Abstract
The effects of the nondominant or secondary ventricle on the Fontan circulation are not known. The present study used cardiac magnetic resonance imaging to investigate the relations between secondary ventricular size and global cardiac performance. The Fontan cross-sectional study collected data from 7 centers participating in the Pediatric Heart Network. Subjects with complete cardiac magnetic resonance imaging data and an identifiable secondary ventricle were included in the analysis. Relationships between body surface area–adjusted parameters of the secondary ventricle (mass, end-diastolic volume, mass/volume ratio, and stroke volume) and the following measures were assessed. These measures included the percentage of predicted peak oxygen consumption and oxygen consumption at the ventilatory anaerobic threshold, ejection fraction of the main ventricular chamber, echocardiographic diastolic function grade, serum B-type natriuretic peptide, primary ventricular end-diastolic pressure, and parent-reported physical functioning summary score on the Child Health Questionnaire. Of the 546 enrolled subjects, 123 (age 12.1 ± 3.3 years, 56% male) had undergone cardiac magnetic resonance imaging, and 38 had achieved maximal aerobic capacity. A larger secondary ventricular end-diastolic volume, lower mass/volume ratio, and greater secondary/total ventricular stroke volume ratio were associated with a greater exercise capacity. No significant relationships were found between the measures of the secondary ventricle and the other outcomes. In conclusion, in children after the Fontan operation, a larger and less hypertrophied secondary ventricle with a greater contribution to stroke volume was associated with a better exercise capacity.
Late heart failure after the Fontan operation continues to be an important clinical challenge, and ventricular dysfunction is thought to be a significant determinant of the long-term outcome.1–6 In single ventricular physiology, although systolic work is performed predominantly by the dominant (primary) ventricle, it has been postulated that through ventricle–ventricle interaction, a larger nondominant (secondary) ventricle might impair function of the primary ventricle.7 However, few studies have evaluated the potential effects of mechanical interaction between the primary and secondary ventricular chambers and its effect on overall cardiac performance in the Fontan circulation.7,8 These studies were hampered by small sample sizes and methodologic challenges. Cardiac magnetic resonance (CMR) imaging allows accurate assessment of the size and systolic function of the primary and secondary ventricles, free of geometric assumptions. We used CMR imaging to investigate the effect of the size and functional contribution of the secondary ventricle on exercise capacity and other parameters of global cardiac performance late after a Fontan operation.
Methods
The present study used data from the Pediatric Heart Network prospective multicenter cross-sectional study of subjects 6 to 18 years old who had undergone a Fontan operation without additional intervention in the 6-month period before enrollment. The design and results of the main study have been previously reported.9,10 The institutional review board at each of the 7 participating centers approved the protocol. Of the subjects enrolled in the main study, those with complete CMR data and an identifiable secondary ventricle were included in the present study.
CMR imaging was performed using a standardized imaging protocol developed by the core laboratory. The study subjects did not undergo CMR imaging as a part of their assessment if they had met any of the following criteria: (1) unable to cooperate without sedation; (2) had a pacemaker, defibrillator, permanent pacemaker lead, or implanted device considered a contraindication according to institutional guidelines; (3) had intravascular occlusion coils deemed to result in excessive image artifact; or (4) were <6 weeks from endovascular device implantation. Imaging was performed using locally available 1.5 T whole body scanners (General Electric, Signa LX or TwinSpeed, Milwaukee, Wisconsin; Philips Intera, Best, The Netherlands; and Siemens Sonata or Maestro, Erlangen, Germany). The standardized imaging protocol included electrocardiographicgated segmented k-space fast (turbo) gradient (14% of studies) or steady state free precession (86% of studies) cine magnetic resonance acquisitions in the vertical and horizontal long-axis planes and contiguous short-axis cine imaging from the atrioventricular junction through the cardiac apex. De-identified CMR data were analyzed using commercially available software (MASS, Medis, Leiden, The Nether-lands) at the core CMR laboratory by a single observer. The dominant ventricle, designated the “primary ventricle,” was considered dominant because of its larger size and that it received most of the ventricular inflow. The smaller ventricle was designated the “secondary ventricle.” When both ventricles were similar in size, the classification of the primary and secondary ventricles was adjudicated according to each ventricle’s contribution to systemic output. The end-diastolic volume (EDV) and end-systolic volume (ESV), mass, and ejection fraction were measured separately for the primary and secondary ventricles using previously described techniques.11 The mass/EDV ratio and stroke volume were calculated. To adjust for body surface area, the ventricular volumes were divided by (body surface area)1.3, as previously described to calculate the indexed EDV and ESV.12–14
