Abstract
Aims
We aimed to study the diagnostic influence of adding a routine cardiovascular ultrasound screening of the cardiac anatomy and function, the pericardium, the pleura and the abdominal great vessels by the new pocket-size ultrasound device (pUS) with grey scale and colour Doppler imaging.
Methods and results
In 119 randomly selected patients admitted to a cardiac unit at a non-university hospital, routinely adding a cardiovascular ultrasonography of only 4.4 min with a pocket-size device corrected the primary diagnosis in 16% of patients. In addition, 29% had the primary diagnosis verified and in 10% an additional important diagnosis was made. Higher age predicted any diagnostic influence of pUS screening with an increase of 61% (P = 0.003) per 10 years of higher age. Overall, the pUS screening had a sensitivity and specificity with respect to detecting at least moderate pathology of 97 and 93%. Positive and negative predictive values were 93 and 87%, respectively. In the sub-group of subjects with a change in the primary diagnosis following pUS there was no false-negative or false-positive findings.
Conclusion
Screening by pUS assessed vascular and cardiac anatomy and function accurately and enabled correction of the diagnosis in 16% of patients admitted to a cardiac unit. In 55% of the participants, the cardiovascular ultrasound screening had important diagnostic influence. We suggest that it would be appropriate to implement strategies and systems for routinely adding an ultrasound cardiovascular examination to patients in cardiac units.
Keywords: Echocardiography, Vscan, Hand-held, Scanner, Workflow, Device
Introduction
Diagnosis and treatment are the key elements in every inpatient's stay. Unfortunately, a correct diagnosis is not always made in time with a possible non-favourable outcome, time delay and patient's suffering as the result.1 Former studies have shown that adding an echocardiographic or cardiovascular ultrasound examination to the usual care diagnostics improve the accuracy of the diagnosis.2–5 The last decade's miniaturization of ultrasound devices capable of offering high-quality recordings has made possible a logistic basis for applying ultrasound examinations in a more routine way. The pocket-size ultrasound devices (pUS) of the last years have the potential to dramatically rearrange physical examinations and diagnosis.6,7 The pUS has been shown to be both accurate and feasible as a tool for cardiac imaging when used by experienced operators.8–10 However, it is not known how the pUS could be used as a tool in a cardiac unit influence diagnosis. In addition, it is uncertain how reliable the pUS examinations are when they are performed at the bedside under non-optimal conditions. We therefore aimed to study the diagnostic influence of routinely adding a bedside pUS cardiovascular screening with examination of the heart, the pericardium, the pleura and the abdominal great vessels. Furthermore, we wanted to study the reliability of the bedside pUS examination, and at last, study predictors of diagnostic influence of the pUS cardiovascular screening.
Methods
Study population
Patients admitted to the unit of cardiovascular medicine at the non-university Levanger Hospital in Norway between March and September 2010 were available for inclusion in this cardiovascular ultrasound study. As the second-call duty at this hospital is served by three cardiologists experienced in echocardiography and abdominal ultrasound and 10 other specialists in internal medicine, patients were only available for inclusion if one of the three cardiologists were on call the day the patients were admitted to the department. Prior to inclusion, patients were examined in the emergency department by a junior and senior resident. From medical history, clinical examination, laboratory tests and goal-directed imaging procedures other than echocardiography the primary diagnosis was made. All of these newly admitted patients who were available at the unit at the cardiologists evening round were included if they consented to participate. In total 119 patients were included in the study and screened by cardiovascular ultrasound.
The study was approved by the Regional Committee for Medical Research Ethics and conducted according to the second Helsinki Declaration. Written informed consent was obtained from all participants. Patients not able to consent were not included.
Diagnostic usefulness of screening with pocket-size ultrasound
Prior to the pUS examination, the primary diagnosis was recorded in the patient's journal files. Secondly, the results of the bedside pUS screening and the cardiologist own opinion of the diagnostic usefulness of the pUS screening were reported according to European Association of Echocardiography (EAE) recommendations.11 Thirdly, the Study Committee, consisting of two internal and one external (Trondheim University Hospital) cardiologists experienced in echocardiography and abdominal ultrasonography, graded the diagnostic usefulness of the bedside pUS screening. From the patients' journal files, the Committee individually graded the diagnostic usefulness as: (i) change in the primary diagnosis, (ii) verification of the primary diagnosis, (iii) added diagnosis important for further treatment or follow-up of the patient or (iv) not useful, depending on the descriptions of diagnosis, findings and therapeutic influence. In case of doubt, the clear majority in the Committee decided the grading of the diagnostic usefulness.
