Introduction
Regenerative medicine refers to the process of creating functional tissues to augment or replace organs lost to age, disease, damage, or congenital defects1. Several technologies brought to bear on this challenge have had notable results. In gastroenterology and hepatology in particular, recent publications have demonstrated pre-clinical success in the transplantation of engineered liver tissue, augmentation of enteric sphincter function by transplantation of smooth muscle, and transplantation of enteric neurons. We will begin by reviewing the basic techniques that underlie these advances, specifically stem cell biology, gene therapy, and engineered biomaterials. We will describe some promising applications of regenerative medicine in dermatology, pulmonology, cardiology, neurology, and urology. And finally we will describe the state of basic scientific and pre-clinical research in regenerative gastroenterology.
The Tools
Stem Cells
Although the concept of regenerative medicine is as old as the myth of Prometheus, its modern era began with seminal discoveries in stem cell biology in the last couple of decades. Stem cells are defined by the capacity for unlimited self-renewal and the ability to differentiate into mature end-organ cells. They are conveniently categorized by provenance (adult, embryonic, fetal, or induced) and according to their developmental potential (totipotent, pluripotent, multipotent). Unipotent cells, or adult progenitor cells, retain the capacity for self-renewal or differentiation into a single cell type (e.g., hepatocytes, skeletal myocytes). In general, in vitro propagation, expansion, and differentiation of these cells remain difficult.
Initial attempts at tissue regeneration focused on naturally occurring stem cells. Although embryonic stem cells (ESC) received great popular attention, and are technically attractive due to their pluripotency, legal and ethical objections have diminished the enthusiasm for their use. Other difficulties include the immunogenicity of transplanted ESC or ESC-derived tissues3,4 and the potential for teratoma formation in vivo5. Although there has been recent progress in defining the molecular basis for this tumor risk6, it is clear that there are significant technical hurdles before human ESC will be ready for clinical use.
Among the promising candidates for regenerative therapy are mesenchymal stem cells, a class of multipotent cell found in several mature and immature organs, including adult adipose tissue and bone marrow, Wharton's jelly, and tooth bud. Their relative developmental flexibility, together with their availability in postnatal (even adult) mammals, lends them unique clinical promise. Yet at present we lack standardized protocols for differentiating these cells into target tissues7,8.
Safety and technical issues with naturally occurring stem cells have prompted the examination of other approaches to regeneration. Somatic cell nuclear transfer (SCNT) and induced pluripotent stem cells are two of the most exciting and revolutionary developments in this field. SCNT entails removing the nucleus from a recipient oocyte and fusing the enucleated oocyte with a mature donor cell, typically a fibroblast. The product is a pluripotent cell containing cytoplasm and mitochondrial DNA from the recipient oocyte and nuclear DNA from the mature donor cell9. SCNT has been used in reproductive cloning of several species (e.g., Dolly the sheep), and as such has been subject to significant controversy and legal debate, particularly with respect to humans. Therapeutic, or “research,” cloning, intended to yield cells or tissues but not whole organisms, has not been free of controversy, but research continues. Nuclear transfer remains a technically challenging procedure with a very low yield (< 1%)9. Some have also raised concerns about the possibility of exploitative sourcing of oocytes should therapeutic cloning find clinical applications10.
The reprogramming of mature somatic cells to assume the behavior of embryonic stem cells is a major scientific breakthrough, suggesting that pluripotent cells might be derived from a patient's own mature tissue, even an easily accessible biopsy site such as the skin. With such an origin, these cells, termed induced-pluripotent stem cells (iPSC), offer a way to bypass most ethical objections to regular embryonic stem cells. The original reprogramming approach by Takahashi and Yamanaka in 2006 used retrovirus-induced expression of transcription factors (Oct3/4, Sox2, c-Myc, and Klf4) first in mouse11, and later in human fibroblasts12. Similarly, Yu et al showed that retroviral expression of OCT4, SOX2, NANOG, and LIN28 induces pluripotency in human fibroblasts13. Concern over the oncogenic potential of retroviruses14–16 has led to a refinement in techniques. It is now possible to produce iPSC without any stable genomic modification to the target cells in both mouse17,18 and human19,20 models. Further exciting developments in the last year include the discovery of induced pluripotency. The original approach to producing iPS cells attempted to return a differentiated cell, such as a fibroblast, to an undifferentiated, pluripotent state (“de-differentiation”) and then “re-differentiate” it into the desired phenotype. However, in 2010 Vierbuchen and colleagues succeeded in bypassing the de-differentiation step and converting mouse fibroblasts directly into neurons having excitable membranes and functional synapses21.