The primary outcome was exercise capacity, measured using ramp cycle ergometry with assessment of the gas exchange. Peak oxygen consumption and oxygen consumption at the ventilatory anaerobic threshold (VAT) were expressed as percentages of the predicted values for age and gender.15 Resting and peak heart rates were measured, and percentage predicted peak heart rate and chronotropic index [(maximal heart rate − heart rate at rest)/(predicted maximal heart rate − heart rate at rest)] were calculated. Oxygen saturation, determined using pulse oximetry, at rest and peak exercise was measured. The exercise methods and results for the entire cross-sectional study have been previously reported.16
To further assess overall cardiac performance, the following outcome measures were also evaluated: primary ventricular ejection fraction, plasma B-type natriuretic peptide (BNP, Shiniogi BNP-32 human assay9), echocardiographic diastolic function grade, primary ventricular end-diastolic pressure measured at cardiac catheterization before the Fontan operation, and physical functioning score from the parent report of the Child Health Questionnaire.17 A core laboratory interpreted the echocardiographic data. Ventricular diastolic function was assessed using measures derived from pulsed Doppler and tissue Doppler imaging. Diastolic function was graded as normal, impaired relaxation, pseudonormalization, or restrictive, as previously described.10 Atrioventricular and semilunar valve regurgitation were qualitatively graded as moderate or greater, if any of the following were present: right, left, or common atrioventricular valve regurgitation was moderate or greater; both right and left atrioventricular valve regurgitation grades were mild; native aortic valve or native pulmonary valve regurgitation was moderate or greater; or both native aortic and native pulmonary valve regurgitation grades were mild.
Before formal statistical analyses, exploratory methods were used to summarize distributional properties of the variables and to assess the evidence in favor of associations. Relations between continuous predictors and outcomes were assessed using scatterplots, with smoothing using generalized additive models. Transformations to continuous outcomes were applied as appropriate to remedy skewness and heteroscedasticity. Unadjusted associations were assessed using correlation statistics (in the case of continuous predictors) and one-way analysis of variance (in the case of categorical predictors). After assessment of bivariate associations, multiple linear regression analysis was used to assess the statistical significance of the associations in the presence of potential confounders. The statistical significance of individual predictors was assessed using Wald and likelihood ratio tests. Statistical significance was evaluated with respect to a type I error probability threshold of 0.05.
Results
A total of 546 subjects were enrolled in the Fontan cross-sectional study cohort. Only those subjects with a complete CMR data set and an identifiable secondary ventricle (n = 123) were included in the present study. Subject characteristics are listed in Table 1.
Table 1.
Patient characteristics (n = 123)
| Characteristic | Value |
|---|---|
| Age at enrollment (years) | 12.1 ± 3.3 |
| Age at Fontan operation (years) | 3.5 ± 2.2 |
| Age at volume unloading surgery (years) | 1.8 ± 1.7 |
| Female (%) | 54 (44%) |
| Diagnosis | |
| Tricuspid atresia | 36 (29%) |
| Double-inlet left ventricle | 22 (18%) |
| Hypoplastic left heart syndrome | 14 (12%) |
| D-loop transposition of great arteries with 2 ventricles, pulmonary stenosis, or pulmonary atresia | 9 (7%) |
| Heterotaxy, double outlet right ventricle, single ventricle | 9 (7%) |
| Pulmonary atresia, intact ventricular septum | 7 (6%) |
| L-loop transposition of great arteries or double-outlet right ventricle with 2 ventricles, pulmonary stenosis, or pulmonary atresia | 6 (5%) |
| Unbalanced atrioventricular canal | 3 (2%) |
| Other | 17 (14%) |
| Morphologic type of secondary ventricle | |
| Left ventricle | 39 (32%) |
| Right ventricle and infundibulum | 56 (46%) |
| Infundibulum alone | 28 (22%) |
| Fontan operation type | |
| Atriopulmonary connection | 24 (20%) |
| Total cavopulmonary connection (intracardiac lateral tunnel) | 71 (58%) |
| Total cavopulmonary connection (extracardiac lateral tunnel) | 13 (11%) |
| Total cavopulmonary connection (extracardiac conduit) | 14 (11%) |
| Other | 1 (1%) |
| Resting oxygen saturation (%) | 94.1 ± 3.6 |
| Peak oxygen saturation (%) | 91.1 ± 5.4 |
| Chronotropic index | 0.68 ± 0.17 |
| Moderate or greater atrioventricular valve regurgitation | 22 (18%) |
| Moderate or greater semilunar valve regurgitation | 4 (6%) |
The morphologic types of primary and secondary ventricles are summarized in Table 1. The secondary ventricle contributed to the systemic circulation (patent connection to the aorta directly or through a ventricular septal defect) in 112 (91%) of the 123 subjects. The body surface area-adjusted values for EDV and ESV, ejection fraction, mass, and mass/volume ratio of the primary and secondary ventricles and the secondary ventricular/total stroke volume ratio are listed in Table 2. As expected, on average, the secondary ventricles were significantly smaller than the primary ventricle but had greater mass/volume ratios. When both ventricles were similar in size, classification into primary and secondary ventricles was adjudicated, as described in the “Methods” section. This sometimes resulted in a secondary ventricle that was slightly larger than the primary ventricle.
