Abstract
Background: Dairy product and calcium consumption have been associated with modifying body fat and body weight in children and adults.
Objective: In overweight adolescent boys and girls, we aimed to determine the effect of the doubling of habitual calcium intake to the recommended intake from dairy or calcium carbonate on energy balance and purported mechanisms including fecal fat excretion, macronutrient use, and parathyroid hormone suppression.
Design: Twenty-five girls with a mean (±SD) BMI (in kg/m2) of 33 ± 5 and 17 boys with a BMI of 28 ± 5, aged 12–15 y, participated in two 3-wk controlled feeding sessions that used a crossover design in random order as a summer research camp. In one session, 756 mg Ca/d was consumed; in the other session, an additional 650 mg Ca/d was provided as dairy or calcium carbonate supplements that were matched to the control in macronutrient content. Total energy and macronutrient intakes were controlled and were the same for the 2 sessions for each subject. Primary outcome measures were energy balance, fecal fat excretion, lipid oxidation, and postprandial energy expenditure.
Results: There were no effects of quantity or source of calcium on energy or fat balance, despite calcium-induced increases (P <0.01) in postprandial serum parathyroid hormone suppression.
Conclusion: These data lend little evidence to support the proposed mechanisms for the relation between an increase in calcium intake from calcium carbonate or dairy and weight loss or weight maintenance in children. This trial was registered at clinicaltrials.gov as NCT00592137.
See corresponding editorial on page 1159.
INTRODUCTION
Effective dietary interventions are needed to combat the increasing prevalence of overweight and obesity in American children, which was recently estimated at 31.9% (1). As recently reviewed (2–4), a higher calcium intake from calcium salts (eg, CaCO3 supplements) and dairy products has been associated with both a decrease in and no change in the incidence of excessive adiposity in adults and children. The evidence includes observational and prospective studies and randomized clinical trials. However, an interpretation of findings from these types of studies is difficult because of incomplete dietary control and variable periods of intervention. Controlled feeding studies of sufficient duration to reach metabolic steady state at a given calcium intake (≥2 wk) are necessary to address the question of the relation of dietary calcium to energy balance because the estimation of dietary intakes greatly underestimates actual intakes. In the population studied in the current study, we showed that self-reported, prestudy diet records underestimated energy intakes by 35 ± 20% (5). In addition, underreporting increased with a higher BMI and was associated with hidden (forgotten) fat intakes, which made it particularly important to study overweight children on controlled diets.
Several mechanisms were proposed to explain how dietary calcium might influence the energy balance and assist with weight control. Higher calcium intake decreases the apparent absorption of fat by increasing the formation of indigestible calcium–fatty acid soaps within the gastrointestinal tract, which results in increased fecal fat excretion (6–11). Dietary calcium may also increase lipid oxidation (12), potentially through the suppression of PTH5 (13). The fasting serum PTH concentration was negatively associated with postprandial whole-body fat oxidation after a 1-y dietary intervention in young women (14). When dairy sources provided part of the calcium intake, increases in lipid use and decreases in weight gain during refeeding after energy restriction were enhanced, possibly because of noncalcium dairy constituents (15).
Rigorous controlled feeding studies to address the role of dairy and calcium intakes in the achievement of appropriate body fat are lacking, especially during formative years. Thus, the aims of this study were to determine the effect of the CaCO3 or dairy calcium intake on energy and fat balances and to evaluate underlying mechanisms of calcium modulation of energy metabolism in overweight adolescent girls and boys. We hypothesized that the doubling of calcium intake by supplementing a strictly controlled diet matched in macronutrient content with a calcium salt or dairy would promote a negative energy balance on a fixed energy intake. We hypothesized that a negative energy balance would result from 1) decreased apparent dietary fat absorption through increased fecal fat excretion and 2) increased resting and TEEs through increased lipid oxidation mediated by decreases in serum PTH concentrations. In addition, we hypothesized there would be no differences that were due to the source of calcium.
SUBJECTS AND METHODS
Study participants
Forty-two overweight adolescents (25 girls and 17 boys), who were recruited from local schools, participated in the study. The recruitment targeted overweight (85th–100th percentiles of BMI-for-age), but otherwise healthy, girls aged 12–14 y and boys aged 13–15 y. These age ranges are the periods of peak skeletal calcium accretion for girls and boys and corresponds to a comparable stage of pubertal stages across sexes (16). A medical history questionnaire, physical examination, and serum biochemistries were used to determine the health status of subjects. Subjects completed 9-d diet records (6 records before camp and 3 records between the 2 sessions), and self-selected nutrient intakes were determined with NDS-R software (version 5.0–3.5; University of Minnesota). Sexual maturation was evaluated by a pediatric endocrinologist using the Tanner stage system (17). Individuals with a history of diabetes mellitus, digestive malabsorption disorders, or bone, liver, or kidney disease were excluded. The institutional review boards of Purdue University and Indiana University School of Medicine approved the study.
Study design
Adolescent, overweight subjects were recruited to participate in two 3-wk metabolic balance sessions in which a strictly controlled diet that contained 2 amounts of calcium were given by using a crossover randomized-order design. The 2 metabolic balance sessions were administered as a summer camp. Participants were all housed in a residence hall on the Purdue University campus, which allowed close supervision of all eating occasions and sample collections. Physical activity was designed to be equivalent between the 2 sessions, which was confirmed with accelerometers worn at the hip (data not shown). All food and beverages were prepared with deionized water, and daily composites were analyzed for energy and selected nutrients. A 4-d-cycle menu of 3 meals and 2 snacks provided 55% of energy from carbohydrates, 15% of energy from protein, 30% of energy from fat, 756 ± 121 mg Ca, 234 ± 36 mg Mg, 2144 ± 99 mg Na, and 1850 ± 352 mg K. Energy intakes were based on energy needs for weight maintenance according to estimated energy-requirement formulas (18). Energy intakes were achieved by providing each subject with a standardized menu adjusted to 1 of 5 energy amounts plus portioned quantities of cookies of a similar macronutrient composition. These sessions were separated by a 3-wk washout period during which time the boys and girls resided at their homes and consumed their unrestricted usual diets.
