Abstract
In plants, light is an important environmental signal that induces photomorphogenesis and interacts with endogenous signals, including hormones. We found that light increased polar auxin transport in dark-grown Arabidopsis (Arabidopsis thaliana) and tomato (Solanum lycopersicum) hypocotyls. In tomato, this increase was induced by low-fluence red or blue light followed by 1 d of darkness. It was reduced in phyA, phyB1, and phyB2 tomato mutants and was reversed by far-red light applied immediately after the red or blue light exposure, suggesting that phytochrome is involved in this response. We further found that the free indole-3-acetic acid (IAA) level in hypocotyl regions below the hook was increased by red light, while the level of conjugated IAA was unchanged. Analysis of IAA synthesized from [13C]indole or [13C]tryptophan (Trp) revealed that both Trp-dependent and Trp-independent IAA biosynthesis were increased by low-fluence red light in the top section (meristem, cotyledons, and hook), and the Trp-independent pathway appears to become the primary route for IAA biosynthesis after red light exposure. IAA biosynthesis in tissues below the top section was not affected by red light, suggesting that the increase of free IAA in this region was due to increased transport of IAA from above. Our study provides a comprehensive view of light effects on the transport and biosynthesis of IAA, showing that red light increases both IAA biosynthesis in the top section and polar auxin transport in hypocotyls, leading to unchanged free IAA levels in the top section and increased free IAA levels in the lower hypocotyl regions.
Indole-3-acetic acid (IAA), the major form of auxin in plants, is involved in a number of developmental processes and allows plants to react to their environment. Consistent with its importance, plants have evolved a complex system to regulate the level of free, active IAA (Normanly et al., 2005). IAA can be actively transported in a polar fashion, maintaining concentration gradients among plant tissues and cells. Biochemical and genetic studies have shown that higher plants synthesize IAA directly from indole by a Trp-independent (TI) pathway, while at least four Trp-dependent (TD) pathways are also potentially operative. IAA can be conjugated to other molecules via covalent bonds, forming an IAA reservoir that can release free IAA via hydrolysis (for review, see Woodward and Bartel, 2005; Seidel et al., 2006). Clearly, multiple IAA regulatory pathways exist in plants, and they function cooperatively to precisely control the level of free IAA, especially when plants perceive environmental changes, such as high temperature (Gray et al., 1998), wounding (Sztein et al., 2002), and cold stress (Shibasaki et al., 2009).
Light also acts as a critical environmental signal that controls plant growth and development. Upon transition from dark growth to light growth, hypocotyl elongation rate decreases, hooks unfold, cotyledons open, and the photosynthetic machinery develops. Such changes are rapid and complex, and a series of photoreceptors are involved in sensing the quantity and quality of light, including phytochromes (red [R] light and far-red [FR] light receptors), cryptochromes (blue [B] and UV-A light receptors), phototropins (B light receptors), and UV-B photoreceptors (Casal et al., 1998; Briggs and Olney, 2001). Once the photoreceptors are activated, the light signals are then transferred to the downstream molecular networks that trigger growth and developmental responses, including networks that involve phytohormones (Kraepiel and Miginiac, 1997).
Interactions between light and auxin have been reported at multiple levels. In dark-grown corn (Zea mays) seedlings, both a single flash and hours of light exposure were found to induce a decrease in free IAA and an increase in conjugated IAA (Bandurski et al., 1977; Jones et al., 1991; Barker-Bridges et al., 1998; Zelená, 2000a), and the effects varied in different tissue types (Zelená, 2000b). After a short period of R light exposure, IAA synthesized from Trp in corn coleoptiles was decreased, and free IAA that diffused out of excised coleoptile tips was greatly reduced (Iino, 1982a; Koshiba et al., 1995; Nishimura et al., 2006), which could result in a reduction of IAA supply to mesocotyl tissues below the coleoptiles (Iino, 1982b). Light effects on auxin levels and metabolism were also found in dicotyledonous plants. Behringer and Davies (1992) reported that after etiolated pea (Pisum sativum) seedlings were exposed to R light for 3 h, the free IAA level stayed constant in upper stem sections but decreased in epidermal peels. When etiolated pea seedlings were exposed to a long period of continuous white light, Symons and Reid (2003) found a transient increase of IAA that started at 24 h, peaked at 48 h, and disappeared at 96 h. In response to decreased R/FR light ratios that mimic a shade condition, IAA biosynthesis and the free IAA level were increased in Arabidopsis (Arabidopsis thaliana) seedlings during the shade avoidance response (Tao et al., 2008). The shade avoidance response also required polar auxin transport (PAT), and the auxin transport facilitator protein PIN-FORMED3 (PIN3) was recently found to play a critical role in this process (Steindler et al., 1999; Keuskamp et al., 2010).