Table 2.
Cardiac magnetic resonance (CMR) data (n = 123)
| Variable | Primary Ventricle | Secondary Ventricle | Secondary/Primary Ventricle Ratio (Median, Range) |
|---|---|---|---|
| End-diastolic volume index (ml/body surface area1.3) | 70.5 ± 22 | 15.3 ± 14 | 0.14 (0.01–1.2) |
| End-systolic volume index (ml/body surface area1.3) | 30 ± 13 | 7.2 ± 7 | 0.16 (0.01–1.35) |
| Secondary ventricular/total stroke volume ratio | NA | NA | 0.12 (0.001–0.56) |
| Ejection fraction (%) | 57.9 ± 10 | 50.8 ± 21 | — |
| Mass index (g/m2.6) | 55.5 ± 19 | 17.3 ± 11 | 0.26 (0.01–1.36) |
| Mass/volume ratio | 0.8 ± 0.3 | 1.9 ± 1.6 | — |
NA = not applicable.
The outcome measures are summarized in Table 3. Exercise capacity was impaired for age and gender, although maximal aerobic capacity (respiratory exchange ratio ≥1.1) was achieved in only a few (38 of 123) of the subjects. On average, primary ventricular ejection fraction and CMR-measured cardiac index were normal, as was the ventricular end-diastolic pressure at the pre-Fontan catheterization. Similarly, the plasma BNP level was normal but with a wide and skewed range of values. The diastolic function grade was abnormal in most subjects. The mean Child Health Questionnaire physical functioning score was lower (47.7 ± 11) than the scores from the normal United States population (53 ± 8.8, p < 0.001).18
Table 3.
Outcome measures (n = 123)
| Measure | Value |
|---|---|
| % Predicted peak oxygen consumption | 62.8 ± 15 |
| % Predicted oxygen consumption at ventilatory anaerobic threshold | 77.1 ± 23 |
| Plasma B-type natriuretic peptide (pg/ml) | 12.6 (4–185) |
| Echocardiographic diastolic function grade | |
| Normal | 24 (33%) |
| Impaired relaxation | 8 (9%) |
| Pseudonormal | 36 (38%) |
| Restrictive | 20 (20%) |
| Pre-Fontan primary ventricular end-diastolic pressure (mm Hg) | 8 ± 3 |
| Physical functioning score (Child Health Questionnaire) | 47.7 ± 11 |
Data are presented as mean ± SD, median (range), or n (%).
