Abstract
Background: Human and animal studies have produced conflicting results with regard to the effect of soy isoflavones on breast cancer risk. This may be due to differences in isoflavone metabolism.
Objective: The objective of this study was to determine whether soy isoflavone phase II metabolism differs between humans and rodents.
Design: Circulating total and unconjugated isoflavone concentrations were determined by mass spectrometry in plasma samples from 7 separate studies: 1) in Sprague-Dawley rats and in 3 strains of mice fed commercial soy-containing diets; 2) in Sprague-Dawley rats gavaged with genistein; 3) in healthy adults who consumed single servings of soy nuts, soy milk, and tempeh; 4) in healthy adults subchronically given soy milk; 5) in healthy women orally administered 50 mg genistein; 6) in healthy women orally administered 20 mg pure S-(-)equol; and 7) in 6-mo-old infants fed soy infant formula and later, at age 3 y, a soy germ isoflavone supplement.
Results: The proportion of unconjugated genistein in plasma from adults and infants who consumed different soy foods, pure genistein, or an isoflavone supplement was <1% in steady state and <2% at peak concentrations. By contrast, rodents fed soy-containing diets conjugate isoflavones less efficiently. The plasma percentages of unconjugated genistein concentrations in Sprague-Dawley rats and C57BL/6, nude, and transgenic AngptL4B6 mice were 4.0 ± 0.6%, 4.6 ± 0.6%, 11.6 ± 0%, and 30.1 ± 4.3%, respectively, which represent 20, 23, 58, and 150 times that in humans.
Conclusion: The markedly higher circulating concentrations of biologically active (unconjugated) genistein in certain strains of mice cast doubt on the value of the use of these rodents for gaining insight into the effects of isoflavones in humans, especially with regard to the effects on breast tissue.
INTRODUCTION
Soy foods have been the subject of rigorous investigation during the past 2 decades because of research that suggested that, independent of nutrient content, they provide important health benefits, including a reduced risk of heart disease (1–3) and breast (4, 5) and prostate (6) cancers and alleviation of menopause-related hot flashes (7). Many of these proposed benefits are attributed to the presence of isoflavones (8–10), a group of diphenolic compounds that are classified more accurately as selective estrogen receptor (ER) modulators (11, 12) because they preferentially bind to and transactivate ER-β (13, 14). The soybean contains 12 different isoflavones: the 3 aglycons genistein, daidzein, and glycitein; their respective β-glycosides of genistin, daidzin, and glycitin; and 3 β-glucosides, each esterified with either malonic or acetic acid (15, 16). Genistein, daidzein, and glycitein and their respective glycosides account for ∼50%, 40%, and 10%, respectively, of total soybean isoflavone content.
Much of the initial interest in the chemopreventive effects of soy can be traced to early rodent research that showed that incorporation of isoflavone-rich soy into the diets of rats inhibited 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth (17). This research was largely responsible for the National Cancer Institute's first allocation of funds for further studies in this area >20 y ago (5). However, despite the proposed benefits, isoflavones and soy foods are not without controversy. Concerns have arisen that the estrogen-like properties of soy isoflavones may be harmful to patients with estrogen-sensitive breast cancer and to women at high risk of development of this disease (18, 19). This concern is based largely on research that showed that genistein stimulates the growth of mammary tumors in athymic ovariectomized mice implanted with MCF-7 cells, an ER-positive human breast cancer cell line (20). In contrast to the effects of genistein, tumor stimulation does not occur in response to daidzein or equol, the bacterially synthesized metabolite of daidzein (21). Furthermore, in this mouse model, products that were more highly processed led to higher circulating concentrations of unconjugated genistein, the aglycon and biologically active forms (22), and were more tumor stimulatory (23). This unconjugated fraction is often referred to incorrectly as the “free” fraction because, in steroid terms, “free” defines the fraction that is non–protein bound, not unconjugated. In fact, soy flour, the least-processed product evaluated, did not stimulate tumor growth. These observations have led some health professionals and organizations to warn women with a history of breast cancer against the use of isoflavone supplements but not soy foods (24).
Whether these results are applicable to humans is unknown, although it is well established that there are interspecies differences in isoflavone metabolism (25). For example, in captive cheetah, small quantities of soy in the diet were shown to cause veno-occlusive disease of the liver and infertility, because felines are deficient in UDP–glucuronyl transferase (26). Phase II metabolism by glucuronidation is a major pathway for the elimination of isoflavones (27) and endogenous steroid hormones (28). Differences in glucuronidation between rats and mice have also been noted (28); the former have much greater capabilities. Most importantly, humans have a high capacity for conjugation of steroid and steroid-like molecules between the intestinal tract and liver, so that, in common with endogenous estrogens, circulating proportions of unconjugated isoflavones are maintained at relatively low concentrations (25, 29–31).
To better understand the clinical relevance of rodent models to the soy/isoflavone breast cancer relation it is necessary to identify whether differences in isoflavone metabolism exist between rodents and humans, particularly as they pertain to circulating concentrations of biologically active unconjugated forms, because such differences would go some way toward the establishment of the relevance of rodent models to humans. To our knowledge, the current research is the first attempt to systematically address this issue. Second, we believe it is the first research to directly and comprehensively evaluate the effects of the food matrix on circulating unconjugated genistein concentrations in human subjects in response to the consumption of differently processed soy products.
SUBJECTS AND METHODS
Seven separate studies are reported that examined the concentrations and proportions of unconjugated isoflavones circulating in the plasma of rodents fed commercial soy-based rodent diet or gavaged with pure genistein and in humans fed different soy foods or pure isoflavones. The human studies included infants at 6 mo and again at 3 y of age and healthy adults.
Animal studies
Study 1
Sprague-Dawley rats (n = 18) were purchased from Charles River Breeding Laboratories and bred in house. Female offspring were maintained for >1 wk on an AIN93G soy-free diet or on a soy-containing Purina 5008 (Ralston Purina) diet. Healthy adult mice were obtained from several suppliers. Athymic (nude) mice (n = 10) were obtained from Harlan Laboratories and C57BL/6 mice (n = 11) from Jackson Laboratories, and the transgenic AngptL4B6 strain of mice (n = 11) were bred at the Cincinnati Children's Hospital Medical Center and were a gift from Sherry Thronton (Cincinnati, OH). All mice had been maintained on the soy-containing rodent diet (Purina 5010) for several weeks. Purina 5008 and 5010 are the most commonly used commercial rodent diets (32). Blood samples (1–3 mL) were obtained by intracardiac stick under anesthesia with isoflurane, followed by carbon dioxide inhalation and sacrifice. All blood samples were centrifuged at 3000 rpm for 10 min, and the plasma was removed and stored at −20°C for analysis of total and unconjugated forms of isoflavones.
Study 2
Four adult Sprague-Dawley rats, 2 males and 2 females, maintained on a soy-free AIN-93 diet, were gavaged with 1 mg pure genistein (3.7 mg/kg BW) in a 1% carboxymethylcellulose solution as a single dose, and blood was obtained by cardiac stick under isoflurane anesthesia 1–2 h later.
For both of these studies, the animals were housed in an Association for the Assessment and Accreditation of Laboratory Animal Care–accredited facility that met or exceeded the Animal Welfare Act requirements, and the study protocols (Sprague-Dawley rats, 5B09057; nude mice, 9D12095; C57BL/6 mice, 9D09077; Angptl4B6 mice, 9D10082) were approved by the Children's Hospital Research Foundation's Animal Use Committee.
