Abstract
Muscle protein synthesis requires energy and amino acids to proceed and can be stimulated by insulin under certain circumstances. We hypothesized that short-term provision of insulin and nutritional energy would stimulate muscle protein synthesis in healthy subjects only if amino acid availability did not decrease. Using stable isotope techniques, we compared the effects on muscle phenylalanine kinetics across the leg of an amino acid-lowering, high-energy (HE, n = 6, 162 ± 20 kcal/h) hyperglycemic hyperlipidemic hyperinsulinemic clamp with systemic insulin infusion to a low-energy (LE, n = 6, 35 ± 3 kcal/h, P < 0.05 vs. HE) euglycemic hyperinsulinemic clamp with local insulin infusion in the femoral artery. Basal blood phenylalanine concentrations and phenylalanine net balance, muscle protein breakdown, and synthesis (nmol·min−1·100 g leg muscle−1) were not different between groups. During insulin infusion, femoral insulinemia increased to a similar extent between groups and blood phenylalanine concentration decreased 27 ± 3% in the HE group but only 9 ± 2% in the LE group (P < 0.01 HE vs. LE). Phenylalanine net balance increased in both groups, but the change was greater (P < 0.05) in the LE group. Muscle protein breakdown decreased in the HE group (58 ± 12 to 35 ± 7 nmol·min−1 ·100 g leg muscle−1) and did not change in the LE group. Muscle protein synthesis was unchanged in the HE group (39 ± 6 to 30 ± 7 nmol·min−1 ·100 g leg muscle−1) and increased (P < 0.05) in the LE group (41 ± 9 to 114 ± 26 nmol·min−1 ·100 g leg muscle−1). We conclude that amino acid availability is an important factor in the regulation of muscle protein synthesis in response to insulin, as decreased blood amino acid concentrations override the positive effect of insulin on muscle protein synthesis even if excess energy is provided.
Muscle protein synthesis requires energy and amino acids to proceed (1, 40) and can be stimulated by insulin under certain circumstances (2). Insulin is a potent stimulus for muscle protein anabolism; however, the mechanisms by which insulin enhances muscle protein anabolism are still debated. A stimulatory effect of insulin on protein synthesis has been demonstrated in various tissues, including skeletal muscle (13, 29). Moreover, in vitro and animal studies, along with recent work in humans (22), have shown that insulin can acutely stimulate muscle protein synthesis by increasing the initiation of mRNA translation (20, 21, 28).
Previous investigations have definitively shown that amino acids provided orally (31, 36) or intravenously (1, 4, 32) stimulate human muscle protein synthesis due to the increase in blood amino acid concentrations. In addition, the amount of energy provided by the meal may also affect muscle protein synthesis (8, 33, 34, 40). For example, the provision of excess energy may compensate for the deleterious effects of stress on protein retention (6, 37). Muscle protein synthesis is an energy-consuming process using ~0.7 kcal/g of protein synthesized, or 240 kcal/day for an average person (41). Thus, for muscle protein synthesis to proceed, the muscle cell should have an adequate energy supply.
The infusion of insulin into human subjects results in the net uptake of amino acids (1, 2, 11, 14, 16, 17, 23–25, 27, 42). Several studies have reported an increase in human muscle protein synthesis in response to insulin (1, 2, 17, 25, 27, 42). However, others have reported an inhibition of muscle proteolysis with no significant changes in muscle protein synthesis (11, 14, 16, 23, 24). We (44) have previously hypothesized that these different results might be explained by differences in amino acid availability. The mode of insulin infusion appears to be a major factor in determining whether or not muscle protein synthesis will be stimulated. For example, systemic insulin infusions decrease blood amino acid concentrations (10, 11, 24, 27, 35) unless amino acids are replaced by an exogenous infusion (15, 17, 24, 25, 27). On the other hand, local insulin infusion into the leg exposes the muscle tissue to relatively high insulin levels while avoiding a major reduction in blood amino acid concentrations (2, 23). Therefore, the studies that report an increase in muscle protein synthesis by insulin infusion also show an increased amino acid delivery to the muscle (1, 2, 15, 17, 25, 27, 42). Interestingly, most studies reporting no change in amino acid delivery and/or a reduction in blood amino acid concentrations with systemic insulin infusion also report that muscle proteolysis is inhibited (11, 16, 23, 24).
We hypothesized that insulin and nutritional energy stimulate skeletal muscle protein synthesis provided that blood amino acid availability is not reduced. To test our hypothesis, we examined the differential effects of an amino acid-lowering systemic insulin infusion with a high-energy clamp (hyperglycemic hyperlipidemic) vs. a local insulin infusion in the femoral artery with a low-energy euglycemic clamp that did not induce major decreases in blood amino acid concentrations.
MATERIALS AND METHODS
Subjects
We studied 12 young subjects (8 men and 4 women) from the Los Angeles metropolitan area. All subjects were healthy and physically active but were not engaged in an exercise training program. Screening of subjects was performed with clinical history, physical examination, and laboratory tests, including complete blood count with differential liver and kidney function tests, coagulation profile, fasting blood glucose, and oral glucose tolerance test (OGTT), hepatitis B and C screening, HIV test, TSH, lipid profile, pregnancy test in women, urinalysis, drug screening, and ECG, and females were not taking oral contraceptives. Only subjects with normal screening results were assigned to one of two groups: high-energy hyperglycemic hyperinsulinemic hyperlipidemic clamp with a systemic insulin infusion (HE) or a low-energy euglycemic hyperinsulinemic clamp with local insulin infusion in the femoral artery (LE). The subjects’ characteristics are summarized in Table 1.
Table 1.