All subjects were randomly assigned to receive a calcium intake of ∼650 mg Ca/d (by analysis, 756 mg Ca/d) during the control period and 1300 mg Ca/d (by analysis, ∼1400 mg Ca/d) during the intervention period. For the intervention, the additional calcium came from either CaCO3 (for one-half of subjects) or dairy calcium (for one-half of subjects). The recommended calcium intake for this age range is 1300 mg Ca/d, and 650 mg Ca/d represents the 20th percentile of intake for adolescent girls and the 16th percentile of intake for adolescent boys (19). To achieve the different calcium intakes, each subject's standardized menus included 2 servings of 1 of 3 chocolate-flavored frozen products that were developed and provided by the Schwann Food Company. Each serving contained an ∼180-kcal energy equivalent in macronutrient content. For the low-calcium diet, the placebo product contained no calcium source and was based on soy protein, vegetable oil, and carbohydrates (ie, sucrose, maltodextrin, glucose, soluble fiber). The product that provided an additional 650 mg Ca/d as CaCO3 was otherwise identical to the placebo. The product that provided an additional 650 mg Ca/d from dairy contained dry milk solids fortified with a milk mineral complex and milk fat and no soy protein or vegetable oil.
Body weight (without shoes or outerwear) for adjustment of metabolic energy intake was measured daily to the nearest 0.1 kg with a calibrated electronic scale. Height was measured to the nearest 0.5 cm with a wall-mounted stadiometer. Dual-energy X-ray absorptiometry (Prodigy; GE Lunar Corp.) was used to measure total-body fat mass, lean tissue mass, fat distribution, and bone mineral density and content for descriptive purposes. The percentage CV on the basis of 10 subjects and measured twice for total-body fat mass and lean tissue mass was 2% and 1%, respectively. Waist circumference was measured midway between the lateral lower rib margin and the iliac crest while the subject was standing. Blood pressure was measured by auscultation as a part of the baseline physical exam. The fasting serum 25(OH)D concentration was measured by using a protein-binding assay (CV: 8.1%) (DiaSorin Inc).
Fat, energy, and nitrogen balance
The first week of each metabolic session was used as an equilibration period, and the last 14 d served as the experimental period. The occasional uneaten food item was stored for later analysis. Urine and stools were collected throughout the study. Urine was collected in acid-washed containers and pooled in 24-h collections that ended with the rising collection of the next day. Daily volumes were measured in triplicate by adjustment of the collection weight with specific gravity with a digital refractometer (Misco). The 14-d mean urinary creatinine excretions were used to adjust urinary excretions to 24-h pools (20). Aliquots acidified with concentrated hydrochloric acid (1% by volume) were frozen at −10°C. Pooled weighted samples were freeze-dried (Vir Tis; SP Industries) and pulverized for later analysis of energy. Fecal samples were collected daily in preweighed containers. The completeness of stool collections was determined by the measurement of recovery of the nonabsorbable fecal marker PEG. One gram of PEG was given in capsules at each meal. Fecal PEG was measured by a turbidometric method (21). The PEG fecal recovery was used as a qualitative measure to evaluate the completeness of fecal collection. Pooled 24-h collections were homogenized with hydrochloric acid and ultra-high-purity water, freeze-dried, and pulverized for later analysis.
Gross energy was determined in diet, urine, and fecal samples by using bomb calorimetry (model 1281; Parr Instruments Co). Gross energy balance or MEI was determined as the gross energy of the diet minus the gross energy in urine minus the gross energy in feces. Only representative urine samples were measured for gross energy because of the very low amounts in urine.
Samples of diet and feces (∼0.5g) were lyophilized and dried to a constant weight at 102°C. Fat was extracted with petroleum ether with an automated soxhlet type system (Ankom X-15; Ankom Technology). Solvent was removed by drying at 90°C for 30 min. The difference between initial and final weights was equivalent to the total fat content of the sample. The apparent fat balance (g/d) was calculated as the total fat intake in the diet minus excretion determined on the last 2 wk of each balance period (did not account for endogenous fat oxidation). Samples were measured for total nitrogen with a LECO analyzer (model FP-528; LECO), and the nitrogen balance was calculated as previously described (28).
Calcium balance
Diet, urine, and feces were analyzed for calcium with an inductively coupled plasma optimal emission spectrometer (Optima 4300; Perkin Elmer Instruments). The calcium balance was calculated as the intake minus excretion during the last 2 wk of each session.
Hormonal response to calcium test meal and biochemistries
During the third week of each metabolic session, the response of the serum PTH concentration to the calcium load and source was tested. A venous catheter was inserted for sequential blood draws and a fasting-state baseline sample was collected. A breakfast that contained 170 g of the assigned defrosted intervention product was administered and contained 20.4 mg Ca in the control product, 654 mg Ca in the CaCO3 product, and 665 mg Ca in the dairy product. Blood draws were taken at 0, 0.5, 1, 3, and 5 h. Serum was analyzed for PTH (1–84) by using an immunoradiometric assay (Nichols Institute). The interassay variation for PTH was 12.6%.
REE, PPEE, and TEE
Energy expenditure and substrate oxidation were determined after the first week in each metabolic period. The fasting REE (30 min after a 15-min acclimation) was measured by using indirect calorimetry (Med Graphics Cardiopulmonary Diagnostics Systems; Med Graphics Corp) as previously reported (22).
The response of the PPEE to the test meal was measured by using indirect calorimetry after consumption within 15 min of a quantity of assigned intervention products that contained 40% of the REE. The macronutrient composition of the 3 test products (ie, the control, CaCO3, and dairy) did not differ and contained 612–654 kcal energy, 98–108 g carbohydrates, 13–14 g fat, and 24–26 g protein. The test meal contained ∼45 mg Ca during the control period and ∼680 mg Ca during the supplementation period.
Substrate oxidation was computed by using published equations (23). Protein oxidation was calculated from the urinary nitrogen excretion during testing. Urine was measured for the total nitrogen content with the LECO analyzer (model FP-528I; LECO).