While iPSC developmental reprogramming undoubtedly has immense scientific and clinical potential, there are major challenges to translating current research into therapies. It has become clear that there are differences between the iPSCs produced by retroviral induction and ESCs. Further, the nature of the reprogramming process is still obscure, and the developmental potential of iPSCs derived by different methods, from different tissues, is unknown22.
Materials Engineering
While stem cells can assume a desired cellular phenotype under the appropriate conditions, their organization into functional tissues also requires the proper spatial architecture and integration with their environment. Mammalian cells depend on biological and mechanical interaction with the extracellular matrix (ECM). For tissue regeneration, various biomaterials can replicate the effects of native ECM and form a 3-D scaffold to maintain proper functional shape and cell-cell orientation, and can be loaded with bioactive factors, e.g., adhesion peptides and growth factors. To be suitable for tissue engineering applications a scaffolding material should provide an appropriate three-dimensional structure for the deposition and growth of cells, mimic normal cell-cell interactions, have limited immunogenicity, and allow for diffusion of oxygen and other nutrients. Collagen23 and alginate24–26 are commonly used, though as with other materials derived from biologic sources, there have been concerns about infectious risk and immunogenicity of these products27. Synthetic materials such as poly(ethylene glycol) (PEG), poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(lacti-co-glycolic acid) (PLGA) have the advantages of industrial production, lack of infectious risk, and decreased immunogenicity. They can also be molded or shaped by advanced fabrication techniques such as electrospinning to allow greater control over the small-scale structure of biomaterials28, which may enhance mechanical properties and mimicry of normal extracellular matrix29. Synthetic modifications to the basic polymer structure can include cross-linking peptides degradable by proteases from migrating cells and protein ligands for cell-surface receptors30,31.
Another source of scaffolding for engineered or regenerative tissue is decellularized natural tissue. A natural animal or cadaveric explant is washed with detergent or otherwise treated to remove cells, DNA, and other antigenic material32,33, and then seeded with cells capable of migrating into the residual matrix. In animal models the technique has been applied to liver34, lung35,36, heart37, and intestinal submucosa38. Probably the most mature clinical area of tissue engineering is skin grafting, with decellularized skin commercially available (e.g., AlloDerm, LifeCell Corporation, Branchburg, NJ).
An exciting alternative to scaffold-based tissue engineering has emerged in “bioprinting,” a collection of processes for depositing living cells into a defined pattern using computer-controlled machines analogous to three-dimensional rapid-prototyping technology. Boland, et al, describe using thermal ink jet printing to deposit neurons in a two-dimensional pattern39. And the Forgacs group has demonstrated bioprinted structures composed of human endothelial cells with chicken cardiomyocytes40, as well as complex, branched tubular structures having concentric layers of fibroblasts and smooth muscle cells41.