Physical characteristics of subjects in HE or LE insulin infusion groups
| Subjects’ Characteristics | HE | LE |
|---|---|---|
| No. of subjects | 6 | 6 |
| Sex | 6M/0F | 2M/4F |
| Age, yr | 35 ± 3* | 23 ± 1 |
| Height, cm | 170 ± 6 | 167 ± 5 |
| Body weight, kg | 81 ± 6* | 59 ± 4 |
| Body mass index, kg/m2 | 27 ± 1* | 21 ± 1 |
| Fat-free mass, kg | 59 ± 4 | 50 ± 5 |
| Body fat, % | 23 ± 1 | 20 ± 3 |
| Leg volume, liters | 9.1 ± 0.5 | 7.9 ± 0.5 |
| Leg muscle mass, kg | 9.5 ± 0.7 | 7.3 ± 0.9 |
Values are means ± SE. HE, high-energy hyperglycemic hyperinsulinemic hyperlipidemic clamp with a systemic insulin infusion; LE, low-energy euglycemic hyperinsulinemic clamp with local insulin infusion in the femoral artery.
P < 0.05 vs. LE.
All subjects gave informed, written consent before participating in the study, which was approved by the Institutional Review Board of the University of Southern California (Los Angeles, CA).
Study design
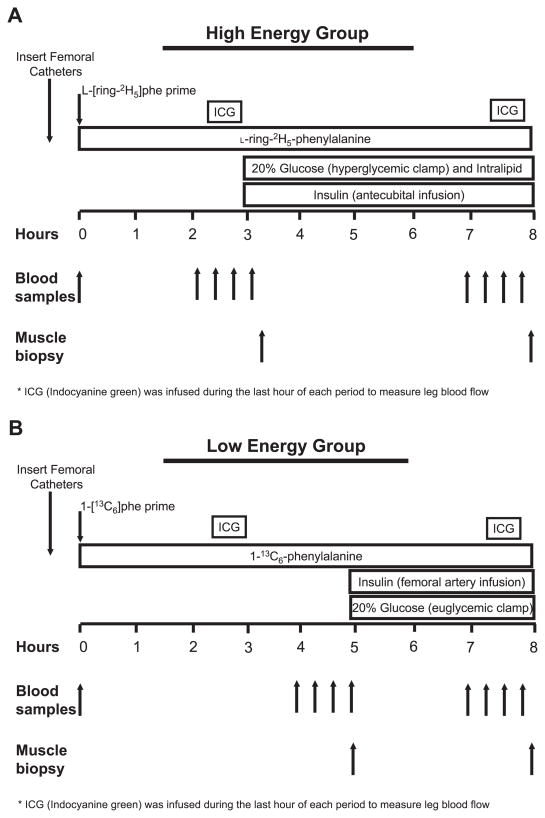
The protocol was designed to measure muscle protein and amino acid kinetics in the postabsorptive basal state and during a systemic or local insulin infusion. The night before the study, each subject was admitted to the General Clinical Research Center of the University of Southern California. Subjects refrained from exercise for 24 h before study participation. At admission, a dual-energy X-ray absorptiometry (DEXA) scan (Hologic QDR 4500W, Bedford, MA) was performed to measure body composition and muscle mass, and a pregnancy test was repeated in women. The subjects were then fed a standard dinner, and a snack was given at 2200. The subjects were studied after an overnight fast under basal conditions and during a hyperinsulinemic clamp; thus, after 2200, the subjects were allowed only water ad libitum until the end of the experiment. The morning of the study, polyethylene catheters were inserted into a forearm vein for tracer and glucose infusion, in a contralateral hand vein that was heated for arterialized blood sampling, and in the femoral artery and vein of one leg for blood sampling. The forearm catheter was also used for lipid and insulin in the HE group. The arterial catheter was also used for the infusion of insulin (in the LE group) and indocyanine green (ICG; Akorn, Buffalo Grove, IL).
After drawing of a blood sample for the measurement of background amino acid enrichments and ICG concentration, a primed continuous infusion of L-[ring-13C6]phenylalanine or L-[ring-2H5]-phenylalanine (Cambridge Isotope Laboratories, Andover, MA) was started (time = 0 h) and maintained at a constant rate until the end of the experiment (Fig. 1). The following priming dose for the labeled phenylalanine was 2 μmol/kg, and the infusion rate was 0.05 μmol·kg−1 ·min−1.
Fig. 1.
Study design for the high-energy and low-energy insulin infusion groups. HE, high-energy hyperglycemic hyperinsulinemic hyperlipidemic clamp with a systemic insulin infusion; LE, low-energy euglycemic hyper-insulinemic clamp with local insulin infusion in the femoral artery.
Subjects were divided into two groups: a systemic insulin infusion with a high nutritional energy infusion (HE) and a local insulin infusion with a low nutritional energy infusion (LE). Both experiments lasted 8 h (Fig. 1), with the HE group undergoing a 5-h insulin and nutritional energy infusion and the LE group undergoing a 3-h insulin and nutritional energy infusion.
At the beginning of the last hour of the basal period, the continuous infusion of ICG was started in the femoral artery (0.5 mg/min) and maintained for 30 min. During ICG infusion, blood samples were taken four times, at 10-min intervals, from the femoral vein and the hand vein to measure ICG concentration (Fig. 1). Subsequently, the next 30 min, four blood samples were taken from the femoral artery and vein and from the hand vein to measure femoral arterial and venous amino acid concentrations and enrichments, glucose concentrations, and insulin concentrations. At the end of the basal period, a first muscle biopsy was taken from the lateral portion of the vastus lateralis from the leg with the femoral catheters, using a 5-mm Bergström biopsy needle, using sterile procedure and local anesthesia with 1% lidocaine injected subcutaneously and on the fascia. The muscle sample (125–375 mg) was rinsed with ice-cold saline and blotted, any visible fat or connective tissue was quickly removed, and it was immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Immediately after the first biopsy, in the LE group a local insulin infusion (0.15 mU·min−1 ·100 ml−1) was initiated into the femoral artery, and in the HE group a systemic insulin infusion (0.5 mU·kg−1 ·min−1) was initiated into a hand vein. After the start of the insulin infusion, blood samples (0.5 ml) were taken every 5–10 min to monitor the plasma glucose concentration. Dextrose (20%) was then infused at a variable rate as necessary to clamp the plasma glucose concentration at the basal value in the LE group and at ~140 mg/dl in the HE group. Intralipid (0.7 ml·kg−1 ·min−1) and heparin (7 U·kg−1 ·min−1) (Baxter, Deerfield, IL) were also infused in the HE group to further increase the amount of nutritional energy in the form of triglycerides and fatty acids.