Human studies
Plasma samples were collected from healthy men and women (age range: 20–65 y) for the determination of plasma conjugated and unconjugated isoflavone concentrations after consumption of different soy foods or isolated isoflavones. Exclusion criteria included subjects with chronic renal, liver, pulmonary, or cardiovascular disease; women taking oral contraceptives or hormone therapy; or anyone who had been administered antibiotics within the 3 mo preceding the study. In each study, participants were asked to abstain from foods that contained soy protein for ≥1 wk before, and during, the study. All human studies were approved by the Institutional Review Board of the Scientific Advisory Committee of the Cincinnati Children's Hospital Medical Center and the Clinical Translational Research Center, and informed consent was obtained from adults or the parents/guardians of each infant. These studies were monitored by a data safety management monitoring board at Cincinnati Children's Hospital Medical Center and were conducted before the requirement for registration of clinical trials.
Adults
Study 3
Healthy women (n = 10) who ranged in age from 25 to 65 y (mean ± SD: 47.1 ± 12.6 y) were recruited beginning in October 1998. After fasting overnight, the subjects consumed (in a randomized crossover study) a single serving of either 250 mL soy milk (Sanitarium Health Foods Company), 46 g tempeh (White Wave) prepared in the form of a burrito, or 10 g toasted soy nuts (Country Life Natural Foods) on 3 separate occasions that were a minimum of 21 d apart. These foods contained 3.5 mg, 5.8 mg, and 3.5 mg of daidzein, respectively, and correspondingly 9.5 mg, 11.2 mg, and 4.1 mg genistein when expressed in aglycone equivalents. Blood samples (5–10 mL) were obtained via an indwelling catheter or by venipuncture ∼6 h postprandially, a time point that was expected to correspond to the peak plasma isoflavone (Cmax) concentrations, on the basis of previous studies of isoflavone pharmacokinetics (29, 33, 34). The blood samples were centrifuged and the plasma separated and frozen immediately at −20°C for later determination by mass spectrometry of plasma unconjugated and conjugated isoflavone concentrations.
Study 4
Healthy men (n = 10) aged 24–44 y (mean ± SD: 30.5 ± 5.6 y) and women (n = 10) aged 21–58 y (mean ± SD: 35.5 ± 14.9 y) were recruited beginning in September 2005, and they consumed 250 mL soy milk (Sanitarium Health Foods Company) in the morning and evening for 3.5 d (35). Blood samples (5–10 mL) were collected by venipuncture ∼6 h after the last serving of soy milk. Blood was centrifuged and the plasma separated and frozen immediately at −20°C for analysis of the total and unconjugated isoflavone concentrations by mass spectrometry.
Study 5
Six healthy premenopausal women (age range: 25–50 y) were recruited during 1993 and were administered a single oral bolus of encapsulated pure genistein (50 mg) taken with a glass of water followed by a meal, and serial blood samples were obtained by venipuncture at timed intervals for up to 48 h. Plasma was analyzed from stored samples from a previously reported study of the pharmacokinetics of pure genistein (29). For the purpose of the current study, the peak plasma unconjugated and conjugated genistein concentrations were determined by mass spectrometry in the samples collected between 4 and 8 h.
Study 6
Twelve healthy adults (6 women, 6 men; age range: 20–51 y) were recruited beginning in September 2007 and were administered by mouth a single 20-mg dose of enantiomeric pure S-(-)[2-13C]equol as described in detail previously (36). The concentration of unconjugated S-(-)[2-13C]equol was measured in plasma by mass spectrometry in samples collected at the time of peak plasma concentration, determined previously for each individual subject (36), and this value was compared with the total plasma S-(-)[2-13C]equol concentration to determine the percentage of unconjugated S-(-)[2-13C]equol.
Studies in infants and children
Study 7
Ten 6-mo-old breastfed infants (4 females, 6 males) were recruited beginning in January 2006 and were fed 118 mL (4 oz) of soy infant formula (Similac Isomil Advanced Soy Formula) in the morning and evening for 3.5 d before the usual breastfeeding. The soy infant formula contained 32 mg total isoflavones/L, composed of 67% genistein, 29% daidzein, and 4% glycitein, which is consistent with the previously reported composition of soy infant formulas (37, 38). On day 4, blood (0.5–1.0 mL) was obtained by venipuncture. When these same children were ∼3 y of age they were given a soy isoflavone supplement twice daily for 3.5 d. The soy isoflavone supplement, made from a soy germ extract, contained a total of 12 mg daidzin/daidzein, 7 mg glycitin/glycitein, and 2.5 mg genistin/genistein according to the manufacturer's analysis (Nature's Bounty Soy Isoflavones by Soy Life). The supplement was in a capsule form opened by the parents, and the powder was sprinkled into the child's favorite food. The plasma concentrations of total and unconjugated isoflavones were determined by mass spectrometry, and the percentage unconjugated fraction of each isoflavone was calculated. The study was approved by the Institutional Review Board of the Cincinnati Children's Hospital Medical Center (protocol 2008-0963), and written informed consent was obtained from the parents or guardians of each infant.
Materials and reagents
S-(-)[2-13C]equol was synthesized as described previously (36). [2,3,4-13C3]genistein, [2,3,4-13C3]daidzein, and (±)[2,3,4-13C3]equol (>99% purity) were purchased from the University of Edinburgh, United Kingdom. Working solutions of these compounds were prepared in ethanol from 1 mg/mL stock solutions. Calibrators and quality-control samples for human and rat plasma analysis were prepared in a charcoal-pretreated plasma pool as a matrix. The calibration standards were prepared at concentrations of 0, 1, 5, 10, 50, 100, 200, and 500 ng/mL for the measurement of total isoflavones and at concentrations of 0, 0.1, 0.2, 0.5, 1, 2, 5, and 10 ng/mL for the measurement of the individual unconjugated isoflavones. Sodium acetate, sodium hydroxide, acetic acid, and sodium bicarbonate, acetone, isopropanol, and all other solvents (HPLC grade) were purchased from Fisher Scientific. Helix pomatia digestive juice (a mixed solution of β-glucuronidase, 96,000 units/mL, and sulfatase, 390 units/mL) was purchased from Sigma-Aldrich.
Analytic techniques
Genistein, daidzein, glycitein, and S-(-)-equol were measured in plasma samples from rats, mice, and humans by liquid chromatography tandem mass spectrometry with multiple reaction monitoring and with the use of stable isotopic–labeled [2,3,4-13C3]genistein, [2,3,4-13C3]daidzein, and [2,3,4-13C3](±)equol as internal standards. Glycitein was measured against [2,3,4-13C3]daidzein in samples from those infants given a soy germ supplement. For the quantification of total and unconjugated isoflavone concentrations, 200 μL and 400 μL, respectively, of plasma were used for the assay. In brief, the method for total isoflavones involved the addition of the stable-labeled internal standard to plasma, incubated with 10 vol of triethylamine sulfate solution at 64°C; solid-phase extraction; overnight hydrolysis of conjugates by a mixed β-glucuronidase and sulfatase preparation (Helix pomatia); reextraction with the use of solid-phase cartridges; and reconstitution in HPLC mobile phase. For the separate determination of unconjugated isoflavones, the same procedure was used, with the omission of the enzyme hydrolysis step. These methods have been described in recent publications (35, 36, 39).
Isoflavones and the metabolite S-(-)-equol were detected and quantified by monitoring the multiple reaction monitoring transition ions, for genistein and [2,3,4-13C3]genistein (m/z 269→133, 272→135, respectively), daidzein and [2,3,4-13C3]daidzein (m/z 253→224, 256→226, respectively), and glycitein (m/z 286→238), and for S-(-)equol and (±)[2,3,4-13C3]equol (m/z 241→121, 244→122, respectively). Plasma concentrations were determined from the peak area ratio of each ion relative to the peak area response from the internal standard and by interpolation of this area ratio against calibration curves plotted for known concentrations of daidzein, genistein, and S-(-)equol. The intraassay and interassay precisions were 4–8%, expressed as a CV for quality control samples with a range of concentrations of 1 to 200 ng/mL. The limit of detection of the assay was 1 pg on column, and the lower limit of quantification was 0.1 ng/mL.