Progress in Other Specialties
Cellular transplantation is becoming a clinically important technology. For example, autologous cultures of keratinocyte stem cells (holoclones) have now been used for more than two decades to restore defects in the skin, mucosa and cornea42–44. Most recently, epidermal stem cells from an adult patient with junctional epidermolysis bullosa were transduced with a functional copy of the laminin 5–b3 gene, mutation of which is responsible for the disease phenotype. Epidermal grafts prepared from these cells were then transplanted onto the patient's legs and resulted in a local cure45. South Korean regulators have recently approved the first stem cell-based therapy for clinical use, injecting bone marrow-derived mesenchymal stem cells into the coronary arteries of patients with coronary ischemia46 A trial of a similar treatment has been reported in the United States46. And there appears to be some promise regarding transplantation of neurons into the CNS. In 1995, Kordower and colleagues reported on a 59-year-old patient with Parkinson's disease in whose brain they implanted fetal brain tissue from several donors47. After 18 months they showed significant survival of neurons expressing tyrosine hydroxylase within the engrafted areas. Wernig, et al, induced iPS cells to differentiate into neurons in vitro, and implanted them into the cerebral ventricles of fetal mice, where they were found to have migrated into widespread areas of the developing brain. Subsequently, the same group injected dopaminergic neurons derived from iPS cells into the brains of Parkinsonian mice and showed survival of the engrafted neurons four weeks after surgery48. Yet risks loom large, as when a 13-year-old boy whose CNS was injected with stem cells derived from fetal brain developed a malignancy of donor origin49.
The tissue engineering approach, while still very much in the pre-clinical stage, has also made remarkable advances. For example, two separate groups, in Boston and New Haven, have constructed engineered lungs. Ott and colleagues perfused decellularized rat lungs with both fetal rat lung cells and human umbilical vein endothelial cells.35 Petersen, et al, likewise perfused a decellularized rat lung with rat lung epithelial and endothelial cells via the pulmonary artery and trachea, respectively36. After maturing the reperfused organs ex vivo, both groups found that the gross histology resembled normal lung, with intact alveoli and capillaries. Furthermore, they were able to transplant the newly formed lungs into recipient rats and demonstrate gas exchange over a period of hours (Figure 1). Lanza and colleagues succeeded in growing a primitive kidney-like structure in cattle50. A somatic cell nucleus was transferred into a donor oocyte using SCNT to create a cloned bovine embryo. Renal cells were isolated from cloned embryos and grown in culture, then seeded into a polycarbonate tube coated with collagen. The devices were implanted into the subcutaneous space and drained into an external reservoir. Histologically, organized tubular structures were observed emanating from the implanted tubes, and terminating in vascular tufts resembling glomeruli. These “neo-kidneys” produced fluid which was relatively concentrated in urea and creatinine. Atala and colleagues have reported on a series of patients in whom they performed bladder augmentation using engineered tissue51. In several patients, decellularized bladder submucosal tissue was used as scaffolding, and in several others a composite scaffold of PGA and collagen was formed to fit the specific patient. In all cases, the scaffold was seeded with the patient's own bladder smooth muscle cells and urothelial cells, and after maturation ex vivo was anastomosed to the native bladder. They were able to document long-term viability of the grafts with preservation of normal three-layer bladder histology, and clinical outcomes similar to those achieved by augmentation with colon.
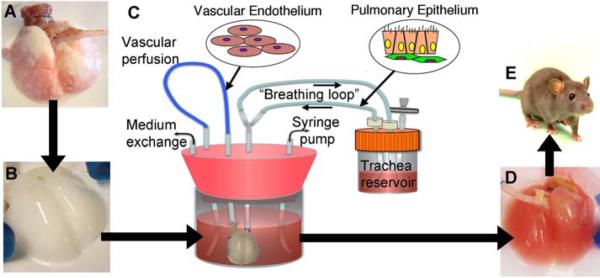
Fig. 1.
Schema for lung tissue engineering. (A) Native adult rat lung is cannulated in the pulmonary artery and trachea for infusion of decellularization solutions. (B) cellular lung matrix is devoid of cells after 2 to 3 hours of treatment. (C) Acellular matrix is mounted inside a biomimetic bioreactor that allows seeding of vascular endothelium into the pulmonary artery and pulmonary epithelium into the trachea. (D) After 4 to 8 days of culture, the engineered lung is removed from the bioreactor and is suitable for implantation into (E) the syngeneic rat recipient36. From Petersen TH, et al. Science 2010 Jul;329(5991):538–541. Reprinted with permission from AAAS.
