Abstract
A key challenge to greater progress in tuberculosis (TB) control is the reservoir of latent TB infection (LTBI), which represents a huge long-lived reservoir of potential TB disease. In parts of Africa, as many as 50% of 15-year-olds and 77%–89% of adults have evidence of LTBI. A second key challenge to TB control is the human immunodeficiency virus (HIV)–associated TB epidemic, and Africa alone accounts for one-quarter of the global burden of HIV-associated TB. HIV co-infection promotes both reactivation TB from LTBI and rapidly progressive primary TB following recent exposure to Mycobacterium tuberculosis. Preventing active TB and tackling latent infection in addition to the Directly Observed Treatment, Short-Course (DOTS) strategy could improve TB control in high-burden settings, especially where there is a high prevalence of HIV co-infection. Current strategies include intensified case finding (ICF), TB infection control, antiretroviral therapy (ART), and isoniazid preventive therapy (IPT). Although ART has been widely rolled out, ICF and IPT have not. A key factor limiting the rollout and effectiveness of IPT and ICF is the limitations of existing tools to both diagnose LTBI and identify those persons most at risk of progressing to active TB. In this review, we examine the obstacles and consider current progress toward the development of new tools to address this pressing global problem.
The targets of the Millennium Development Goals and associated Stop TB Partnership are to halt and reverse the increasing incidence of tuberculosis (TB) and halve the 1990 prevalence and mortality rates by 2015 [1]. Global incidence has been falling since 2004 (albeit at a slow rate of <1% per year), and good progress has been made toward attaining the prevalence and mortality goals in most regions. However, the overall global targets may be missed largely due to mortality and prevalence rates in sub-Saharan Africa and the eastern European region [2]. Moreover, the additional long-term goal to eliminate TB as a public health problem by 2050 (a target TB incidence of <1 case per 1 million population) will require far more rapid progress with a mean reduction in global TB incidence of 16% per year over the next 40 years [3]. Even if the Global Plan to Stop TB were successfully implemented, it is estimated that TB incidence would decrease by only ∼6% annually, meaning that global incidence rates in 2050 would remain 100-fold higher than the elimination target [4].
One of the key challenges to greater progress in TB control is the reservoir of latent TB infection (LTBI), which is estimated to affect one-third of the world’s population [5]. This represents a huge long-lived reservoir of potential TB disease. Mathematical modeling suggests that TB elimination cannot be reached by case finding and case management strategies alone [6, 7] and that more rapid progress in TB control requires implementation of additional interventions to target this huge reservoir. A second key challenge to TB control is the human immunodeficiency virus (HIV)–associated TB epidemic. The Directly Observed Treatment, Short-Course (DOTS) strategy has proved to be completely insufficient to address this, and in many African countries, HIV has led to 3–5-fold increases in TB notification rates since 1990 [8]. The south and east of the continent have been worst affected by the HIV infection epidemic, where preexisting TB incidence and LTBI prevalence rates were high [9]. Approximately 1% of the population is estimated to develop TB each year in South Africa and Swaziland. More than two-thirds of TB cases are HIV-associated in these and many other countries in Africa, including Malawi, Kenya, Tanzania, Uganda, and Rwanda [2]. South Africa alone accounts for one-quarter of the global burden of HIV-associated TB [2]. This epidemic is fueled by very high rates of LTBI and high ongoing prevailing rates of infection. In parts of South Africa, as many as 50% of 15-year-olds [10] and 77%–89% of adults have evidence of LTBI [11, 12]. HIV co-infection leads to high rates of both reactivation TB and rapidly progressive primary TB following recent exposure to Mycobacterium tuberculosis.
To address the HIV-associated TB epidemic, preventive interventions are needed in addition to the DOTS strategy. These include intensified case finding (ICF), TB infection control, antiretroviral therapy (ART), and isoniazid preventive therapy (IPT) [13–15]. To date, ART is the only one of these that has been implemented at scale, having been provided for >5.3 million people in low- and middle-income countries by the end of 2009 [16]. In contrast, only 0.2% of eligible individuals received IPT in 2008, according to the World Health Organization (WHO) [8]. To galvanize greater momentum in the implementation of these interventions, the WHO launched in 2008 the “3 I’s” policy, which calls for IPT, ICF, and infection control to be scaled up in parallel with ART [17]. However, the potential benefits of IPT and ICF on the African TB epidemic have yet to be realized. Many factors underlie the very limited use of IPT and ICF, especially to address the HIV-associated TB epidemic in resource-limited settings. Many of these factors relate to the restricted capacity of health systems to implement and sustain these strategies. Some of the countries face an absolute and critical shortage of health personnel, and most face weak public health facilities and infrastructure. In addition to these factors, another key factor is the inherent limitation in the current tools to both diagnose LTBI accurately and identify those individuals who are most at risk of progressing to active TB. In this review, we examine the obstacles and consider current progress toward the development of new tools to address this pressing global problem.
THE NATURAL HISTORY OF LTBI AND THE BASIS FOR PREVENTIVE TREATMENT
LTBI is a clinical state that is currently defined by immunological evidence of M. tuberculosis infection accompanied by an absence of clinical and radiographic evidence of TB-related symptoms and pathology [18, 19]. Little is known about the anatomical location, number, and metabolic state in vivo of the infecting tubercle bacilli in LTBI [18, 19]. LTBI is biologically important because it represents a state of long-term bacillary containment, but the correlates and mediators of this immune control are incompletely understood. The geographic distribution of LTBI largely mirrors that of active TB, with the highest prevalence in Sub-Saharan Africa and the Indian subcontinent [10–12, 20].
Immunocompetent adults with tuberculin skin test (TST) conversion after exposure to persons with infectious TB cases have a 5% risk of progression to active TB disease in the 2 years following exposure, with a further 5% residual lifetime risk of progression [21]. TB control policy in low-burden regions targets recent contacts for preventive therapy on the basis of this 5% early risk. Several host factors, including age of <5 years, HIV co-infection, diabetes mellitus, smoking, undernutrition, chronic renal failure, and iatrogenic immunosuppression, increase the risk of progression to active TB in remotely infected individuals to levels much higher than 5% over several decades [22]. In low-burden high-resource regions, persons with these endogenous risk factors are therefore targeted for preventive therapy (IPT) regardless of whether their infection was acquired recently or remotely. Untreated HIV infection with advanced immunosuppression has been reported to increase the risk to 5%–8% per annum in a low-burden setting [23, 24], but rates as high as 25% per annum have been recorded among individuals with advanced immunodeficiency living in the worst affected communities of South Africa [25]. Even after ART-induced immune reconstitution, the risk remains 4–5-fold higher than in HIV-uninfected persons [26–29]. Therefore, in high-burden settings, the key target groups for LTBI diagnosis are recent case contacts, children <5 years old, and all people living with HIV infection.
IPT is effective in both HIV-positive and HIV-negative individuals [30, 31]. In HIV-infected individuals, IPT is associated with an overall reduction in TB risk of 33% and a 64% risk reduction in those with positive TSTs [31]. The observations that TST positivity is so strongly predictive of likely benefit of IPT and that highly exposed (and likely infected) individuals with cutaneous anergy to tuberculin derive no benefit from IPT suggests that the efficacy of IPT may possibly be dependent in part on preserved immune responses [32]. However, data are lacking on the efficacy of secondary IPT at different CD4 cell counts, with only one study reporting a lack of an interaction between CD4 cell count and IPT efficacy [33]. Additional studies on the influence of CD4 cell count on the effectiveness of chemoprophylaxis are required.