Only 38 subjects achieved their maximal aerobic capacity (peak respiratory exchange ratio ≥1.1), and the analyses of exercise capacity were limited to these subjects. The subjects who achieved their maximal aerobic capacity were older (age 14.4 ± 3 vs 11.1 ± 3 years, p < 0.001) and had a greater chronotropic index (0.78 ± 0.13 vs 0.62 ± 0.16, p < 0.001) than those who had not achieved their maximal aerobic capacity. No differences were seen in the gender distribution, age at Fontan operation, morphologic type of secondary ventricle, type of Fontan operation, oxygen saturation, or valve regurgitation. The relationships between the CMR parameters of the secondary ventricle and measures of exercise capacity for those who achieved the maximal aerobic capacity are shown in Figure 1 and listed in Table 4. After adjusting for potential confounders (i.e., age, chronotropic index, and atrioventricular valve regurgitation), a greater secondary/primary ventricular EDV ratio, greater secondary ventricular/total stroke volume ratio, and lower log-transformed secondary ventricular mass/volume ratio were associated with a greater percent predicted peak oxygen consumption and percent predicted oxygen consumption at VAT. The analyses were also repeated using secondary ventricular EDV index, mass index, and stroke volume index, instead of the ratios of these values for the secondary and primary ventricles and yielded similar results. No association was found between exercise capacity and the secondary/primary ventricular mass ratio. A repeat analysis after excluding 1 subject in whom the secondary ventricle was not contributing to the systemic circulation yielded similar results. When analyses involving VAT were repeated to also include those patients who had not achieved maximal aerobic capacity, no significant relations between the parameters of the secondary ventricle and VAT were found.
Figure 1.
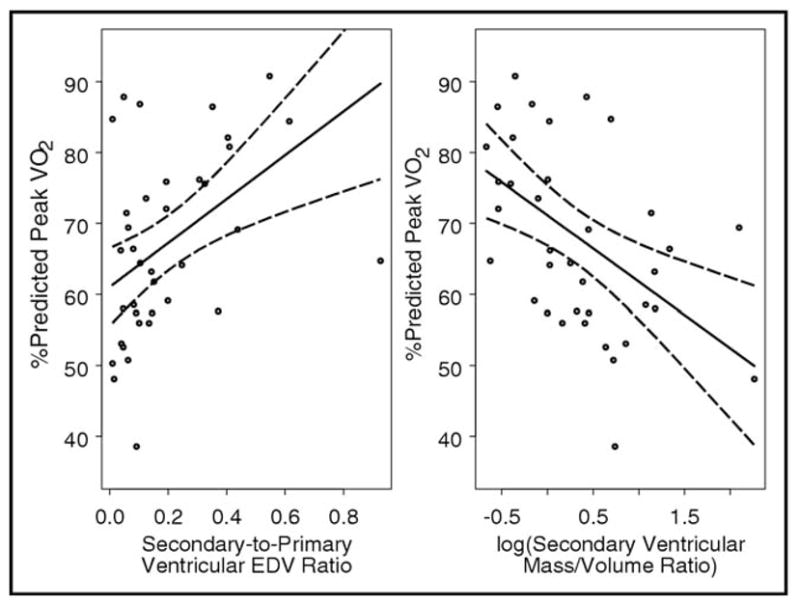
Relation between percentage of predicted peak oxygen consumption and (A) secondary/primary end-diastolic volume ratio (n = 38; R = 0.49; p = 0.002) and (B) log-transformed secondary chamber mass/volume ratio (n = 37; R = −0.58; p = 0.002). Both graphs show linear regression fits with 95% point-wise confidence bands and were restricted to subjects who achieved maximal aerobic capacity (respiratory exchange ratio ≥1.1).
Table 4.
Correlation between cardiac magnetic resonance (CMR) parameters of secondary ventricle and exercise capacity (maximal aerobic capacity group, n = 38)
| Variable | Percentage of Predicted Peak Oxygen Consumption
|
Percentage of Predicted Peak Oxygen Consumption at VAT
|
||
|---|---|---|---|---|
| R | p Value | r | p Value | |
| Secondary/primary ventricular end-diastolic volume ratio | 0.49 | 0.002 | 0.42 | 0.01 |
| Secondary/primary ventricular mass ratio | 0.29 | 0.09 | 0.21 | 0.2 |
| log (Secondary ventricular mass/volume ratio) | −0.58 | 0.002 | −0.4 | 0.01 |
| Secondary ventricular/total stroke volume ratio | 0.45 | 0.006 | 0.35 | 0.04 |
CMR parameters of the secondary ventricle were not significantly associated with the following outcome measures: ejection fraction of the primary ventricle, log BNP, pre-Fontan primary ventricular end-diastolic pressure, Child Health Questionnaire physical functioning score, or echocardiographic diastolic function grade. These relations were not influenced by age or ventricular type.