Regenerative Medicine and Neurogastroenterology
Several neuromuscular diseases of the gastrointestinal tract offer potential targets for regenerative therapies, because the clinical impairment is related to loss or dysfunction of neurons, smooth muscle, or other tissues within the anatomically intact bowel. These include GERD and fecal incontinence, which are often myopathic; neuropathic diseases such as achalasia and Hirschsprung's disease; and problems such as diabetic gastroparesis, which has been associated with loss of interstitial cells of Cajal (ICC). Congenital and acquired forms of intestinal pseudoobstruction result from either neuropathic or myopathic processes, and in some cases appear related to a loss of ICC. In all these diseases, pharmacologic therapies have been relatively unsuccessful, probably because, in the absence of a functional enteric nervous system, a systemically delivered drug cannot stimulate the complex pattern of muscular contraction required for effective peristalsis or other motor programs in the GI tract. In addition, these agents have so far not been targeted with sufficient specificity and have been fraught with toxicity.
Current Status
Initial attempts at regeneration focused on neural stem cells. Nitric oxide (NO) is an important neurotransmitter in the relaxation of normal enteric smooth muscle, and knockout mice lacking the neuronal isoform of nitric oxide synthase (nNOS) have hypertrophic antral musculature, impaired gastric emptying, and dilated stomachs52. Micci, et al, showed that this phenotype could be partially rescued by transplantation of neuronal stem cells (NSCs). NSCs were isolated from the subventricular zone (SVZ) of the CNS of embryonic mice without the nNOS knockout. These NSCs were injected into the pylorus of nNOS−/− mice. Transplanted cells differentiated into both glia and neurons after one week, and nNOS expression was found in the pylori of recipient but not control animals. In an ex vivo preparation, the relaxation induced by electrical field stimulation was enhanced in recipient muscle. Prior to sacrifice, recipient mice were shown to empty a liquid meal from the stomach more completely than controls53.
Recent studies have also demonstrated promising results in animal models of myopathic disease. Pasricha and colleagues used cultured cells derived from skeletal muscle (MDCs) in both rats and beagles54. Beagle LES was injected by endoscopic approach with MDCs; after four weeks the injected cells appeared well integrated into the native muscle. Every dog individually had an increase in LES pressures. One dog found to have gastroesophageal reflux prior to injection appeared improved afterward.
On the tissue-engineering front, Bitar's group has demonstrated a technique for construction of internal anal sphincters (IACs) using isolated IAC smooth muscle cells. Smooth muscle cells were isolated from human IAC surgical specimens, and grown in an annular culture dish coated with fibrin gel55. Over several weeks in culture, SMCs grew into a dense ring and spontaneously aligned along the circumferential lines of force. Their biochemical phenotype resembled native IAS smooth muscle. More recently, this group has shown that bioengineered human IAS tissue can be innervated with immortal neurons and, after subcutaneous transplantation, preserves the integrity and physiology of myogenic and neuronal components56.
While these studies demonstrate the promise of both the tissue-engineering and cell-transplantation approaches to regeneration in the GI tract, much work remains to optimize the methods. Two active areas of investigation are protocols for optimizing survival and differentiation of transplanted cells, and the best source of neural progenitor cells. Neural transplantation trials have been dogged by poor graft survival57, both because of immunologically mediated rejection58 and also because of non-immune promotion of apoptosis in the implanted neurons59. In the context of the gut, Micci, et al, found that inhibition of apoptosis, but not immunosuppression, significantly increased graft survival 1 week after implantation60.
Post-migratory enteric neural precursor cells (ENPs), which can be isolated from adult bowel, are presumably more committed to an enteric neural fate and may be more responsive to the environmental cues that normally act on developing enteric neurons. Kruger, at al, formed an enriched population of multipotent neural progenitors by sorting cells of the outer muscle layer and myenteric plexus and selecting for high p75 expression61. Stem cells were identified in both immature and adult rats, though the yield of colony-forming cells declined significantly from P22 to adulthood, and furthermore the developmental potential appeared to become more restricted with age, with a bias toward the formation of glia rather than neurons. In humans, Metzger and colleagues were able to obtain proliferating neural progenitors from endoscopic mucosal biopsies. When implanted into aganglionic chick embryo or aganglionic bowel from patients with Hirschsprung's disease, these cells migrated into and colonized the recipient bowel. This very exciting demonstration raises the hope that we are not far from the ability to harvest, proliferate in culture, and re-transplant autologous neural stem cells in a clinical setting.