Cardiovascular screening
The cardiovascular screening was performed with a pUS, Vscan (GE Vingmed Ultrasound, Horten, Norway). The device weighs 390 g, including the phased-arrayed probe, which is sized 135 × 73 × 28 mm. The device offers two-dimensional grey scale and colour Doppler imaging, with a movable colour Doppler sector. The bandwidth with a range of 1.7–3.8 MHz is automatically adjusted. An algorithm enables automatic storage and looping of a cardiac cycle without ECG signal.12 The length of recordings of other structures is predefined and limited to 2 s. Patient identification was performed by voice recording and the automatically assessed examination number. All images and recordings were saved on the device's micro-SD card, and later transferred to a computer by commercial software (Gateway; GE Vingmed Ultrasound).
The cardiovascular screening by pUS was performed at the bedside with patients in left-lateral decubitus and supine position. Assessment of left ventricular (LV) global and regional function, right ventricular (RV) size and function, valvular anatomy and function and the pericardium were assessed from parasternal long- and short-axis and apical four-chamber, two-chamber and long-axis views. Global LV and RV functions were classified online by visual assessment as (i) normal/near normal, (ii) moderate dysfunctional or (iii) severe dysfunctional, while regional LV function was classified as (i) no regional dysfunction or (ii) regional dysfunction present. Valvular anatomy and function were classified as (i) normal/near normal anatomy and function, (ii) moderate pathology/dysfunction or (iii) severe pathology/dysfunction by visual assessment from grey scale and colour Doppler imaging. The most severe valvular pathology was used in the analyses. Pericardial effusion was classified as (i) not present or (ii) present. The size of the left atrium was measured on grey-scale parasternal long-axis measurement images by the device's caliper mode. An attempt was made in order to do the measurement at end systole. From the subxiphoid position, the abdominal aorta and inferior vena cava were assessed by grey-scale imaging. The abdominal aorta was assessed distally to the bifurcation and classified as (i) no abdominal aortic aneurysm present and (ii) abdominal aortic aneurysm present, depending on whether the diameter exceeded 35 mm or not. Maximal dimension was measured in case of doubt by visual assessment. The inferior vena cava diameter was measured end expiratory within 2 cm from the right atrium orifice and respiratory variation was assessed to estimate the right atrium filling pressure.13 All measurements of size were done on the pUS. With patients in the supine position the pleura was assessed by grey-scale imaging from left and right lateral views, and the amount of pleural effusion was classified as (i) no pleural effusion, (ii) insignificant or moderate pleural effusion or (iii) significant pleural effusion.14 All recordings were saved on the pUS and the time used for the screening was calculated as the time from start to end of the examination.
Validation of pUS screening
A high-end echocardiographic examination was performed by a Vivid 7 (GE Vingmed Ultrasound) scanner. One of the four experienced cardiologists other than the one who performed the pUS screening performed the examination. They were blinded to the result of the pUS examination. The same cardiovascular structures as described above were measured and classified according to the guidelines of the EAE.15–19 Ejection fraction was measured by Simpson's rule from apical four-chamber and two-chamber views, dimensions were measured by motion mode from parasternal recordings.18 Valvular pathology was graded according to the recommendations from the EAE.15–17 In addition, imaging techniques as computer tomography, magnetic resonance imaging or ultrasound were used by usual care at the Department of Radiology. For the analyses in the patients who underwent both echocardiographic and radiologic examinations, the radiologists grading of pleural effusion and size of the abdominal aorta was preferred compared with the echocardiography. At last all examinations were graded as described for pUS.
Statistics
As the different echocardiographic and anthropometric measures partly deviated from normal distribution, the basic characteristics are presented as mean ± standard deviation (SD) and range. Comparison of continuous variables between groups was done by the non-parametric Mann–Whitney U test of independent samples, and proportions between groups were analysed by the χ2 test or Fisher's exact test. The Spearmans rho (r) is used for comparison of the grading of pathology between the pUS and the high-end echocardiographic or radiologic examinations. Data are presented as r [95% confidence interval (CI)] where the 95% CI is analysed by determining the bootstrap distribution randomly re-sampling the study population 10 000 times. For comparison of continuous variables between the pUS and the high-end examinations Pearson's rho (r) was used, respectively. In order to assess predictors of influence of the pUS screening, logistic regression analyses were used. Diagnostic influence, graded as diagnostic usefulness or no diagnostic usefulness, was used as the dependent variable, and the age and known increased risk of cardiovascular disease were used as explanation variables. As there was a linear relationship between the diagnostic usefulness and increasing age, age was entered into the analyses as a continuous variable. Increased risk was classified as present if the patients had any known cardiovascular disease, hypertension or diabetes. Sample size of around 100 participants was estimated by expecting a change in the main diagnosis of at least 8–10% points, in addition to a more pronounced proportion in which the diagnose was verified or another important diagnose was added. However, from these estimates we expected only around 50–70% power to detect significant predictors of diagnostic usefulness of pUS screening with respect to change in the main diagnosis, and some underpowered analyses with respect to detecting predictors of any diagnostic usefulness as well (SamplePower, SPSS, Inc., Chicago, IL, USA). All the statistical analyses were performed using SPSS for Windows (version 18.0, SPSS, Inc.).