The measurement of unconjugated S-[2-13C]equol in human plasma collected from a pharmacokinetic study of S-[2-13C]equol was performed exactly as described previously for total S-[2-13C]equol, with the exclusion of the enzyme hydrolysis step and after derivatization with dansyl chloride before HPLC–electrospray tandem mass spectrometry analysis (36, 39).
All samples were analyzed with the Waters Quattro (Premier/Micro) ultra HPLC system coupled to electrospray tandem mass spectrometry. Chromatographic separation of the individual isoflavones was achieved on a reverse-phase Acquity C18 ultra HPLC BEH column (100 × 1.0 mm internal diameter, 1.7 μm; Waters). A gradient mobile phase was used with a binary solvent system, which changed from 100% solvent A to 40% solvent A over 10 min, then to 100% solvent B over 2 min, and then, after a 2-min hold, to 100% solvent A over a 3-min period, and this was held for 3 min. The total run time was 20 min, and the flow rate was 0.1 mL/min. Solvent A consisted of 950 mL water/L and 50 mL methanol/L that contained 2 mmol ammonium acetate/L; solvent B consisted of methanol that contained 2 mmol ammonium acetate/L. The injection volume was 10 μL. The optimal signal for the ion pair of daidzein, genistein, and S-(-)equol was achieved in negative ion mode with the use of the following instrument settings: capillary voltage, 1.0 kV; extractor voltage, 1 V; radio frequency lens voltage, 0.2 V; entrance, −1; exit, 0; source temperature, 120°C; desolvation temperature, 400°C; desolvation gas flow, 600 L/h; and cone gas flow, 50 L/h. Cone voltage, collision energy, and ion dwell time were optimized for each isoflavone; helium was used as the collision gas. Data were acquired and processed with Masslynx 4.1 software (Waters).
Statistical methods
Plasma isoflavone concentrations were expressed as ng/mL and the percentage unconjugated isoflavone composition determined by division of the unconjugated plasma concentration by the total, expressed as a percentage value. Group data were expressed as means ± SEMs, unless otherwise stated. Statistical analysis was performed on the relative percentage of isoflavone compositions only.
Study 1
All possible pairwise comparisons were made for the plasma percentage unconjugated concentrations of S-(-)equol, daidzein, and genistein between the 3 different strains of mice and the Sprague-Dawley rats with the use of an ANOVA procedure for each endpoint at α = 0.05. Pairwise comparisons were performed with the Tukey's honestly significant difference test to control for multiple comparisons. The data were log transformed to meet the assumptions of the analysis of variance.
Study 2
Only descriptive data are presented for this study. No statistical testing was done.
Study 3
The percentage unconjugated fractions for daidzein and genistein were compared between the 3 foods with the use of ANOVA procedures, with terms for subject and diet at α = 0.05. Pairwise comparisons were done with Tukey's honestly significant difference test to control for multiple comparisons. The data were log transformed to meet the assumptions of the ANOVA.
Study 4
To compare the plasma percentage unconjugated genistein and daidzein between men and women, a 2-independent-sample t test with the assumption of unequal variances was used. To satisfy the assumptions of normality, a log transformation was used for each endpoint.
Studies 5–7
Only descriptive data and/or graphical displays are presented for these studies. No statistical testing was done.
RESULTS
Plasma total isoflavone concentrations in different species of rodents fed commercial soy-containing diets
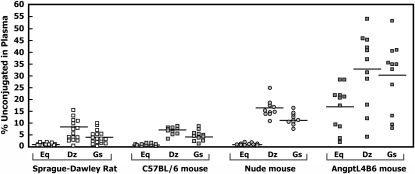
Total and unconjugated genistein, daidzein, and S-(-)equol concentrations in the plasma collected from 18 adult female Sprague-Dawley rats maintained on a soy-free AIN-93G diet were below the detection limits of the method (data not shown). In Sprague-Dawley rats that consumed a commercial Purina 5008 soy-containing diet, and in each strain of mouse fed a similar soy-based Purina 5010 diet, S-(-)equol was the predominant isoflavan in plasma, consistent with previously reported data on the metabolism of isoflavones in rodents (32, 40). The plasma total (conjugated + unconjugated) concentration of the metabolite S-(-)equol far exceeded that of its precursor daidzein, and also genistein, in all rodents (Table 1). Whereas the absolute concentration of total S-(-)equol was high in the C57BL/6 mice, the unconjugated S-(-)equol concentration in plasma was relatively low and was similar to that in the Sprague-Dawley rat and the athymic mouse (Table 1). When expressed as a proportion of the total, the percentage unconjugated S-(-)equol in plasma was relatively low in these 3 rodents, consistent with efficient phase II conjugation of S-(-)equol (Figure 1). By contrast, the soy isoflavones genistein and daidzein were less efficiently conjugated, as evident by the higher proportions of genistein and daidzein circulating in unconjugated forms. Striking was the relatively high proportions of genistein and daidzein in unconjugated forms in the athymic and AngptL4B6 transgenic mice, when compared with either the Sprague-Dawley rat or the C57BL/6 mouse (P < 0.01 for all, Table 1).
TABLE 1.
Comparison of the steady state plasma concentrations of S-(-)equol, daidzein, and genistein in the plasma of adult Sprague-Dawley rats and in 3 different strains of mice that were fed commercial soy-containing diets1
| Sprague-Dawley rat | C57BL/6 mouse | Nude mouse | AngptL4B6 mouse | |
| Total no. (sex no.) | 18 (18 F) | 11 (11 F) | 10 (10 F) | 11 (6 F, 5 M) |
| Total, conjugated + unconjugated (ng/mL) | ||||
| S-(-)Equol | 864 ± 156 | 1742 ± 223 | 1054 ± 124 | 843 ± 83 |
| Daidzein | 71 ± 22 | 180 ± 37 | 398 ± 32 | 175 ± 31 |
| Genistein | 126 ± 41 | 152 ± 30 | 298 ± 24 | 177 ± 30 |
| Unconjugated (ng/mL) | ||||
| S-(-)Equol | 10 ± 2 | 11 ± 2 | 13 ± 2 | 150 ± 30 |
| Daidzein | 7 ± 2 | 14 ± 3 | 63 ± 7 | 52 ± 13 |
| Genistein | 6 ± 2 | 7 ± 1 | 35 ± 4 | 52 ± 13 |
| Unconjugated fractions as a proportion of total (%) | ||||
| S-(-)Equol | 1.1 ± 0.1 | 0.7 ± 0.1 | 1.2 ± 0.12 | 17.0 ± 2.9 3–5 |
| Daidzein | 8.1 ± 1.1 | 7.4 ± 0.76 | 16.1 ± 1.267 | 32.7 ± 4.734 |
| Genistein | 4.0 ± 0.6 | 4.6 ± 0.6 | 11.6 ± 0.937 | 30.1 ± 4.3348 |
Values are group means ± SEMs unless indicated otherwise. All possible pairwise comparisons between the strains of mice and the Sprague-Dawley rat were performed on the unconjugated fractions by using Tukey's honestly significant difference test. P values were adjusted to control for multiple comparisons. Data were log transformed for analysis to meet the assumptions of the ANOVA.
Significantly different from C57BL/6 mouse:2 P ≤ 0.05,4 P < 0.0001, 7P ≤ 0.01.
Significantly different from rat: 3P < 0.0001, 6P < 0.01.
Significantly different from nude mouse:5 P < 0.0001, 8P ≤ 0.05.
FIGURE 1.