At the beginning of the last hour of the insulin and nutritional energy infusion, ICG was again infused to measure leg blood flow, and blood samples were taken as described for the basal period. Given the different insulin infusion routes (femoral artery in LE and forearm vein in HE), insulin concentrations were measured at different sites in the two groups. Specifically, in the LE group, insulin concentrations were measured both in the arterialized hand vein and in the femoral vein to determine the systemic and femoral insulin concentrations, respectively. In the HE group, we determined insulin concentrations in the femoral arterial blood only, which represented both the systemic and the femoral insulin exposure due to the fact that insulin was infused systemically. At the end of the infusion period, before the tracer, nutritional energy, and insulin infusions were stopped, a second muscle biopsy was taken as described above. The same incision was used to obtain each muscle biopsy; however, the biopsy needle was inclined at different angles each time so that the second biopsy was taken ~2 in. (~5 cm) apart from the first.
Analysis
Plasma insulin concentrations were determined by radio-immunoassay (Diagnostic Products, Los Angeles, CA). Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA) at λ = 805 nm (19). Plasma glucose concentration was measured using an automated glucose analyzer (YSI, Yellow Springs, OH).
Sulfosalicylic acid (15%) was used to precipitate and separate plasma amino acids, and the supernatant was eluted through a cation exchange column with 4 M NH4OH (43). Concentrations and enrichments of blood phenylalanine were determined on their tert-butyldimethylsilyl derivatives (t-BDMS) using an appropriate internal standard, with gas chromatography-mass spectrometry (GC-MS; 6890 Plus GC, 5973N MSD/DS, 7683 autosampler; Agilent Technologies, Palo Alto, CA) as previously described (43).
Muscle tissue samples were ground, and intracellular free amino acids and muscle proteins were extracted as previously described (43). Intracellular free concentrations and enrichments of phenylalanine were determined by GC-MS using an appropriate internal standard (43). Mixed-muscle protein-bound phenylalanine enrichment was analyzed by GC-MS after protein hydrolysis and amino acid extraction (43), using the external standard curve approach (7).
Calculations
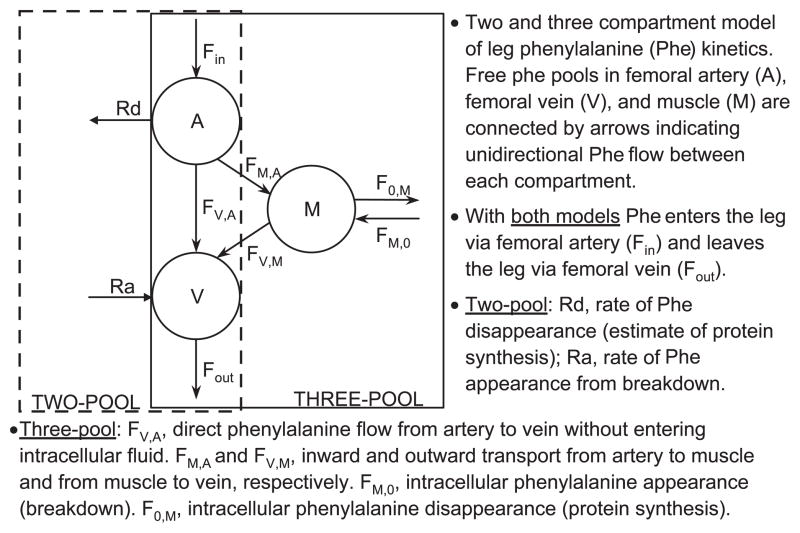
The kinetics of muscle phenylalanine were calculated using two different arteriovenous methods: the traditional two-pool model (43) and the three-pool model (3). We used both models because each of them provides unique information regarding muscle amino acid kinetics (Fig. 2). Additionally, although the three-pool model provides more detailed information regarding intracellular amino acid kinetics, this is a fairly new method and is used by only a few groups. The two-pool model has been used by a number of research groups, thus allowing for a comparison of our results with data collected by others. The three-pool model is an expansion of the two-pool model and relies not only on the measurement of the amino acid enrichments and concentrations in the femoral artery and vein but also on the direct measurement of the amino acid enrichment in the free tissue water. This allows for the direct measurement of the amino acid intracellular utilization for protein synthesis and release from protein breakdown. In addition, it is possible to calculate the rate of phenylalanine transport from the artery into the tissue and from the tissue into the venous blood.
Fig. 2.
Protein kinetic models.
The two-pool and three-pool models share the following parameters:
| (1) |
| (2) |
| (3) |
The other kinetic parameters of the two-pool method were calculated as follows:
| (4) |
| (5) |
| (6) |
CA and CV are the blood amino acid concentrations in the femoral artery and vein, respectively; Ra and Rd are rate of appearance and disappearance, respectively; EA and EV are the amino acid enrichments, expressed as tracer-to-tracee ratio, in the femoral arterial and venous blood, respectively; BF is leg blood flow, as calculated from the steady-state ICG concentration values in the femoral and wrist veins, as previously described (19). Kinetic data are expressed per 100 grams of lean leg mass (assumed to be primarily muscle), which was assessed via DEXA.
The specific parameters of the three-pool model were calculated as follows:
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
where EM is the amino acid enrichment, expressed as tracer/tracee ratio, in the muscle.