Discussion
The role of the secondary ventricle in patients with single ventricular physiology who have undergone a Fontan operation has been debated due to conflicting data. It has been speculated that through ventricle–ventricle interaction, a large secondary ventricle may impair the function of the primary ventricle.7 Using tagged CMR imaging, Kurotobi et al7 showed that a larger secondary ventricle was associated with impaired regional shortening, asynchronous contraction, and greater end-diastolic pressure of the primary ventricle. Fogel et al19 also used tagged CMR imaging to demonstrate that in patients with a hypoplastic right ventricle, left ventricular strain, radial motion, and twisting were abnormal. In contrast, Wisler et al8 did not find a consistent relation between the size of the hypoplastic left ventricle and echocardiographic measurements of global right ventricular function in patients with hypoplastic left heart syndrome. Although these studies have shown that the secondary ventricle can affect regional functioning of the primary ventricle, the effect of such ventricle–ventricle interactions on global cardiovascular performance has not been studied.
In the current study, we used the large multicenter cross-sectional database of the Pediatric Heart Network to evaluate the effects of the secondary ventricle on several measures of global cardiovascular performance. In the subjects who achieved maximal effort on exercise testing, a larger secondary/primary ventricular end-diastolic volume ratio, larger secondary ventricular/total stroke volume ratio, and lower secondary ventricular mass/volume ratio were modestly associated with greater exercise capacity. We found no relations between the CMR parameters of the secondary ventricle and the other outcome measures.
The finding of greater exercise capacity in subjects with a larger and less hypertrophied secondary ventricle with a greater contribution to stroke volume challenges the hypothesis that a larger secondary ventricle impairs the functioning of the primary ventricle in the Fontan circulation. In contrast, our results support the notion that contribution from the secondary ventricle might help augment the work performed by the primary ventricle.
Another finding of interest was the lack of associations between secondary ventricular size and parameters of diastolic function of the primary ventricle, including echocardiographic diastolic function grade and end-diastolic pressure. Abnormal diastolic function is common after a Fontan operation, and evidence is growing that it might be associated with adverse early outcomes.20 Although diastolic dysfunction was common in our study group, no evidence was seen that a larger secondary ventricle was associated with a worse diastolic function grade of the primary ventricle.
The findings of the present study might have implications for the treatment of children with a functional single ventricle. The observation that a greater exercise capacity was associated with a larger secondary ventricle lends support to management strategies that use surgical and transcatheter therapies to maximize growth of the secondary ventricle, even in patients likely to undergo a Fontan operation.21,22 It has recently been shown that in patients with borderline hypoplasia of left heart structures, primary left ventricular rehabilitation with endocardial fibroelastosis resection and mitral and aortic valvuloplasty results in improved left ventricular systolic and diastolic performance and decreased right ventricular pressures after biventricular repair.21 Although data on the long-term outcomes after these interventions are not available, our results suggest the possibility that improved growth of the secondary ventricle might be beneficial even if biventricular repair is not feasible and a Fontan operation is ultimately performed.
The subjects of the present study were relatively healthy late survivors of a Fontan operation who were a minority of the entire Fontan cross-sectional study data set. Although we studied several measures of global cardiovascular performance, regional myocardial function was not assessed. The number of subjects with adequate exercise data was relatively small, and this precluded meaningful analyses of subgroups such as stratification by ventricular morphology. Finally, invasive measurement of ventricular end-diastolic pressure was available only from the pre-Fontan evaluation and might not have accurately reflected their current status.
Acknowledgments
This study was supported by U01 grants HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland.