Although there is some debate over the ideal treatment length and the drug regimen of choice, isoniazid monotherapy for 6 months is the most widely used treatment for LTBI globally, and resource-poor countries use isoniazid monotherapy almost exclusively [34]. The duration of the effectiveness of IPT is a key issue, especially in an environment where TB is endemic and individuals are continually reexposed, such as the South African gold mines, where there is a 10% annual risk of infection [35]. Data from HIV-negative populations have recorded 19 years protection of in a low-burden setting where TB was epidemic (∼2500 cases per 100 000 population per year) [36]. However, protection is much shorter (6 months–2 years) in HIV-positive populations in settings with high TB burden [31, 37–39]. This is likely to result from high prevailing reinfection pressures; the higher the risk of reinfection, the more limited the durability of preventive effect following completion of the prophylaxis regimen. A recent study Botswana found that the beneficial impact of IPT in HIV-infected individuals with positive TSTs was lost within a few months of discontinuing a 6-month course of IPT; in contrast, those receiving long-term therapy had marked persisting suppression of TB incidence rates over 3 years [39]. These data have underpinned a conditional recommendation for potential use of long-term IPT in high-burden settings [40].
POOR IMPLEMENTATION OF IPT IN HIGH-BURDEN, LOW-RESOURCE SETTINGS
It is estimated that only 0.2% of eligible individuals globally received IPT in 2008 [2]. In a recent WHO report aiming to measure implementation of IPT policy recommendations, 21 (51%) of 41 countries (representing all WHO regions) were found to have a national IPT policy, but only 6 (28%) of 21 countries had achieved nationwide implementation [41]. Implementation of IPT has been hindered by the concerns over the reliability with which active TB can be excluded and the associated concerns over potential generation of isoniazid resistance [41, 42]. A large individual patient meta-analysis of almost 10 000 patients has been conducted to develop a symptom screening algorithm with high negative predictive value to rule out active TB [43]. This may go some way toward alleviating concerns, but is not yet widely known, and will still require health personnel time to administer. Concerns over resistant strains are not substantiated by the data shown in a number of studies and review articles [42, 44]. This viewpoint has been further supported by the WHO following their 2010 guidelines meeting on preventative therapy and case finding for TB in people living with HIV infection [40].
A further major barrier to implementation to date has also been the requirement for the use of TST to identify those individuals who will benefit from IPT. The TST is logistically problematic for a number of reasons including the lack of highly trained health care workers who can accurately administer and read the test, the need for a return visit to read the test result, the cost of purified protein derivative (PPD), and the requirement of continuous refrigeration [41]. The latest WHO recommendation is to scale up IPT in all people living with HIV infection in countries with high TB burden, regardless of TST status, degree of immunosuppression, or prevalence of LTBI [40]. In order to overcome the barrier imposed by the TST, the WHO recommendation notes that TSTs should be performed where possible but should not be a prerequisite for IPT initiation. However, this nontargeted use of IPT is far from ideal and is presenting national policy makers with a dilemma. Because only TST-positive individuals have been shown to benefit from IPT, failure to use the TST will result in large numbers of individuals unnecessarily receiving therapy with no benefit to themselves. It could be argued that this is far too inefficient and costly a strategy for high-burden, low-resource countries because the prevalence of TST positivity is generally not high in HIV-infected populations due to their immunodeficiency, which contributes to test failure. For example, in studies by Karam et al [45] in Senegal and Mosimaneostile et al [46] in Botswana, only ∼20% of HIV-infected individuals were TST positive, suggesting that only 1 in 5 HIV-infected people would potentially derive benefit from IPT rollout in those settings.
Conversely, if IPT is given without prior selection using the TST, most patients treated would derive no benefit and yet be subject to unnecessary risks of drug toxicity such as hepatitis and neuropathy [47]. Absolute risks per individual are very low, but these are an important consideration for a large-scale intervention. Moreover, this risk may be greater if IPT is given as a long-term intervention, as is now an option under current WHO guidelines [40]. Although 10%–20% of individuals receiving IPT may experience transient elevations of serum aminotransferase levels, clinical hepatitis is much less common and is rarely fatal if recommendations for surveillance are followed [48, 49]. Levels of clinical isoniazid-associated hepatitis are reported to range from 0.36% to 1.7% in both HIV-negative and HIV-positive populations [30, 50–52]. Hospitalization rates are reported to range from 0.01% to 0.02% of individuals, whereas mortality rates range from 0% to 0.03% [51, 53–57]. In theory, all individuals receiving IPT, especially those receiving ART concomitantly, should have their liver function regularly monitored to ensure that aminotransferase levels do not exceed 3–5 times the normal values. This may prove to be difficult in high-burden, low-resource settings. However, current WHO recommendations support symptom screening as opposed to laboratory monitoring for isoniazid-associated hepatitis surveillance [40]. In Botswana (where the national rollout of IPT in people living with HIV infection has been implemented), the government has introduced a policy of monthly follow-up meetings where individuals are educated on the symptoms of hepatitis [52].
DIAGNOSIS: THE GATEKEEPER TO EFFECTIVE LTBI TREATMENT
The conundrum facing policy makers at present is that the TST is currently the best means for identifying those patients who will benefit from IPT and yet the difficulties of using this test in the field in low-resource settings has been a key reason for the poor scale-up of IPT [41]. Thus, although revised WHO guidelines dispense with TST evaluation as a prerequisite for IPT, the nontargeted use of IPT is far from ideal and especially so when recent data provide the rationale for use of long-term therapy. Novel and improved diagnostics for LTBI that are predictive of the benefit of IPT are urgently required. In the remainder of this article, we review the current tools and emerging developments in the diagnosis of LTBI.
The TST has been used for >100 years [18]. The test measures a delayed-type hypersensitivity response to PPD from the supernatant of liquid cultures of M. tuberculosis. Many of the antigens in PPD are shared by M. tuberculosis, Mycobacterium bovis, M. Bovis bacille Calmette-Guérin (BCG), and several species of mycobacteria. Unfortunately, the TST has low specificity and low sensitivity. For example, false positive results are frequently observed in individuals who have been vaccinated with BCG, and false negative results are observed in individuals with impaired cellular immunity. In particular, TST sensitivity is greatly reduced in HIV–co-infected individuals due to HIV-induced cutaneous anergy resulting in false negatives [11, 58].
Interferon γ (IFN-γ) release assays (IGRAs), which were recently introduced as novel assays for the detection of LTBI, show increased sensitivity and specificity in comparison with TST. Both types of commercially available IGRAs (T-SPOT.TB, based on an enzyme-linked immunospot [ELISPOT] platform; and QuantiFERON-TB Gold In-Tube [QFN-GIT], based on enzyme-linked immunosorbent assay [ELISA] technology) measure M. tuberculosis antigen–specific IFN-γ production by T cells primed by in vivo exposure to the pathogen. These assays are based on 2 highly immunodominant antigens (CFP-10 [Rv3874] and ESAT-6 [Rv3875]) from the region of difference 1 (RD1), a section of the genome that is present in M. tuberculosis but deleted from all strains of BCG and most environmental mycobacteria. These tests are therefore not confounded by prior vaccination with BCG and have much higher diagnostic specificity than TST. These IGRAs also probably have higher sensitivity than TST, especially in the setting of HIV co-infection in active TB [59–63]. Because there is no gold standard diagnostic for LTBI, it is not possible to evaluate the sensitivity and specificity of novel assays directly. Two main markers have been used as a surrogate for LTBI in order to assess assay sensitivity: active TB and the degree of exposure to infectious cases [5, 64–66]. Assay specificity is measured by assessing numbers of negative results in healthy BCG-vaccinated individuals at low risk of TB infection [67].