Additionally, we calculated the intracellular amino acid availability as the sum of transport into the muscle FM,A and the intracellular rate of appearance FM,O.
| (12) |
It is also possible to calculate the efficiency of amino acid utilization for muscle protein synthesis as follows:
| (13) |
We also calculated the fractional synthetic rate (FSR) of mixed-muscle proteins during the clamp by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEP/t) and using the precursor-product model to calculate the synthesis rate:
| (14) |
where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, and EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Data are expressed as percent per hour.
Leg glucose utilization was calculated as net glucose uptake across the leg:
| (15) |
The energy content provided during the infusion was calculated on the basis of the Atwater general factors for dietary carbohydrates (4 kcal/g) and lipids (9 kcal/g), which provides the net metabolizable energy value available to the body.
Statistical analysis
All values are expressed as means ± SE. Comparisons were performed using analysis of variance with repeated measures, the effects being subject, group (HE, LE), and time (basal, insulin) and using JMP statistical software v. 4.0.5 (SAS Institute). In addition, analysis of covariance was performed using sex and body mass index (BMI) as covariates to account for any potential influence of these two variables on the response of muscle protein metabolism to the treatments. Post hoc testing was performed using the Tukey-Kramer when appropriate. The two-tailed unpaired Student’s t-test was used to compare subject characteristics, FSR, and energy delivered during the clamp. Significance was set at P ≤ 0.05.
RESULTS
Subjects’ characteristics
The demographic and physical characteristics of each group (LE and HE) are shown in Table 1. The HE group was slightly older, had a greater body weight compared with the LE group, and had more male subjects. As a result, the HE group had a significantly greater BMI (kg/m2, P < 0.05). However, leg volume and leg muscle mass were not significantly different between groups. In addition, there were no differences in the area under the curve for basal glucose concentrations during the OGTT between the HE and LE groups (data not shown). Thus insulin sensitivity for glucose metabolism was not different between groups.
Blood flow
There were no differences in basal leg blood flow (ml·min−1 ·100 ml leg−1) between HE and LE (3.0 ± 0.4 vs. 4.2 ± 1.0, respectively; Table 2). During insulin infusion, blood flow increased (P < 0.05) in both the HE and LE groups, with no significant differences between groups (3.9 ± 0.5 vs. 6.9 ± 1.5, respectively).
Table 2.
Glucose and insulin concentrations, leg glucose uptake, and blood flow at baseline and during HE or LE insulin infusion
| HE | LE | ||
|---|---|---|---|
| Glucose concentrations, mmol/l | |||
| Femoral artery | Basal | 5.39 ± 0.20 | 4.86 ± 0.15 |
| Clamp | 8.26 ± 0.14*† | 4.81 ± 0.18 | |
| Femoral vein | Basal | 5.26 ± 0.21 | 4.78 ± 0.15 |
| Clamp | 7.65 ± 0.25*† | 4.57 ± 0.18 | |
| Leg glucose uptake, μmol·min−1·100 ml leg−1 | |||
| Basal | 0.36 ± 0.04 | 0.24 ± 0.09 | |
| Clamp | 2.48 ± 0.96† | 1.29 ± 0.25† | |
| Insulin, μU/ml | |||
| Femoral | Basal | 5.5 ± 1.1 | 11.7 ± 0.5 |
| Clamp | 40.0 ± 4.0†‡ | 48.3 ± 6.9†‡ | |
| Wrist vein | Basal | ND | 12.1 ± 0.4 |
| Clamp | ND | 19.7 ± 1.1† | |
| Blood flow, ml ·min−1·100 ml leg−1 | |||
| Basal | 3.0 ± 0.4 | 3.9 ± 0.5 | |
| Clamp | 4.2 ± 1.0 | 6.9 ± 1.5 | |
| Energy, kcal | |||
| 808 ± 104* | 106 ± 10 | ||
Values are means ± SE. Nutritional energy provision is shown for the clamp period.
P < 0.05 vs. LE;
P < 0.05 vs. basal;
P < 0.05 vs. wrist vein.
Insulin and glucose concentrations
There were no basal differences in femoral vein or artery and wrist vein insulin concentrations among the two groups (Table 2). Systemic and local insulin infusions increased (P < 0.001) femoral insulin concentration in both groups, with no differences between groups (Table 2). Also, the systemic insulin concentrations increased significantly (P < 0.001) in both groups, but such an increase was much smaller in the LE group than in the HE group, with a significant time-by-group interaction (P < 0.001).
The results of glucose concentrations are summarized in Table 2. Plasma arterial and venous glucose concentrations were not different between the two groups during the basal period. Blood glucose concentrations were significantly higher in the HE group than in the LE group during the clamp (P < 0.001) due to the study design (hyperglycemic vs. euglycemic clamp).
Net leg glucose uptake (Table 2) was not different between groups during the basal period and significantly increased during the clamp in both groups (P < 0.05), with no significant differences between groups.
Nutritional energy
During the clamp, the amount of energy provided intravenously in the form of carbohydrate and lipid was nearly eightfold higher in the HE group compared with the LE group (Table 2). In addition, the amount of energy provided per hour was also significantly greater (P < 0.05) in the HE group (162 ± 21 kcal/h) compared with the LE group (35 ± 3 kcal/h).
Amino acid enrichments and concentrations
The average amino acid concentrations and enrichments in the femoral artery and vein and in the muscle are reported in Table 3. During the basal period, phenylalanine concentrations in the femoral artery and vein and in the muscle tissue were not different between groups. Phenylalanine arterial concentrations significantly decreased (P < 0.01) in both groups during the clamp, but to a significantly greater extent in the HE group (27 ± 3%) compared with the LE group (9 ± 2%, P < 0.05).
Table 3.