Results
Study population
Table 1 shows basic characteristics of the 119 study participants (45 women and 74 men). Mean ± SD (range) age was 67 ± 15 (25–85) years among women and 66 ± 14 (20–89) years among men, with no significant difference (P = 0.31). The distribution of age departed from the normal distribution and was positively skewed (Figure 1), P < 0.001. Each of the pUS measurements was obtained in at least 78% of the participants, and complete visualization of the abdominal aorta and the inferior vena cava diameter had lowest feasibility. Except for these latter measurements, all structures were assessed to satisfaction in at least 98% of the participants. A total of 65% had previous known atrial fibrillation, hypertension, diabetes or any kind of known cardiovascular disease and these participants were classified as at increased cardiovascular risk. The time used for the bedside pUS screening was 4.4 ± 1.7 (2.0–13) min.
Table 1.
Basic characteristics of 119 study participants
| Mean ± SD (range) | |
|---|---|
| Age (years) | 66.5 ± 14.2 (20–89) |
| Women [n (%)] | 45 (38) |
| Height (cm) | 172 ± 9 (146–189) |
| Weight (kg) | 80 ± 15 (45–122) |
| Body mass index (kg/m2) | 27.4 ± 4.9 (17–44) |
| Systolic blood pressure (mmHg) | 146 ± 31 (58–250) |
| Diastolic blood pressure (mmHg) | 80 ± 20 (32–161) |
| Heart rate (bpm) | 81 ± 24 (29–150) |
| Atrial fibrillation [n (%)] | 19 (16) |
| Known hypertension [n (%)] | 44 (37) |
| Known diabetes [n (%)] | 19 (16) |
| Known myocardial infarction [n (%)] | 34 (29) |
| Known angina [n (%)] | 26 (22) |
| Known heart failure [n (%)] | 10 (8) |
| Known peripheral vessel disease [n (%)] | 9 (8) |
| Known stroke [n (%)] | 8 (7) |
| Increased cardiovascular risk [n (%)] | 77 (65) |
| Known cancer [n (%)] | 4 (3) |
Increased cardiovascular risk; previous known atrial fibrillation, hypertension, diabetes or any kind of known cardiovascular disease.
Figure 1.
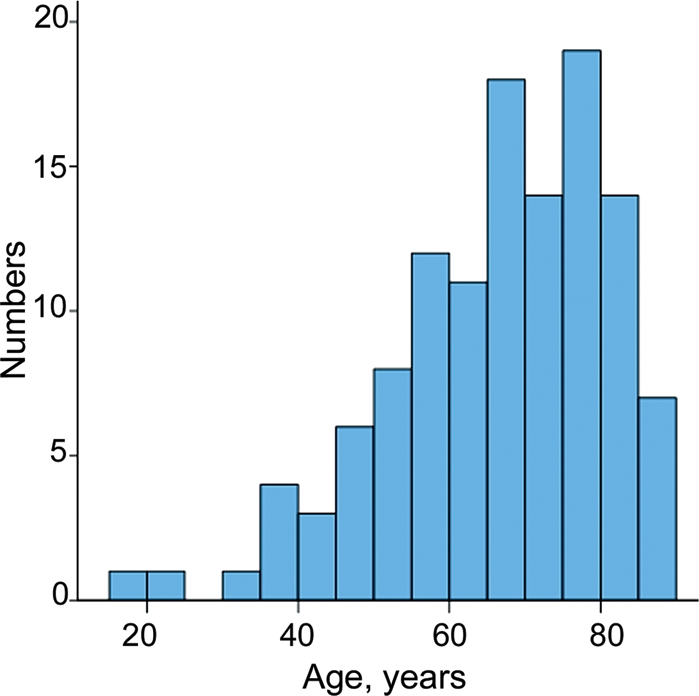
Age distribution of the 119 participants. Distribution deviated significantly from normal distribution (P < 0.001).
Diagnostic usefulness of screening with pocket-size ultrasound
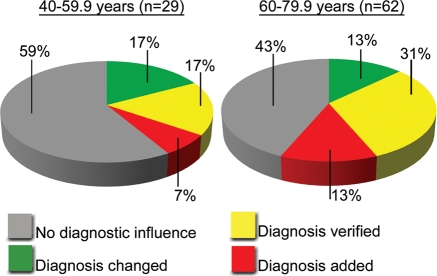
The diagnostic usefulness of bedside cardiovascular ultrasound screening with the pocket-size Vscan is shown in Table 2 and Figures 2 and3. In 19 (16%) participants the primary diagnosis was changed following pUS. In a total of 65 (55%) patients there was diagnostic usefulness, classified as either change in primary diagnosis, verification of primary diagnosis or adding a diagnosis important for treatment or follow-up of the patient. In Table 3 basic characteristics, the primary diagnosis, the findings and the correct diagnosis after pUS screening are listed for the 19 patients, with a change in the primary diagnosis following the pUS screening. Figure 4 show the electrocardiogram and an echocardiographic image of one of the patients who had the diagnosis changed.