IGRAs have been shown to correlate well with TB exposure in low- and medium-burden regions in both well-defined outbreaks and community-based contact investigations [66, 68–81]. However, few studies have been performed in high-burden settings; those published to date have shown a correlation of IGRA results with TB exposure in community-based contact investigations [74, 78, 81–86]. A recent study performed among people living with HIV infection in Zimbabwe reported that the ELISPOT IGRA platform was able to identify a higher level of LTBI in case contacts of sputum-smear–positive cases compared with case contacts of controls [85].
Active TB has also been used as a surrogate for LTBI, and a number of reports have indicated that IGRAs have a high sensitivity for active TB. A recent systematic review reported that the ELISPOT IGRA platform had a pooled sensitivity of 90% (range, 83%–100%), whereas the latest generation ELISA platform (QFN-GIT) has a pooled sensitivity of 70% (range, 64%–94%), and the diagnostic sensitivity of ELISPOT assays is less adversely affected by HIV co-infection than is TST in both adults and children [87]. A recent systematic review by Diel et al [88] reported a pooled sensitivity of 70% for the TST, 81% for the QFN-GIT, and 88% for the T-SPOT.TB (or 84%, 89%, and 89%, respectively, when the analysis was restricted to developed countries). Compared to the study by Pai et al [87], this systematic review only evaluated studies that used accepted gold standards for the diagnosis of active TB disease (ie, culture, polymerase chain reaction, and/or histologic examination).
Although IGRA sensitivity in active TB is not yet good enough to use as a test to rule out suspected active TB, it is an improvement on the TST, suggesting by extrapolation that IGRAs are more sensitive in LTBI. This tentative conclusion is consistent with the results of studies that have used TB exposure as a surrogate. Importantly, IGRAs have consistently been shown to be substantially more specific than TST in both BCG-vaccinated and unvaccinated populations. Pai et al [87] reported a pooled specificity of the ELISA platform (QFN-GIT) of 96% (range, 89%–100% for all ELISA platforms), whereas the pooled specificity of the ELISPOT IGRA platform was 93% (range, 85%–100%). Diel et al [88] report a specificity of 99% for the QFN-GIT and 86% for the T-SPOT.TB. Unfortunately, it is difficult to assess the specificity of these assays in high-burden settings due to the lack of unexposed BCG-vaccinated controls.
A KEY OBSTACLE TO WIDER TREATMENT OF LTBI IS THE PREDICTIVE VALUE OF DIAGNOSTIC TESTS
IGRAs will be clinically useful only if they have prognostic value for TB. The prognostic value of positive IGRA test results has recently been reported in a number of studies based in low- and high-burden settings and in most key risk groups including children and HIV-positive individuals [89–98]. However, the available estimates of the prognostic power of IGRAs from published longitudinal clinical outcome studies to date are heterogeneous and vary between low- and high-burden settings [90, 99]. Diel et al [99] performed a large study in a low-burden setting and reported that the positive predictive value of the ELISA-based IGRA in recent TB contacts (14.6%) was significantly superior to that of the TST (2.3%; cutoff, of 5 mm) in children as well as in adults. Other studies in low- and medium-burden settings have also found ELISA-based and ELISPOT-based IGRAs to be prognostic for development of active TB in recent TB contacts, but in general the prognostic power of the IGRA was not substantially stronger than that of the TST in these studies [90, 94, 95].
In high-burden settings, the prognostic power of IGRAs is less clear because individuals with remote infection may also appear to be IGRA positive. In addition, de novo community transmission may occur in household contacts, which would alter the accuracy of any studies. For example, Hill et al [91], in a study from the Gambia, reported that the ELISPOT IGRA platform was not prognostic of active TB disease. A more recent larger study, performed in Senegal, suggests somewhat better prognostic power of the ELISPOT IGRA platform [95]. To conclude, although few prospective studies have been performed, current reports indicate that IGRAs are predictive of active TB and may be significantly more predictive than the TST in low-burden settings. However, the prognostic power is not as impressive in high-burden settings, and the difference between it and the TST is minimal. Further prospective studies are clearly needed, especially in high-burden settings and especially among people living with HIV infection, in whom the prognostic power of IGRAs has to date only been shown in one small study in a low-burden setting [93]. Interestingly, there are benefits to doing both available tests (TST and IGRA) together, because the predictive power of a dual positive result is greater than that of a positive result of each test alone [90, 95].
The natural history of LTBI as elucidated by the TST reflects the 5% risk of progression to active TB in recently exposed HIV-negative adults with positive TST results, which requires treatment of 20 persons for every case of TB averted (ie, number needed to treat [NNT], 20). The key question is therefore how much stronger is the prognostic power of IGRAs compared with that of TSTs and to what extent can the NNT be reduced to <20. Based on the published evidence above, the prognostic value of current IGRAs would not meaningfully reduce the NNT in high-prevalence regions because the vast majority of people (>80%) who have a positive IGRA result (or TST result or even both) do not develop TB. In addition, the value of IGRAs for predicting benefit from IPT has not yet been comprehensively evaluated in resource-poor settings.
However, even if the prognostic capability of IGRAs could be improved, their deployment is severely limited by their cost and need for laboratory infrastructure. The operating characteristics of the current platforms for LTBI diagnosis are major obstacles to the treatment of LTBI. This means that in many parts of Africa, IGRAs can only be deployed where there are hospital facilities, in contrast to the HIV test, which can be performed at stand-alone clinics and peripheral health centers. The current need for patients, or their blood samples, to travel to hospital laboratory facilities in resource-limited settings is a major obstacle, and ultimately a point-of-care diagnostic for LTBI is required in order to fully address the TB crisis across Africa.
IMPROVED DIAGNOSTICS FOR LTBI: WHAT IS IN THE PIPELINE?
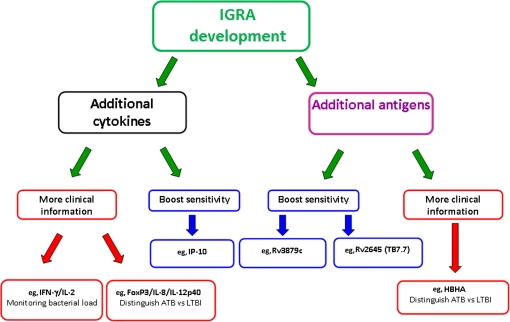
There is an obvious need for new diagnostic tests with a far greater prognostic value than those currently available. This may be possible, in part, by the further development of IGRAS. There are 2 main strategies to improve on current IGRAs: the first involves the measurement of additional cytokine levels, and the second is incorporation of additional antigens (recently summarized by Lalvani et al [100]) (Figure 1). The use of additional M. tuberculosis–specific antigens has already been shown to increase test sensitivity (without compromising specificity) when Rv2645 (TB7.7) was added to the ELISA platform to create QFN-GIT [97, 101]. The addition of RV3879c to the ELISPOT platform was also shown to increase test sensitivity in the ELISPOTPLUS compared with the standard ELISPOT (T-SPOT.TB) [102]. Further research into the diagnostic utility of additional antigens is required, including putative latent phase antigens such as Rv2031c and heparin-binding hemagglutinin [103–110].
Figure 1.
