Plants and microbial pathogens are engaged in an endless arms race. For the interactions between plants and biotrophic pathogens (feeding on living plant tissue), the central concept is the activation of the plant defense response upon pathogen perception and the subversion of immunity by virulence factors produced by successful pathogens. The first layer of the plant innate immune system is based on the recognition of pathogen- or microbe-associated molecular patterns, which leads to a signal transduction cascade and eventually defense gene expression (Zipfel, 2009). Pathogen-associated molecular pattern-triggered immunity (PTI), broadly referred as the general defense response, restricts the growth of the vast majority of potential pathogens encountered by plants. However, successful pathogens produce virulence factors to effectively suppress PTI. For example, gram-negative bacteria inject type III secreted effectors (T3SEs) to inhibit PTI in plant cells (Block et al., 2008; Galán, 2009). As a counteraction, nucleotide-binding leucine-rich repeat (NB-LRR) proteins have evolved to perceive specific T3SEs and elicit a robust and localized programmed cell death, called effector-triggered immunity (ETI; Chisholm et al., 2006; Jones and Dangl, 2006; Boller and He, 2009). Both PTI and ETI also induce systemic acquired resistance (SAR), which is a long-lasting immunity protecting the plants from secondary infection by a broad range of microbial pathogens (Durrant and Dong, 2004).
PTI, ETI, and SAR all involve extensive transcription reprogramming. While there is substantial progress in our understanding of the early events in the defense response, including pathogen recognition and the initiation of signal transduction, significant gaps remain between these early events and the downstream transcription reprogramming. Furthermore, although many genes that are differentially expressed during PTI and ETI are the same, the transcriptional changes are stronger and more sustained during ETI (Tao et al., 2003; Wang et al., 2011b). It has become clear that the tight control of the kinetics and expression levels of defense genes is critical for plant immunity. However, the underlying molecular mechanisms are largely unknown.
Chromatin configuration allows or prevents protein access to specific DNA regions and regulates essential cellular processes such as DNA replication, DNA repair, and transcription (Clapier and Cairns, 2009). Chromatin dynamics is orchestrated by ATP-dependent chromatin-remodeling complexes and histone-modifying enzymes. In conjunction with other coregulators, these chromatin remodelers modify histone-DNA interaction and regulate transcription at specific genomic loci. In this review, we summarize the experimental evidence supporting a role of chromatin configuration in regulating the plant immune response, especially the kinetics of defense gene expression. Modulation of chromatin configuration as a strategy employed by bacterial virulence proteins to subvert plant immunity is also discussed.
HISTONE-MODIFYING ENZYMES AND HISTONE MODIFICATIONS
Eukaryotic chromatin structure consists of multiple superimposed layers. DNA wound around the core histone octamers forms the fundamental packaging units called nucleosomes. The N-terminal tails of the core histones are subjected to posttranslational modifications, including acetylation, methylation, phosphorylation, ubiquitination, glycosylation, sumoylation, and ADP ribosylation (Fuchs et al., 2006; Berger, 2007; Bannister and Kouzarides, 2011; Hottiger, 2011). Together, histone-modifying enzymes generate a “histone code” in specific gene regions, leading to changes in chromatin configuration and gene expression.
Upon perception of pathogens, the mitogen-activated protein kinase (MAPK) signaling cascade is activated, leading to the phosphorylation of downstream nuclear targets including defense-related WRKY transcription factors and ethylene-responsive regulators (Andreasson and Ellis, 2010). Unlike in animals (Clayton and Mahadevan, 2003), direct histone phosphorylation by MAPK has not been reported in plants. Nonetheless, various histone-modifying enzymes and chromatin remodelers are changed at transcriptional and/or posttranslational levels in response to pathogen infection (Table I). These changes lead to the establishment of specific histone codes and the subsequent transcription reprogramming of defense-related genes.
Table I. Chromatin-related proteins known for or potentially involved in resistance against bacterial infection.
| Type | Gene | Phenotype or Function | Expression Change upon PtoDC3000 Infectiona | Reference |
| Core ATPase belonging to the SNF2 family | SYD/SPLAYED/CHR3 (AT2G28290) | SNF2 subfamily member; regulating MYC2, VSP, PDF1.2, and PR1 gene expression; directly shown to be recruited to promoters of MYC2 and VSP | No change | Walley et al. (2008) |
| BRM/BRAHMA/CHR2 (AT2G46020) | SNF2 subfamily member; regulating SA-responsive PR1, PR2, and PR5 gene expression | No change | Bezhani et al. (2007) | |
| PIE1/CHR13 (AT3G12810) | SWR1 subfamily member; interacting with ACTIN-RELATED PROTEIN6 and SERRATED LEAVES, forming a large complex; negatively regulating defense against PtoDC3000; involved in H2A.Z histone replacement; regulating the expression of EDS5, PR1, NIMIN1, WRKY18, and WRKY38 | No change | March-Díaz et al. (2008) | |
| DDM1/CHR1 (AT5G66750) | LSH subfamily member; maintaining DNA methylation and possibly regulating immunity through gene silencing | Down-regulated | Li et al. (2010b) | |
| Homologous recombination related | SSN1/RAD51D (AT1G07745) | Forming a protein complex with the Leu-rich nuclear protein SNI1; positively regulating SAR and defense against P. syringae pv maculicola ES4326 | Up-regulated | Durrant et al. (2007) |
| SSN2 (AT4G33925) | No change | Song et al. (2011) | ||
| SSN3/BRCA2A (AT4G00020) | No change | Wang et al. (2010b) | ||
| Histone-modifying enzymes | HDA19/HD1 (AT4G38130) | Histone deacetylase; regulating the transcriptional activation activity of WRKY38 and WRKY62; also regulating the expression of DND1 and DND2, which negatively regulate defense against PtoDC3000 | Up-regulated | Clough et al. (2000); Jurkowski et al. (2004); Kim et al. (2008b); Zhu et al. (2010) |
| SRT2 (At5G09230) | Histone deacetylase; negatively regulating defense by suppressing SA biosynthetic genes PAD4, EDS5, and SID2 | Down-regulated | Wang et al. (2010a) | |
| SDG8 (AT1G77300) | Histone methyltransferase; positively regulating defense against P. syringae pv maculicola ES4326, PtoDC3000, and strains expressing the type III effectors AvrB or AvrRpm1 | No change | Palma et al. (2010) | |
| ATX1/SDG27 (AT2G31650) | Histone methyltransferase; positively regulating defense against PtoDC3000 by enhancing the expression of WRKY70 | No change | Alvarez-Venegas et al. (2007) | |
| Others | SRFR1 (AT4G37460) | Tetratricopeptide repeat domain-containing protein; suppressing AvrRps4-mediated ETI; involved in NB-LRR protein accumulation and negatively regulating defense gene expression | No change | Kim et al. (2009, 2010); Kwon et al. (2009) |
| ELP2 (At1G49540) | Elongator subunit 2; positively regulating the kinetics of PTI and ETI | No change | DeFraia et al. (2010) |
Expression data are based on published results or, when not available, extracted from the Bio-Array Resource for Plant Biology (http://esc4037-shemp.csb.utoronto.ca/welcome.htm) based on change in gene expression at 24 h post inoculation of PtoDC3000 (108 colony-forming units mL−1).