The Future: Rebuilding the Gut
Malabsorption from short-gut syndrome or intestinal pseudo-obstruction due to neuronal and/or muscle failure are among the most intractable problems in gastroenterology. While intestinal transplantation is an option for some patients, an even more attractive approach would be to use bioengineering to reconstruct entire segments of the gastrointestinal tract. Kuwahara and colleagues have shown that murine ES cells, differentiating in vitro, can form a structure having a lumen, possessing an inner columnar epithelial layer and an outer layer of smooth muscle, separated by an intermediate layer of connective tissue62. These structures also contain c-Kit-immunoreactive cells resembling ICC, which form an interconnected network, and what appear to be ganglia composed of PGP9.5-immunoreactive cells, consistent with neurons. Ueda, et al, have similarly shown that murine iPSCs form gut-like structures in culture, having both neurons and ICC, and engaging in coordinated rhythmic contractions similar to peristalsis63. Thus there is the intriguing possibility that we may ultimately be capable of recapitulating intestinal organogenesis in vitro using pluripotent cells of human, and perhaps autologous, derivation.
Conclusion
Recent years have seen an explosion in the science of stem cells, biomaterials, and tissue engineering. Novel clinical applications will follow, perhaps within the next five years, possibly even regeneration of the enteric neuromuscular system in motility disorders, for which treatments have so far been unsatisfactory. Neural stem cell trials will expand from the CNS to the enteric nervous system. We also expect to see trials of myocyte injection and the implantation of engineered sphincters, initially for such conditions as achalasia, gastroesophageal reflux disease and fecal incontinence. In the future we may see functional bowel segments engineered for implantation.
Despite these exciting possibilities, fundamental questions remain in both basic and clinical science. What processes underly the re-induction of pluripotency in differentiated cells? What will be the best source of cells for implantation and organ regeneration: tissue banks, autologous iPS, autologous adult stem cells, or directed donors? How do we optimize cell survival and developmental fate after transplantation? What are the best biomaterials and how do we standardize production of scaffolding and other structural components? How do we mitigate the risk of neoplasia associated with the use of pluripotent cells?
Acknowledgments
Supported by NIH grant DK080920.
Supported by NIH grant 5T32DK007056
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anon [Accessed June 28, 2011];Regenerative medicine - Wikipedia, the free encyclopedia. Available at: http://en.wikipedia.org/wiki/Regenerative_medicine.
