adjusted for age, race/ethnicity, total energy intake.
RR (95% CI) adjusted for age, race/ethnicity, total energy intake, income, education, smoking, alcohol intake, family history of diabetes, use of postmenopausal hormone therapy, systolic blood pressure, diastolic blood pressure, BMI, physical activity, and an interaction term between quintiles of yogurt intake and time.
Linear trends were tested by using the median value of each category as an ordinal variable.
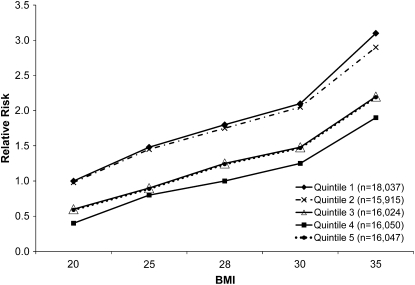
Tests of interaction did not reveal effect modification by race, physical activity, or family history of diabetes. However, there was a modest degree of effect modification by BMI, with the lower risk associated with low-fat dairy product consumption being somewhat more pronounced in women with higher BMI (compared with the first quintile of low-fat dairy foods, P-interaction = 0.008 between the 4th quintile of low-fat dairy foods and BMI; P-interaction = 0.046 between the 5th quintile of low-fat dairy foods and BMI). The effect of this interaction is subtle and best illustrated by example. If a woman with a BMI of 20 kg/m2 and low-fat dairy product intake in the lowest quintile is considered the reference case and has a RR of 1.0 for new-onset diabetes, the RR for a woman with a similar BMI and low-fat dairy food intake in the 4th quintile is 0.5 and in the 5th quintile is 0.6. For a woman with a BMI of 35 kg/m2 and low-fat dairy food intake in the lowest quintile, the RR is 3.1 for new-onset diabetes, and the RR for a woman with a similar BMI and low-fat dairy food intake in the 4th quintile is 1.9 and in the 5th quintile is 2.2 (Fig. 1).
FIGURE 1.
RR of incident diabetes in postmenopausal women by quintile of low-fat dairy product intake at various levels of BMI.
Discussion
Our results show that low-fat dairy product and yogurt consumption is associated with a lower risk of incident type 2 diabetes in postmenopausal women followed for roughly 8 y. The recommended intake of dairy foods for women over age 50 y is three 8-oz. cups (250 g) of milk (or equivalent) per day (25). This corresponds roughly to the median intake in the 5th quintile for both low-fat dairy and total dairy foods. Therefore, another way of interpreting these results is that relative to intake of the recommended amount, intake far less than the recommended amount is associated with increased diabetes risk. Unlike previous studies, the WHI measured weight change between baseline and 3 y of follow-up. The reduced risk was not mediated by an effect of low-fat dairy foods on weight, because there was no association of low-fat dairy food intake with weight change. We also observed a modest interaction of low-fat dairy food intake with BMI, which has not been reported in previous studies.
Previous research on dairy products and the risk of type 2 diabetes has also suggested a lower risk in men (10) and both black (12) and white women (12). As in the current study, which was restricted to women in the postmenopausal years, the lower risk of diabetes was found only for consumption of low-fat dairy products. In the study of middle-aged and older men, the only specific dairy food that was significantly associated with a lower risk of diabetes was skim or low-fat milk (10). In the study of middle-aged women in the WHI, the only specific dairy food that had a significant inverse relationship with diabetes was yogurt (11). The confirmation of this finding in the WHI suggests that the association was not due to chance. Because yogurt consumption is associated with other healthy behaviors, such as physical activity, not smoking, and consuming a diet high in fiber (26), it is important to adjust for potential confounders, as was done in both of these studies.
A potential mechanism by which low-fat dairy food intake could lower the risk of type 2 diabetes may relate to the effect of milk proteins on release of gut hormones such as glucose-dependent insulinotropic polypeptide and glucagon-like peptide, hormones secreted from the gut that augment insulin secretion and slow the absorption of nutrients (27). However, because milk proteins are similar in low- and high-fat milk, the differential association of low-fat dairy products with diabetes risk would not be explained by this mechanism. Calcium, magnesium, vitamin D, and the low glycemic load of dairy products have also been postulated to lower the risk of diabetes (12, 28). However, because adjustment for each of these did not materially change our results, it is unlikely that they account for the findings. Another possibility is that women who drink more milk are less likely to have high intake of other foods, particularly sweetened beverages, that increase diabetes risk in women (29, 30). However, the findings did not change after adjustment for intake of soft drinks or fruit juice.
Given that there is no clear mechanism for the inverse association of low-fat dairy product or yogurt consumption and type 2 diabetes, it is worth considering the possibility that our findings result from unmeasured or residual confounding despite extensive adjustment for other dietary variables and risk factors for diabetes. In fact, adjustment for dietary fat intake did attenuate the inverse association more than adjustment for other dietary variables. Another potential limitation of our study is that diabetes incidence was measured by self-report. However, concordance of treated diabetes self-report with medication inventories and medical records was good. Strengths of the study include the large size of the WHI-OS, comprehensive data on the characteristics of the participants, and repeated measures using an FFQ with detailed questions about low-fat dairy products.