Table 2.
Diagnostic influence of bedside cardiovascular screening by pocket-size ultrasound in 119 study participants
| Diagnostic influence | Number (%) |
|---|---|
| Change in primary diagnosis | 19 (16%) |
| Verification of primary diagnosis | 34 (29) |
| Additional diagnosis made | 12 (10) |
| No diagnostic usefulness | 54 (45) |
Figure 2.
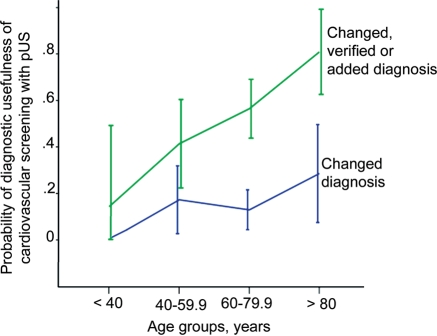
Diagnostic influence of cardiovascular ultrasound screening according to age. The probability of diagnostic influence of cardiovascular screening by pocket-size ultrasound according to the pre-stratified age groups. Error bars refer to 95% confidence interval.
Figure 3.
Diagnostic influence of cardiovascular ultrasound screening of participants 40–79.9 years. Diagrams show diagnostic influence of cardiovascular screening by pocket-size ultrasound according to the pre-stratified age groups 40–59.9 (left) and 60–79.9 years (right).
Table 3.
Characteristics and findings of the 19 participants with a change in primary diagnosis after cardiovascular screening with pocket-size ultrasound
| Characteristics | Primary diagnosis | Main findings at bedside screening with pocket-size cardiovascular ultrasound | Changed diagnosis |
|---|---|---|---|
| M, 75 years, HT, MI, op. CABG | Dyspnea | AAA 56 mm, PE, PlE | PE, AAA |
| W, 82 years, HT, AP, op. AVR | Dyspnea | PlE | PlE, severe amount |
| M, 78 years, asthma | Chest pain | Aortic valve stenosis/insufficiency | Aortic stenosis |
| W, 83 years, HT, COPD | Dyspnea LAI? | PlE, moderate valvular insufficiencies, LV dysfunction | Heart failure |
| M, 75years, AFIB, AP, PerVes, COPD | Heart failure | Normal LV and RV function | COPD |
| W, 60 years, HT | Chest pain | LVH | Hypertensive heart disease |
| W, 64 years, HT | Chest pain | Dissection of thoracic and abdominal aorta | Aortic dissection |
| W, 59 years, HT, DM | NSTEMI | Dilated and dysfunctional LV, multi-valvular pathology | Heart failure |
| M, 70 years, AFIB | AFIB | Anterior wall dysfunction | NSTEMI |
| W, 58 years, HT | Chest pain | Anteroseptal wall dysfunction | NSTEMI |
| W, 80 years, HT, DM, PerVes | Pneumonia | Dilated and dysfunctional LV | Heart failure |
| W, 67 years, AFIB, HT, DM | AFIB | Severe LA dilatation, dilated IVC, PlE | Heart failure |
| M, 62 years, MI, AP | Dyspnea | Global and regional LV dysfunction, severe MR | Heart failure (MR) |
| W, 81 years, HT, PerVes, strokea | Chest pain | LVH, moderate MI, severe dilated IVC | Hypervolemia (HF) |
| W, 81 years, HT, op. | Pneumonia | RV dilatation and dysfunction, dilated IVC | Ac. cor pulmonale (PuE?) |
| M, 53 years, asthma | NSTEMI? | Severe LV dilatation and dysfunction | DCM |
| W, 56 years, asthma | PuE | Inferior wall dysfunction | NSTEMI |
| M, 87 years, MI, AP, HF | Heart failure | Severe AS, PlE, near normal LV function | Severe AS |
| W, 81 years, MI | Dizziness | AAA 100 mm | AAA |
AAA, abdominal aortic aneurysm; AFIB, atrial fibrillation; AP, angina; AS, aortic stenosis; AVR, aortic valve replacement; DCM, dilated cardiomyopathy; DM, diabetes mellitus; HF, heart failure; HT, hypertension; IVC, inferior vena cava; LA, left atrium; LAI, lower airway infection; LV, left ventricular; LVH, left ventricular hypertrophy; MI, myocardial infarction, MR, mitral regurgitation; NSTEMI, non-ST-elevation myocardial infarction; op., recent surgery; PE, pericardial effusion; PlE, pleural effusion; PuE, pulmonary embolism; COPD, chronic obstructive pulmonic disease; PerVes, peripheral vessel disease.
aExcessive salt intake.