What does the future hold? New approaches and next-generation interferon γ (IFN- γ) release assays (IGRAs). Two main strategies are available to improve on current IGRAs: the first involves the measurement of additional cytokine levels, and the second involves the incorporation of additional antigens [93]. The latter has already been shown to increase test sensitivity (without compromising specificity) when Rv2645 (TB7.7) was added to the enzyme-linked immunosorbent assay platform to create QuantiFERON-TB Gold In-Tube [97, 101] and when RV3879c was added to the enzyme-linked immunospot (ELISPOT) platform to create ELISPOTPLUS [102]. Further research into the diagnostic utility of additional antigens is required, including that of heparin-binding hemagglutinin (HBHA) [103–110]. The other strategy to improve IGRAs has also been investigated, and Ruhwald et al [111] have reported that measuring IFN-γ inducible protein 10 (IP-10) levels in addition to IFN-γ levels significantly improved diagnostic sensitivity for active tuberculosis (ATB) by 4% over QuantiFERON-TB Gold In-Tube test alone (while only slightly decreasing specificity from 100% to 98%; P < .009). The simultaneous measurement of interleukin 2 (IL-2) levels has also raised hopes for improving on IGRAs, because IFN- γ and IL-2 T-cell cytokine secretion profiles have been shown to correlate with pathogen load and the successful response to tuberculosis treatment [112–114]. Ultimately, all of these novel approaches urgently need to be validated in large prospective studies to quantify their clinical utility and evaluate their prognostic power in both low- and high-burden settings. IL-8, interleukin 8; IL-12p40, interleukin 12p40; LTBI, latent tuberculosis infection.
A number of studies have investigated the use of additional cytokines and chemokines downstream of IFN-γ such as IFN-γ inducible protein 10 (IP-10 or CXCL10) and monocyte chemoattractant protein. For example, Ruhwald et al [111] reported that measuring plasma IP-10 levels in addition to IFN-γ levels significantly improved diagnostic sensitivity for active TB by 4% over that of the QFN-GIT test alone (while only slightly decreasing specificity from 100% to 98%; P < .009). Further work on this finding needs to be performed in order to confirm its validity as a test for latent M. tuberculosis, including correlating the performance of this assay with epidemiologically well-defined exposure to TB, followed by establishment of the prognostic value for the development of active TB as has been done for IGRAs [90, 91, 100, 111]. However, Ruhwald et al [115] recently demonstrated a plasma IP-10 correlation with TB exposure in a pediatric population in Nigeria.
The simultaneous measurement of interleukin 2 (IL-2) has also shown hope to improve on IGRAs, because IFN-γ and IL-2 T-cell cytokine secretion profiles have been shown to correlate with pathogen load and the successful response to TB treatment [112–114]. This dynamic relationship between dual IFN-γ and IL-2 T-cells and bacterial load is supported by recent studies by Sutherland et al [116] and Caccomo et al [117], and Casey et al [118] and the presence of IL-2 secretion (with or without IFN-γ secretion) by M. tuberculosis–specific T cells is consistently associated with LTBI compared with active TB and, together with studies of HIV and TB co-infection, suggests this may represent a correlate of protective immunity [114, 116, 119, 120]. However, trifunctional M. tuberculosis–specific T cells that additionally secrete tumor necrosis factor α T cells seem to predominate in active TB disease compared with treated active TB and LTBI, serving more as a marker of higher bacterial load [75, 116, 117, 120]. Importantly, functional signatures of T cells may serve as biomarkers of disease stage, helping to identify individuals with LTBI and aiding the development of the next generation of IGRAs. Additional approaches may also aid the development of the next generation of IGRAs. For example, Wu et al [121] have reported that they could differentiate between latent and active TB by measuring mRNA expression levels for interleukin 8, FOXP3, and interleukin 12β in response to ESAT-6 stimulation. Recent genomewide microarray studies have identified gene-expression profiles that correlate well with both active TB and LTBI and suggest new potential biomarkers [122–124]. All of these novel approaches mentioned above now urgently need to be validated in large prospective studies to quantify their clinical utility and evaluate their prognostic power in both low- and high-burden settings.
The traditional paradigm that distinguishes latent infection from active disease as distinct binary states is overly simplistic. Granulomas are not fixed inert structures, as previously described, but they are very active and have constantly changing dynamic structures of metabolically active tissues [125]. It is thought to be likely that a continuous spectrum of states exists both in the same individual and between different individuals, with varying degrees of immune control and mycobacterial bacillary load [125, 126], and that HIV infection profoundly shifts this spectrum in favor of bacillary replication [32]. In light of this new paradigm, new biomarkers are required that can more precisely define these disease states and assess the probability of progression of M. tuberculosis infection to active TB disease.
Ultimately a highly predictive, rapid, reliable, and cheap point-of-care diagnostic for LTBI is needed to enable the highly targeted rollout of preventative therapy. In the meantime, until a powerfully prognostic affordable point-of-care LTBI diagnostic is developed, IGRAs remain the most accurate available reference standard, especially in HIV-positive populations. However, the use of the TST can be optimized in resource-limited settings by calibrating TST readings to line up with IGRA results from assays performed on a small sample of the target population. This optimization of TST interpretation has been tried and tested in Turkey [127] and Zimbabwe [128].
NOVEL APPROACHES TO AID THE EFFECTIVE IMPLEMENTATION OF IPT AND ITS CONTRIBUTION TO GLOBAL TB CONTROL
Even with improved diagnostics for LTBI, there are important changes that should be made to aid the effective implementation of IPT and its contribution to global TB control.
Inclusion of IPT Within ICF Programs
First, LTBI treatment and diagnosis should be incorporated into ICF programs. Because there is a prerequisite to exclude active TB (by symptom screening with or without chest radiography) before initiating a treatment regimen for LTBI, it is important to highlight that IPT should not be seen as a separate intervention but as part of the basic package of care for persons living with HIV infection, which includes ICF. IPT and ICF can act in synergy to reduce TB risk in persons living with HIV infection [6]. TB case finding will itself reduce the prevalence of active undiagnosed TB, which is a key determinant of TB transmission at a population level. The costs of strengthening health systems can therefore logically be shared between the following interventions; ICF, IPT, infection control, and ART rollout. Although this review has placed an emphasis on HIV-infected populations, in considering LTBI diagnosis in regions with a high burden of TB, we must not forget those with recent infection regardless of HIV status (household contacts) and children <5 years old (both at high risk of progression to active TB). All would benefit from the inclusion of IPT within ICF programs.
Rational Strategy of IPT and ART Rollout Based on CD4 Cell Count
A rational strategy based on CD4 cell count for IPT rollout in populations of individuals living with HIV infection should be implemented where CD4 cell counts can be readily measured using current technology. [12]. The rationale behind this recommendation is that it is more difficult to rule out active TB in HIV-infected individuals with low CD4 cell counts, and so isoniazid should be provided to those individuals with higher CD4 cell counts (>350 × 106 cells/L) and ART (plus delayed isoniazid) to those with lower CD4 cell counts (<350 × 106 cells/L) [12, 15]. This viewpoint is supported by a WHO meta-analysis which showed that the sensitivity of current symptoms (cough of >24 hours duration, fever, night sweats, and weight loss) as a tool for active TB diagnosis has a lower negative predictive value in individuals with a low CD4 cell count, leading to a much higher prevalence of undiagnosed TB [12, 129–131]. If ART is used as the primary tool to combat LTBI in HIV-infected individuals with low CD4 cell counts, then it will unmask any subclinical TB within the first few months of treatment, reducing the risk of providing IPT to those with active TB. This is supported by the notion that waning immune function may limit the durability of isoniazid, and so delaying IPT (for a suggested 3 months) until CD4 cell count has increased, and potentially TST reversion has occurred, will increase the benefits that can be derived from IPT [12]. ART has been shown to reduce TB incidence by 67% in patients with low CD4 cell counts (irrespective of TST status) [12, 39]. Importantly, there is an additive benefit of both ART and IPT, and so when sufficient immune reconstitution has taken place, individuals should be given both treatment regimens [132, 133]. More data from randomized controlled trials are needed to help drive policy development in this area, and new international guidelines need to be formulated on the concomitant use of ART and IPT. This is also important because hepatitis is an adverse effect of both IPT and ART (particularly nevirapine- and ritonavir-boosted protease inhibitors) [52, 134]. Unfortunately, the above proposal is a technology-dependent and rather complex algorithm that will require human resource numbers and health system infrastructure, which are absent in most high-burden areas. Many HIV-infected patients in high-burden settings access ART without the benefit of CD4 cell testing. Until CD4 cell testing becomes at least as close to the point of care as HIV testing, continent-wide IPT rollout through this strategy will be extremely challenging.