Histone Acetylation
Acetylation of histone H3 and H4 usually associates with active genes (Berger, 2007). The level of acetylation is balanced by histone acetyltransferases and histone deacetylases (HDACs). Two HDACs have been reported to regulate plant immunity. HISTONE DEACETYLASE19 (HDA19) is induced in Arabidopsis (Arabidopsis thaliana) upon infection of the hemibiotrophic bacterial pathogen Pseudomonas syringae pv tomato strain DC3000 (PtoDC3000). As a positive regulator of defense, HDA19 interacts with and represses the activity of two transcription factors, WRKY38 and WRKY62, which negatively regulate the expression of Pathogenesis-Related (PR) genes (Kim et al., 2008b). Enhanced resistance to PtoDC3000 and repression of WRKY activities are abolished in an HDA19 catalytic mutant, suggesting that the deacetylase activity is required for HDA19-mediated plant defense (Kim et al., 2008b). Another HDAC in Arabidopsis, SIRTUIN2 (SRT2), suppresses the expression of salicylic acid (SA) biosynthetic genes (Wang et al., 2010a). SA is a crucial signaling molecule for resistance against P. syringae and other biotrophic pathogens (Vlot et al., 2009). SRT2 is down-regulated upon PtoDC3000 infection, thereby promoting SA production and the expression of defense-related genes (Wang et al., 2010a).
Histone Methylation
Comparing to acetylation, the effect of methylation is more diverse. Methylation of H3 can either enhance or repress transcription depending on the specific Lys or Arg residues that are modified and the number of methyl groups that are attached to these residues (Berger, 2007). Two histone methyltransferases are reported to regulate plant immunity. The histone methyltransferase ARABIDOPSIS HOMOLOG OF TRITHORAX (ATX1) is a positive regulator of basal resistance against a type III secretion system-deficient mutant strain of PtoDC3000. ATX1 positively regulates the expression of WRKY70, which is a key transcription factor regulating genes involved in SA and jasmonate/ethylene defense signaling pathways (Alvarez-Venegas et al., 2006, 2007). Another histone methyltransferase, SET DOMAIN GROUP8 (SDG8), is a positive regulator of PtoDC3000-triggered defense. SDG8 regulates the expression of NB-LRR genes in a gene-specific manner (Palma et al., 2010). For example, LAZARUS5 (LAZ5) and RESISTANCE TO P. SYRINGAE PV MACULICOLA1 (RPM1) are positively regulated by SDG8, which does not affect the transcription of RESISTANT TO P. SYRINGAE2 (RPS2) and RPS4. Consistently, an activating mark trimethylation of H3K36 is enriched at the LAZ5 locus during pathogen infection (Palma et al., 2010).
Although altered resistance has been reported for histone modification mutant plants, targeting specificity and the corresponding chromatin signatures associated with the targeted defense-related loci remain undefined for these histone-modifying enzymes. Despite a simple “on-or-off” histone code, a complex language of histone modifications is likely generated at defense genes upon pathogen infection, leading to a dynamic transcriptional regulation of immunity.
ATP-DEPENDENT CHROMATIN-REMODELING COMPLEXES
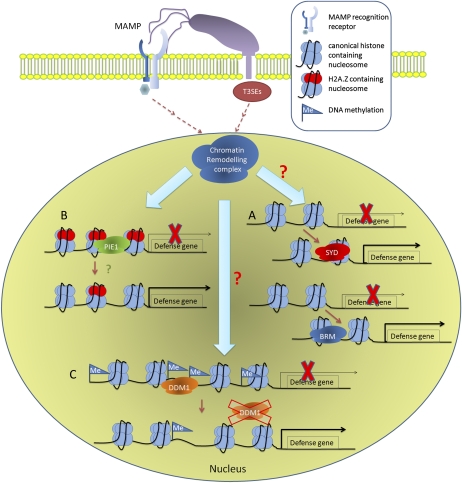
ATP-dependent chromatin-remodeling factors are multisubunit complexes with a catalytic subunit containing a conserved SUCROSE-NONFERMENTING2 (SNF2) ATPase domain. Other domains adjacent to the ATPase domain make each chromatin-remodeling ATPase unique (Mohrmann and Verrijzer, 2005). The specificity of remodeling complexes is also determined by the accompanying subunits of the ATPase. In Arabidopsis, 42 SNF2 family ATPases have been annotated and categorized into 24 distinct subfamilies (Flaus et al., 2006). Among them, SPLAYED (SYD) and BRAHMA (BRM) of the SNF2 subfamily, PHOTOPERIOD-INDEPENDENT EARLY FLOWERING1 (PIE1) of the SWI/SNF-RELATED1 (SWR1) subfamily, and DECREASED DNA METHYLATION1 (DDM1) of the LYMPHOID-SPECIFIC HELICASE (LSH) subfamily have been reported to regulate plant immunity (Fig. 1).
Figure 1.