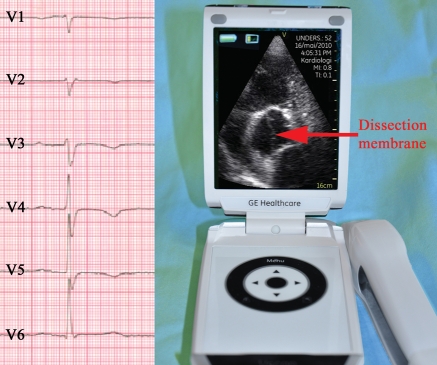
Figure 4.
Clinical case of a patient with change in diagnosis after examination with pUS. A 64-year-old woman was admitted with chest pain suspect of coronary ischaemia. Electrocardiogram showed T-wave inversions in precordial leads (left figure). Bedside screening with pocket-sized device revealed dissection of the ascending aorta (right figure; modified five-chamber view), aneurysm of the ascending aorta and aortic regurgitation. Dissection membrane was also visible in the abdominal aorta. She was transferred to the regional university hospital for surgery. See Supplementary data online, video loops.
Age and known increased risk of cardiovascular disease of the study participants differed significantly between those with any diagnostic influence of the pUS screening and those without. Mean age was almost 10 years higher in those where pUS screening influenced the diagnosis (P < 0.001) with mean ± SD (range) 70.9 ± 11.7 (38–89) compared to 61.2 ± 15.2 (20–85). The proportion of participants with increased cardiovascular risk, assessed as previous known atrial fibrillation, hypertension, diabetes, angina, myocardial infarction, heart failure, peripheral vessel disease or stroke, was 75% in those with any diagnostic usefulness of pUS screening compared to 52% in the other group (P= 0.007). Figure 2 shows the probability and 95% CI of diagnostic usefulness of pUS screening according to pre stratified age groups (<40, 40–59.9, 60–79.9 and >80 years). Figure 3 illustrates diagnostic influence of the pUS screening according to the two age groups of participants between 40 and 80 years. In logistic regression analyses 10 years higher age was found to increase the probability of any diagnostic influence of pUS screening with 61% (P= 0.003) adjusted for increased cardiovascular risk as present or absent (Table 4), but the corresponding 33% (P= 0.2) increased probability of change in primary diagnosis was not significant. Correspondingly, increased cardiovascular risk, assessed as present or absent, did not show significant increased probability of changed primary diagnosis or any diagnostic use when adjusted for age (P≥ 0.14). However, as shown in Table 4 there was a clear trend towards increased probability of diagnostic usefulness of cardiovascular bedside screening with pUS in those with higher cardiovascular risk. The other basic characteristics did not predict diagnostic usefulness of the cardiovascular screening with pUS.
Table 4.
Predictors of any diagnostic influence of bedside cardiovascular ultrasound screening
| OR | 95% CI OR | P-value | |
|---|---|---|---|
| Age per 10 yearsa | 1.72 | (1.27–2.32) | <0.001 |
| Any increased cardiovascular riskb | 2.84 | (1.31–6.18) | 0.008 |
| Age per 10 yearsc | 1.61 | (1.17–2.21) | 0.003 |
| Any increased cardiovascular riskd | 1.89 | (0.81–4.39) | 0.14 |
Any usefulness is changed primary diagnosis, verified diagnosis or additional diagnosis.
CI, confidence interval; OR, odds ratio.
aNot adjusted for cardiovascular risk,
bNot adjusted for age,
cAdjusted for cardiovascular risk
dAdjusted for age.
Validation of pUS screening
Validation of pUS screening was tested in a sample of 90 (76%) of the population. The correlation coefficient was1.0 for the grading of pericardial effusion and detection of abdominal aortic aneurysm, 0.94 (CI: 0.88–0.99) and 0.92 (CI: 0.83–0.99) for the grading of global and regional LV functions, respectively, 0.84 (CI: 0.60–1.0) for the grading of RV size and function, 0.89 (CI: 0.81–0.95) for the grading of valvular function, 0.67 (CI: 0.54–0.79) for the assessment of end-expiratory size of the inferior vena cava and 0.66 (CI: 0.51–0.78) for left atrium. All correlations were very highly significant (all P< 0.001). The inferior vena cava and the complete abdominal aorta were available for comparison in 81 (90%) and 59 (66%), respectively. All other structures were feasible in at least 97% of the participants.
In analyses of the 90 subjects that had been re-examined with at least one of the high-end reference methods, the sensitivity and specificity of the pUS examination with respect to detecting moderate or severe pathology of LV global and regional functions, RV function, valvular function and dilatation of the left atrium, detection of pericardial or pleural effusion as well as abdominal aortic aneurysms was 97 and 93%. The corresponding positive and negative predictive values were 93 and 87%, respectively. In the 19 subjects with change in the primary diagnosis following pUS there was no misclassification at all.