Percentages of S-(-)equol, daidzein, and genistein in the unconjugated form in plasma of Sprague-Dawley rats (n = 18) fed a soy-containing diet (Purina 5008) and in C57BL/6 (n = 11), athymic (n = 10), and transgenic AngptL4B6 (n = 11) mice fed a soy-containing diet (Purina 5010). The horizontal lines represent the group mean values. Dz, daidzein; Eq, S-(-)equol; Gs, genistein.
Plasma unconjugated genistein concentrations in Sprague-Dawley rats after oral administration of pure genistein
In study 2, the oral administration of 3.7 mg genistein/kg body weight as a single dose to 4 adult Sprague-Dawley rats resulted in mean (±SEM) plasma total and unconjugated genistein concentrations of 124 ± 34 and 3.6 ± 1.4 ng/mL, respectively, 1–2 h after gavage. The proportion of genistein that was present in unconjugated form was 3.1% ± 0.8% and was similar to that observed when the animals were fed a soy-containing Purina 5008 diet (4.0% ± 0.6%).
Plasma unconjugated isoflavone in healthy adults after consumption of different soy foods
Cmax isoflavone concentrations measured in 10 healthy adults (Study 3) 4–6 h after oral ingestion of a single serving of soy nuts (10g), soy milk (250 mL), or tempeh (46 g) were extremely low or, in some subjects, below the lower limit of quantification of the method, which indicated extensive phase II metabolism of isoflavones (Table 2). The mean proportion of genistein in unconjugated form in plasma at the Cmax was ∼2% of the total plasma isoflavone concentration and was not significantly different between the 3 different soy foods. The percentage unconjugated daidzein was significantly higher for women who consumed soy nuts than for those who consumed either soy milk (P = 0.023) or tempeh (P = 0.032). There was no difference for soy milk compared with tempeh. The bacterially derived metabolite S-(-)equol was not detected in any of the plasma samples at 4–6 h after oral ingestion, which is consistent with previous data that showed that equol formation is a time-dependent process that typically requires 12–36 h after isoflavone consumption (41).
TABLE 2.
Peak plasma total and unconjugated daidzein and genistein concentrations and the relative percentage composition of the unconjugated fraction in 10 healthy women 4–6 h after consumption of a single serving of soy nuts, soy milk, and tempeh on 3 separate occasions1
| Plasma isoflavones | 10 g Soy nuts | 250 mL Soy milk | 46 g Tempeh |
| Total concentration (ng/mL) | |||
| Daidzein | 30.2 ± 4.9 | 26.6 ± 3.5 | 10.7 ± 1.7 |
| Genistein | 39.0 ± 6.8 | 43.9 ± 7.4 | 19.6 ± 3.3 |
| Unconjugated concentration (ng/mL) | |||
| Daidzein | 0.6 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.1 |
| Genistein | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.4 ± 0.2 |
| Unconjugated fractions as a proportion of total (%) | |||
| Daidzein | 2.1 ± 0.4 | 0.9 ± 0.22 | 0.9 ± 0.32 |
| Genistein | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.7 ± 0.5 |
All values are means ± SEMs. All possible pairwise comparisons between foods were performed by using Tukey's honestly significant difference test. P values were adjusted to control for multiple comparisons. Data were log transformed for analysis to meet the assumptions of the ANOVA.
Significantly different from soy nuts, P ≤ 0.03.
Under steady state conditions after the intake of soy milk (250 mL twice daily) for 3.5 d (study 4), the plasma concentrations of unconjugated genistein and daidzein in 20 healthy adults (10 women and 10 men) were extremely low (Table 3). There were no significant sex differences between the percentage unconjugated genistein or daidzein concentrations (P > 0.66), and consequently these data were pooled. The plasma total genistein concentration was consistently higher than the total daidzein concentration in all subjects, in accord with previously published data (29, 33, 34).
TABLE 3.
Steady state plasma unconjugated and total genistein, daidzein, and S-(-)equol concentrations and the relative proportions (%) circulating in unconjugated form measured in samples taken from 20 healthy adults (n = 10 women, n = 10 men) after 3.5 d of soy milk consumption (2 × 250 mL/d)
| Unconjugated | Total | Percentage unconjugated | |||||||
| Subject number | Genistein | Daidzein | Equol | Genistein | Daidzein | Equol1 | Genistein | Daidzein | Equol |
| ng/mL | ng/mL | % | |||||||
| Men | |||||||||
| 1M | 0.72 | 0.49 | ND2 | 170 | 56 | 38 | 0.42 | 0.87 | — |
| 2M | 0.35 | 0.52 | ND | 164 | 45 | ND | 0.21 | 1.14 | — |
| 3M | 0.85 | 0.39 | ND | 240 | 49 | ND | 0.35 | 0.81 | — |
| 4M | 0.00 | 0.51 | ND | 81 | 49 | ND | 0.00 | 1.03 | — |
| 5M | 0.40 | 0.59 | ND | 220 | 61 | ND | 0.18 | 0.97 | — |
| 6M | 0.10 | 2.45 | ND | 178 | 51 | ND | 0.00 | 4.79 | — |
| 7M | 0.31 | 0.22 | ND | 117 | 44 | 15 | 0.26 | 0.50 | — |
| 8M | 0.10 | 0.10 | ND | 121 | 33 | ND | 0.08 | 0.31 | — |
| 9M | 1.83 | 2.06 | ND | 234 | 109 | ND | 0.78 | 1.88 | — |
| 10M | 0.00 | 0.10 | ND | 58 | 45 | ND | 0.00 | 0.22 | — |
| Means ± SEMs | 0.47 ± 0.18 | 0.74 ± 0.26 | — | 158.3 ± 20.0 | 54.3 ± 6.6 | 26.3 ± 5.2 | 0.25 ± 0.07 | 1.25 ± 0.42 | |
| Women | |||||||||
| 11F | 0.10 | 0.10 | ND | 104 | 25 | ND | 0.10 | 0.41 | — |
| 12F | 0.36 | 0.34 | ND | 94 | 24 | ND | 0.38 | 1.39 | — |
| 13F | 1.37 | 1.22 | ND | 236 | 110 | ND | 0.58 | 1.11 | — |
| 14F | 0.10 | 0.24 | ND | 65 | 32 | ND | 0.15 | 0.74 | — |
| 15F | 0.66 | 0.63 | ND | 164 | 55 | ND | 0.40 | 1.15 | — |
| 16F | 0.10 | 0.21 | ND | 126 | 56 | 69 | 0.08 | 0.38 | — |
| 17F | 0.00 | 0.10 | ND | 11 | 4 | ND | 0.46 | 2.69 | — |
| 18F | 0.00 | 0.24 | ND | 97 | 44 | ND | 0.05 | 0.55 | — |
| 19F | 1.55 | 1.60 | ND | 424 | 129 | ND | 0.37 | 1.24 | — |
| 20F | 0.00 | 0.10 | ND | 147 | 87 | 25 | 0.03 | 0.11 | — |
| Means ± SEMs | 0.44 ± 0.18 | 0.48 ± 0.17 | — | 146.7 ± 36.2 | 56.7 ± 12.8 | 9.4 ± 7.1 | 0.26 ± 0.06 | 0.98 ± 0.23 | — |
| All subjects combined | |||||||||
| Means ± SEMs | 0.46 ± 0.12 | 0.61 ± 0.15 | — | 152 ± 20 | 56 ± 6.8 | 12.2 ± 4.9 | 0.2 ± 0.05 | 1.1 ± 0.2 | — |
Only 4 subjects produced measureable concentrations of total S-(-)equol, equivalent to 20% of the adults being defined as “equol producers.”
ND, not detected or below the limit of detection of the method.