Possible mechanisms of ATP-dependent chromatin remodelers in regulating plant immunity upon bacterial infection. Chromatin remodelers are activated or repressed in response to pathogen perception by unknown pathways. A, SYD, and possibly BRM, are recruited to target loci and induces the expression of defense-related genes. B, PIE1 mediates H2A.Z deposition at target loci in the absence of pathogens to suppress defense gene expression. Upon pathogen infection, these genes might be activated due to a decrease of H2A.Z occupancy level. C, DNA methylation-associated gene silencing on defense genes might be alleviated due to the down-regulation of DDM1 at the transcriptional level upon pathogen infection. MAMP, Microbe-associated molecular pattern.
SYD and BRM have unique and overlapping targets, including some defense-related genes (Bezhani et al., 2007; Walley et al., 2008). The SA-responsive gene PR1 is up-regulated in the syd-2 mutant upon PtoDC3000 infection, suggesting that SYD is a negative regulator of the SA pathway. However, syd-2 mutant plants did not exhibit altered resistance against PtoDC3000 (Walley et al., 2008). This could be due to the functions of T3SEs produced by PtoDC3000, which may mask potential SYD-regulated resistance. Various SA-responsive genes, including PR1, are also up-regulated in the brm-101 mutant plants. However, BRM has not been examined for its phenotype in plant resistance. It would be interesting to use the type III secretion system-deficient mutant of P. syringae and strains triggering ETI to study the role of SYD and BRM in basal and effector-triggered immunity. Moreover, since SYD and BRM each has unique targets and protein partners, characterization of the double mutant will also be necessary to understand the role of the SNF2 subfamily ATPases in plant response to pathogen infection.
So far, the underlying mechanisms by which SYD and BRM regulate plant immunity remain largely unknown. SYD is recruited to the promoters of its target defense-related genes (Walley et al., 2008), but insufficient evidence is available to show that BRM is directly associated with defense-related gene regions. BRM contains a bromodomain and three DNA-binding regions with different binding specificities. A BRM mutant without the bromodomain and two of the DNA-binding regions showed an intermediate phenotype between the wild type and the null mutant, suggesting that these domains are required for the full function of BRM (Farrona et al., 2007). The bromodomain has been found in many chromatin-remodeling proteins and is believed to act as a functional unit for protein-protein interactions (Zeng and Zhou, 2002). Intriguingly, the bromodomains of histone acetyltransferase-associated coactivators mediate direct interactions of these coactivators with histone, especially via the acetylated Lys residues (Dhalluin et al., 1999). BRM also interacts with H3 and H4 in vitro; however, its preferential binding to acetylated histones was not found (Farrona et al., 2007). Further study on the binding specificity of the bromodomain may help in constructing a mechanistic model of the functionality of BRM in plant immunity.
PIE1 is a negative regulator of plant resistance against PtoDC3000. Various defense-related genes that are repressed in the absence of the pathogen are constitutively expressed in the pie1-5 mutant plants (March-Díaz et al., 2008). PIE1 interacts with the histone variant H2A.Z and was shown to be required for the deposition of H2A.Z at the PIE1-regulated loci (Deal et al., 2007; March-Díaz et al., 2008). Although deposition of H2A.Z at defense-related loci by PIE1 has not been reported, both pie1-5 and the H2A.Z mutant plants show enhanced resistance to PtoDC3000 (March-Díaz et al., 2008). H2A.Z is usually found in nucleosomes flanking the transcription start sites and has been implicated in regulating the transcription of heat-responsive genes (Kumar and Wigge, 2010). It is likely that PIE1 deposits H2A.Z at the targeted defense-related loci and suppresses transcription in the absence of pathogen infection.
DDM1 contains a conserved SNF2 ATPase domain. Without a known methyltransferase domain or demonstrated methyltransferase activity (Jeddeloh et al., 1999), DDM1 is required to maintain DNA methylation, probably by modulating the access of DNA methyltransferases or DNA demethylases to the genome via its chromatin-remodeling activity (Brzeski and Jerzmanowski, 2003; Zemach et al., 2005). ddm1 mutant plants exhibit global DNA hypomethylation, which leads to decreased gene silencing (Vongs et al., 1993). Although the role of DDM1 in the plant response to bacterial pathogens remains to be elucidated, another protein, ARGONAUTE4, which regulates small RNA-mediated DNA methylation and gene silencing, is a positive regulator of plant defense against PtoDC3000 (Agorio and Vera, 2007). A recent study (Li et al., 2010b) showed slightly increased (although not statistically significant) susceptibility of the ddm1-8 mutant plants upon infection of the obligate biotrophic oomycete pathogen Hyaloperonospora arabidopsidis Noco2. This is consistent with the idea that DDM1 may act as a positive regulator of plant resistance against biotrophic bacterial pathogens.
OTHER CHROMATIN-ASSOCIATED PROTEINS
Other chromatin-associated proteins have also been identified to regulate plant immunity. In genetic screens for the SUPPRESSOR OF SNI1 (SSN), three genes, RAD51D/SSN1, BRCA2A/SSN3, and SSN2, were identified (Durrant et al., 2007; Wang et al., 2010b; Song et al., 2011). Arabidopsis SSN knocked out mutants exhibit reduced PR gene expression and dampened resistance against the infection of P. syringae pv maculicola ES4326. SSN2 contains a SWI2/SNF2 and MuDR domain and is mainly located to the nucleus (Song et al., 2011). RAD51D directly interacts with SSN2 and forms a complex with BRCA2A, suggesting that these three SSNs work together in one protein complex. Upon induction by SA, this protein complex can be recruited by TGA transcription factor(s) to the promoters of PR genes and regulate their transcription (Wang et al., 2010b; Song et al., 2011). BRCA2 promotes RAD51-ssDNA assembly in human (Jensen et al., 2010). During homologous DNA recombination, the RAD51-ssDNA nucleoprotein filament associates with and stimulates the chromatin-remodeling activity of RAD54, which is the core catalytic ATPase subunit of an ATP-dependent chromatin remodeler in yeast (Alexeev et al., 2003; Jensen et al., 2010). Although the identification of an RAD54 homolog was not reported in the genetic screen for SSNs, RAD51D, and possibly BRCA2A and SSN2, may associate with a chromatin-remodeling complex for the transcriptional regulation of SNI1 and other PR genes.