Discussion
In 119 randomly selected patients admitted to a cardiac unit at a non-university hospital routinely adding a cardiovascular ultrasonography of only 4.4 min with a pocket-size device corrected the primary diagnosis in 16% of patients. In addition, 29% had the primary diagnosis verified and in 10% an additional important diagnosis was made. Thus, in only 45% of the participants the cardiovascular ultrasound screening had no diagnostic influence.
Study population
Median age was 69 years, and as shown in Figure 1 the distribution of age was positively skewed. The basic characteristics are in line with those published in former studies 3,5,20 and thus, it might reflect the everyday clinical setting at cardiac departments.
Diagnostic usefulness of pUS screening
The diagnostic influence of routinely adding a pocket-size cardiovascular ultrasound examination performed by experts are remarkable, but still in line with previous publications on screening of patient groups by larger mobile ultrasound devices.3–5 The cost-benefit of a screening programme depends on the accuracy of the method used and the prevalence of pathology in the population.21,22 This study, as well as others, shows the high prevalence of underlying disease among inpatients with suspected cardiac or internal medical diseases.3–5 Furthermore, it underlines how difficult it is to make a correct diagnosis based on medical history, clinical examination, laboratory tests and routine imaging alone.1–5 Even though many of the incorrect diagnosis would have been corrected during the patient's stay, usual care practice would have lead to a significant time delay as well as a probable misdiagnosing. The accuracy of pUS screening presented is in line with recent publications performed under more optimized conditions compared with examinations performed bedside.8–10 We therefore suggest that screening with cardiovascular ultrasound in addition to usual care examinations should be recommended in patients admitted to a cardiac unit to optimize diagnostic accuracy and inpatient workflow. The use of pUS devices is quick, accurate and cheap, and thus, it has the potential to dramatically change the inpatient and outpatient workflow.6,7,10,23–25
Not surprisingly patient's age and level of cardiovascular risk predicted the diagnostic usefulness of the cardiovascular pUS screening. Former studies in comparable populations are scarce,2 but these findings are in line with the conceptual fundamentals of screening programmes, as the risk of diseases influences the outcome.21,26 Increased risk of cardiovascular disease assessed as known atrial fibrillation, known hypertension, known diabetes or any kind of known cardiovascular disease did not significantly predict diagnostic influence of pUS screening when adjusted for age. However, there was a clear trend. This may be explained by the fact that this study was not optimally powered to detect different predictors of diagnostic influence of pUS screening. As shown by the results, the diagnostic influence of cardiovascular screening with pUS seems to be apparent also at those at lowest age, and this advocates screening examinations in similar populations without further restriction with respect to age or risk. Similarly, former publications have also recommended echocardiographic screening in outpatient populations.2,5,10,24 The benefit of the screening procedure must be weighed against the possible harm. This refers particularly to possible false positive findings 22 as diagnostic ultrasound have no acknowledged side effects.27 The high-positive and -negative predictive values in this study indicate that screening programmes of similar populations may be cost beneficial.
Validation
There was a very high accuracy of pUS screening compared with high-end echocardiography or radiologic examinations for assessment of aortic aneurysms, pericardial effusion, LV global and regional size and function, RV size and function and valvular function. Assessment of size of the left atrium and end-expiratory diameter of the vena cava was fair and in line with a recent publication.8 With respect to these two structures, there are some methodological aspects that might have influenced the results. As the use of ECG cables is unpractical on pUS devices, the exact timing in the cardiac cycle may be non-optimal on the pUS device as it depends on visual assessment. However, the high accuracy with respect to detecting abdominal aortic aneurysms as well as a almost perfect correlation for aortic dimension with r= 0.99 (95% CI: 0.98–1.0) in those where aortic dimension was measured with pUS indicates that the non-optimal accuracy of measuring size of the left atrium and inferior vena cava is influenced by timing of the measurement in the cardiac or respiratory cycle. In addition, the time delay from the pUS screening to the high-end echocardiography was mean (SD) 16 h,12 which may have influenced the repeatability of the inferior vena cava measurements, due to physiological reasons and treatment during the time period.
Conclusion
In this study we found that a quick cardiovascular ultrasound screening by pUS assessed vascular and cardiac structures’ size and function accurately and enabled correction of the diagnosis in 16% of patients admitted to a cardiac unit. In addition, several patients had their diagnosis verified or an additional diagnosis important for treatment or follow-up made. We suggest that implementing strategies and systems for routinely adding an ultrasound cardiovascular examination to patients in cardiologic units are appropriate.