The TEE was measured by using doubly labeled water administered after the 1-wk equilibration period of each metabolic session as previously described (5).
Analysis and sample-size calculations
A priori power calculations for a ≥80% power determined a final sample size of 15 subjects in each of the crossover groups for the 2 dietary calcium sources. This was based on predicted responses of the 3 primary response variables, ie, fecal fat excretion (6, 24), lipid oxidation (12), and PPEE (25). We had a >80% power to detect changes with an calcium intake of 0.57 g/d for fecal fat excretion, 0.045 g/min for lipid oxidation, and 0.06 kcal/min for PPEE, or <1 SD. We assumed SDs of 0.75 g/d for fecal fat excretion, 0.05 g/min for lipid oxidation, and 0.08 kcal/min for PPEE. The experimental SDs were within 15% of these assumed values.
Statistical analyses were performed with SAS computer software (version 9.2; SAS Institute Inc). Response variables were evaluated with a linear model associated with a 2-period crossover design (ANOVA) by using PROC GLM and PROC MIXED models (SAS, version 9.2; SAS Institute Inc) in which the subject was considered a random effect. t tests were performed to compare low- and high-calcium diets and calcium sources. For the PPEE, a square root transform was used to standardize the variance. Post hoc analyses were performed by using Tukey's multiple-comparison adjustment. PTH concentrations in response to a meal were assessed with a 2-period crossover-design linear model with repeated measures and included sex as a covariate. In addition, the total change in PTH from baseline was quantified by using the AUC, which was calculated by using the trapezoidal rule, and data were expressed as picograms per mL · h−1, and differences were assessed by the PROC MIXED model (SAS, version 9.2; SAS Institute Inc) as previously described. Significance was assigned at P ≤ 0.05.
RESULTS
Baseline characteristics
Of the 25 girls and 17 boys who completed the first metabolic-balance session, 26 subjects were white, 8 subjects were black, 7 subjects were Hispanic, and 1 subject was a Pacific Islander. Compared with boy, girls were more sexually mature but of a similar chronological age (Table 1). Boys had a lower total-body bone mineral density and content than did girls. Girls were heavier with higher body fat than were boys. All girls were overweight (BMI >95th percentile), whereas 4 boys were at risk of being overweight (BMI of 85th–95th percentiles), and 2 boys had a healthy body weight at the time of enrollment despite being classified as overweight at screening. Girls had a lower REE than did boys. Girls had lower serum 25(OH)D but higher serum PTH concentrations than did boys. Other measures were not different between sexes.
TABLE 1.
Subject characteristics at baseline1
| Dairy intervention |
CaCO3 intervention |
P values |
||||
| Girls (n = 12) | Boys (n = 10) | Girls (n = 13) | Boys (n = 7) | By intervention | By sex | |
| Age (y) | 13.3 ± 0.7 | 13.7 ± 0.6 | 13.5 ± 0.9 | 13.8 ± 0.8 | 0.65 | 0.20 |
| Tanner stage | 4.8 ± 0.6 | 3.5 ± 1.2 | 4.7 ± 0.8 | 3.9 ± 1.2 | 0.51 | <0.01 |
| Height (cm) | 161.7 ± 4.0 | 161.2 ± 7.0 | 160.8 ± 6.6 | 165.8 ± 7.8 | 0.60 | 0.34 |
| Weight (kg) | 87.5 ± 12.3 | 69.5 ± 14.8 | 85.0 ± 17.8 | 79.8 ± 14.5 | 0.45 | 0.01 |
| BMI (kg/m2) | 33.5 ± 5.1 | 26.6 ± 4.5 | 32.6 ± 5.0 | 29.1 ± 5.6 | 0.56 | <0.01 |
| Body fat (%) | 45.1 ± 4.3 | 34.0 ± 9.0 | 45.1 ± 5.1 | 40.3 ± 8.7 | 0.18 | <0.01 |
| Waist circumference (cm) | 105.7 ± 12.6 | 91.2 ± 14.7 | 101.8 ± 13.9 | 101.0 ± 10.4 | 0.59 | 0.05 |
| REE (kcal/d) | 1607 ± 312 | 1782 ± 353 | 1407 ± 328 | 1800 ± 232 | 0.19 | 0.01 |
| Total-body aBMD (g/cm2) | 1.13 ± 0.1 | 1.04 ± 0.06 | 1.19 ± 0.1 | 1.06 ± 0.1 | 0.05 | <0.01 |
| Total-body BMC (g) | 2522 ± 285 | 2192 ± 349 | 2746 ± 373 | 2418 ± 285 | 0.03 | <0.01 |
| Systolic BP (mm Hg) | 121 ± 15 | 120 ± 12 | 111 ± 10 | 127 ± 11 | 0.39 | 0.14 |
| Diastolic BP (mm Hg) | 72 ± 10 | 71 ± 3 | 68 ± 9 | 71 ± 8 | 0.47 | 0.68 |
| Serum | ||||||
| 25(OH)D (ng/mL) | 16.3 ± 5.2 | 26.4 ± 4.2 | 21.9 ± 9.4 | 28.3 ± 9.2 | 0.54 | 0.02 |
| PTH (pg/mL) | 28.1 ± 12.8 | 18.8 ± 3.0 | 32.5 ± 16.5 | 25.5 ± 9.7 | 0.12 | 0.02 |
| Calcium (mg/dL) | 9.4 ± 0.5 | 9.6 ± 0.4 | 9.4 ± 0.3 | 9.2 ± 0.4 | 0.21 | 0.87 |
| Reported dietary intake, energy (kcal/d) | 1942 ± 745 | 2329 ± 542 | 1650 ± 407 | 1862 ± 426 | 0.04 | 0.06 |
| Calcium (mg/d) | 746 ± 349 | 893 ± 481 | 828 ± 295 | 712 ± 271 | 0.83 | 0.77 |
All values are means ± SDs. Analyses reflect a t test comparison by using the PROC TTEST model (version 9.2; SAS Institute Inc). aBMD, areal bone mineral density; BMC, bone mineral content; BP, blood pressure; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone; REE, resting energy expenditure.