Free phenylalanine concentrations and enrichments in femoral artery and vein and in muscle tissue at baseline and during HE or LE insulin infusion
| HE | LE | ||
|---|---|---|---|
| Phenylalanine concentrations, μmol/l | |||
| Artery | Basal | 56± 3 | 55± 2 |
| Clamp | 41± 2*† | 50± 2† | |
| Vein | Basal | 63± 3 | 60± 2 |
| Clamp | 42± 3*† | 48± 2† | |
| Muscle | Basal | 86± 5 | 89± 11 |
| Clamp | 73± 3 | 85± 6 | |
| Phenylalanine enrichments (tracer/tracee) | |||
| Artery | Basal | 0.068± 0.004 | 0.069± 0.001 |
| Clamp | 0.085± 0.005*† | 0.079± 0.003† | |
| Vein | Basal | 0.051± 0.004 | 0.057± 0.003 |
| Clamp | 0.069± 0.005*† | 0.068± 0.003† | |
| Muscle | Basal | 0.049± 0.005 | 0.046± 0.004 |
| Clamp | 0.066± 0.006*† | 0.049± 0.004 | |
Values are means ± SE.
P < 0.05 vs. LE;
P < 0.05 vs. basal.
Phenylalanine enrichment in the femoral artery and vein were not different between groups in the basal or clamp periods (Table 3). During the clamp, phenylalanine enrichment in the artery and vein significantly increased in both groups (P < 0.05), and there was a significant time-by-group interaction (P < 0.05) due to a larger increase in the HE group. Phenylalanine enrichment in the muscle tissue was not different between groups in the basal period, and it significantly increased during the insulin infusion only in the HE group (P < 0.05).
Amino acid kinetics
Leg and muscle phenylalanine kinetics are shown in Table 4. All kinetic parameters were not different between groups in the basal period.
Table 4.
Leg phenylalanine kinetics at baseline and during HE or LE insulin infusion
| Phenylalanine Kinetics | HE | LE | |
|---|---|---|---|
| Fin | Basal | 176 ± 34 | 247 ± 36 |
| Clamp | 154 ± 45* | 424 ± 104 | |
| Fout | Basal | 196 ± 36 | 271± 40 |
| Clamp | 159 ± 49 | 409 ± 99 | |
| Leg Ra | Basal | 56 ± 11 | 58 ± 14 |
| Clamp | 33 ± 7 | 73 ± 18 | |
| Leg Rd | Basal | 37 ± 5 | 34 ± 8 |
| Clamp | 28 ± 5* | 89 ± 23† | |
| FM,A | Basal | 156± 38 | 139 ± 34 |
| Clamp | 150 ± 45 | 198 ± 53 | |
| FV,M | Basal | 176 ± 40 | 163 ± 38 |
| Clamp | 155 ± 49 | 183 ± 48 | |
| FV,A | Basal | 20 ± 17 | 109 ± 38 |
| Clamp | 4 ± 20* | 226 ± 59 | |
| Efficiency, % | Basal | 19 ± 2 | 23 ± 3 |
| Clamp | 19 ± 5* | 46 ± 6† | |
| IC availability | Basal | 215 ± 42 | 235 ± 47 |
| Clamp | 185 ± 50 | 298 ± 72 |
Values are means ± SE in nmol·min−1·100 g leg muscle mass−1, except for efficiency (%). See text for definitions.
P < 0.05 vs. LE;
P < 0.05 vs. basal.
Phenylalanine delivery to the leg (Fin) significantly decreased only in the HE group (P < 0.05), whereas the phenylalanine outflow (Fout) from the leg did not change significantly in either group.
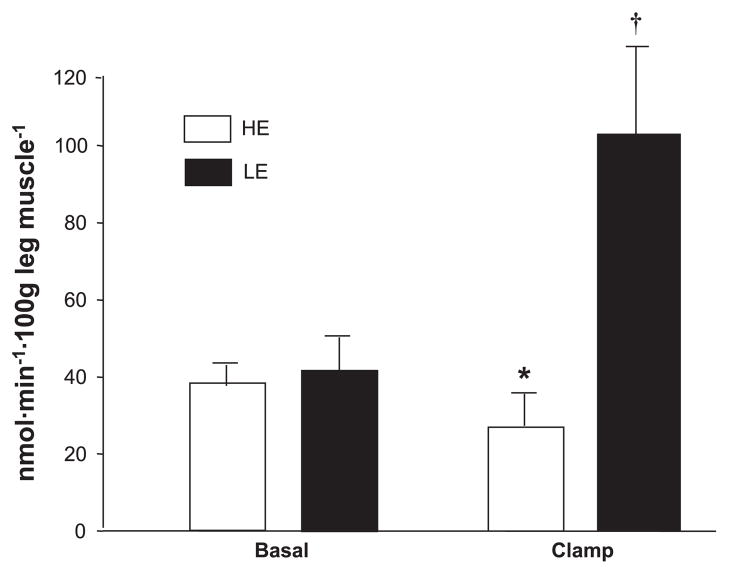
With the two-pool model, the leg rate of appearance (leg Ra) of phenylalanine, an index of leg muscle protein breakdown, did not significantly change during the clamp in either group. The leg rate of disappearance (leg Rd) of phenylalanine, an index of leg muscle protein synthesis, significantly increased only in the LE group, with a time-by-group interaction (P < 0.01).
With the three-pool model, phenylalanine transport into the muscle (FM,A) and out of the muscle (FV,M) did not change significantly during the clamp in either group.
The efficiency of phenylalanine utilization for protein synthesis did not change in the HE group during the clamp, whereas it increased significantly (P < 0.001) in the LE group (Table 4).