Limitations
The main limitation of this study is that the bedside cardiovascular pUS screening was performed by consultant cardiologists experienced in echocardiography as well as in abdominal ultrasonography. How these findings correspond to non-expert use of pUS have to be proved. Secondly, we studied patients admitted to a non-university hospital without a catheterization laboratory. Patients with ST elevation in pre-hospital electrocardiograms or cardiogenic shock were directed to the regional university hospital with catheterization laboratory facilities and were not included in the acute phase of the disease. Thus, the study results may not be generalized to such patient populations. However, the complete cardiovascular pUS of 4 min makes it possible to do a fast cardiovascular ultrasound screening also in such patients without significant time delay, and the evolving attention to the so-called stress cardiomyopathies are examples of a potential benefit of following the same strategies also in this patient group.28 The possible usefulness in such a population needs to be proved. The intention was to validate all examinations, but due to internal logistics 29 (24%) of the pUS was not validated by high-end examinations. Out of these patients the numbers with changed diagnosis, verified diagnosis, added important diagnosis and no diagnostic usefulness of pUS was 1, 2, 2 and 24, respectively. For those of diagnostic importance, these findings were verified on the same recording, but these examinations were excluded from the validation analyses. No data are available from those who did not consent to participate. There was only around 50–70% power to detect significant predictors of diagnostic usefulness of pUS screening with respect to change in main diagnosis, and some underpowered analyses with respect to detecting predictors of any diagnostic usefulness as well.
Supplementary data
Supplementary data are available at European Journal of Echocardiography online.
Funding
This study is funded by the Nord-Trøndelag Health Trust, Norway and MI Lab and Department of Circulation and Medical Imaging, Norwegian University of Science and Technology, Norway. No financial support from industry, such as a free scanner, was provided.
Supplementary Material
Acknowledgements
B.O.H. and H.D. hold position in the MI Lab, a Centre of Research-based Innovation that is funded by the Research Council of Norway and industry. One of the industry partners is GE Vingmed Ultrasound. The Centre has a total budget of ∼124 millions NOK for the 8 years period from 2007 to 2014, and the contribution from GE Vingmed Ultrasound to this budget is ∼7 million NOK (∼6%).
Conflict of interest: none declared.
References
- 1.Burton JL, Underwood J. Clinical, educational, and epidemiological value of autopsy. The Lancet. 2007;369:1471–80. doi: 10.1016/S0140-6736(07)60376-6. [DOI] [PubMed] [Google Scholar]
- 2.Kimura BJ, Shaw DJ, Agan DL, Amundson SA, Ping AC, DeMaria AN. Value of a cardiovascular limited ultrasound examination using a hand-carried ultrasound device on clinical management in an outpatient medical clinic. Am J Cardiol. 2007;100:321–5. doi: 10.1016/j.amjcard.2007.02.104. doi:10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 3.de Groot-de Laat LE, ten Cate FJ, Vourvouri EC, van Domburg RT, Roelandt JRTC. Impact of hand-carried cardiac ultrasound on diagnosis and management during cardiac consultation rounds. Eur J Echocardiogr. 2005;6:196–201. doi: 10.1016/j.euje.2004.09.013. doi:10.1111/j.1574-6941.2002.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 4.Spencer K, Anderson A, Bhargava A, Bales A, Sorrentino M, Furlong K, et al. Physician-performed point-of-care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient. J Am Coll Cardiol. 2001;37:2013–8. doi: 10.1016/s0735-1097(01)01288-8. doi:10.4319/lo.2009.55.2.0885. [DOI] [PubMed] [Google Scholar]
- 5.Vourvouri EC, Schinkel AFL, Roelandt JRTC, Boomsma F, Sianos G, Bountioukos M, et al. Screening for left ventricular dysfunction using a hand-carried cardiac ultrasound device. Eur J Heart Fail. 2003;5:767–74. doi: 10.1016/s1388-9842(03)00155-7. doi:10.1111/j.1462-2920.2008.01849.x. [DOI] [PubMed] [Google Scholar]
- 6.Roelandt JR. Ultrasound stethoscopy. Eur J Intern Med. 2004;15:337–47. doi: 10.1016/j.ejim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Sicari R, Galderisi M, Voigt J-U, Habib G, Zamorano JL, Lancellotti P, et al. The use of pocket-size imaging devices: a position statement of the european association of echocardiography. Eur J Echocardiogr. 2011;12:85–7. doi: 10.1093/ejechocard/jeq184. doi:10.1016/0924-7963(96)00018-8. [DOI] [PubMed] [Google Scholar]