More Effective Pharmacological or Immunological Interventions Against LTBI
The design of TB preventative therapy has to be improved, because currently there is an incomplete protective effect, even in TST-positive individuals [39]. In addition, 6 months of preventative therapy is far too long, and there is an urgent need for novel drugs that act faster. Current IPT regimens do not provide long-term benefit in settings where TB is endemic and/or epidemic, and so novel therapeutic regimens for the treatment of latent M. tuberculosis infection must be identified. This is exceptionally challenging because relatively little is known about the biology of dormant bacilli in vivo. The ideal scenario would be an ultrashort course of a safe, tolerable regimen for the treatment of latent infection that is able to cause life-long sterilization. Evaluation of combinations of new and existing drugs in mouse models of LTBI are required to identify potential short, sterilizing regimens for evaluation in clinical trials, including for the treatment of latent infection with multidrug-resistant and extensively drug-resistant (XDR) TB.
An alternative strategy to novel drugs is therapeutic vaccination in persons with existing LTBI, which appears to be the mechanism of action of Mycobacterium vaccae in a recent large study in Tanzania in a population with high prevalence of LTBI and HIV co-infection [135]. Alternatively, IPT could be combined with an adjunctive therapeutic TB vaccine (such as RUTI, which is made of detoxified fragmented M. tuberculosis that is delivered in liposomes) or a booster vaccine such as the Aeras Ad35 TB vaccine to stimulate TB-specific immunity to improve durability of IPT or possibly to reduce the duration of IPT. Both of these suggested strategies are currently being evaluated. A further alternative is to use a combination of IPT or other drugs with immunotherapeutic agents such as thalidomide analogs to enhance activity of the preventive therapy and promote sterilization [136].
CONCLUSION
The current lack of a rapid point-of-care diagnostic test for LTBI with sufficient prognostic power for development of active TB is reflected in the latest WHO recommendation to scale-up IPT in all people living with HIV infection regardless of TST status [40]. This may be an inefficient and costly strategy for high-burden, low-resource countries with high numbers of individuals living with HIV infection. Research to develop more powerfully prognostic tests of LTBI that are suitable for use in developing countries is a public health priority. In order to support effective chemoprophylaxis for LTBI, research into treatments that are shorter-acting and longer-lasting must take place. In the interim period, the WHO policy must be supported where possible because IPT for HIV-positive persons and child contacts <5 years of age, with or without TST or IGRA results, remains an important public health measure in high-burden countries. To aid IPT implementation (and its effective contribution to global TB control), a number of important changes to IPT policy should be considered, including a strategy of IPT and ART rollout based on CD4 cell count (where possible) and the inclusion of IPT rollout within ICF programs.
Notes
Financial support.
This work was supported by the Wellcome Trust (funding to S. D. L.); KwaZulu-Natal Research Institute for Tuberculosis and HIV (K-RITH) (funding to V. O. K.); and the Howard Hughes Medical Institute (funding to V. O. K.). A. L. is a Wellcome Senior Research Fellow in Clinical Science and a National Institutes of Health Research Senior Investigator.
Supplementary sponsor.
This article was published as part of a TB Diagnostics Supplement sponsored by the KwaZulu-Natal Research Institute for Tuberculosis and HIV (K-RITH), the Harvard University Center for AIDS Research (CFAR), the Albert Einstein College of Medicine, and the Einstein-Montefiore CFAR.
Potential conflicts of interest.
A. L. is listed as the inventor for several patents underpinning T-cell–based diagnosis. The ESAT-6/CFP-10 interferon c enzymelinked immunospot assay was commercialized by an Oxford University spin-off company (T-SPOT.TB; Oxford Immunotec, Abingdon, UK) in which the University of Oxford and A. L. have minority shares of equity and royalty entitlements. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.United Nations. The Millenium development goals report. New York, NY: United Nations; 2008. 2008. http://www.un.org/millenniumgoals/. Accessed 1 September 2011. [Google Scholar]
- 2.World Health Organization (WHO) Global tuberculosis control: a short update to the 2009 report. Geneva: WHO; 2009. http://www.who.int/tb/publications/global_report/2009/update/tbu_9.pdf. Accessed 1 March 2010. [Google Scholar]
- 3.Lonnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–6. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Watt CJ, Hosseini SM, Lonnroth K, Williams BG, Dye C. The global epidemiology of tuberculosis. In: Schaaf HS, Zumla IA, editors. Tuberculosis—a comprehensive clinical reference. Edinburgh: Elsevier; 2009. [Google Scholar]
- 5.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement: global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 6.Dye C, Williams BG. Eliminating human tuberculosis in the twenty-first century. J R Soc Interface. 2008;5:653–62. doi: 10.1098/rsif.2007.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Raddad LJ, Sabatelli L, Achterberg JT, et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci U S A. 2009;106:13980–5. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) Global tuberculosis control 2010 report. Geneva: WHO; 2010. www.who.int/tb/publications/global_report/2010/en/index.html. Accessed 15 March 2011. [Google Scholar]
- 9.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–33. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14:406–12. [PMC free article] [PubMed] [Google Scholar]
- 11.Hanifa Y, Grant AD, Lewis J, Corbett EL, Fielding K, Churchyard G. Prevalence of latent tuberculosis infection among gold miners in South Africa. Int J Tuberc Lung Dis. 2009;13:39–46. [PubMed] [Google Scholar]
- 12.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis. 2010;10:489–98. doi: 10.1016/S1473-3099(10)70078-5. [DOI] [PubMed] [Google Scholar]
- 13.Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nat Rev Immunol. 2005;5:819–26. doi: 10.1038/nri1704. [DOI] [PubMed] [Google Scholar]
- 14.Lawn SD, Edwards DJ, Wood R. Reducing the burden of tuberculosis presenting during the initial months of antiretroviral therapy in resource-limited settings. Clin Infect Dis. 2010;50:124–5. doi: 10.1086/649007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic—when will we act? Lancet. 2010;375:1906–19. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Geneva, Switzerland: WHO; 2010. http://www.who.int/hiv/pub/2010progressreport/summary_en.pdf. Accessed 1 September 2011. [Google Scholar]
- 17.World Health Organization (WHO) WHO three I’s meeting: report of a joint WHO HIV/AIDS and TB Department meeting. Geneva, Switzerland: WHO; 2008. http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf. Accessed 1 September 2011. [Google Scholar]
- 18.Mack U, Migliori GB, Sester M, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33:956–73. doi: 10.1183/09031936.00120908. [DOI] [PubMed] [Google Scholar]
- 19.Sridhar S, Pollock K, Lalvani A. Redefining latent tuberculosis. Future Microbiology. doi: 10.2217/fmb.11.82. In Press. [DOI] [PubMed] [Google Scholar]
- 20.Pareek M, Abubakar I, White PJ, Garnett GP, Lalvani A. Eur Respir J. 2011;37:1175–82. doi: 10.1183/09031936.00105810. [DOI] [PubMed] [Google Scholar]