INTERPLAY AMONG HISTONE-MODIFYING ENZYMES, ATP-DEPENDENT CHROMATIN REMODELERS, AND OTHER REGULATORS
It is generally accepted that while histone-modifying enzymes modify histone posttranslationally, ATP-dependent chromatin remodelers use the energy generated by ATP hydrolysis to move or eject nucleosomes along DNA (Hargreaves and Crabtree, 2011; Kasten et al., 2011). They are unlikely to work independently. Whether histone modifications, such as methylation and acetylation, and chromatin remodeling occur sequentially is still unresolved (Neely and Workman, 2002). Recently, the p65 subunit of the NF-κB protein complex was found to be reversibly methylated and demethylated by the histone methylase NSD1 and the demethylase FBXL11 (Lu et al., 2010), suggesting that histones are not the only substrates of histone-modifying enzymes. It would be exciting to investigate the possible roles of histone-modifying enzymes in regulating other transcription regulators, including transcription factors and chromatin-remodeling proteins.
POTENTIAL ROLE OF CHROMATIN REMODELERS IN REGULATING THE KINETICS OF DEFENSE GENE EXPRESSION
Without specialized immune cells, plants must tightly control the immune response so that the allocation of available resources is finely balanced with other biological processes, such as reproduction and growth (Kliebenstein and Rowe, 2008). The ability to switch fast between alternate states of gene transcription, therefore, is fundamental to an economic and efficient resistance. How plants regulate the timing of these switches remains a major challenge. Recent evidence suggests that chromatin-remodeling complexes might play an important role in determining the kinetics of defense-related gene expression in plants.
SRFR1 (for SUPPRESSOR OF RPS4-RLD1) was identified as a negative regulator of defense-related gene expression in Arabidopsis (Kim et al., 2009, 2010; Kwon et al., 2009). SRFR1 is located in both cytoplasm and nucleus and possibly possesses dual functions. In the cytoplasm, SRFR1 associates with the NB-LRR protein complexes and regulates NB-LRR protein accumulation (Kim et al., 2010; Li et al., 2010a). In the nucleus, SRFR1 suppresses defense gene expression and possibly sets up a threshold to switch on the immune response. Interestingly, SRFR1 shares sequence similarity with the yeast corepressor protein Ssn6, which is recruited by transcription factors to specific target genes. In yeast, Ssn6 primes these genes for rapid derepression by directing the preacetylation of nucleosomes (Desimone and Laney, 2010). It remains to be determined whether SRFR1 associates with chromatin-remodeling complexes and/or other transcription factors at specific gene regions.
Arabidopsis ELONGATOR SUBUNIT2 (ELP2) was recently shown to regulate the kinetics of defense gene expression (DeFraia et al., 2010). Elongator complex associates with RNA polymerase II and regulates transcription through various processes, including histone modification (Otero et al., 1999; Winkler et al., 2002). In Atelp2 mutant plants, induction of defense-related genes during PTI and ETI is delayed and/or decreased, suggesting that AtELP2 is a positive regulator of immunity by accelerating the induction of defense-related genes (DeFraia et al., 2010). It will be interesting to test whether AtELP2 triggers histone modification or other chromatin configuration changes at its target genes.
BACTERIAL PATHOGENS MANIPULATE CHROMATIN-REMODELING PROCESSES TO FACILITATE INFECTION
Microbial pathogens employ a variety of strategies to suppress defense and successfully infect plants. The conserved nature of chromatin-remodeling complexes makes them attractive targets through which pathogens subvert innate immunity. This is particularly important for the biotrophic and hemibiotrophic pathogens, because they maintain a rather stable symbiotic relationship with the hosts at least at the early stages of the infection.
The first example of virulence proteins directly modulating plant chromatin remodeling is the HC-toxin produced by the maize (Zea mays) fungal pathogen Cochliobolus carbonum. HC-toxin inhibits histone deacetylase activity, leading to hyperacetylation of histones during infection (Brosch et al., 1995). An HC-toxin reductase can detoxify HC-toxin and readily confer resistance of maize to the pathogen, suggesting that HC-toxin is a major virulence determinant in this interaction (Johal and Briggs, 1992). Thus far, how the inhibition of histone deacetylase activity promotes pathogen infection is not well understood. Since C. carbonum is a necrotrophic pathogen, HC-toxin could simply promote host cell death by targeting a fundamental cellular process.
Agrobacterium tumefaciens Virulence Proteins VirE2 and 6b
The Agrobacterium-Arabidopsis interaction represents an excellent example of the direct interaction between bacterial virulence factors and host chromatin-associating proteins. Agrobacterium causes plant diseases by transferring a segment of its own DNA (T-DNA) into the nucleus of plant cells. The T-DNA, carrying oncogenes coding for auxin and cytokinin biosynthetic enzymes, is then integrated into the plant genome. These phytohormones promote plant cell division and enlargement; thereby inducing uncontrolled growth of plant tissues to form galls. T-DNA also encodes opine biosynthetic enzymes, which produce opine that can be used as a nutrient source by Agrobacterium (Escobar and Dandekar, 2003). It is evident that multiple chromatin-related proteins, including core histones, histone-modifying enzymes, histone chaperones, and chromatin assembly proteins, function in the process of T-DNA integration (Lacroix and Citovsky, 2009; Gelvin, 2010).