The mean (±SEM) unconjugated genistein and daidzein concentrations were extremely low at 0.46 ± 12 and 0.61 ± 0.15 ng/mL, respectively, which represented 0.2% and 1.1%, respectively, of the total plasma isoflavones. S-(-)equol was detected in the plasma of 4/20 adults, which represents a prevalence of “equol producers” of 20% (35). The plasma unconjugated S-(-)equol concentrations were below the lower limit of quantification of the method.
Extent of conjugation of genistein in healthy adults after administration of genistein
The mean (±SEM) peak plasma total and unconjugated isoflavone concentrations in 6 healthy adults measured 3–5 h after oral administration of 50 mg pure genistein (study 5) were 349 ± 68 and 5.2 ± 0.8 ng/mL, respectively. The proportion of genistein circulating in unconjugated form when the pure compound was administered was 1.7 ± 0.4%, similar to that after healthy adults consumed soy foods, but lower than the percentage unconjugated genistein in the plasma of Sprague-Dawley rats gavaged with genistein (4.0 ± 0.6%) or fed a soy-containing Purina 5008 rodent diet.
Unconjugated S-(-)equol in healthy adults after administration of [2-13C]S-(-)equol stable isotopic–labeled tracer
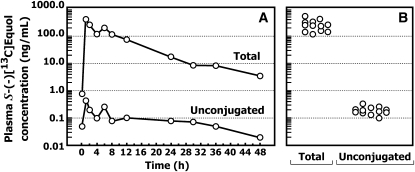
In study 6, a stable isotopic–labeled tracer was administered to 12 healthy adults to define the pharmacokinetics of S-(-)equol. Plasma total [2-13C]S-(-)equol concentrations were reported previously (36). The typical time course for plasma appearance and disappearance of total and unconjugated [2-13C]S-(-)equol concentrations after a single oral administration of 20 mg of pure [2-13C]S-(-)equol to a healthy adult man is shown in Figure 2. In these same samples, the unconjugated [2-13C]S-(-)equol concentrations were very low at all time points when compared with total [2-13C]S-(-)equol concentrations. On the basis of these values, the proportion of unconjugated [2-13C]S-(-)equol relative to the total ranged from 0.10% to 0.83% over the entire 48-h period in the example shown, which indicates extensive conjugation of S-(-)equol after oral administration. The mean (±SEM) total and unconjugated [2-13C]S-(-)equol concentrations in plasma collected from the 12 healthy subjects at the time of peak plasma concentrations were 238 ± 30 and 0.21 ± 0.02 ng/mL, respectively. The unconjugated fraction of [2-13C]S-(-)equol was calculated to account for only 0.09% ± 0.02% of the total [2-13C]S-(-)equol in plasma at the time of peak concentrations, which confirms its efficient conjugation.
FIGURE 2.
A: Typical plasma total and unconjugated [2-13C]S-(-)equol concentration time curve (ng/mL) in a healthy adult after the oral administration of 20 mg pure [2-13C]S-(-)equol. B: Plasma total and unconjugated [2-13C]S-(-)equol concentrations in 12 healthy adults (6 women, 6 men) measured at the time of peak plasma concentration.
Plasma unconjugated isoflavones in early life of infants who consumed soy isoflavones
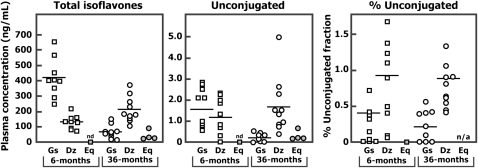
Plasma isoflavone concentrations measured in 10 infants fed 236 mL (8 oz) of soy infant formula daily at 6 mo of age, and in the same infants at age 3 y after they took an isoflavone supplement made from soy germ, are shown in Figure 3. In 6-mo-old infants, the total plasma genistein concentrations were higher than the daidzein concentrations, which is consistent with the isoflavone composition of the soy infant formula (37, 38, 42). Data from one infant was excluded because it was deemed noncompliant based on a low concentration of total genistein and daidzein (25 and 6 ng/mL, respectively). The mean (±SEM) plasma total genistein and daidzein concentrations for the remaining 9 infants were 419 ± 42 and 131 ± 14 ng/mL, respectively. The mean plasma unconjugated genistein and daidzein concentrations in these infants were extremely low (1.6 ± 0.3 and 1.2 ± 0.3 ng/mL, respectively). The mean (±SEM) unconjugated genistein and daidzein fraction in plasma accounted for only 0.4 ± 0.1% and 0.9 ± 0.2%, respectively, of total plasma isoflavones, which was similar to that of adults who consumed soy foods and indicates that efficient conjugation of isoflavones occurs early in life.
FIGURE 3.
Plasma total and unconjugated genistein, daidzein, and equol concentrations (ng/mL) and the proportion circulating in unconjugated form in the same 10 infants measured at 6 mo of age after consumption of soy infant formula for 3.5 d (118 mL twice daily) and again at 3 y of age after consumption of a soy isoflavone supplement made from soy germ for 3.5 d. Horizontal lines represent group means. Dz, daidzein; Eq, equol; Gs, genistein.
At age 3 y, after each child had been administered an isoflavone supplement made from soy germ, the plasma concentration of total daidzein was much higher than that of genistein, which is consistent with the predominance of daidzein in soy germ (Figure 3) (15, 43). The mean (±SEM) plasma total genistein and daidzein concentrations were 63 ± 14 and 210 ± 27 ng/mL, respectively. As observed at 6 mo of age, these same children had very low concentrations of unconjugated genistein (0.2 ± 0.1 ng/mL) and daidzein (1.7 ± 0.4 ng/mL). The proportions of genistein and daidzein circulating in the unconjugated form were consequently extremely low, representing 0.2 ± 0.1% and 0.8 ± 0.1%, respectively, of total plasma isoflavones, which was similar to that measured in the same infants at age 6 mo when fed soy infant formula. S-(-)equol was not detected in any of the samples from the 6-mo-old infants but was shown in 4 of 10 infants at age 3 y. Because soy germ contains appreciable amounts of glycitin/glycitein, the mean (±SEM) plasma total and unconjugated glycitein concentrations were measured in these samples only, and were 23.3 ± 3.7 and 1.9 ± 0.2 ng/mL, respectively. When expressed as percentage of the total isoflavones in plasma, the fraction of glycitein that was unconjugated was 1.9 ± 0.2%, which was higher than for either daidzein or genistein.
DISCUSSION
Isoflavones, in common with endogenous estrogens, circulate in blood and are excreted in the urine of humans and rodents, predominantly as glucuronides and, to a lesser extent, sulfates (27, 44, 45). Because the conjugated forms of isoflavones are considered to have relatively little or no biological activity (46), the extent of phase II metabolism has a significant effect on the physiologic effects of these soybean constituents. Conjugation of isoflavones takes place within the enterocyte during absorption (47) and therefore it is perhaps not surprising that in some rodent studies in which isoflavones are injected subcutaneously, a route of administration that bypasses first-pass metabolism, numerous deleterious effects have been observed that are not seen in humans who consume soy foods or isoflavone supplements.
One of the most important and controversial findings from rodent studies is the stimulatory effect of genistein on the growth of existing mammary tumors in ovariectomized athymic mice injected with MCF-7 cells (20). In addition, in this model, differently processed isoflavone-containing products, despite containing similar amounts of genistein, differentially affected tumor growth (23), and the greatest stimulatory effects from processing were associated with higher plasma concentrations of unconjugated genistein. More specifically, we estimated from published data, peak plasma concentrations (1 h after ingestion) of unconjugated genistein in ovariectomized Balb/c nude mice to be 80, 155, 175, 230, and 270 nmol/L in response to diets that contained 750 ppm genistein (present in the diet primarily as glucosides) in the form of soy flour plus mixed isoflavones, soy molasses, Novasoy, mixed isoflavones alone, and genistein, respectively (22). Thus, there was a 3-fold difference between the 2 processing extremes (soy flour compared with isolated genistein) and a 2-fold difference between soy flour and a very widely used commercially available isoflavone supplement (Novasoy). Importantly, the processing effect on tumor growth was perfectly correlated with circulating concentrations of unconjugated genistein (r2 = 0.96, P < 0.001). That is, the more processed forms of soy led to higher unconjugated genistein concentrations and greater tumor growth (22). These findings have led organizations such as the American Cancer Society to advise against the use of isoflavone supplements by women with a history of estrogen-sensitive breast cancer (24).