Twenty-three girls and 15 boys completed both sessions of the study. Two girls and 2 boys who dropped out after the first session were homesick or had conflicting family plans.
Dietary intakes were closely monitored by camp counselors at each meal. The average fecal PEG recovery was 72%.
Energy and fat balance and fecal calciumndashfatty acid soap formation
There were no observed differences in energy (MEI), apparent fat, or nitrogen balances because of calcium treatment from either CaCO3 or dairy calcium in adolescent boys and girls in either the whole group or stratified by BMI, although the calcium balance was significantly improved with calcium supplementation regardless of the source (Table 2). Similarly, the TEE and MEI were not different with calcium supplementation whether adjusted for body weight or not. There was no significant difference in changes in body weight between the control and intervention sessions.
TABLE 2.
Effect of calcium supplementation from 2 different sources on energy, fat, nitrogen, and calcium balance1
| Dairy calcium (n = 21) |
CaCO3 (n = 17) |
||||
| Control | Dairy | Control | CaCO3 | P values (calcium amount) | |
| Gross energy intake (kcal/d) | 2740 ± 123 | 2765 ± 123 | 2703 ± 131 | 2632 ± 129 | 0.68 |
| Gross fecal energy (kcal/d) | 126 ± 9 | 132 ± 9 | 133 ± 10 | 127 ± 9 | 0.98 |
| MEI (kcal/d)2 | 2589 ± 121 | 2607 ± 121 | 2547 ± 130 | 2480 ± 127 | 0.67 |
| TEE (kcal/d) | 2999 ± 94 | 3007 ± 93 | 3028 ± 107 | 3063 ± 103 | 0.68 |
| Nitrogen intake (mg N ⋅ kg−1 ⋅ d−1) | 178 ± 11 | 176 ± 11 | 169 ± 11 | 168 ± 11 | 0.13 |
| Urinary nitrogen (mg N ⋅ kg−1 ⋅ d−1) | 116 ± 49 | 116 ± 9 | 111 ± 10 | 109 ± 10 | 0.67 |
| Fecal nitrogen (mg N ⋅ kg−1 ⋅ d−1) | 15 ± 1 | 15 ± 1 | 12 ± 1 | 12 ± 1 | 0.66 |
| Nitrogen balance (mg N ⋅ kg−1 ⋅ d−1) | 42 ± 5 | 41 ± 5 | 42 ± 5 | 42 ± 5 | 0.83 |
| Fat intake (g/d) | 77.8 ± 3.2 | 76.5 ± 3.2 | 77.3 ± 3.4 | 77.8 ± 3.4 | 0.56 |
| Fecal fat excretion (g/d) | 3.3 ± 0.2 | 3.0 ± 0.2 | 3.2 ± 0.3 | 3.1 ± 0.2 | 0.40 |
| Apparent fat balance (g/d) | 70.4 ± 3.7 | 73.4 ± 3.7 | 74.2 ± 4.0 | 73.0 ± 3.9 | 0.40 |
| Calcium intake (mg/d) | 786 ± 8 | 1461 ± 7 | 788 ± 8 | 1483 ± 8 | <0.01 |
| Fecal calcium excretion (mg/d) | 484 ± 54 | 817 ± 54 | 524 ± 60 | 877 ± 57 | <0.01 |
| Corrected urinary calcium (mg/d) | 67.6 ± 8.3 | 91.6 ± 8.3 | 61.0 ± 8.8 | 73.0 ± 8.7 | <0.01 |
| Calcium balance (mg/d) | 234 ± 54 | 553 ± 54 | 203 ± 60 | 533 ± 57 | <0.01 |
| PTH (pg/mL) | 27.6 ± 2.3 | 23.6 ± 2.3 | 26.9 ± 2.6 | 26.0 ± 2.5 | 0.15 |
| Weight loss (kg) | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.9 ± 0.9 | 0.60 |
All values are means ± SEMs. For control values, subjects were matched to their assigned intervention. There were no significant differences because of the calcium source as analyzed by ANOVA. MEI, metabolizable energy intake; PTH, parathyroid hormone; TEE, total energy expenditure.
MEI (energy balance) = gross energy intake − fecal energy − urine energy. Urine energy was 25 kcal/d on the basis of an average of a subset of urine samples from the study.
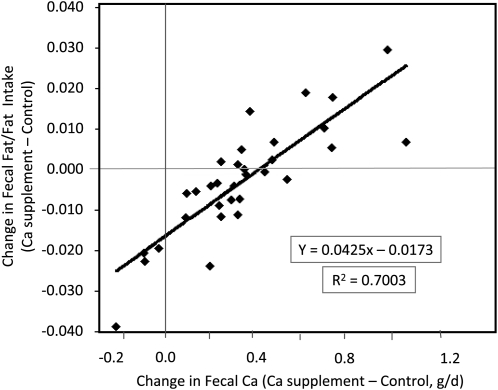
Dairy calcium and CaCO3 supplementation increased (P < 0.01) fecal and urine calcium excretions and calcium retention but not fecal fat excretion or retention. Differences in calcium excretion and retention between supplementation and control periods were not affected by the calcium source (dairy compared with CaCO3). When the calcium source was disregarded and data were pooled, the change in fecal calcium excretion from control to calcium-supplemented diets predicted the change in fecal fat as a fraction of the intake (R2 = 0.70, P < 0.01) (Figure 1). The change in fecal fat became positive with changes in fecal calcium amounts >0.4 g/d. The relation was not affected by body weight, BMI, or fat intake.
FIGURE 1.
Changes in fat excretion compared with changes in fecal calcium (in g/d) with increased calcium intake from either dairy or CaCO3 supplementation in adolescents (closed diamonds; n = 32).