References
- 1.Vitarelli A, Conde Y, Cimino E, D’Angeli I, D’Orazio S, Ventriglia F, Bosco G, Colloridi V. Quantitative assessment of systolic and diastolic ventricular function with tissue Doppler imaging after Fontan type of operation. Int J Cardiol. 2005;102:61–69. doi: 10.1016/j.ijcard.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Eicken A, Fratz S, Gutfried C, Balling G, Schwaiger M, Lange R, Busch R, Hess J, Stern H. Hearts late after Fontan operation have normal mass, normal volume, and reduced systolic function: a magnetic resonance imaging study. J Am Coll Cardiol. 2003;42:1061–1065. doi: 10.1016/s0735-1097(03)00986-0. [DOI] [PubMed] [Google Scholar]
- 3.Fogel MA, Weinberg PM, Chin AJ, Fellows KE, Hoffman EA. Late ventricular geometry and performance changes of functional single ventricle throughout staged Fontan reconstruction assessed by magnetic resonance imaging. J Am Coll Cardiol. 1996;28:212–221. doi: 10.1016/0735-1097(96)00111-8. [DOI] [PubMed] [Google Scholar]
- 4.Milanesi O, Stellin G, Colan SD, Facchin P, Crepaz R, Biffanti R, Zacchello F. Systolic and diastolic performance late after the Fontan procedure for a single ventricle and comparison of those undergoing operation at <12 months of age and at >12 months of age. Am J Cardiol. 2002;89:276–280. doi: 10.1016/s0002-9149(01)02227-5. [DOI] [PubMed] [Google Scholar]
- 5.Akagi T, Benson LN, Green M, Ash J, Gilday DL, Williams WG, Freedom RM. Ventricular performance before and after Fontan repair for univentricular atrioventricular connection: angiographic and radio-nuclide assessment. J Am Coll Cardiol. 1992;20:920–926. doi: 10.1016/0735-1097(92)90194-r. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury UK, Airan B, Kothari SS, Talwar S, Saxena A, Singh R, Subramaniam GK, Pradeep KK, Patel CD, Venugopal P. Specific issues after extracardiac Fontan operation: ventricular function, growth potential, arrhythmia, and thromboembolism. Ann Thorac Surg. 2005;80:665–672. doi: 10.1016/j.athoracsur.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Kurotobi S, Sano T, Naito H, Matsushita T, Takeuchi M, Kogaki S, Arisawa J, Matsuda H, Okada S. Regional ventricular systolic abnormalities caused by a rudimentary chamber in patients with univentricular hearts. Am J Cardiol. 1998;82:86–92. doi: 10.1016/s0002-9149(98)00244-6. [DOI] [PubMed] [Google Scholar]
- 8.Wisler J, Khoury PR, Kimball TR. The effect of left ventricular size on right ventricular hemodynamics in pediatric survivors with hypoplastic left heart syndrome. J Am Soc Echocardiogr. 2007;21:464–469. doi: 10.1016/j.echo.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Sleeper LA, Anderson P, Hsu DT, Mahony L, McCrindle BW, Roth SJ, Saul JP, Williams RV, Geva T, Colan SD, Clark BJ. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152:427–433. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ., III Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 12.Gutgesell HP, Rembold CM. Growth of the human heart relative to body surface area. Am J Cardiol. 1990;65:662–668. doi: 10.1016/0002-9149(90)91048-b. [DOI] [PubMed] [Google Scholar]
- 13.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 14.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 15.Cooper DM, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis. 1984;129:S47–S48. doi: 10.1164/arrd.1984.129.2P2.S47. [DOI] [PubMed] [Google Scholar]
- 16.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 17.Landgraf JM, Abetz L, Ware JE. The Child Health Questionnaire (CHQ) User’s Manual. Boston: HealthAct; 1999. [Google Scholar]
- 18.Baker AL, Gauvreau K, Newburger JW, Sundel RP, Fulton DR, Jenkins KJ. Physical and psychosocial health in children who have had Kawasaki disease. Pediatrics. 2003;111:579–583. doi: 10.1542/peds.111.3.579. [DOI] [PubMed] [Google Scholar]
- 19.Fogel MA, Weinberg PM, Gupta KB, Rychik J, Hubbard A, Hoffman EA, Haselgrove J. Mechanics of the single left ventricle: a study in ventricular-ventricular interaction. II. Circulation. 1998;98:330–338. doi: 10.1161/01.cir.98.4.330. [DOI] [PubMed] [Google Scholar]
- 20.Garofalo CA, Cabreriza SE, Quinn TA, Weinberg AD, Printz BF, Hsu DT, Quaegebeur JM, Mosca RS, Spotnitz HM. Ventricular diastolic stiffness predicts perioperative morbidity and duration of pleural effusions after the Fontan operation. Circulation. 2006;114:I56–I61. doi: 10.1161/CIRCULATIONAHA.105.001396. [DOI] [PubMed] [Google Scholar]
- 21.Emani SM, Bacha EA, McElhinney DB, Marx GR, Tworetzky W, Pigula FA, Del Nido PJ. Primary left ventricular rehabilitation is effective in maintaining two-ventricle physiology in the borderline left heart. J Thorac Cardiovasc Surg. 2009;138:1276–1282. doi: 10.1016/j.jtcvs.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McElhinney DB, Marshall AC, Wilkins-Haug LE, Brown DW, Benson CB, Silva V, Marx GR, Mizrahi-Arnaud A, Lock JE, Tworetzky W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120:1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]