- 8.Galderisi M, Santoro A, Versiero M, Lomoriello VS, Esposito R, Raia R, et al. Improved cardiovascular diagnostic accuracy by pocket size imaging device in non-cardiologic outpatients: the NaUSiCa (Naples Ultrasound Stethoscope in Cardiology) study. Cardiovasc Ultrasound. 2010;8:51. doi: 10.1186/1476-7120-8-51. doi:10.1128/AEM.70.3.1506-1513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prinz C, Voigt J-U. Diagnostic accuracy of a hand-held ultrasound scanner in routine patients referred for echocardiography. J Am Soc Echocardiogr. 2011;24:132–4. doi: 10.1016/j.echo.2010.10.017. doi:10.1016/j.virol.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Cardim N, Fernandez Golfin C, Ferreira D, Aubele A, Toste J, Cobos MA, et al. Usefulness of a new miniaturized echocardiographic system in outpatient cardiology consultations as an extension of physical examination. J Am Soc Echocardiogr. 2011;24:117–24. doi: 10.1016/j.echo.2010.09.017. doi:10.1046/j.1529-8817.1999.3550941.x. [DOI] [PubMed] [Google Scholar]
- 11.Evangelista A, Flachskampf F, Lancellotti P, Badano L, Aguilar R, Monaghan M, et al. European association of echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur J Echocardiogr. 2008;9:438–48. doi: 10.1093/ejechocard/jen174. [DOI] [PubMed] [Google Scholar]
- 12.Aase SA, Snare SR, Dalen H, Støylen A, Orderud F, Torp H. Echocardiography without electrocardiogram. Eur J Echocardiogr. 2011;12:3–10. doi: 10.1093/ejechocard/jeq112. doi:10.4319/lo.1995.40.4.0730. [DOI] [PubMed] [Google Scholar]
- 13.Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D, Vasaiwala S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr. 2007;20:857–61. doi: 10.1016/j.echo.2007.01.005. doi:10.4319/lo.1996.41.4.0783. [DOI] [PubMed] [Google Scholar]
- 14.Vignon P, Chastagner C, Berkane V, Chardac E, François B, Normand S, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33:1757–63. doi: 10.1097/01.ccm.0000171532.02639.08. doi:10.1073/pnas.1007615107. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner H, Hung J, Bermejo J, Chambers J, Evangelista A, Griffin B, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. doi:10.1038/21119. [DOI] [PubMed] [Google Scholar]
- 16.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease) Eur J Echocardiogr. 2010;11:307–32. doi: 10.1093/ejechocard/jeq031. doi:10.3354/ame009203. [DOI] [PubMed] [Google Scholar]
- 17.Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E, et al. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease) Eur J Echocardiogr. 2010;11:223–44. doi: 10.1093/ejechocard/jeq030. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–93. doi: 10.1093/ejechocard/jep007. doi:10.1126/science.1081867. [DOI] [PubMed] [Google Scholar]
- 20.Martin LD, Howell EE, Ziegelstein RC, Martire C, Whiting-O'Keefe QE, Shapiro EP, et al. Hand-carried ultrasound performed by hospitalists: does it improve the cardiac physical examination? Am J Med. 2009;122:35–41. doi: 10.1016/j.amjmed.2008.07.022. doi:10.1016/j.femsec.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 21.de Koning HJ. Assessment of nationwide cancer-screening programmes. Lancet. 2000;355:80–1. doi: 10.1016/S0140-6736(99)00419-5. doi:10.1111/j.1462-2920.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 22.Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364:749–57. doi: 10.1056/NEJMra0909487. doi:10.3354/ame045171. [DOI] [PubMed] [Google Scholar]
- 23.Badano LP, Nucifora G, Stacul S, Gianfagna P, Pericoli M, Del Mestre L, et al. Improved workflow, sonographer productivity, and cost-effectiveness of echocardiographic service for inpatients by using miniaturized systems. Eur J Echocardiogr. 2009;10:537–42. doi: 10.1093/ejechocard/jen341. doi:10.1111/j.1462-2920.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- 24.Atherton JJ. Screening for left ventricular systolic dysfunction: is imaging a solution? JACC Cardiovasc Imaging. 2010;3:421–8. doi: 10.1016/j.jcmg.2009.11.014. doi:10.1038/ismej.2008.130. [DOI] [PubMed] [Google Scholar]
- 25.Roelandt J. A personal ultrasound imager (ultrasound stethoscope): a revolution in the physical cardiac diagnosis! Eur Heart J. 2003;23:523–7. doi: 10.1053/euhj.2001.2800. doi:10.3354/ame01190. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142:198–202. doi: 10.7326/0003-4819-142-3-200502010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, et al. ACC/AHA Guidelines for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee on clinical application of echocardiography) Developed in collaboration with the American Society of Echocardiography. Circulation. 1997;95:1686–744. doi: 10.1161/01.cir.95.6.1686. doi:10.3354/ame028157. [DOI] [PubMed] [Google Scholar]
- 28.Hurst RT, Prasad A, Askew Iii JW, Sengupta PP, Tajik AJ. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging. 2010;3:641–9. doi: 10.1016/j.jcmg.2010.01.009. doi:10.1016/S0580-9517(01)30046-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.