The results of the current research show, to our knowledge for the first time, that processing does not affect circulating unconjugated genistein concentrations in humans as it does in nude mice. As shown in Table 2, there were no differences in the proportion of genistein circulating in the biologically active unconjugated form in women after the consumption of 3 differently processed soy foods. The percentages of unconjugated genistein in the circulation after consumption of a single serving of soy nuts, soy milk, and tempeh were 1.5 ± 0.3%, 1.5 ± 0.3%, and 1.7 ± 0.5%, respectively, and this was at the time of peak concentrations. Of the 3 soy foods, the least processed is soy nuts, which contain isoflavones almost exclusively in glycoside form (9). Tempeh, because it is fermented, contains higher proportions of isoflavones as aglycons (9), whereas the soy milk used in the current study was made from isolated soy protein (16) and contained isoflavones almost exclusively as glycosides.
Not only were there no differences shown in the proportion of plasma genistein in the unconjugated form between the 3 soy foods, but there was also no difference in the unconjugated genistein concentrations in women who consumed soy foods (1.5–1.7%) or pure genistein (1.7 ± 0.1%). On the basis of the results in athymic mice, the expected order from highest to lowest proportion of unconjugated genistein concentrations would have been pure compound > tempeh or soy milk > soy nuts. Thus, at least with regard to the effect on Cmax, unconjugated genistein concentrations in the food matrix are not relevant. The same was true for the Sprague-Dawley rat: no difference was observed in percentage unconjugated genistein when rats were fed pure genistein or genistein that was derived from genistin in a soy-containing commercial diet.
Another major finding of the current research is that the capacity to conjugate isoflavones differs markedly between rats and mice, as well as between rodents and humans. In this study we focused on the Sprague-Dawley rat, the animal most used in chemically induced models of breast cancer, and 3 strains of mice: the widely used C57BL/6 strain; the athymic (nude) mouse, which is commonly used in cancer research; and the less commonly used transgenic strain of AngptL4B6 mouse, which is used in angiogenesis and arthritis research. The steady state percentages of unconjugated genistein concentration in plasma from Sprague-Dawley rats and C57BL/6, nude, and transgenic AngptL4B6 mice strains fed soy-containing diets were 4.0 ± 0.6%, 4.6 ± 0.6%, 11.6 ± 0.9%, and 30.1 ± 4.3%, respectively, concentrations that were ∼20, 23, 58, and 150 times those in humans (the mean percentage of genistein in human plasma is 0.2% in steady state; Table 3). These findings provide the strongest evidence to date that humans have a much greater capacity for conjugating isoflavones than do rodents. This difference is most pronounced between humans and the nude and transgenic AngptL4B6 strains of mice, and this finding is highly relevant to models of breast cancer because genistein-glucuronide has ∼50-fold less binding affinity for the ER than does genistein (46).
Concentrations of unconjugated isoflavones in women remain low even after repeated isoflavone exposure to a soy food. This finding is consistent with the findings from other laboratories (25, 30), as well as from our previously published data (29). Likewise, the high unconjugated genistein concentrations in mice observed in the current study have also been observed by others (48–50).
We report, for the first time to our knowledge, on the unconjugated isoflavone concentrations in the plasma of infants, and our findings confirm efficient phase II metabolism as with adults. The unconjugated fractions of genistein and daidzein in 6-mo-old infants exposed subchronically to high concentrations of isoflavones from soy infant formula (37, 38, 42) were very low: 0.4 ± 0.1% and 0.9 ± 0.2%, respectively. Low concentrations were also observed at age 3 y, when the very same infants were challenged for 3.5 consecutive days with an isoflavone supplement made from soy germ. The proportion of genistein and daidzein circulating in unconjugated form was extremely low: 0.2 ± 0.1% and 0.8 ± 0.1%, respectively. These data further show that the phase II metabolism of isoflavones is similar in response to the ingestion of 2 different soy products, soy milk and an isoflavone supplement. It had been stated previously that unconjugated isoflavones were undetectable in the plasma of infants fed soy infant formulas, but this was likely due to the limited sensitivity of the methods used for measurement at that time (51). Nevertheless, the current findings show that there is extensive conjugation of isoflavones by infants, an observation that is relevant, given the continuing debates over the safety of isoflavones in soy infant formulas (52, 53). Circulating concentrations of total isoflavones in the plasma of 3-mo-old infants exceed endogenous estrogens by up to 22,000 times, and plasma isoflavone concentrations are typically an order of magnitude higher than those of adults who consume similar daily intakes of isoflavones from soy foods (37, 38, 52, 54). Despite this very high exposure to soy isoflavones in early life, the proportion of the biologically active unconjugated fraction of isoflavones is maintained at a very low concentration.
Consistent throughout these studies has been the observation of a strong correlation between isoflavone structure and extent of conjugation. Irrespective of the source of the isoflavone, food compared with supplement/pure isoflavone, or the animal species examined, the rank order in efficiency of conjugation was S-(-)equol > genistein > daidzein > glycitein. The metabolite S-(-)equol was the most efficiently conjugated of all the isoflavans studied and this is an important finding with regard to its safety now that it is available as a dietary supplement for women's health (39, 55, 56) and is under development as a potential pharmaceutical agent (57, 58). This indicates differences in substrate specificity for the UDP–glucuronosyl transferases that conjugate isoflavones predominantly with glucuronic acid (27, 44).
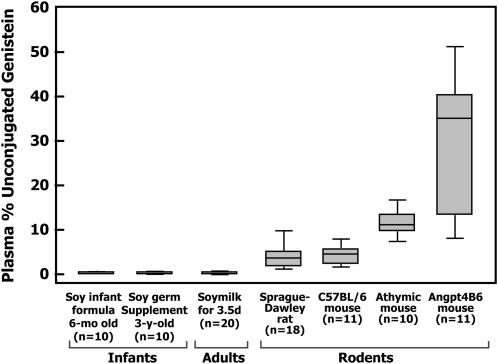
The current findings, which for genistein, are summarized in Figure 4, have potentially important implications for the understanding of the relation between isoflavone exposure and breast cancer risk. First, they suggest that rodents, and especially certain strains of mice, including most importantly nude mice, may not be especially useful for gaining insight into the health effects of isoflavones in humans, because circulating concentrations of genistein, the biologically active form, are so much higher in the former than in the latter. In fact, it would be very unlikely that the high unconjugated plasma concentrations of genistein shown in mice would occur in humans who consume soy foods or isoflavone supplements, given the very high capacity for phase II metabolism of isoflavones. Second, the findings suggest that, given similar genistein exposures, conclusions that supplements and highly processed forms of soy will affect breast tissue and breast cancer risk differently from less processed soy foods do not appear justified. That is, if isoflavones increase breast cancer risk and/or recurrence in women with a history of breast cancer, it does not matter whether the exposure occurs via whole soy food or soy extracts. The converse of that is also true: if consumption of isoflavones from soy foods poses no risk to breast cancer patients, the consumption of similar amounts of isoflavones from supplements would also appear to be safe.
FIGURE 4.