PPEE
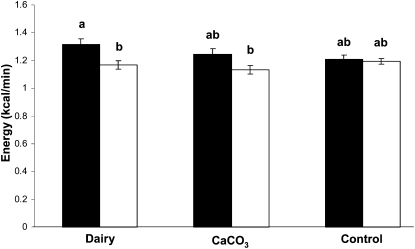
The average PPEE over 240 min expressed as the difference from baseline energy expenditure at rest (ie, REE) was higher (P < 0.05) in boys who consumed the dairy product (1.318 ± 0.037 kcal/min) than in girls who consumed the dairy product (1.167 ± 0.030 kcal/min) or the CaCO3 product (1.134 ± 0.030 kcal/min; P < 0.05) (Figure 2). In boys, but not in girls, the PPEE after consumption of the dairy product tended to be higher than that after consumption of the control product (1.318 ± 0.037 compared with 1.211 ± 0.026 kcal/min, respectively; P < 0.07).
FIGURE 2.
Mean (±SEM) values of postprandial energy expenditure in overweight male (solid bars; n = 16) and female (open bars; n = 24) adolescents after consumption of either a dairy product (n = 9 males and 12 females) or a CaCO3 product (n = 7 males and 12 females) and a control product (n = 16 males and 24 females). Different superscript letters represent significant differences at P < 0.05 (ANOVA).
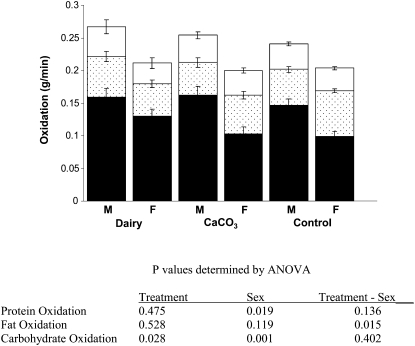
Postprandial protein oxidation was higher in boys than in girls (0.043 ± 0.002 compared with 0.034 ± 0.001 g/min, respectively) (Figure 3). Although the interaction was not significant, a sex difference in protein-oxidation rates was more pronounced with dairy-based products than with CaCO3-based products (0.045 ± 0.004 g/min in boys compared with 0.031 ± 0.003 in girls; P < 0.06). Regarding postprandial fat oxidation, responses among treatments differed by sex (sex-treatment interaction, P < 0.02) and were reflected by lower rates in girls after consumption of the dairy-based product (0.050 ± 0.006 g/min) than after consumption of the control product (0.070 ± 0.004 g/min) but not after consumption of the CaCO3 product (0.050 ± 0.005 g/min). There were no differences between treatments for boys. No other variable affected fat-oxidation rates. Carbohydrate oxidation was higher in the dairy-based treatment (0.145 ± 0.009 g/min) than in the control treatment (0.133 ± 0.009 g/min) but not with CaCO3 products (0.123 ± 0.006 g/min). Carbohydrate oxidation was higher in boys than in girls (0.164 ± 0.012 compared with 0.100 ± 0.009 g/min, respectively). No treatment-by-sex interactions were observed with carbohydrate oxidation.
FIGURE 3.
Mean (±SEM) values of postprandial substrate oxidation (open bars denote protein, dotted bars denote fat, and solid bars denote carbohydrate) in overweight male (n = 16) and female (n = 24) adolescents after consumption of either a dairy-based product (n = 9 males and 12 females) or a CaCO3 product (n = 7 males and 12 females) and a control product (n = 16 males and 24 females). Values were compared by using ANOVA.
Serum PTH response to calcium test meal
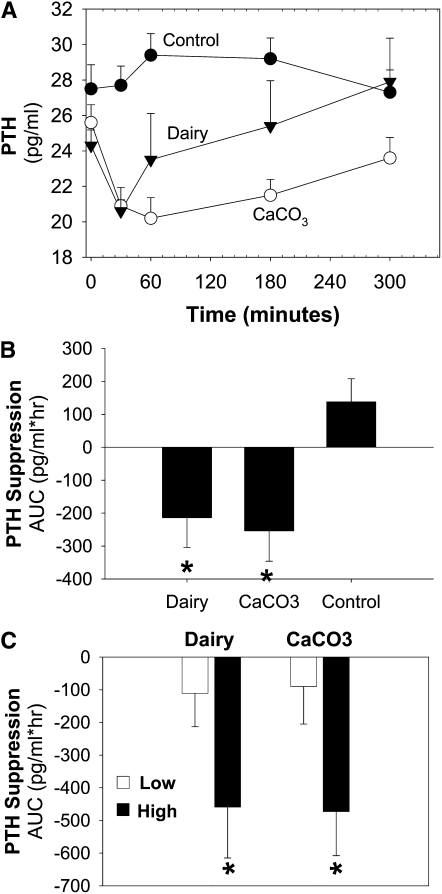
Calcium supplementation reduced postprandial serum PTH relative to the control in response to a test meal comprised of the intervention product (main effect of treatment P = 0.0003; Figure 4A). Both CaCO3 and dairy supplementation suppressed PTH from baseline and compared with the control, and there were no differences in the suppression of PTH between calcium and dairy supplementation (Figure 4B). When basal PTH concentrations were included in the PROC MIXED model (SAS, version 9.2; SAS Institute Inc), basal PTH concentrations (P < 0.01) predicted the postprandial serum PTH response. To explore this relation, participants were categorized into 2 groups on the basis of their basal PTH concentrations (mean PTH concentration: 25.7 pg/mL) into a lower half (basal PTH concentration <25.7 pg/mL) or an upper half (basal PTH concentration ≥25.7 pg/mL). Serum PTH was significantly suppressed with calcium supplementation and was observed in subjects with higher basal PTH concentrations (main effect of time P = 0.02; main effect of treatment P = 0.01) but not in subjects with lower basal PTH concentrations (main effect of time P = 0.86; main effect of treatment P = 0.17). Results were similar when 25(OH)D and BMI were included in the model for either higher basal PTH or lower basal PTH concentrations. The results were also expressed as the AUC, and neither CaCO3 nor dairy suppressed PTH in the group with lower basal PTH concentrations, but both meal challenges suppressed PTH in the group with higher basal PTH concentrations (Figure 4C). Therefore, the response of serum PTH to increased calcium from either carbonate or dairy was not different, but the PTH response was dependent on the basal concentration of PTH.
FIGURE 4.