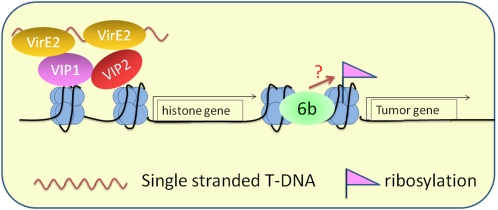
The Agrobacterium virulence protein VirE2 has been shown to modulate the chromatin functions and facilitate T-DNA integration (Fig. 2). VirE2-INTERACTING PROTEIN1 (VIP1) directly interacts with various core histones, such as H2A, in Arabidopsis (Li et al., 2005). VIP1 may act as a bridge to reinforce the association of VirE2 with plant nucleosome. In this way, VirE2 may facilitate T-DNA integration by directing the T-DNA complex to host chromatin (Lacroix et al., 2008). Furthermore, another VirE2-interacting protein, VIP2, may regulate histone gene transcription (Anand et al., 2007). Consistent with this finding, several histone genes are up-regulated upon Agrobacterium infection (Veena et al., 2003). It is intriguing to speculate that VirE2 or other Agrobacterium effectors may modulate histone gene expression to facilitate infection. For example, VirE3 has been shown to be a transcription activator (García-Rodriguez et al., 2006).
Figure 2.
A model for the Agrobacterium effectors VirE2 and 6b in modulating chromatin configurations and gene expression in the plant nucleus. VirE2 directly interacts with VIP1 and VIP2, which also interact with each other. VIP1 facilitates the association of T-DNA complexes with histones and thereby promotes T-DNA integration. VIP2 may activate histone gene expression. 6b directly interacts with histone H3 and possesses an ADP-ribosyltransferase activity. 6b could potentially modulate histone modification and induce genes involved in abnormal cell growth.
The virulence protein 6b of Agrobacterium contributes to the induction of abnormal plant growth and tumor formation (Tinland et al., 1990). 6b physically associates with several Arabidopsis proteins in the nucleus, including key components of the microRNA pathway and the core histone H3 (Terakura et al., 2007; Wang et al., 2011a). 6b has been proposed to act as a histone chaperone, which works together with other chromatin remodelers to affect nucleosome assembly, histone displacement, and transcription in a gene-specific manner (Terakura et al., 2007). Recent structural analysis suggests that 6b possesses an ADP-ribosyltransferase activity (Wang et al., 2011a). Since 6b directly interacts with H3, it will be interesting to examine whether H3 can be modified by 6b and how this potential 6b-mediated ribosylation of H3 might impact transcription.
T3SEs
T3SEs are well-studied virulence proteins produced by gram-negative bacteria to suppress host immunity (Galán, 2009). After being delivered into the host cytoplasm, some T3SEs can enter the nucleus either by the nuclear localization sequence in the effector sequence or via interaction with host nuclear proteins. Some of these nucleus-localized T3SEs have been shown to modulate transcription in the host. For example, the transcription activator-like (TAL) effectors produced by Xanthomonas species bind to specific promoter sequences and directly activate gene expression to promote bacterial infection and disease symptoms (Boch and Bonas, 2010). So far, TAL effectors have only been found in Xanthomonas species, and direct transcriptional regulation has not been reported for the other nucleus-localized T3SEs produced by plant pathogens. It remains to be determined whether any of these effectors interfere with chromatin-associated functions to indirectly modulate host gene expression.
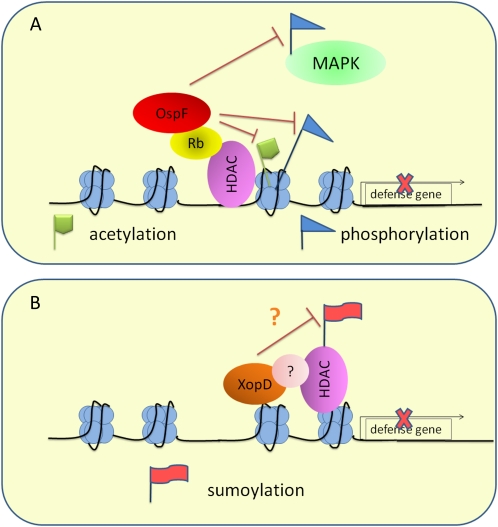
The type III effector OspF of the animal pathogen Shigella flexneri remodels host chromatin by inducing dephosphorylation and deacetylation of H3, which leads to decreased expression of specific immunity-related genes (Arbibe et al., 2007). This function is accomplished through the interaction of OspF with host retinoblastoma protein, which has been linked to histone modification (Zurawski et al., 2009; Fig. 3A). OspF also has the phospho-Thr lyase activity that targets the phosphorylated MAPKs in the nucleus (Li et al., 2007). The plant pathogen P. syringae produces an OspF-like T3SE, HopAI1, which possesses the same phospho-Thr lyase activity. HopAI1 also disrupts defense signal transduction by directly inactivating MAPKs in plants (Zhang et al., 2007). Since the nuclear localization of HopAI1 has not been reported, whether HopAI1 could also target nuclear MAPKs and/or modulate histone modifications in plant cells remains unknown.
Figure 3.
T3SEs suppress defense gene expression by modulating host chromatin configuration. A, OspF from the animal pathogen S. flexneri induces the dephosphorylation of MAPK and the dephosphorylation and deacetylation of histone, leading to reduced transcription of defense-related genes. The histone modification changes induced by OspF is dependent on its interaction with the retinoblastoma protein (Rb), which could interact with histone-modifying enzymes such as HDAC. B, XopD from the plant pathogen X. campestris pv vesicatoria has SUMO protease activity and acts as a transcriptional repressor. Through its EAR domain, XopD may associate with and modify histone-modifying enzymes, such as HDAC, or other chromatin-remodeling proteins that are substrates for sumoylation.
Another T3SE with possible chromatin-remodeling activity is XopD from Xanthomonas campestris pv vesicatoria, which has small ubiquitin-like modifier (SUMO) protease activity. Genome-wide proteomic study in Arabidopsis identifies diverse SUMO substrates, including histone-modifying enzymes, chromatin-remodeling complex components, and defense-related transcription factors (Miller et al., 2010; van den Burg and Takken, 2010). A loss-of-function mutant of a SUMO E3 ligase, SIZ1, shows elevated accumulation of SA and constitutive defense response in Arabidopsis (Lee et al., 2007), implicating a role of sumoylation in defense regulation. XopD is located to the subnuclear foci in plant cells. In addition to the SUMO protease activity, XopD also contains an ERF-associated amphiphilic repressor motif (EAR), which is responsible for the transcriptional repression of defense- and senescence-related genes by XopD (Kim et al., 2008a). The EAR domain of the auxin regulator Aux/IAA directly interacts with the corepressor TOPLESS (TLP), which associates with HDA19 (Szemenyei et al., 2008). Since both TLP and HDA19 are substrates for sumoylation (Miller et al., 2010), it is intriguing to speculate that XopD could recruit and/or modify chromatin-remodeling proteins and regulate their activities through its SUMO protease activity (Fig. 3B). Identification of XopD-interacting proteins will help test this possibility.