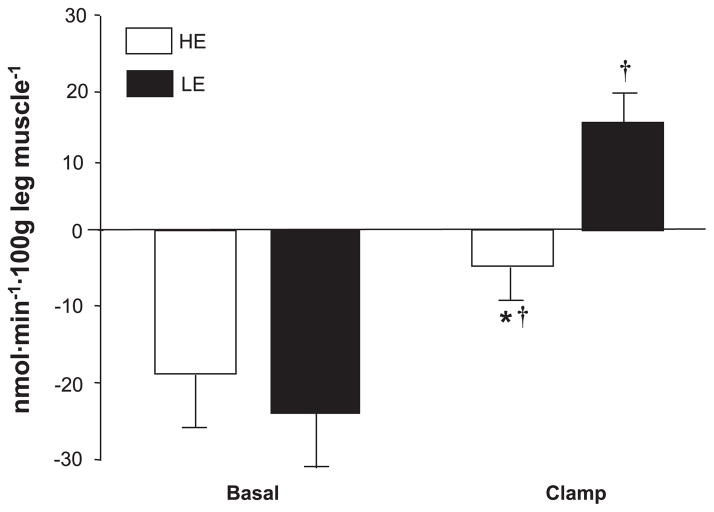
There was a significant time effect for phenylalanine net balance (NB; P < 0.01; Fig. 3), which was mostly due to a large increase (P < 0.05) in the LE group, in which NB also became positive, indicating a shift from net muscle protein catabolism to net muscle protein deposition.
Fig. 3.
Phenylalanine net balance (NB) across the leg for HE and LE insulin infusion groups. Values are means ± SE. *P < 0.05 vs. LE; †P < 0.05 vs. basal.
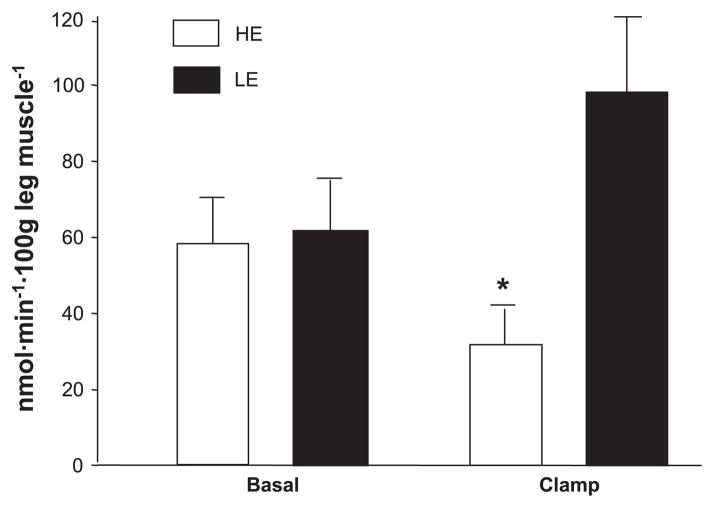
Intracellular amino acid availability remained unchanged during the clamp period and was not different between groups (Table 4). Phenylalanine release from protein breakdown (FM,O) was reduced in the HE group compared with the LE group during the clamp, with a significant time-by-group interaction (Fig. 4). Phenylalanine FO,M, which is a measure of phenylalanine utilization for muscle protein synthesis, increased significantly only in the LE group (time-by-group interaction, P < 0.01), but it did not change in the HE group during the clamp (Fig. 5). FSR of mixed-muscle proteins during the clamp was also significantly higher (0.114 ± 0.015 vs. 0.045 ± 0.003%/h, P < 0.05) in the LE and HE groups, respectively. Overall, muscle protein synthesis was ~2.5-fold higher during the clamp in the LE group vs. the HE group, and this was confirmed by all three methods we used for measuring muscle protein synthesis (i.e., 2-pool, 3-pool, and precursor-product model).
Fig. 4.
Release of phenylalanine from muscle protein breakdown (FM,0), at baseline and during infusion of insulin for HE and LE insulin infusion groups. Values are means ± SE. *P < 0.05 vs. LE.
Fig. 5.
Utilization of phenylalanine for muscle protein synthesis (F0,M), at baseline and during infusion of insulin for HE and LE insulin infusion groups. Values are means ± SE. *P < 0.05 vs. LE; †P < 0.05 vs. basal.
DISCUSSION
Our results indicate that amino acid availability is the predominant factor regulating the stimulation of human muscle protein synthesis. In particular, if amino acid availability decreases during an insulin infusion, muscle protein synthesis is not stimulated even in the presence of a large amount of nutritional energy; thus overall net balance fails to reach a positive value (HE group). However, if amino acid availability is not reduced, as we have shown by infusing insulin locally into the femoral artery, insulin and a small amount of energy can significantly increase the rate of muscle protein synthesis and improve net balance (LE group). Our data provide evidence that the stimulation of muscle protein synthesis by insulin is dependent on amino acid availability; however, the role of insulin is not only permissive because it can directly stimulate muscle protein synthesis if blood amino acid concentrations are maintained at the baseline value. Interestingly, when amino acid availability is not reduced during exposure to insulin, muscle protein breakdown is not altered. Thus maintenance of blood amino acid concentrations during the local insulin infusion, even in the absence of nutritional energy, results in net protein deposition, as indicated by the significant shift in net balance from a net catabolic state under basal conditions to a net anabolic state during the hyperinsulinemic clamp. However, if a systemic insulin infusion is used and the blood amino acid concentrations decrease, muscle protein synthesis is not stimulated, muscle protein breakdown is reduced, and phenylalanine balance remains in a net catabolic state. This reduction in breakdown is most likely due to the decreased amino acid availability and subsequent conservation response by the muscle tissue.
Our data may provide an explanation for the conflicting studies previously published on the effects of insulin on human skeletal muscle protein synthesis. Those studies reporting an increase in muscle protein synthesis during hyperinsulinemia also show an increased amino acid delivery to the muscle (1, 2, 17, 25, 27, 42), whereas those reporting no change or a decrease in amino acid delivery also reported either a decrease or no change in muscle protein synthesis (11, 16, 23, 24, 27). The differences in amino acid delivery were primarily due to a general decrease in blood amino acid levels, which were dependent on the mode of insulin infusion (i.e., systemic vs. local) and/or the concomitant infusion of exogenous amino acids. There has only been one insulin infusion study in which amino acid delivery was increased to a limb and muscle protein synthesis was not stimulated (14). Therefore, the results of the present study suggest that these discrepancies can be explained by differences in amino acid availability.