- 21.Blower SM, McLean AR, Porco TC, et al. The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med. 1995;1:815–21. doi: 10.1038/nm0895-815. [DOI] [PubMed] [Google Scholar]
- 22.Lawn SD, Zumla A. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 23.Selwyn PA, Hartel D, Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–50. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 24.Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301:1535–7. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Ayles H, Egwaga S, et al. Potential utility of empiric tuberculosis treatment for patients with advanced HIV in high HIV-TB burden settings. Int J Tuberc Lung Dis. 2011;15:287–95. [PubMed] [Google Scholar]
- 26.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 27.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 28.Girardi E, Sabin CA, d’Arminio Monforte A, et al. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41:1772–82. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 29.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23:1717–25. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smieja MJ, Marchetti CA, Cook DJ, Smaill FM. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev. 2000:CD001363. doi: 10.1002/14651858.CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawn SD, Wood R, Wilkinson RJ. Changing concepts of “latent tuberculosis infection” in patients living with HIV infection. Clin Dev Immunol. 2011;2011:980594. doi: 10.1155/2011/980594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Churchyard GJ, Fielding K, Charalambous S, et al. Efficacy of secondary isoniazid preventive therapy among HIV-infected Southern Africans: time to change policy? AIDS. 2003;17:2063–70. doi: 10.1097/00002030-200309260-00007. [DOI] [PubMed] [Google Scholar]
- 34.Apers L, Lynen L, Worodria W, Colebunders R. Review editorial: prevention of tuberculosis in resource-poor countries with increasing access to highly active antiretroviral treatment. Trop Med Int Health. 2005;10:1209–14. doi: 10.1111/j.1365-3156.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 35.Corbett EL, Mallory KF, Grant AD, Churchyard GJ, De Cock KM. HIV-1 infection and risk of tuberculosis after rifampicin treatment. Lancet. 2001;357:957–8. doi: 10.1016/S0140-6736(05)71656-1. [DOI] [PubMed] [Google Scholar]
- 36.Comstock GW, Baum C, Snider DE., Jr Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am Rev Respir Dis. 1979;119:827–30. doi: 10.1164/arrd.1979.119.5.827. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JL, Okwera A, Hom DL, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS. 2001;15:2137–47. doi: 10.1097/00002030-200111090-00009. [DOI] [PubMed] [Google Scholar]
- 38.Quigley MA, Mwinga A, Hosp M, et al. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS. 2001;15:215–22. doi: 10.1097/00002030-200101260-00011. [DOI] [PubMed] [Google Scholar]
- 39.Samandari T. In: Proceedings of the 40th Union World Conference on Lung Health. (Cancun, Mexico). Paris, France: International Union Against Tuberculosis and Lung Disease; 2009. Preliminary results of the Botswana isoniazid preventative therapy (IPT) clinical trial (6 vs 36 months) [Google Scholar]
- 40.World Health Organization (WHO) WHO guidelines for intensified case finding and isoniazid preventive therapy for tuberculosis for people living with HIV in resource constrained settings. Geneva, Switzerland: WHO; 2010. http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. Accessed 1 September 2011. [Google Scholar]
- 41.Date AA, Vitoria M, Granich R, Banda M, Fox MY, Gilks C. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ. 2010;88:253–9. doi: 10.2471/BLT.09.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12:744–51. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Halsema CL, Fielding KL, Chihota VN, et al. Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. AIDS. 2010;24:1051–5. doi: 10.1097/QAD.0b013e32833849df. [DOI] [PubMed] [Google Scholar]
- 45.Karam F, Mbow F, Fletcher H, et al. Sensitivity of IFN-gamma release assay to detect latent tuberculosis infection is retained in HIV-infected patients but dependent on HIV/AIDS progression. PLoS One. 2008;3:e1441. doi: 10.1371/journal.pone.0001441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosimaneotsile B, Mathoma A, Chengeta B, et al. Isoniazid tuberculosis preventive therapy in HIV-infected adults accessing antiretroviral therapy: a Botswana experience, 2004–2006. J Acquir Immune Defic Syndr. 2010;54:71–7. doi: 10.1097/QAI.0b013e3181c3cbf0. [DOI] [PubMed] [Google Scholar]
- 47.Gilroy SA, Rogers MA, Blair DC. Treatment of latent tuberculosis infection in patients aged ≥35 years. Clin Infect Dis. 2000;31:826–9. doi: 10.1086/314037. [DOI] [PubMed] [Google Scholar]
- 48.American Thoracic Society. Medical Section of the American Lung Association: treatment of tuberculosis and tuberculosis infection in adults and children. Am Rev Respir Dis. 1986;134:355–63. doi: 10.1164/arrd.1986.134.2.355. [DOI] [PubMed] [Google Scholar]
- 49.Scharer L, Smith JP. Serum transaminase elevations and other hepatic abnormalities in patients receiving isoniazid. Ann Intern Med. 1969;71:1113–20. doi: 10.7326/0003-4819-71-6-1113. [DOI] [PubMed] [Google Scholar]
- 50.Kopanoff DE, Snider DE, Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991–1001. doi: 10.1164/arrd.1978.117.6.991. [DOI] [PubMed] [Google Scholar]
- 51.Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin: a meta-analysis. Chest. 1991;99:465–71. doi: 10.1378/chest.99.2.465. [DOI] [PubMed] [Google Scholar]
- 52.Tedla Z, Nyirenda S, Peeler C, et al. Isoniazid-associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in HIV-infected adults in Botswana. Am J Respir Crit Care Med. 2010;182:278–85. doi: 10.1164/rccm.200911-1783OC. [DOI] [PubMed] [Google Scholar]
- 53.Millard PS, Wilcosky TC, Reade-Christopher SJ, Weber DJ. Isoniazid-related fatal hepatitis. West J Med. 1996;164:486–91. [PMC free article] [PubMed] [Google Scholar]
- 54.Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis clinic. JAMA. 1999;281:1014–8. doi: 10.1001/jama.281.11.1014. [DOI] [PubMed] [Google Scholar]
- 55.Salpeter SR. Fatal isoniazid-induced hepatitis: its risk during chemoprophylaxis. West J Med. 1993;159:560–4. [PMC free article] [PubMed] [Google Scholar]
- 56.Snider DE, Jr, Caras GJ. Isoniazid-associated hepatitis deaths: a review of available information. Am Rev Respir Dis. 1992;145:494–7. doi: 10.1164/ajrccm/145.2_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 57.Diel R, Schaberg T, Loddenkemper R, Welte T, Nienhaus A. Enhanced cost-benefit analysis of strategies for LTBI screening and INH chemoprevention in Germany. Respir Med. 2009;103:1838–53. doi: 10.1016/j.rmed.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Shafer RW, Edlin BR. Tuberculosis in patients infected with human immunodeficiency virus: perspective on the past decade. Clin Infect Dis. 1996;22:683–704. doi: 10.1093/clinids/22.4.683. [DOI] [PubMed] [Google Scholar]
- 59.Chapman AL, Munkanta M, Wilkinson KA, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002;16:2285–93. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 60.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004;364:2196–203. doi: 10.1016/S0140-6736(04)17592-2. [DOI] [PubMed] [Google Scholar]