Comparison of the plasma proportion of unconjugated genistein expressed as a proportion of the total genistein under steady state conditions in 6-mo-old infants fed soy milk, in the same infants at 3 y of age given isoflavones from a soy germ supplement, in adults who consumed soy milk, in adult Sprague-Dawley rats fed commercial soy-containing diet (Purina 5008), and in 3 different strains of mice fed a soy-containing diet (Purina 5010). Study data are displayed as box plots in which the lines inside the boxes represent the median or 50th percentile of the data; the boxes extend from the 25th to the 75th percentile, an interval referred to as the interquartile range. The lines that emerge from the boxes extend to the upper and lower adjacent values (ie, the largest data point ≤75th percentile plus 1.5 times the interquartile range and the smallest data point ≥25th percentile minus 1.5 times the interquartile range, respectively).
In any event, recently published clinical and epidemiologic data do not support concerns that isoflavones are contraindicated for breast cancer patients or women at high risk of breast cancer. Clinical studies show that in healthy postmenopausal subjects and breast cancer patients, isoflavone exposure, whether from foods or supplements, does not affect breast tissue density or breast cell proliferation (59, 60). This lack of effect of isoflavones contrasts with the known effects of estrogen and progestin use, which increases breast tissue density (61), breast cell proliferation (62), and breast cancer risk (63). Furthermore, the epidemiologic literature suggests that soy consumption either has no effect on, or improves the prognosis of, postmenopausal ER+ breast cancer patients. These data include 4 prospective studies, 2 from China (64, 65) and 2 from the United States (66, 67). Importantly, soy intake was shown not to inhibit the efficacy of tamoxifen and actually enhanced the efficacy of the aromatase inhibitor, anastrozole (65). The lack of interaction with tamoxifen and the favorable interaction with anastrozole contrast with findings from the athymic mouse model (68, 69) that are widely cited as evidence of the harmful effects of isoflavones (20, 68, 70).
In conclusion, the major findings of the current research that marked differences in phase II metabolism of isoflavones exist between rodents and humans cast doubt on the value of the use of certain strains of mice for gaining insight into the effects of isoflavones, and in particular genistein, in humans, especially with regard to the effects of isoflavones on breast tissue and tumor progression. Our findings, combined with the results of recently published clinical and epidemiologic data, suggest that recommendations that breast cancer patients and women at high risk of development of breast cancer avoid isoflavone exposure are no longer justified.
Acknowledgments
We acknowledge the support of Tracy Glauser, Cincinnati Children's Hospital Medical Center, who performed the data safety monitoring for the humans studies. These findings were presented at the 9th International Symposium on the Role of Soy in Preventing and Treating Chronic Disease, held in Washington, DC, October 8–10, 2010.
The authors’ responsibilities were as follows— KDRS: principal investigator; KDRS, NMB, and MJM: conception and design of the research studies; NMB and SLL: performance of animal studies; NMB and SLL: screening, recruitment and enrollment of human study subjects; JEH: overseeing and monitoring of human studies; SLL: technical work; XZ: mass spectrometry analytical work; ECK: statistical analysis of the data; KDRS, MJM, and NMB: writing of the manuscript; and KDRS, NMB, XZ, SLL, JEH, ECK, and MGJ: reading of, and providing input on, the final manuscript. KDRS has intellectual property on S-(-)equol licensed by the Cincinnati Children's Hospital Medical Center, Cincinnati, OH, to industries and is a consultant to the Sanitarium Health Food Company, Berkeleyvale, NSW, Australia. MJM is a consultant to the soy industry. None of the other authors had a conflict of interest or anything to disclose, financially or otherwise.
REFERENCES
- 1.Cassidy A, Hooper L. Phytoestrogens and cardiovascular disease. J Br Menopause Soc 2006;12:49–56 [DOI] [PubMed] [Google Scholar]
- 2.Li SH, Liu XX, Bai YY, Wang XJ, Sun K, Chen JZ, Hui RT. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: a meta-analysis of randomized placebo-controlled trials. Am J Clin Nutr 2010;91:480–6 [DOI] [PubMed] [Google Scholar]
- 3.Messina M, Lane B. Soy protein, soybean isoflavones, and coronary heart disease risk: where do we stand? Future Lipidol 2007;2:55–74 [Google Scholar]
- 4.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer 2008;98:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messina M, Wu AH. Perspectives on the soy-breast cancer relation. Am J Clin Nutr 2009;89:1673S–9S [DOI] [PubMed] [Google Scholar]
- 6.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr 2009;89:1155–63 [DOI] [PubMed] [Google Scholar]
- 7.Bolaños R, Del Castillo A, Francia J. Soy isoflavones versus placebo in the treatment of climacteric vasomotor symptoms: systematic review and meta-analysis. Menopause 2010;17:660–6 [PubMed] [Google Scholar]
- 8.Setchell KDR, Borriello SP, Kirk DN, Axelson M. Non-steroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr 1984;40:569–78 [DOI] [PubMed] [Google Scholar]
- 9.Coward L, Barnes NC, Setchell KDR, Barnes S. Genistein, daidzein, and their β-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J Agric Food Chem 1993;41:1961–7 [Google Scholar]
- 10.Franke AA, Custer LJ, Wang W, Shi CY. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proc Soc Exp Biol Med 1998;217:263–73 [DOI] [PubMed] [Google Scholar]
- 11.Brzezinski A, Debi A. Phytoestrogens: the “natural” selective estrogen receptor modulators? Eur J Obstet Gynecol Reprod Biol 1999;85:47–51 [DOI] [PubMed] [Google Scholar]
- 12.Setchell KDR. Soy isoflavones–benefits and risks from nature's selective estrogen receptor modulators (SERMs). J Am Coll Nutr 2001;20:354S–62S; discussion 81S-83S [DOI] [PubMed] [Google Scholar]
- 13.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JÅ. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998;139:4252–63 [DOI] [PubMed] [Google Scholar]
- 14.Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med 2008;74:1656–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy PA, Barua K, Hauck CC. Solvent extraction selection in the determination of isoflavones in soy foods. J Chromatogr B Analyt Technol Biomed Life Sci 2002;777:129–38 [DOI] [PubMed] [Google Scholar]
- 16.Setchell KDR, Cole SJ. Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. J Agric Food Chem 2003;51:4146–55 [DOI] [PubMed] [Google Scholar]
- 17.Barnes S, Grubbs C, Setchell KDR, Carlson J. Soybeans inhibit mammary tumors in models of breast cancer. Prog Clin Biol Res 1990;347:239–53 [PubMed] [Google Scholar]
- 18.Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology 2008;16:219–26 [DOI] [PubMed] [Google Scholar]
- 19.Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr 2010;140:2326S–34S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr 2001;131:2957–62 [DOI] [PubMed] [Google Scholar]
- 21.Ju YH, Fultz J, Allred KF, Doerge DR, Helferich WG. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis 2006;27:856–63 [DOI] [PubMed] [Google Scholar]
- 22.Allred CD, Twaddle NC, Allred KF, Goeppinger TS, Churchwell MI, Ju YH, Helferich WG, Doerge DR. Soy processing affects metabolism and disposition of dietary isoflavones in ovariectomized BALB/c mice. J Agric Food Chem 2005;53:8542–50 [DOI] [PubMed] [Google Scholar]
- 23.Allred CD, Allred KF, Ju YH, Goeppinger TS, Doerge DR, Helferich WG. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis 2004;25:1649–57 [DOI] [PubMed] [Google Scholar]