It is also apparent that the utilization of different kinetic models for calculating muscle protein turnover can affect the conclusions of these experiments. We used the two arteriovenous models available to measure human muscle protein synthesis: the two-pool (43) and the three-pool (3) model. Because most previous studies had utilized the two-pool model (11, 14, 16, 17, 23, 25, 27), this allowed us to compare our data with the others’. However, this method cannot detect increases in intracellular amino acid recycling from proteolysis into synthesis, because it measures only the utilization of plasma amino acids for synthesis and the release into the blood of amino acids coming from proteolysis. On the other hand, the three-pool model allows for the measurement of the intracellular synthesis and breakdown rates, thus including the recycling (3). Both methods indicated that the local infusion of insulin induced a significant increase in muscle protein synthesis, whereas the systemic insulin infusion, even with a very large amount of nutritional energy provided, did not significantly affect any of the muscle protein metabolic parameters.
Excess energy intake results in a corresponding increase in lean body mass (12), which occurs when the rate of protein synthesis exceeds that of protein breakdown over time (30). However, our findings may not necessarily reflect physiological conditions, as the current study design utilized local and systemic infusions of insulin and nutrients as opposed to ingestion of a meal. For instance, when excess energy in the form of carbohydrate is ingested, either with or without an increase in protein intake, protein breakdown is reduced (9, 18, 26, 40) probably due to the increase in plasma insulin concentrations. Additionally, when 40 g of mixed amino acids are combined with 40 g of carbohydrates, muscle protein synthesis is increased to a greater extent (38) compared with when mixed amino acids are supplied alone (39), which may be a result of dual activation of intracellular energy-sensing and -signaling pathways. It remains to be seen whether increasing energy availability during hyperaminoacidemia without hyperinsulinemia will further stimulate muscle protein synthesis.
In addition, other data suggest that insulin may exert protein-specific effects (5) that were not measured in the present and previous human studies (1, 2, 11, 14, 16, 17, 23–25, 27, 42). Thus future studies should also assess the effects of the insulin modulation of amino acid availability on the synthesis of specific muscle proteins, the role of nutritional energy in combination with amino acid availability in the regulation of human skeletal muscle protein synthesis, and the role of insulin-induced increases in blood flow, perfusion, and amino acid delivery to muscle.
In summary, our study suggests that, in healthy young subjects, an increase in amino acid availability during short-term exposure to physiological hyperinsulinemia is an important factor in stimulating muscle protein synthesis. In fact, if amino acid concentrations decrease during hyperinsulinemia muscle protein synthesis will not be stimulated even in the presence of abundant nutritional energy. We conclude that the anabolic action of insulin in young subjects is dependent on amino acid availability and that insulin not only acts in a permissive role but can directly stimulate muscle protein synthesis if blood amino acid concentrations are maintained.
Acknowledgments
We thank Jeanine Cordero for technical assistance, the study volunteers for their patience and dedication, and all the nurses and personnel of the General Clinical Research Center of the University of Southern California for their help with the conduct of the clinical portion of this study.
GRANTS
This study was supported by Grant no. R01 AR-049877 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases (B. B. Rasmussen), Zumberge Research and Innovation Fund (B. B. Rasmussen), Grant no. R01 AG-18311 from the National Institute on Aging, (E. Volpi), Grant no. S10 RR-16650 from the Shared Instrumentation Grant Program, National Center for Research Resources (E. Volpi), and Grant no. M01 RR-43 from the General Clinical Research Branch, National Center for Research Resources, National Institutes of Health.
References
- 1.Bennet WM, Connacher AA, Scrimgeour CM, Jung RT, Rennie MJ. Euglycemic hyperinsulinemia augments amino acid uptake by human leg tissues during hyperaminoacidemia. Am J Physiol Endocrinol Metab. 1990;259:E185–E194. doi: 10.1152/ajpendo.1990.259.2.E185. [DOI] [PubMed] [Google Scholar]
- 2.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab. 1995;268:E75–E84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 4.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 5.Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes. 2001;50:2652–2658. doi: 10.2337/diabetes.50.12.2652. [DOI] [PubMed] [Google Scholar]
- 6.Butterfield GE, Gates J, Fleming S, Brooks GA, Sutton JR, Reeves JT. Increased energy intake minimizes weight loss in men at high altitude. J Appl Physiol. 1992;72:1741–1748. doi: 10.1152/jappl.1992.72.5.1741. [DOI] [PubMed] [Google Scholar]
- 7.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–009 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 8.Calloway DH, Spedor H. Nitrogen balance as related to caloric intake and protein intake in active young men. Am J Clin Nutr. 1954;2:405–411. doi: 10.1093/ajcn/2.6.405. [DOI] [PubMed] [Google Scholar]
- 9.Clugston GA, Garlick PJ. The response of protein and energy metabolism to food intake in lean and obese man. Human Nutr Clin Nutr. 1982;36C:57–70. [PubMed] [Google Scholar]
- 10.De Feo P, Volpi E, Lucidi P, Cruciani G, Reboldi G, Siepi D, Mannarino E, Santeusanio F, Brunetti P, Bolli GB. Physiological increments in plasma insulin concentrations have selective and different effects on synthesis of hepatic proteins in normal humans. Diabetes. 1993;42:995–1002. doi: 10.2337/diab.42.7.995. [DOI] [PubMed] [Google Scholar]