- 61.Clark SA, Martin SL, Pozniak A, et al. Tuberculosis antigen-specific immune responses can be detected using enzyme-linked immunospot technology in human immunodeficiency virus (HIV)-1 patients with advanced disease. Clin Exp Immunol. 2007;150:238–44. doi: 10.1111/j.1365-2249.2007.03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rangaka MX, Wilkinson KA, Seldon R, et al. Effect of HIV-1 infection on T-cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175:514–20. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 63.Raby E, Moyo M, Devendra A, et al. The effects of HIV on the sensitivity of a whole blood IFN-gamma release assay in Zambian adults with active tuberculosis. PLoS One. 2008;3:e2489. doi: 10.1371/journal.pone.0002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131:1898–906. doi: 10.1378/chest.06-2471. [DOI] [PubMed] [Google Scholar]
- 65.Houk VN, Baker JH, Sorensen K, Kent DC. The epidemiology of tuberculosis infection in a closed environment. Arch Environ Health. 1968;16:26–35. doi: 10.1080/00039896.1968.10665011. [DOI] [PubMed] [Google Scholar]
- 66.Lalvani A, Pathan AA, Durkan H, et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357:2017–21. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 67.Lalvani A, Pareek M. A 100 year update on diagnosis of tuberculosis infection. Br Med Bull. 2010;93:69–84. doi: 10.1093/bmb/ldp039. [DOI] [PubMed] [Google Scholar]
- 68.Shams H, Weis SE, Klucar P, et al. Enzyme-linked immunospot and tuberculin skin testing to detect latent tuberculosis infection. Am J Respir Crit Care Med. 2005;172:1161–8. doi: 10.1164/rccm.200505-748OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168–73. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 70.Dominguez J, Ruiz-Manzano J, De Souza-Galvao M, et al. Comparison of two commercially available gamma interferon blood tests for immunodiagnosis of tuberculosis. Clin Vaccine Immunol. 2008;15:168–71. doi: 10.1128/CVI.00364-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soysal A, Millington KA, Bakir M, et al. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet. 2005;366:1443–51. doi: 10.1016/S0140-6736(05)67534-4. [DOI] [PubMed] [Google Scholar]
- 72.Connell TG, Ritz N, Paxton GA, Buttery JP, Curtis N, Ranganathan SC. A three-way comparison of tuberculin skin testing, QuantiFERON-TB Gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624. doi: 10.1371/journal.pone.0002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diel R, Loddenkemper R, Meywald-Walter K, Gottschalk R, Nienhaus A. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold In Tube assay, and T-Spot.TB test in contact investigations for tuberculosis. Chest. 2009;135:1010–8. doi: 10.1378/chest.08-2048. [DOI] [PubMed] [Google Scholar]
- 74.Nicol MP, Davies MA, Wood K, et al. Comparison of T-SPOT.TB assay and tuberculin skin test for the evaluation of young children at high risk for tuberculosis in a community setting. Pediatrics. 2009;123:38–43. doi: 10.1542/peds.2008-0611. [DOI] [PubMed] [Google Scholar]
- 75.Richeldi L, Ewer K, Losi M, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med. 2004;170:288–95. doi: 10.1164/rccm.200403-307OC. [DOI] [PubMed] [Google Scholar]
- 76.Zellweger JP, Zellweger A, Ansermet S, de Senarclens B, Wrighton-Smith P. Contact tracing using a new T-cell-based test: better correlation with tuberculosis exposure than the tuberculin skin test. Int J Tuberc Lung Dis. 2005;9:1242–7. [PubMed] [Google Scholar]
- 77.Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004;170:65–9. doi: 10.1164/rccm.200402-232OC. [DOI] [PubMed] [Google Scholar]
- 78.Hill PC, Brookes RH, Adetifa IM, et al. Comparison of enzyme-linked immunospot assay and tuberculin skin test in healthy children exposed to Mycobacterium tuberculosis. Pediatrics. 2006;117:1542–8. doi: 10.1542/peds.2005-2095. [DOI] [PubMed] [Google Scholar]
- 79.Detjen AK, Keil T, Roll S, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007;45:322–8. doi: 10.1086/519266. [DOI] [PubMed] [Google Scholar]
- 80.Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent tuberculosis diagnosis in children by using the QuantiFERON-TB Gold In-Tube test. Pediatrics. 2009;123:30–7. doi: 10.1542/peds.2007-3618. [DOI] [PubMed] [Google Scholar]
- 81.Chun JK, Kim CK, Kim HS, et al. The role of a whole blood interferon-gamma assay for the detection of latent tuberculosis infection in Bacille Calmette-Guerin vaccinated children. Diagn Microbiol Infect Dis. 2008;62:389–94. doi: 10.1016/j.diagmicrobio.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 82.Hill PC, Brookes RH, Fox A, et al. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in the Gambia. Clin Infect Dis. 2004;38:966–73. doi: 10.1086/382362. [DOI] [PubMed] [Google Scholar]
- 83.Nakaoka H, Lawson L, Squire SB, et al. Risk for tuberculosis among children. Emerg Infect Dis. 2006;12:1383–8. doi: 10.3201/eid1209.051606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okada K, Mao TE, Mori T, et al. Performance of an interferon-gamma release assay for diagnosing latent tuberculosis infection in children. Epidemiol Infect. 2008;136:1179–87. doi: 10.1017/S0950268807009831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mutsvangwa J, Millington KA, Chaka K, et al. Identifying recent Mycobacterium tuberculosis transmission in the setting of high HIV and TB burden. Thorax. 2010;65:315–20. doi: 10.1136/thx.2009.124891. [DOI] [PubMed] [Google Scholar]
- 86.Connell TG, Curtis N, Ranganathan SC, Buttery JP. Performance of a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax. 2006;61:616–20. doi: 10.1136/thx.2005.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–84. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest. 2010;137:952–68. doi: 10.1378/chest.09-2350. [DOI] [PubMed] [Google Scholar]
- 89.Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008;177:1164–70. doi: 10.1164/rccm.200711-1613OC. [DOI] [PubMed] [Google Scholar]
- 90.Bakir M, Millington KA, Soysal A, et al. Prognostic value of a T-cell-based, interferon-gamma biomarker in children with tuberculosis contact. Ann Intern Med. 2008;149:777–87. doi: 10.7326/0003-4819-149-11-200812020-00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hill PC, Jackson-Sillah DJ, Fox A, et al. Incidence of tuberculosis and the predictive value of ELISPOT and Mantoux tests in Gambian case contacts. PLoS One. 2008;3:e1379. doi: 10.1371/journal.pone.0001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doherty TM, Demissie A, Olobo J, et al. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J Clin Microbiol. 2002;40:704–6. doi: 10.1128/JCM.40.2.704-706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aichelburg MC, Rieger A, Breitenecker F, et al. Detection and prediction of active tuberculosis disease by a whole-blood interferon-gamma release assay in HIV-1-Infected individuals. Clin Infect Dis. 2009;48:954–62. doi: 10.1086/597351. [DOI] [PubMed] [Google Scholar]
- 94.Kik SV, Franken WPJ, Mensen M, et al. Predictive value for progression to tuberculosis by IGRA and TST in immigrant contacts. Eur Respir J. 2010;35:1346–53. doi: 10.1183/09031936.00098509. [DOI] [PubMed] [Google Scholar]
- 95.Leung CC, Yam WC, Yew WW, et al. T-Spot.TB outperforms tuberculin skin test in predicting tuberculosis disease. Am J Respir Crit Care Med. 2010;182:834–40. doi: 10.1164/rccm.200912-1875OC. [DOI] [PubMed] [Google Scholar]
- 96.Yoshiyama T, Harada N, Higuchi K, Sekiya Y, Uchimura K. Use of the QuantiFERON-TB Gold test for screening tuberculosis contacts and predicting active disease. Int J Tuberculosis Lung Dis. 2010;14:819–27. [PubMed] [Google Scholar]