CONCLUSION AND PROSPECTS
The activation and suppression of innate immunity are central principles of plant-pathogen interaction. PTI and ETI involve large-scale gene expression changes in a quantitative manner. However, a significant gap of knowledge lies between the recognition of microbe-associated molecular patterns or effectors and the downstream transcription reprogramming. Emerging evidence suggests chromatin remodeling as an integral component linking these two events. Transcription regulation requires combined actions of sequence-specific DNA-binding transcription factors and other coregulatory chromatin-remodeling machineries. Current data suggest that different chromatin-remodeling proteins control different sets of defense-related genes, probably upon the perception of specific elicitors. The specificity of chromatin-remodeling proteins with regard to their role in immunity may allow the integration of signals from different elicitors and generate the kinetic and quantitative regulation of defense gene expression.
The role of chromatin remodeling in plant immunity was not appreciated until recently. The rich resources available for the model system Arabidopsis-P. syringae provide an excellent opportunity to investigate the general function of chromatin-associating proteins in plant immunity. Systemic analyses of known chromatin-remodeling proteins by screening the corresponding Arabidopsis mutants for an altered resistance phenotype will provide a fundamental understanding of the significance and universality of chromatin-remodeling-mediated transcription regulation in innate immunity. However, as a fundamental biological process, evaluating the contribution of chromatin remodeling to a specific function could be challenging, since the knockout mutants will likely exhibit rather pleiotropic deficiencies. Therefore, it is important to determine the target specificities of chromatin remodelers using chromatin immunoprecipitation sequencing, which is much more feasible and efficient with the development of the next-generation sequencing technology. Furthermore, the genome-wide charting of histone modifications, before and after pathogen infection, will also provide important hints on the role of chromatin remodelers in plant resistance.
Bacterial pathogens have evolved a fascinating number of virulence proteins, mainly T3SEs and toxins, to subvert plant immunity. In recent years, a great deal of effort has been invested to elucidate the molecular basis for T3SE-mediated suppression of innate immunity. This knowledge has greatly contributed to our basic understanding of bacterial pathogenicity and plant immunity. However, except for the TAL effectors, the functions of the nucleus-targeting T3SEs are largely unknown. It will be particularly interesting to examine whether any of these T3SEs directly target chromatin-remodeling processes and influence transcription at specific defense-related loci. This information will establish a clearer picture of chromatin configuration as a new battlefield between plants and bacterial pathogens.
Acknowledgments
We are grateful to Dr. Xuemei Chen and two anonymous reviewers for critical reading of and constructive suggestions on the manuscript.
References
- Agorio A, Vera P. (2007) ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 19: 3778–3790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeev A, Mazin A, Kowalczykowski SC. (2003) Rad54 protein possesses chromatin-remodeling activity stimulated by the Rad51-ssDNA nucleoprotein filament. Nat Struct Biol 10: 182–186 [DOI] [PubMed] [Google Scholar]
- Alvarez-Venegas R, Abdallat AA, Guo M, Alfano JR, Avramova Z. (2007) Epigenetic control of a transcription factor at the cross section of two antagonistic pathways. Epigenetics 2: 106–113 [DOI] [PubMed] [Google Scholar]
- Alvarez-Venegas R, Sadder M, Hlavacka A, Baluska F, Xia Y, Lu G, Firsov A, Sarath G, Moriyama H, Dubrovsky JG, et al. (2006) The Arabidopsis homolog of trithorax, ATX1, binds phosphatidylinositol 5-phosphate, and the two regulate a common set of target genes. Proc Natl Acad Sci USA 103: 6049–6054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Krichevsky A, Schornack S, Lahaye T, Tzfira T, Tang Y, Citovsky V, Mysore KS. (2007) Arabidopsis VIRE2 INTERACTING PROTEIN2 is required for Agrobacterium T-DNA integration in plants. Plant Cell 19: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson E, Ellis B. (2010) Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci 15: 106–113 [DOI] [PubMed] [Google Scholar]
- Arbibe L, Kim DW, Batsche E, Pedron T, Mateescu B, Muchardt C, Parsot C, Sansonetti PJ. (2007) An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat Immunol 8: 47–56 [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. (2011) Regulation of chromatin by histone modifications. Cell Res 21: 381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL. (2007) The complex language of chromatin regulation during transcription. Nature 447: 407–412 [DOI] [PubMed] [Google Scholar]
- Bezhani S, Winter C, Hershman S, Wagner JD, Kennedy JF, Kwon CS, Pfluger J, Su Y, Wagner D. (2007) Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell 19: 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block A, Li G, Fu ZQ, Alfano JR. (2008) Phytopathogen type III effector weaponry and their plant targets. Curr Opin Plant Biol 11: 396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch J, Bonas U. (2010) Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol 48: 419–436 [DOI] [PubMed] [Google Scholar]
- Boller T, He SY. (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324: 742–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch G, Ransom R, Lechner T, Walton JD, Loidl P. (1995) Inhibition of maize histone deacetylases by HC toxin, the host-selective toxin of Cochliobolus carbonum. Plant Cell 7: 1941–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzeski J, Jerzmanowski A. (2003) Deficient in DNA methylation 1 (DDM1) defines a novel family of chromatin-remodeling factors. J Biol Chem 278: 823–828 [DOI] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124: 803–814 [DOI] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR. (2009) The biology of chromatin remodeling complexes. Annu Rev Biochem 78: 273–304 [DOI] [PubMed] [Google Scholar]