Mean (±SEM) values of the effect of dietary calcium source on serum PTH response to a test meal in 38 adolescents. A: Serum PTH was assessed after a meal challenge comprising an intervention product (main effect of treatment, P = 0.0003; time by treatment interaction, P = 0.02). PTH amounts are indicated for the control (closed circles; n = 38), dairy (closed triangles; n = 19), and CaCO3 (open circles; n = 19). B: Results were quantified and expressed as the AUC from baseline. *Significant difference between zero and the control group. C: Subjects were categorized by basal PTH concentrations [basal PTH concentration <25.7 pg/mL (Low: dairy, n = 14; carbonate, n = 11); basal PTH concentration ≥25.7 pg/mL (High: dairy, n = 5; carbonate, n = 8]. *Significant difference between zero and low-basal-PTH group given the same intervention product. PTH, parathyroid hormone.
DISCUSSION
In this randomized-ordered, double-blinded, crossover trial, we observed no capacity of the doubling of calcium intake whether given as dairy calcium or as CaCO3 as part of a controlled diet to promote metabolic changes that would improve body weight or composition in overweight adolescent boys and girls. Putative mechanisms for this potential effect, including the regulation of PTH release, fecal fat excretion, and fat oxidation, were measured and did not lead to a negative energy or fat balance. These results suggested that, under conditions when the energy intake is controlled and not reduced for weight loss, dietary calcium does not influence the energy balance in adolescents. The evidence (2, 3) for a benefit of dietary calcium or dairy on body weight is either a false positive because of an uncontrolled energy intake or because they act as an appetite suppressant in free-living populations or the benefit is restricted to weight loss.
One of the primary hypotheses related to the relation of calcium intake and body fat is that the formation of calcium–fatty acid soaps in the lower gut may decrease the energy that is available for uptake as was shown in vitro by X-ray–diffraction studies (6, 26). In our study, the doubling of calcium intake through supplementation significantly increased fecal calcium excretion, but the intervention did not alter the mean fat excretion. However, we showed a significant positive relation between the change in fecal calcium and fecal fat excretion, which suggests that characteristics unrelated to diet influenced the rate of calcium and fat excretion. Overweight adolescents in this study had a wide range of changes in fecal calcium in response to the doubling of calcium intake that varied from −9% to 150%. Because fecal calcium represents unabsorbed calcium, the wide range in changes in fecal calcium represented a wide range in calcium absorption. A small change in fecal calcium when the calcium intake was doubled would have occurred under conditions of high-calcium absorption that augments skeletal calcium accretion. As individuals mature, skeletal calcium accretion decreases, and decreased calcium absorption leads to increased fecal calcium. In support of this, the change in fecal calcium correlated with the height-for-age percentile (r = 0.43, P < 0.01). The fecal fat excretion increased only in individuals with large changes in fecal calcium when calcium intake increased. When the change in fecal calcium was low (ie, <0.4 g/d), the fecal fat excretion decreased. This result suggests that high calcium absorption may facilitate fat absorption and contribute to weight gain, and only when a change in fecal calcium is >0.4 g/d will the fecal fat excretion increase. We previously reported the relation between fecal calcium excretion and calcium intake and observed that such a large increase in fecal calcium could occur at very high calcium intakes (ie, >1500 mg/d) (24, 27) or after completion of puberty. Calcium retention is also influenced by BMI. The augmentation of skeletal calcium accretion in response to dietary calcium is greater in adolescents with a high BMI, which was consistent with the high BMI of these subjects (28).
Short-term studies in adults have shown significant increases in fecal fat with calcium supplementation by using crossover designs (6, 10). A meta-analysis of 15 substudies (29), including only 1 study in children (8), showed that an increase in dietary calcium by 1241 mg/d increased the daily fecal fat by 5.2 g. In one crossover study in adults of low calcium (500 mg/d; one-half of the requirement and mostly from dairy products) and normal protein (15% of the energy intake), high calcium (1800 mg/d) and normal protein, or high calcium and high protein (23% of the energy intake), the fecal fat excretion increased ∼2.5-fold (8.2 g/d) on the high-calcium, normal-protein diet (10). This effect increased the energy excretion by ∼83 kcal/d, which the authors calculated could lead to weight reduction of 3.5 kg/y. These findings were reproduced in a second short-term study (11). Our change in calcium excretion was one-third that reported by Jacobson (10) and should have resulted in a detectable change of ∼1.64–2.7 g fecal fat/d if our subjects had similar calcium binding as they reported. The Jacobson study involved adults in whom calcium-absorption efficiency was much less than in pubertal children (30). The higher fecal calcium in adults than in pubertal children who consumed similar calcium may explain the differences in fecal fat excretion. We directly compared adolescents and young adults on a controlled diet containing ∼1300 mg/d and showed that the amount of daily fecal calcium was greater in young adults than in adolescents (1061 ± 142 compared with 901 ± 117 mg/d, respectively; P < 0.01), which resulted in greater calcium retention in teens than in young adults (326 ± 111 compared with 73 ± 107 mg/d, respectively; P < 0.01) (24). These results were consistent with the observation that 10 early pubertal girls who resided in a boarding school and received bread with added calcium phosphate (2.286 g/d total calcium) had double the fecal fat (4.93 ± 0.73 compared with 2.44 ± 0.61; P < 0.01) of 8 girls in a control school (1.1295 g total calcium/d) (8). In contrast, a long-term (16 wk) study in adults by Lupton et al (31) showed no effect of supplementation with 2 g CaCO3 or 3 g calcium citrate on fecal fat excretion. Ditscheid et al (32) also showed no effect of 4 wk of calcium phosphate supplementation on fecal fat excretion compared with that of a placebo period.
In contrast to the stoichiometric relation of calcium–fatty acid binding, metabolic changes such as increased lipid oxidation and use may be mediated by dietary calcium–induced hormonal changes, specifically the PTH–vitamin D axis. We showed that calcium supplementation, regardless of the source, resulted in a significant postprandial PTH suppression and was higher in children with higher basal PTH concentrations.