3We conclude that a diet high in low-fat dairy products is associated with lower diabetes risk in postmenopausal women, particularly those who are obese. The consistency of the association of low-fat dairy food intake with lower type 2 diabetes risk between studies and by age, race, and sex is striking. Further research into potential mechanisms and dietary patterns characterized by consumption of low-fat dairy foods is needed.
Acknowledgments
K.L.M., I.H.d.B., B.V.H., S.L., J.E.M., Y.M-R., L.S.P., J.M.S., and L.F.T. designed and conducted research; F.W. analyzed data; K.L.M. and F.W. wrote the paper, and K.L.M. had final responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the National Heart, Lung, and Blood Institute, NIH, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. Drs. Margolis and Wei received grant funding from the General Mills Bell Institute of Health and Nutrition.
Literature Cited
- 1.American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 2.Rosell M, Hakansson NN, Wolk A. Association between dairy food consumption and weight change over 9 y in 19 352 perimenopausal women. Am J Clin Nutr. 2006;84:1481–8 [DOI] [PubMed] [Google Scholar]
- 3.Zemel MB. Mechanisms of dairy modulation of adiposity. J Nutr. 2003;133:S252–6 [DOI] [PubMed] [Google Scholar]
- 4.Teegarden D. The influence of dairy product consumption on body composition. J Nutr. 2005;135:2749–52 [DOI] [PubMed] [Google Scholar]
- 5.Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA study. JAMA. 2002;287:2081–9 [DOI] [PubMed] [Google Scholar]
- 6.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr. 2005;82:523–30 [DOI] [PubMed] [Google Scholar]
- 7.Elwood PC, Pickering JE, Fehily AM. Milk and dairy consumption, diabetes and the metabolic syndrome: the Caerphilly prospective study. J Epidemiol Community Health. 2007;61:695–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler S, Hamman R, Lachin J, Walker E, Nathan D, The Diabetes Prevention Program Research Group Reduction in the incidence of Type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuomilehto J, Lindstrom J, Erikkson J, Valle T, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. Prevention of Type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50 [DOI] [PubMed] [Google Scholar]
- 10.Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med. 2005;165:997–1003 [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Choi HK, Ford E, Song Y, Klevak A, Buring JE, Manson JE. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care. 2006;29:1579–84 [DOI] [PubMed] [Google Scholar]
- 12.van Dam RM, Hu FB, Rosenberg L, Krishnan S, Palmer JR. Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care. 2006;29:2238–43 [DOI] [PubMed] [Google Scholar]
- 13.Design of the Women's Health Initiative clinical trial and observational study The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- 14.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–17 [DOI] [PubMed] [Google Scholar]
- 15.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77 [DOI] [PubMed] [Google Scholar]
- 16.Langer RD, White E, Lewis C, Kotchen J, Hendrix S, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21 [DOI] [PubMed] [Google Scholar]
- 17.Patterson RE, Kristal A, Tinker L, Carter R, Bolton M, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87 [DOI] [PubMed] [Google Scholar]
- 18.Henderson MM, Kushi L. Thompson D, Gorbach S, Clifford C, Insull W, Moskowitz M, Thompson R. Feasibility of a randomized trial of low-fat diet for the prevention of breast cancer: dietary compliance in the Women's Health Trial Vanguard Study. Prev Med. 1990;19:115–33 [DOI] [PubMed] [Google Scholar]
- 19.Bowen D, Clifford C, Coates R, Evans M, Feng Z, Fouad M, George V, Gerace T, Grizzle J, Hall W, et al. The Women's Health Trial Feasibility Study in Minority Populations: design and baseline descriptions. Ann Epidemiol. 1996;6:507–19 [DOI] [PubMed] [Google Scholar]
- 20.The Women’s Health Initiative. Scientific Resources Web site [cited 2011 Aug 19]. Available from: http://www.whiscience.org/data/forms/whi/F60v1_6.pdf
- 21.Margolis KL, Lihong Q, Brzyski R, Bonds DE, Howard BV, Kempainen S, Simin L, Robinson JG, Safford MM, Tinker LT, et al. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5:240–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu FB, Stampfer M, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–40 [DOI] [PubMed] [Google Scholar]
- 23.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26 [Google Scholar]
- 24.Willett WC. Nutritional epidemiology. New York, Oxford University Press; 1998 [Google Scholar]
- 25.Dietary Guidelines Advisory Committee Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. Washington, DC: USDA, Agricultural Research Service; 2010 [Google Scholar]
- 26.Mossavar-Rahmani Y, Garland C, Caan B, Herbert JR, Wodarski LA, Vitolins MZ, Himes JH, Parker LM. Yogurt consumption is associated with healthy behavior in postmenopausal women. Clin J Womens Health. 2002;2:128–34 [Google Scholar]
- 27.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–53 [DOI] [PubMed] [Google Scholar]
- 28.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–6 [DOI] [PubMed] [Google Scholar]
- 29.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168:1487–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–34 [DOI] [PubMed] [Google Scholar]