- Clayton AL, Mahadevan LC. (2003) MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett 546: 51–58 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK, Jr, Bent AF. (2000) The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA 97: 9323–9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal RB, Topp CN, McKinney EC, Meagher RB. (2007) Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 19: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFraia CT, Zhang X, Mou Z. (2010) Elongator subunit 2 is an accelerator of immune responses in Arabidopsis thaliana. Plant J 64: 511–523 [DOI] [PubMed] [Google Scholar]
- Desimone AM, Laney JD. (2010) Corepressor-directed preacetylation of histone H3 in promoter chromatin primes rapid transcriptional switching of cell-type-specific genes in yeast. Mol Cell Biol 30: 3342–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. (1999) Structure and ligand of a histone acetyltransferase bromodomain. Nature 399: 491–496 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X. (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Wang S, Dong X. (2007) Arabidopsis SNI1 and RAD51D regulate both gene transcription and DNA recombination during the defense response. Proc Natl Acad Sci USA 104: 4223–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar MA, Dandekar AM. (2003) Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci 8: 380–386 [DOI] [PubMed] [Google Scholar]
- Farrona S, Hurtado L, Reyes JC. (2007) A nucleosome interaction module is required for normal function of Arabidopsis thaliana BRAHMA. J Mol Biol 373: 240–250 [DOI] [PubMed] [Google Scholar]
- Flaus A, Martin DM, Barton GJ, Owen-Hughes T. (2006) Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res 34: 2887–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J, Demidov D, Houben A, Schubert I. (2006) Chromosomal histone modification patterns: from conservation to diversity. Trends Plant Sci 11: 199–208 [DOI] [PubMed] [Google Scholar]
- Galán JE. (2009) Common themes in the design and function of bacterial effectors. Cell Host Microbe 5: 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodríguez FM, Schrammeijer B, Hooykaas PJ. (2006) The Agrobacterium VirE3 effector protein: a potential plant transcriptional activator. Nucleic Acids Res 34: 6496–6504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin SB. (2010) Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu Rev Phytopathol 48: 45–68 [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Crabtree GR. (2011) ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res 21: 396–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottiger MO. (2011) ADP-ribosylation of histones by ARTD1: an additional module of the histone code? FEBS Lett 585: 1595–1599 [DOI] [PubMed] [Google Scholar]
- Jeddeloh JA, Stokes TL, Richards EJ. (1999) Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat Genet 22: 94–97 [DOI] [PubMed] [Google Scholar]
- Jensen RB, Carreira A, Kowalczykowski SC. (2010) Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 467: 678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johal GS, Briggs SP. (1992) Reductase activity encoded by the HM1 disease resistance gene in maize. Science 258: 985–987 [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Jurkowski GI, Smith RK, Jr, Yu IC, Ham JH, Sharma SB, Klessig DF, Fengler KA, Bent AF. (2004) Arabidopsis DND2, a second cyclic nucleotide-gated ion channel gene for which mutation causes the “defense, no death” phenotype. Mol Plant Microbe Interact 17: 511–520 [DOI] [PubMed] [Google Scholar]
- Kasten MM, Clapier CR, Cairns BR. (2011) SnapShot: Chromatin remodeling: SWI/SNF. Cell 144: 310.e1 [DOI] [PubMed] [Google Scholar]
- Kim JG, Taylor KW, Hotson A, Keegan M, Schmelz EA, Mudgett MB. (2008a) XopD SUMO protease affects host transcription, promotes pathogen growth, and delays symptom development in Xanthomonas-infected tomato leaves. Plant Cell 20: 1915–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Lai Z, Fan B, Chen Z. (2008b) Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell 20: 2357–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Gao F, Bhattacharjee S, Adiasor JA, Nam JC, Gassmann W. (2010) The Arabidopsis resistance-like gene SNC1 is activated by mutations in SRFR1 and contributes to resistance to the bacterial effector AvrRps4. PLoS Pathog 6: e1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kwon SI, Bhattacharjee S, Gassmann W. (2009) Regulation of defense gene expression by Arabidopsis SRFR1. Plant Signal Behav 4: 149–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Rowe HC. (2008) Ecological costs of biotrophic versus necrotrophic pathogen resistance, the hypersensitive response and signal transduction. Plant Sci 174: 551–556 [Google Scholar]
- Kumar SV, Wigge PA. (2010) H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140: 136–147 [DOI] [PubMed] [Google Scholar]
- Kwon SI, Kim SH, Bhattacharjee S, Noh JJ, Gassmann W. (2009) SRFR1, a suppressor of effector-triggered immunity, encodes a conserved tetratricopeptide repeat protein with similarity to transcriptional repressors. Plant J 57: 109–119 [DOI] [PubMed] [Google Scholar]
- Lacroix B, Citovsky V. (2009) Agrobacterium aiming for the host chromatin: host and bacterial proteins involved in interactions between T-DNA and plant nucleosomes. Commun Integr Biol 2: 42–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix B, Loyter A, Citovsky V. (2008) Association of the Agrobacterium T-DNA-protein complex with plant nucleosomes. Proc Natl Acad Sci USA 105: 15429–15434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Nam J, Park HC, Na G, Miura K, Jin JB, Yoo CY, Baek D, Kim DH, Jeong JC, et al. (2007) Salicylic acid-mediated innate immunity in Arabidopsis is regulated by SIZ1 SUMO E3 ligase. Plant J 49: 79–90 [DOI] [PubMed] [Google Scholar]
- Li H, Xu H, Zhou Y, Zhang J, Long C, Li S, Chen S, Zhou JM, Shao F. (2007) The phosphothreonine lyase activity of a bacterial type III effector family. Science 315: 1000–1003 [DOI] [PubMed] [Google Scholar]