- 2.Grinnemo K-H, Kumagai-Braesch M, Månsson-Broberg A, et al. Human embryonic stem cells are immunogenic in allogeneic and xenogeneic settings. Reprod. Biomed. Online. 2006;13(5):712–724. doi: 10.1016/s1472-6483(10)60663-3. [DOI] [PubMed] [Google Scholar]
- 3.Grinnemo K-H, Sylvén C, Hovatta O, Dellgren G, Corbascio M. Immunogenicity of human embryonic stem cells. Cell Tissue Res. 2008;331(1):67–78. doi: 10.1007/s00441-007-0486-3. [DOI] [PubMed] [Google Scholar]
- 4.Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 5.Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat. Biotechnol. 2009;27(3):281–287. doi: 10.1038/nbt.1527. [DOI] [PubMed] [Google Scholar]
- 6.Kuo TK, Ho JH, Lee OK. Mesenchymal stem cell therapy for nonmusculoskeletal diseases: emerging applications. Cell Transplant. 2009;18(9):1013–1028. doi: 10.3727/096368909X471206. [DOI] [PubMed] [Google Scholar]
- 7.Vemuri MC, Chase LG, Rao MS. Mesenchymal stem cell assays and applications. Methods Mol. Biol. 2011;698:3–8. doi: 10.1007/978-1-60761-999-4_1. [DOI] [PubMed] [Google Scholar]
- 8.Hipp J, Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4(1):3–11. doi: 10.1007/s12015-008-9010-8. [DOI] [PubMed] [Google Scholar]
- 9.Anon United Nations Declaration on Human Cloning. United Nations General Assembly 59th Session; 2005; Available at: http://daccessods.un.org/access.nsf/Get?Open&DS=A/RES/59/280&Lang=E. [Google Scholar]
- 10.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 13.Montini E, Cesana D, Schmidt M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009;119(4):964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modlich U, Baum C. Preventing and exploiting the oncogenic potential of integrating gene vectors. J. Clin. Invest. 2009;119(4):755–758. doi: 10.1172/JCI38831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118(9):3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 17.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D, Kim C-H, Moon J-I, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagy A, Nagy K. The mysteries of induced pluripotency: where will they lead? Nat. Methods. 2010;7(1):22–24. doi: 10.1038/nmeth.f.292. [DOI] [PubMed] [Google Scholar]
- 22.Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89(5):338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 23.Wong M. Biopolymer Methods in Tissue Engineering. Vol 238. Humana Press; New Jersey: 2003. [Accessed June 29, 2011]. Alginates in Tissue Engineering; pp. 77–86. Available at: http://www.springerlink.com.laneproxy.stanford.edu/content/x4172q1863v70177/ #section=95582&page=1. [Google Scholar]
- 24.Dvir-Ginzberg M, Gamlieli-Bonshtein I, Agbaria R, Cohen S. Liver tissue engineering within alginate scaffolds: effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng. 2003;9(4):757–766. doi: 10.1089/107632703768247430. [DOI] [PubMed] [Google Scholar]
- 25.Dar A, Shachar M, Leor J, Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002;80(3):305–312. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Hillas PJ, Báez JA, et al. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18(2):103–119. doi: 10.2165/00063030-200418020-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ashammakhi N, Ndreu A, Nikkola L, Wimpenny I, Yang Y. Advancing tissue engineering by using electrospun nanofibers. Regen Med. 2008;3(4):547–574. doi: 10.2217/17460751.3.4.547. [DOI] [PubMed] [Google Scholar]
- 28.Dahlin RL, Kasper FK, Mikos AG. Polymeric Nanofibers in Tissue Engineering. [Accessed June 29, 2011];Tissue Eng Part B Rev. 2011 doi: 10.1089/ten.teb.2011.0238. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21699434. [DOI] [PMC free article] [PubMed]
- 29.Lin C-C, Anseth KS. Cell–cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing β-cell function. Proceedings of the National Academy of Sciences. 2011;108(16):6380–6385. doi: 10.1073/pnas.1014026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 31.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badylak SF, Taylor D, Uygun K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. [Accessed June 29, 2011];Annu Rev Biomed Eng. 2010 doi: 10.1146/annurev-bioeng-071910-124743. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21417722. [DOI] [PMC free article] [PubMed]
- 33.Uygun BE, Soto-Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010;16(7):814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010;16(8):927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 35.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329(5991):538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ott HC, Matthiesen TS, Goh S-K, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 37.Wu S, Liu Y, Bharadwaj S, Atala A, Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32(5):1317–1326. doi: 10.1016/j.biomaterials.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Boland T, Xu T, Damon B, Cui X. Application of inkjet printing to tissue engineering. Biotechnol J. 2006;1(9):910–917. doi: 10.1002/biot.200600081. [DOI] [PubMed] [Google Scholar]
- 39.Jakab K, Norotte C, Damon B, et al. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng Part A. 2008;14(3):413–421. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- 40.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30(30):5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallico GG, 3rd, O'Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med. 1984;311(7):448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 42.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 43.Romagnoli G, De Luca M, Faranda F, et al. Treatment of posterior hypospadias by the autologous graft of cultured urethral epithelium. N. Engl. J. Med. 1990;323(8):527–530. doi: 10.1056/NEJM199008233230806. [DOI] [PubMed] [Google Scholar]
- 44.Mavilio F, Pellegrini G, Ferrari S, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat. Med. 2006;12(12):1397–1402. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- 45.Jung H, Hyun-young Y. South Korea back in stem cell spotlight with new treatment. Reuters. 2011 Available at: http://www.reuters.com/article/2011/07/07/us-korea-stemcell-idUSTRE76610C20110707.