The enhanced thermogenic and carbohydrate oxidation responses to the dairy intervention, but not to CaCO3, in boys, but not in girls, suggested a sex-related difference in how dairy is used in adolescents. Perhaps this difference was related to the higher vitamin D status in boys given that the serum 25(OH)D status predicted the change in thermogenesis (P < 0.01) in a multisite dairy-intervention trial on weight loss (12). Still, the increased oxidation did not lead to changes in the fat or energy balance (MEI or TEE). These results support the findings of other studies that showed no relation between calcium intake and substrate oxidation or energy expenditure even when serum 1,25 di-hydroxy vitamin D, and presumably PTH, was changed (9, 33, 34). This lack of a relation between calcium intake and energy expenditure or substrate oxidation contrasts with the positive correlation between calcium or dairy intake and fat oxidation by other authors (11, 35–37). In a study by Melanson et al (36), high dairy-induced increases in fat oxidation only occurred with energy restriction.
The strengths of this study included the rigorously controlled metabolic study of sufficient duration to be in steady state, the crossover design on 2 very different but practical calcium intakes and 2 main sources of dietary calcium, the blinded and aesthetically similar intervention products, and robust outcome measures. This study had several limitations. The cohort was a convenience sample, and the duration was acute; hence, effects of the intervention on long-term changes in body weight and composition could not be determined. The sample size was relatively small to observe changes in energy or fat balance, although it was adequate to find significant differences in the calcium balance. It is possible that calcium supplementation may be effective in assisting with weight loss or increasing fecal fat excretion in younger or older subjects who are in a period of slower growth when calcium absorption and excretion are not regulated for pubertal growth. Finally, although a careful control of dietary intake was necessary for testing most of our hypotheses, it did not allow us to test the hypothesis that an increased calcium or dairy intake reduces the self-selected energy intake.
In conclusion, calcium supplementation did not affect the energy or fat balance in overweight adolescents. No mechanisms measured in this study support previous observations that dietary calcium affects energy balance that would lead to changes in body weight if energy intake and physical activity were controlled. Our data suggest that there may be a threshold for increasing fecal fat excretion with higher calcium intakes, but during periods of high calcium absorption the addition of a calcium supplement leads to decreases in fecal fat excretion. Future research is necessary to determine whether a negative fat balance could be induced with higher calcium intakes than those studied here and on energy-reducing diets.
Acknowledgments
The authors’ responsibilities were as follows—CMW, WWC, DT, BAC, BRM, and LAD: study concept and design; CMW, WWC, BRM, RS, MMB, JWA, TSH, LAD, and MP: acquisition of data; CMW, WWC, DT, BAC, BRM, RS, and JWA: analysis and interpretation of data; CMW and YH: drafting of the manuscript; CMW, WWC, DT, BAC, BRM, JWA, TSH, DAS, LAD, and MP: critical revision of the manuscript for important intellectual content; BRM, BAC, and YH: statistical analysis; CMW, WWC, DT, BAC, and TSH: obtainment of funding; and BRM, MMB, and JWA: study supervision. CMW is on the advisory boards of Pharmavite, Nestle, and Sara Lee. None of the sponsors had a role in study design, data analysis, or preparation of the manuscript. None of the authors had a personal or financial conflict of interest.
Footnotes
Abbreviations used: MEI, metabolizable energy intake; PEG, polyethylene glycol; PPEE, postprandial energy expenditure; PTH, parathyroid hormone; REE, resting energy expenditure; TEE, total energy expenditure; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA 2008;299:2401–5 [DOI] [PubMed] [Google Scholar]
- 2.Van Loan M. The role of dairy foods and dietary calcium in weight management. J Am Coll Nutr 2009;28:120S–9S [DOI] [PubMed] [Google Scholar]
- 3.Astrup A, Chaput J-P, Gilbert J-A, Lorensen JK. Dairy beverages and energy balance. Physiol Behav 2010;100:67–75 [DOI] [PubMed] [Google Scholar]
- 4.Onakpoya IJ, Perry R, Zhang J, Ernst E. Efficacy of calcium supplementation for management of overweight and obesity: systematic review of randomized clinical trials. Nutr Rev 2011;69:335–43 [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Martin BR, Hickey Y, Teegarden D, Campbell WW, Craig BA, Schoeller DA, Kerr DA, Weaver CM. Comparison of self-reported energy intake and measured metabolizable energy intake with total energy expenditure in overweight teens. Am J Clin Nutr 2009;89:1744–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denke MA, Fox MM, Schulte MC. Short-term dietary calcium fortification increases fecal saturated fat content and reduces serum lipids in men. J Nutr 1993;123:1047–53 [DOI] [PubMed] [Google Scholar]
- 7.Drenick EJ. The influence of ingestion of calcium and other soap-forming substances on fecal fat. Gastroenterology 1961;41:242–4 [PubMed] [Google Scholar]
- 8.Lutwak L, Laster L, Gitelman HJ, Fox M, Whedon D. Effects of high dietary calcium and phosphorus on calcium, phosphorus, nitrogen and fat metabolism in children. Am J Clin Nutr 1964;14:76–82 [DOI] [PubMed] [Google Scholar]
- 9.Shahkhalili Y, Murste C, Meirim I, Duruz E, Guinchard S, Cavadini C, Acheson K. Calcium supplementation of chocolate: effect of cocoa butter digestibility and blood lipids in humans. Am J Clin Nutr 2001;73:246–52 [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen R, Lorenzen JK, Tourbo S, Krog-Mikkelsen I, Astrup A. Effect of short term high dietary calcium intake on 24-h energy expenditure, fat oxidation, and fecal fat excretion. Int J Obes (Lond) 2005;29:292–301 [DOI] [PubMed] [Google Scholar]