- 24.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, McTiernan A, Rock CL, Thompson C, Gansler T, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin 2006;56:323–53 [DOI] [PubMed] [Google Scholar]
- 25.Gu L, House SE, Prior RL, Fang N, Ronis MJ, Clarkson TB, Wilson ME. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr 2006;136:1215–21 [DOI] [PubMed] [Google Scholar]
- 26.Setchell KDR, Gosselin SJ, Welsh MB, Johnston JO, Balistreri WF, Kramer LW, Dresser BL, Tarr MJ. Dietary estrogens–a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology 1987;93:225–33 [DOI] [PubMed] [Google Scholar]
- 27.Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos 2000;28:298–307 [PubMed] [Google Scholar]
- 28.Dutton GJ. The influence of sex, species and strain on glucuronidation. CRC Press: Boca Raton, FL, 1980 [Google Scholar]
- 29.Setchell KDR, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr 2001;131:1362S–75S [DOI] [PubMed] [Google Scholar]
- 30.Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, Heizer WD, Thomas BF, Hill JM, Crowell JA, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr 2002;75:126–36 [DOI] [PubMed] [Google Scholar]
- 31.Fischer L, Mahoney C, Jeffcoat AR, Koch MA, Thomas BE, Valentine JL, Stinchcomb T, Boan J, Crowell JA, Zeisel SH. Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer 2004;48:160–70 [DOI] [PubMed] [Google Scholar]
- 32.Brown NM, Setchell KDR. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest 2001;81:735–47 [DOI] [PubMed] [Google Scholar]
- 33.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr 1998;67:867–72 [DOI] [PubMed] [Google Scholar]
- 34.Setchell KDR, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr 2003;133:1027–35 [DOI] [PubMed] [Google Scholar]
- 35.Setchell KDR, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr 2006;136:2188–93 [DOI] [PubMed] [Google Scholar]
- 36.Setchell KDR, Zhao X, Jha P, Heubi JE, Brown NM. The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am J Clin Nutr 2009;90:1029–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 1997;350:23–7 [DOI] [PubMed] [Google Scholar]
- 38.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr 1998;68(Suppl):1453S–61S [DOI] [PubMed] [Google Scholar]
- 39.Setchell KDR, Zhao X, Shoaf SE, Ragland K. The pharmacokinetics of S-(-)equol administered as SE5-OH tablets to healthy postmenopausal women. J Nutr 2009;139:2037–43 [DOI] [PubMed] [Google Scholar]
- 40.Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe B, Zimmer-Nechemias L, Brown NM, Lund TD, et al. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr 2005;81:1072–9 [DOI] [PubMed] [Google Scholar]
- 41.Setchell KDR, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr 2010;140:1363S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy P, Tongtong S, Buseman G, Barua K. Isoflavones in soy-based infant formulas. J Agric Food Chem 1997;45:4635–8 [Google Scholar]
- 43.Clerici C, Setchell KDR, Battezzati PM, Pirro M, Giuliano V, Asciutti S, Castellani D, Nardi E, Sabatino G, Orlandi S, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr 2007;137:2270–8 [DOI] [PubMed] [Google Scholar]
- 44.Shelnutt SR, Cimino CO, Wiggins PA, Badger TM. Urinary pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein. Cancer Epidemiol Biomarkers Prev 2000;9:413–9 [PubMed] [Google Scholar]
- 45.Pritchett LE, Atherton KM, Mutch E, Ford D. Glucuronidation of the soyabean isoflavones genistein and daidzein by human liver is related to levels of UGT1A1 and UGT1A9 activity and alters isoflavone response in the MCF-7 human breast cancer cell line. J Nutr Biochem 2008;19:739–45 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J Nutr 1999;129:399–405 [DOI] [PubMed] [Google Scholar]
- 47.Setchell KDR, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr 2002;76:447–53 [DOI] [PubMed] [Google Scholar]
- 48.Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J Nutr 1997;127:263–9 [DOI] [PubMed] [Google Scholar]
- 49.Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. (Published erratum appears in Cancer Res 1999;59:1388.) Cancer Res 1998;58:3833–8 [PubMed] [Google Scholar]
- 50.Santell RC, Kieu N, Helferich WG. Genistein inhibits growth of estrogen-independent human breast cancer cells in culture but not in athymic mice. J Nutr 2000;130:1665–9 [DOI] [PubMed] [Google Scholar]
- 51.Huggett AC, Pridmore S, Malnoe A, Haschke F, Offord EA. Phyto-oestrogens in soy-based infant formula. [letter; comment] Lancet 1997;350:815–6 [DOI] [PubMed] [Google Scholar]
- 52.Badger TM, Ronis MJ, Hakkak R, Rowlands JC, Korourian S. The health consequences of early soy consumption. J Nutr 2002;132:559S–65S [DOI] [PubMed] [Google Scholar]
- 53.Badger TM, Gilchrist JM, Pivik RT, Andres A, Shankar K, Chen JR, Ronis MJ. The health implications of soy infant formula. Am J Clin Nutr 2009;89:1668S–72S [DOI] [PubMed] [Google Scholar]
- 54.Franke AA, Custer LJ, Tanaka Y. Isoflavones in human breast milk and other biological fluids. Am J Clin Nutr 1998;68(Suppl):1466S–73S [DOI] [PubMed] [Google Scholar]
- 55.Tousen Y, Ezaki J, Fujii Y, Ueno T, Nishimuta M, Ishimi Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: a pilot randomized, placebo-controlled trial. Menopause 2011;18(5):563–74 [DOI] [PubMed] [Google Scholar]
- 56.Aso T. Equol improves menopausal symptoms in Japanese women. J Nutr 2010;140:1386S–9S [DOI] [PubMed] [Google Scholar]
- 57.Schwen R, Jackson R, Proudlock R. Genotoxicity assessment of S-equol in bacterial mutation, chromosomal aberration, and rodent bone marrow micronucleus tests. Food Chem Toxicol 2010;48:3481–5 [DOI] [PubMed] [Google Scholar]
- 58.Jackson RL, Greiwe JS, Desai PB, Schwen RJ. Single-dose and steady-state pharmacokinetic studies of S-equol, a potent nonhormonal, estrogen receptor beta-agonist being developed for the treatment of menopausal symptoms. Menopause 2011;18:185–93 [PubMed] [Google Scholar]
- 59.Messina MJ, Wood CE. Soy isoflavones, estrogen therapy, and breast cancer risk: analysis and commentary. Nutr J 2008;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update 2010;16:745–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lundström E, Wilczek B, von Palffy Z, Soderqvist G, von Schoultz B. Mammographic breast density during hormone replacement therapy: effects of continuous combination, unopposed transdermal and low-potency estrogen regimens. Climacteric 2001;4:42–8 [PubMed] [Google Scholar]
- 62.Conner P, Soderqvist G, Skoog L, Graser T, Walter F, Tani E, Carlstrom K, von Schoultz B. Breast cell proliferation in postmenopausal women during HRT evaluated through fine needle aspiration cytology. Breast Cancer Res Treat 2003;78:159–65 [DOI] [PubMed] [Google Scholar]
- 63.Collins JA, Blake JM, Crosignani PG. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update 2005;11:545–60 [DOI] [PubMed] [Google Scholar]
- 64.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA 2009;302:2437–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang X, Zhang Q, Wang S, Huang X, Jin S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ 2010;182:1857–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guha N, Kwan ML, Quesenberry CP, Jr, Weltzien EK, Castillo AL, Caan BJ. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat 2009;118:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caan BJ, Natarajan L, Parker BA, Gold EB, Thomson CA, Newman VA, Rock CL, Pu M, Al Delaimy WK, Pierce JP. Soy food consumption and breast cancer prognosis. Cancer Epidemiol Biomarkers Prev 2011;20:854–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ju YH, Doerge DR, Allred KF, Allred CD, Helferich WG. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res 2002;62:2474–7 [PubMed] [Google Scholar]
- 69.Ju YH, Doerge DR, Woodling KA, Hartman JA, Kwak J, Helferich WG. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis 2008;29:2162–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ju YH, Allred KF, Allred CD, Helferich WG. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis 2006;27:1292–9 [DOI] [PubMed] [Google Scholar]