- 97.Lienhardt C, Fielding K, Hane AA, et al. Evaluation of the prognostic value of IFN-γ release assay and tuberculin skin test in household contacts of infectious tuberculosis cases in Senegal. PLoS One. 2010;5:e10508. doi: 10.1371/journal.pone.0010508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.del Corral H, París SC, Marín ND, et al. IFN-gamma response to Mycobacterium tuberculosis, risk of infection and disease in household contacts of tuberculosis patients in Colombia. PLoS One. 2009;4:e8257. doi: 10.1371/journal.pone.0008257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and positive predictive value of a whole-blood IGRA for developing active TB—an update. Am J Respir Crit Care Med. 2011;183:88–95. doi: 10.1164/rccm.201006-0974OC. [DOI] [PubMed] [Google Scholar]
- 100.Lalvani A, Millington KA. T-cell interferon-gamma release assays: can we do better? Eur Respir J. 2008;32:1428–30. doi: 10.1183/09031936.00148308. [DOI] [PubMed] [Google Scholar]
- 101.Mahomed H, Hughes EJ, Hawkridge T, et al. Comparison of Mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int J Tuberc Lung Dis. 2006;10:310–6. [PubMed] [Google Scholar]
- 102.Dosanjh DP, Hinks TS, Innes JA, et al. Improved diagnostic evaluation of suspected tuberculosis. Ann Intern Med. 2008;148:325–36. doi: 10.7326/0003-4819-148-5-200803040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hougardy JM, Schepers K, Place S, et al. Heparin-binding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis. PLoS One. 2007;2:e926. doi: 10.1371/journal.pone.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vincenti D, Carrara S, De Mori P, et al. Identification of early secretory antigen target-6 epitopes for the immunodiagnosis of active tuberculosis. Mol Med. 2003;9:105–11. [PMC free article] [PubMed] [Google Scholar]
- 105.Leyten EM, Lin MY, Franken KL, et al. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect. 2006;8:2052–60. doi: 10.1016/j.micinf.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 106.Demissie A, Leyten EM, Abebe M, et al. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin Vaccine Immunol. 2006;13:179–86. doi: 10.1128/CVI.13.2.179-186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geluk A, Lin MY, van Meijgaarden KE, et al. T-cell recognition of the HspX protein of Mycobacterium tuberculosis correlates with latent M. tuberculosis infection but not with M. bovis BCG vaccination. Infect Immun. 2007;75:2914–21. doi: 10.1128/IAI.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goletti D, Butera O, Vanini V, et al. Response to Rv2628 latency antigen associates with cured tuberculosis and remote infection. Eur Respir J. 2010;36:135–42. doi: 10.1183/09031936.00140009. [DOI] [PubMed] [Google Scholar]
- 109.Goletti D, Raja A, Syed Ahamed Kabeer B, et al. Is IP-10 an accurate marker for detecting M. tuberculosis-specific response in HIV-infected persons? PLoS one. 2010;5:e12577. doi: 10.1371/journal.pone.0012577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Locht C, Rouanet C, Hougardy JM, Mascart F. How a different look at latency can help to develop novel diagnostics and vaccines against tuberculosis. Expert Opin Biol Ther. 2007;7:1665–77. doi: 10.1517/14712598.7.11.1665. [DOI] [PubMed] [Google Scholar]
- 111.Ruhwald M, Bodmer T, Maier C, et al. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur Respir J. 2008;32:1607–15. doi: 10.1183/09031936.00055508. [DOI] [PubMed] [Google Scholar]
- 112.Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–23. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 113.Millington KA, Innes JA, Hackforth S, et al. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007;178:5217–26. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lalvani A, Millington KA. T cells and tuberculosis: beyond interferon-gamma. J Infect Dis. 2008;197:941–3. doi: 10.1086/529049. [DOI] [PubMed] [Google Scholar]
- 115.Ruhwald M, Petersen J, Kofoed K, et al. Improving T-cell assays for the diagnosis of latent TB infection: potential of a diagnostic test based on IP-10. PLoS One. 2008;3:e2858. doi: 10.1371/journal.pone.0002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. 2009;39:723–9. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 117.Caccamo N, Guggino G, Joosten SA, et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40:2211–20. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 118.Casey R, Blumenkrantz D, Millington KA, et al. Enumeration of functional T-cell subsets by fluorescence-immunospot defines signatures of pathogen burden in tuberculosis. PLoS One. 2010;5:e15619. doi: 10.1371/journal.pone.0015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Day CL, Mkhwanazi N, Reddy S, et al. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J Infect Dis. 2008;197:990–9. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilkinson KA, Wilkinson RJ. Polyfunctional T cells in human tuberculosis. Eur J Immunol. 2010;40:2139–42. doi: 10.1002/eji.201040731. [DOI] [PubMed] [Google Scholar]
- 121.Wu B, Huang C, Kato-Maeda M, et al. Messenger RNA expression of IL-8, FOXP3, and IL-12beta differentiates latent tuberculosis infection from disease. J Immunol. 2007;178:3688–94. doi: 10.4049/jimmunol.178.6.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maertzdorf J, Repsilber D, Parida SK, et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 2011;12:15–22. doi: 10.1038/gene.2010.51. [DOI] [PubMed] [Google Scholar]
- 124.Mistry R, Cliff JM, Clayton CL, et al. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. J Infect Dis. 2007;195:357–65. doi: 10.1086/510397. [DOI] [PubMed] [Google Scholar]
- 125.Barry CE, 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–55. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17:183–8. doi: 10.1016/j.tim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Bakir M, Dosanjh DP, Deeks JJ, et al. Use of T cell-based diagnosis of tuberculosis infection to optimize interpretation of tuberculin skin testing for child tuberculosis contacts. Clin Infect Dis. 2009;48:302–12. doi: 10.1086/595847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dodd PJ, Millington KA, Ghani AC, et al. Interpreting tuberculin skin tests in a population with a high prevalence of HIV, tuberculosis, and nonspecific tuberculin sensitivity. Am J Epidemiol. 2010;171:1037–45. doi: 10.1093/aje/kwq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reid MJ, Shah NS. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis. 2009;9:173–84. doi: 10.1016/S1473-3099(09)70043-X. [DOI] [PubMed] [Google Scholar]
- 130.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–9. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 131.Getahun H. Proceedings of the 40th Union World Conference on Lung Health (Cancun, Mexico) Paris, France: International Union Against Tuberculosis and Lung Disease; 2009. Meta-analysis to inform the development of a standardised approach for TB screening in HIV-infected patients. [Google Scholar]
- 132.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–8. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Golub JE, Pronyk P, Mohapi L, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23:631–6. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murphy RA, Sunpath H, Kuritzkes DR, Venter F, Gandhi RT. Antiretroviral therapy-associated toxicities in the resource-poor world: the challenge of a limited formulary. J Infect Dis. 2007;196(suppl 3):S449–56. doi: 10.1086/521112. [DOI] [PubMed] [Google Scholar]
- 135.von Reyn CF, Mtei L, Arbeit RD, et al. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010;24:675–85. doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Churchyard GJ, Kaplan G, Fallows D, Wallis RS, Onyebujoh P, Rook GA. Advances in immunotherapy for tuberculosis treatment. Clin Chest Med. 2009;30:769–82, ix. doi: 10.1016/j.ccm.2009.08.009. [DOI] [PubMed] [Google Scholar]