- 11.Bendsen NT, Hother A-L, Jensen SK, Lorenzen JK, Astrup A. Effect of dairy calcium on fecal fat excretion: a randomized crossover trial. Int J Obes (Lond) 2008;32:1816–24 [DOI] [PubMed] [Google Scholar]
- 12.Teegarden D, White K, Lyle RM, Zemel MB, Van Loan M, Matkovic V, Craig B, Schoeller D. Calcium and dairy product modulation of lipid utilization and energy expenditure. Obesity (Silver Spring) 2008;16:1566–72 [DOI] [PubMed] [Google Scholar]
- 13.Gunther CW, Lyle RM, Legowski PA, James JM, McCabe LD, McCabe GP, Peacock M, Teegarden D. Fat oxidation and its relation to serum parathyroid hormone in young women enrolled in a 1-y dairy calcium intervention. Am J Clin Nutr 2005;82:1228–34 [DOI] [PubMed] [Google Scholar]
- 14.Gunther CW, Legowski PA, Lyle RM, McCabe GP, Eagan MS, Peacock M, Teegarden D. Dairy products do not lead to alterations in body weight or fat mass in young women in a 1 y intervention. Am J Clin Nutr 2005;81:751–6 [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Zemel MB. Calcium and dairy products inhibit weight an fat regain during ad libitum consumption following energy restriction in Ap2-Agouti transgenic mice. J Nutr 2004;134:3054–60 [DOI] [PubMed] [Google Scholar]
- 16.Bailey DA, McKay HA, Mirwald RL, Crocker PRE, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan bone mineral accrual study. J Bone Miner Res 1999;14:1672–9 [DOI] [PubMed] [Google Scholar]
- 17.Tanner JM. Growth and adolescence. 2nd ed. Oxford, United Kingdom: Blackwell Scientific, 1962 [Google Scholar]
- 18.Institute of Medicine Dietary Reference Intake for energy, carbohydrate, fibre, fat, fatty acids, cholesterol, protein, and amino acids. Food and Nutrition Board. Washington, DC: National Academy Press, 2002 [Google Scholar]
- 19.Institute of Medicine Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board. Washington, DC: National Academy Press, 1997 [Google Scholar]
- 20.Weaver CMC. Five clinical approaches for studying calcium metabolism and its relationship to disease :Weaver CM, Heaney RP, Calcium in human health. Totowa, NJ: Humana Press, 2006:65–81 [Google Scholar]
- 21.Allen LH, Raynolds WL, Margen S. Polyethylene glycol 4000 as a continuously administered non-absorbable fecal marker in human nutrition experiments. Am J Clin Nutr 1979;32:427–40 [DOI] [PubMed] [Google Scholar]
- 22.Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr 2008;88:1322–9 [DOI] [PubMed] [Google Scholar]
- 23.Jéquier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr 1987;7:187–208 [DOI] [PubMed] [Google Scholar]
- 24.Weaver CM, Martin BR, Plawecki KL, Peacock M, Wood OB, Smith DL, Wastney ME. Differences in calcium metabolism between adolescent and adult females. Am J Clin Nutr 1995;61:577–81 [DOI] [PubMed] [Google Scholar]
- 25.Alper CM, Mattes RD. Effects of chronic peanut consumption on energy balance and hedonics. Int J Obes Relat Metab Disord 2002;26:1129–37 [DOI] [PubMed] [Google Scholar]
- 26.Jandacek RJ. The solubilization of calcium soaps by fatty acids. Lipids 1991;26:250–3 [Google Scholar]
- 27.Braun M, Martin BR, Kern M, McCabe GP, Peacock M, Jiang Z, Weaver CM. Calcium retention in adolescent boys on a range of controlled calcium intakes. Am J Clin Nutr 2006;84:414–8 [DOI] [PubMed] [Google Scholar]
- 28.Hill KM, Braun MM, Egan KA, Martin BR, McCabe LD, Peacock M, McCabe, Weaver CM. Obesity augments calcium-induced increases in skeletal calcium accretion in adoelscents. J Clin Endocrin Metab (Epub ahead of print 13 April 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen R, Lorenzen JK, Svith CR, Bartelo EM, Melanson EL, Saris WH, Tremblay A. Astrup. Effect of calcium from dairy and dairy supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev 2009;10:475–86 [DOI] [PubMed] [Google Scholar]
- 30.Wastney ME, Martin BR, Peacock M, Smith D, Jiang X-Y, Jackman LA, Weaver CM. Changes in calcium kinetics in adolescent girls induced by high calcium intake. J Clin Endocrinol Metab 2000;85:4470–5 [DOI] [PubMed] [Google Scholar]
- 31.Lupton JR, Steinbach G, Chang WC, O'Brien BC, Wiese S, Stoltzfus CL, Golber GA, Wargovich MJ, McPherson S, Winn RJ. Calcium supplementation modifies the relative amounts of bile acids in bile and affects key aspects of human colon physiology. J Nutr 1996;126:1421–8 [DOI] [PubMed] [Google Scholar]
- 32.Ditscheid B, Keller S, Jahreis G. Cholesterol metabolism is affected by calcium phosphate supplementation in humans. J Nutr 2005;135:1678–82 [DOI] [PubMed] [Google Scholar]
- 33.Boon N, Hul GB, Viguerie N, Sicard A, Langin D, Saris WH. Effects of diets with various calcium contents on 24-h energy expenditure, fat oxidation, and adipose tissue message RNA expression of lipid metabolism-related proteins. Am J Clin Nutr 2005;82:1244–52 [DOI] [PubMed] [Google Scholar]
- 34.Bortolotti M, Rudelle S, Schneiter P, Vidal H, Loison E, Tappy L, Acheson KJ. Dairy calcium supplementation in overweight or obese persons: its effect on markers of fat metabolism. Am J Clin Nutr 2008;88:877–85 [DOI] [PubMed] [Google Scholar]
- 35.Melanson EL, Short TA, Schneider J, Donahoo WT, Grunwald GK, Hill JO. Relation between calcium intake and fat oxidation in adult humans. Int J Obes Relat Metab Disord 2003;27:196–203 [DOI] [PubMed] [Google Scholar]
- 36.Melanson EL, Donaho WT, Dong F, Ida T, Zemel MB. Effect of low- and high-calcium dairy-based diets on macronutrient oxidation in humans. Obes Res 2005;13:2102–12 [DOI] [PubMed] [Google Scholar]
- 37.Cummings NK, James AP, Soares MJ. The acute effects of different sources of dietary calcium on postprandial energy metabolism. Br J Nutr 2006;96:138–44 [DOI] [PubMed] [Google Scholar]