- 11.Denne SC, Liechty EA, Liu YM, Brechtel G, Baron AD. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol Endocrinol Metab. 1991;261:E809–E814. doi: 10.1152/ajpendo.1991.261.6.E809. [DOI] [PubMed] [Google Scholar]
- 12.Forbes GB. Lean body mass-body fat interrelationships in humans. Nutr Rev. 1987;45:225–231. doi: 10.1111/j.1753-4887.1987.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 13.Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. 1988;254:579–584. doi: 10.1042/bj2540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelfand RA, Barrett EJ. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987;80:1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 16.Heslin MJ, Newman E, Wolf RF, Pisters PW, Brennan MF. Effect of hyperinsulinemia on whole body and skeletal muscle leucine carbon kinetics in humans [erratum appears in Am J Physiol Endocrinol Metab 265 (1), following table of contents, 1993] Am J Physiol Endocrinol Metab. 1992;262:E911–E918. doi: 10.1152/ajpendo.1992.262.6.E911. [DOI] [PubMed] [Google Scholar]
- 17.Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin’s effect to stimulate protein synthesis in the human forearm. Am J Physiol Endocrinol Metab. 1998;274:E1067–E1074. doi: 10.1152/ajpendo.1998.274.6.E1067. [DOI] [PubMed] [Google Scholar]
- 18.Hoffer LJ, Yang RD, Matthews DE, Bistrian BR, Bier DM, Young VR. Effects of meal consumption on whole body leucine and alanine kinetics in young adult men. Br J Nutr. 1985;53:31–38. doi: 10.1079/bjn19850007. [DOI] [PubMed] [Google Scholar]
- 19.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 20.Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol Cell Physiol. 1998;274:C221–C228. doi: 10.1152/ajpcell.1998.274.1.C221. [DOI] [PubMed] [Google Scholar]
- 21.Kimball SR, Jefferson LS, Fadden P, Haystead TA, Lawrence JC., Jr Insulin and diabetes cause reciprocal changes in the association of eIF-4E and PHAS-I in rat skeletal muscle. Am J Physiol Cell Physiol. 1996;270:C705–C709. doi: 10.1152/ajpcell.1996.270.2.C705. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Wu Y, Nicklas EW, Jahn LA, Price WJ, Barrett EJ. Unlike insulin, amino acids stimulate p70S6K but not GSK-3 or glycogen synthase in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E523–E528. doi: 10.1152/ajpendo.00146.2003. [DOI] [PubMed] [Google Scholar]
- 23.Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest. 1992;90:2348–2354. doi: 10.1172/JCI116124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moller-Loswick AC, Zachrisson H, Hyltander A, Korner U, Matthews DE, Lundholm K. Insulin selectively attenuates breakdown of nonmyofibrillar proteins in peripheral tissues of normal men. Am J Physiol Endocrinol Metab. 1994;266:E645–E652. doi: 10.1152/ajpendo.1994.266.4.E645. [DOI] [PubMed] [Google Scholar]
- 25.Newman E, Heslin MJ, Wolf RF, Pisters PW, Brennan MF. The effect of systemic hyperinsulinemia with concomitant amino acid infusion on skeletal muscle protein turnover in the human forearm. Metab Clin Exper. 1994;43:70–78. doi: 10.1016/0026-0495(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 26.Nissen S, Haymond MW. Changes in leucine kinetics during meal absorption: effects of dietary leucine availability. Am J Physiol Endocrinol Metab. 1986;250:E695–E701. doi: 10.1152/ajpendo.1986.250.6.E695. [DOI] [PubMed] [Google Scholar]
- 27.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52:1377–1385. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- 29.Pain VM, Garlick PJ. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974;249:4510–4514. [PubMed] [Google Scholar]
- 30.Rasmussen BB, Phillips SM. Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev. 2003;31:127–131. doi: 10.1097/00003677-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6:358–362. [PMC free article] [PubMed] [Google Scholar]
- 33.Reeds PJ, Fuller MF, Cadenhead A, Lobley GE, McDonald JD. Effects of changes in the intakes of protein and non-protein energy on whole-body protein turnover in growing pigs. Br J Nutr. 1981;45:539–546. doi: 10.1079/bjn19810132. [DOI] [PubMed] [Google Scholar]
- 34.Reeds PJ, Wahle KW, Haggarty P. Energy costs of protein and fatty acid synthesis. Proc Nutr Soc. 1982;41:155–159. doi: 10.1079/pns19820025. [DOI] [PubMed] [Google Scholar]
- 35.Tessari P, Inchiostro S, Biolo G, Vincenti E, Sabadin L. Effects of acute systemic hyperinsulinemia on forearm muscle proteolysis in healthy man. J Clin Invest. 1991;88:27–33. doi: 10.1172/JCI115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab. 2001;281:E197–E206. doi: 10.1152/ajpendo.2001.281.2.E197. [DOI] [PubMed] [Google Scholar]
- 37.Todd KS, Butterfield GE, Calloway DH. Nitrogen balance in men with adequate and deficient energy intake at three levels of work. J Nutr. 1984;114:2107–2118. doi: 10.1093/jn/114.11.2107. [DOI] [PubMed] [Google Scholar]
- 38.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 40.Welle S, Matthews DE, Campbell RG, Nair KS. Stimulation of protein turnover by carbohydrate overfeeding in men. Am J Physiol Endocrinol Metab. 1989;257:E413–E417. doi: 10.1152/ajpendo.1989.257.3.E413. [DOI] [PubMed] [Google Scholar]
- 41.Welle S, Nair KS. Relationship of resting metabolic rate to body composition and protein turnover. Am J Physiol Endocrinol Metab. 1990;258:E990–E998. doi: 10.1152/ajpendo.1990.258.6.E990. [DOI] [PubMed] [Google Scholar]
- 42.Wolf RF, Heslin MJ, Newman E, Pearlstone DB, Gonenne A, Brennan MF. Growth hormone and insulin combine to improve whole-body and skeletal muscle protein kinetics. Surgery. 1992;112:284–292. [PubMed] [Google Scholar]
- 43.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. New York: Wiley-Liss; 1992. [Google Scholar]
- 44.Wolfe RR, Volpi E. The Endocrine Pancreas and Regulation of Metabolism. New York: Oxford Univ. Press; 2000. [Google Scholar]