- Li J, Krichevsky A, Vaidya M, Tzfira T, Citovsky V. (2005) Uncoupling of the functions of the Arabidopsis VIP1 protein in transient and stable plant genetic transformation by Agrobacterium. Proc Natl Acad Sci USA 102: 5733–5738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li S, Bi D, Cheng YT, Li X, Zhang Y. (2010a) SRFR1 negatively regulates plant NB-LRR resistance protein accumulation to prevent autoimmunity. PLoS Pathog 6: e1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tessaro MJ, Li X, Zhang Y. (2010b) Regulation of the expression of plant resistance gene SNC1 by a protein with a conserved BAT2 domain. Plant Physiol 153: 1425–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Jackson MW, Wang B, Yang M, Chance MR, Miyagi M, Gudkov AV, Stark GR. (2010) Regulation of NF-kappaB by NSD1/FBXL11-dependent reversible lysine methylation of p65. Proc Natl Acad Sci USA 107: 46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March-Díaz R, García-Domínguez M, Lozano-Juste J, León J, Florencio FJ, Reyes JC. (2008) Histone H2A.Z and homologues of components of the SWR1 complex are required to control immunity in Arabidopsis. Plant J 53: 475–487 [DOI] [PubMed] [Google Scholar]
- Miller MJ, Barrett-Wilt GA, Hua Z, Vierstra RD. (2010) Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in Arabidopsis. Proc Natl Acad Sci USA 107: 16512–16517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann L, Verrijzer CP. (2005) Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes. Biochim Biophys Acta 1681: 59–73 [DOI] [PubMed] [Google Scholar]
- Neely KE, Workman JL. (2002) Histone acetylation and chromatin remodeling: which comes first? Mol Genet Metab 76: 1–5 [DOI] [PubMed] [Google Scholar]
- Otero G, Fellows J, Li Y, de Bizemont T, Dirac AM, Gustafsson CM, Erdjument-Bromage H, Tempst P, Svejstrup JQ. (1999) Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol Cell 3: 109–118 [DOI] [PubMed] [Google Scholar]
- Palma K, Thorgrimsen S, Malinovsky FG, Fiil BK, Nielsen HB, Brodersen P, Hofius D, Petersen M, Mundy J. (2010) Autoimmunity in Arabidopsis acd11 is mediated by epigenetic regulation of an immune receptor. PLoS Pathog 6: e1001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Durrant WE, Wang S, Yan S, Tan EH, Dong X. (2011) DNA repair proteins are directly involved in regulation of gene expression during plant immune response. Cell Host Microbe 9: 115–124 [DOI] [PubMed] [Google Scholar]
- Szemenyei H, Hannon M, Long JA. (2008) TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386 [DOI] [PubMed] [Google Scholar]
- Tao Y, Xie Z, Chen W, Glazebrook J, Chang HS, Han B, Zhu T, Zou G, Katagiri F. (2003) Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15: 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakura S, Ueno Y, Tagami H, Kitakura S, Machida C, Wabiko H, Aiba H, Otten L, Tsukagoshi H, Nakamura K, et al. (2007) An oncoprotein from the plant pathogen Agrobacterium has histone chaperone-like activity. Plant Cell 19: 2855–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinland B, Rohfritsch O, Michler P, Otten L. (1990) Agrobacterium tumefaciens T-DNA gene 6b stimulates rol-induced root formation, permits growth at high auxin concentrations and increases root size. Mol Gen Genet 223: 1–10 [DOI] [PubMed] [Google Scholar]
- van den Burg HA, Takken FL. (2010) SUMO-, MAPK-, and resistance protein-signaling converge at transcription complexes that regulate plant innate immunity. Plant Signal Behav 5: 1597–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena JH, Jiang H, Doerge RW, Gelvin SB. (2003) Transfer of T-DNA and Vir proteins to plant cells by Agrobacterium tumefaciens induces expression of host genes involved in mediating transformation and suppresses host defense gene expression. Plant J 35: 219–236 [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Vongs A, Kakutani T, Martienssen RA, Richards EJ. (1993) Arabidopsis thaliana DNA methylation mutants. Science 260: 1926–1928 [DOI] [PubMed] [Google Scholar]
- Walley JW, Rowe HC, Xiao Y, Chehab EW, Kliebenstein DJ, Wagner D, Dehesh K. (2008) The chromatin remodeler SPLAYED regulates specific stress signaling pathways. PLoS Pathog 4: e1000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Gao F, Wu J, Dai J, Wei C, Li Y. (2010a) Arabidopsis putative deacetylase AtSRT2 regulates basal defense by suppressing PAD4, EDS5 and SID2 expression. Plant Cell Physiol 51: 1291–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Soyano T, Machida S, Yang JY, Jung C, Chua NH, Yuan YA. (2011a) Molecular insights into plant cell proliferation disturbance by Agrobacterium protein 6b. Genes Dev 25: 64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Durrant WE, Song J, Spivey NW, Dong X. (2010b) Arabidopsis BRCA2 and RAD51 proteins are specifically involved in defense gene transcription during plant immune responses. Proc Natl Acad Sci USA 107: 22716–22721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Barnaby JY, Tada Y, Li H, Tör M, Caldelari D, Lee DU, Fu XD, Dong X. (2011b) Timing of plant immune responses by a central circadian regulator. Nature 470: 110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler GS, Kristjuhan A, Erdjument-Bromage H, Tempst P, Svejstrup JQ. (2002) Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc Natl Acad Sci USA 99: 3517–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach A, Li Y, Wayburn B, Ben-Meir H, Kiss V, Avivi Y, Kalchenko V, Jacobsen SE, Grafi G. (2005) DDM1 binds Arabidopsis methyl-CpG binding domain proteins and affects their subnuclear localization. Plant Cell 17: 1549–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Zhou MM. (2002) Bromodomain: an acetyl-lysine binding domain. FEBS Lett 513: 124–128 [DOI] [PubMed] [Google Scholar]
- Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, et al. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1: 175–185 [DOI] [PubMed] [Google Scholar]
- Zhu Z, Xu F, Zhang Y, Cheng YT, Wiermer M, Li X, Zhang Y. (2010) Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc Natl Acad Sci USA 107: 13960–13965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C. (2009) Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol 12: 414–420 [DOI] [PubMed] [Google Scholar]
- Zurawski DV, Mumy KL, Faherty CS, McCormick BA, Maurelli AT. (2009) Shigella flexneri type III secretion system effectors OspB and OspF target the nucleus to downregulate the host inflammatory response via interactions with retinoblastoma protein. Mol Microbiol 71: 350–368 [DOI] [PMC free article] [PubMed] [Google Scholar]