- 46.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, Autologous CD34+ Cell Therapy for Refractory Angina. [Accessed July 11, 2011];Circulation Research. 2011 doi: 10.1161/CIRCRESAHA.111.245993. Available at: http://circres.ahajournals.org/content/early/2011/07/07/CIRCRESAHA.111.245993.abstract. [DOI] [PMC free article] [PubMed]
- 47.Kordower JH, Freeman TB, Snow BJ, et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N. Engl. J. Med. 1995;332(17):1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 48.Wernig M, Zhao J-P, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 2008;105(15):5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6(2):e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanza RP, Chung HY, Yoo JJ, et al. Generation of histocompatible tissues using nuclear transplantation. Nat. Biotechnol. 2002;20(7):689–696. doi: 10.1038/nbt703. [DOI] [PubMed] [Google Scholar]
- 51.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 52.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119(3):766–773. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- 53.Micci M-A, Kahrig KM, Simmons RS, et al. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology. 2005;129(6):1817–1824. doi: 10.1053/j.gastro.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 54.Pasricha PJ, Ahmed I, Jankowski RJ, Micci M-A. Endoscopic injection of skeletal muscle-derived cells augments gut smooth muscle sphincter function: implications for a novel therapeutic approach. Gastrointest. Endosc. 2009;70(6):1231–1237. doi: 10.1016/j.gie.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Somara S, Gilmont RR, Dennis RG, Bitar KN. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology. 2009;137(1):53–61. doi: 10.1053/j.gastro.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 56.Raghavan S, Gilmont RR, Miyasaka EA, et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology. 2011;141(1):310–319. doi: 10.1053/j.gastro.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenstein JM. Why do neural transplants survive? An examination of some metabolic and pathophysiological considerations in neural transplantation. Exp. Neurol. 1995;133(1):1–6. doi: 10.1006/exnr.1995.1001. [DOI] [PubMed] [Google Scholar]
- 58.Capetian P, Döbrössy M, Winkler C, Prinz M, Nikkhah G. To be or not to be accepted: the role of immunogenicity of neural stem cells following transplantation into the brain in animal and human studies. [Accessed July 1, 2011];Semin Immunopathol. 2011 doi: 10.1007/s00281-011-0272-x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21533909. [DOI] [PubMed]
- 59.Mahalik TJ, Hahn WE, Clayton GH, Owens GP. Programmed cell death in developing grafts of fetal substantia nigra. Exp. Neurol. 1994;129(1):27–36. doi: 10.1006/exnr.1994.1144. [DOI] [PubMed] [Google Scholar]
- 60.Micci M-A, Pattillo MT, Kahrig KM, Pasricha PJ. Caspase inhibition increases survival of neural stem cells in the gastrointestinal tract. Neurogastroenterol. Motil. 2005;17(4):557–564. doi: 10.1111/j.1365-2982.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- 61.Kruger GM, Mosher JT, Bixby S, et al. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35(4):657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuwahara M, Ogaeri T, Matsuura R, et al. In vitro organogenesis of gut-like structures from mouse embryonic stem cells. Neurogastroenterol. Motil. 2004;16(Suppl 1):14–18. doi: 10.1111/j.1743-3150.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- 63.Ueda T, Yamada T, Hokuto D, et al. Generation of functional gut-like organ from mouse induced pluripotent stem cells. Biochemical and Biophysical Research Communications. 2010;391(1):38–42. doi: 10.1016/j.bbrc.2009.10.157. [DOI] [PubMed] [Google Scholar]