Close interaction between light and auxin transport was inferred from the work of Jensen et al. (1998). The elongation of 7-d light-grown Arabidopsis hypocotyls was greatly inhibited by the PAT inhibitor N-1-naphthylphthalamic acid (NPA), but 7-d dark-grown plants lacked this inhibitory response. Their studies using different light quality and photoreceptor mutants suggested that both phytochrome and cryptochrome were involved in the light-dependent NPA effects on hypocotyl elongation. A later report further supported this finding: when the transport of [3H]IAA was quantified in dark- and light-grown Arabidopsis hypocotyls, auxin transport in dark-grown seedlings was lower than in low-light-grown seedlings, and NPA reduced [3H]IAA transport in light-grown seedlings but not in dark-grown seedlings (Rashotte et al., 2003). Partially consistent with their report, Shinkle et al. (1998) found that 30 or 50 h of continuous dim R light increased the transport of [3H]IAA in etiolated cucumber (Cucumis sativus) hypocotyl segments. However, the transport of [3H]IAA in dark-grown cucumber hypocotyls was greatly inhibited by NPA, suggesting that the lack of NPA inhibition on auxin transport in etiolated Arabidopsis hypocotyls was either species specific or nondetectable by the assay applied. Although these studies established a link between light and auxin transport, they did not address the questions of whether auxin transport affected the level of free IAA or if light changed auxin metabolism in addition to changing auxin transport.
To better understand light effects on PAT in etiolated seedlings, we established a sensitive [3H]IAA transport assay with low background noise and found that PAT in etiolated Arabidopsis and tomato (Solanum lycopersicum) hypocotyls was detectable and inhibited by NPA. Light increased PAT in both Arabidopsis and tomato hypocotyl sections, but only in tomato could the increase be induced by low fluences of R or B light followed by a dark period. We also found that this low-fluence response in tomato involved phytochromes but not cryptochrome. In addition to the increase in transport, IAA biosynthesis and free IAA levels were changed by low-fluence R light in a tissue-dependent manner, and the level of free IAA was a consequence of both the biosynthesis and transport of IAA.
RESULTS
Measurement of PAT in Hypocotyls of Etiolated Seedlings
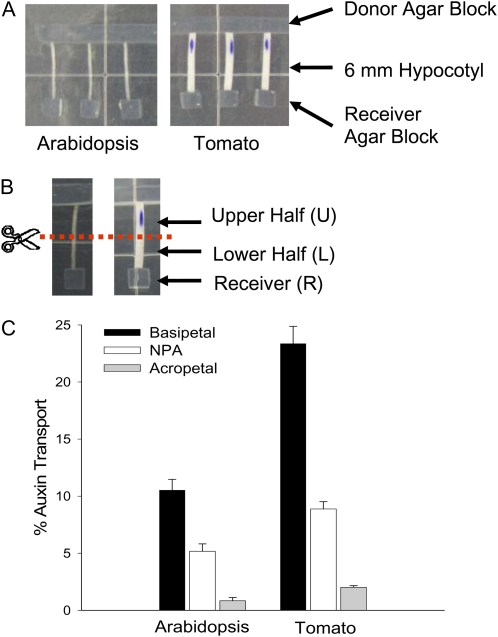
It was reported that the transport of IAA in dark-grown Arabidopsis hypocotyls was very low and not inhibited by NPA (Rashotte et al., 2003), suggesting that a very sensitive method is necessary to measure PAT in etiolated seedlings. Thus, we modified the method described by Rashotte et al. (2003) to increase the signal-to-noise ratio, and we established a sensitive assay that allowed us to measure PAT in excised etiolated Arabidopsis hypocotyl sections. As shown in Figure 1A, 6-mm hypocotyl sections were used to measure PAT, and [3H]IAA tracer was added in the donor agar block adjacent to the apical end of the section while a receiver agar block was placed adjacent to the basal end of the section. After a 3-h transport period, the hypocotyl section was cut into halves (Fig. 1B), and the basipetal transport of IAA was determined as a percentage of radioactivity in the basal section and the receiver block divided by the total radioactivity in the tissue plus the receiver block.
Figure 1.
Etiolated Arabidopsis and tomato (cv Ailsa Craig) seedlings had measurable basipetal PAT in excised hypocotyl sections. A, The PAT assay. The donor agar block contained 10−7 m [3H]IAA; 10 μm NPA was added to the receiver agar block in the NPA control. B, Radioactivity in the upper half (U), lower half (L), and receiver (R) was determined separately at the end of the transport period. C, PAT in 6-d dark-grown Arabidopsis and 4-d dark-grown tomato hypocotyls after a 3-h transport period (n = 10). Similar results were also obtained using 8-d dark-grown Arabidopsis plants. Percentage auxin transport equals the percentage of radioactivity in L and R divided by the sum of L, R, and U. Error bars represent se. [See online article for color version of this figure.]
As shown in Figure 1C, about 10% of the [3H]IAA taken up by the etiolated Arabidopsis hypocotyl section was transported to the basal end of the hypocotyl section. This transport was reduced by at least 50% when 10 μm of the transport inhibitor NPA was added to the receiver agar block, indicating that the measured transport of [3H]IAA was polar and inhibited by NPA. In the acropetal control, auxin transport was as low as 1% of the total, showing a low amount of diffusion of [3H]IAA in the assay, and percentage PAT was determined by subtracting the apparent acropetal auxin transport from the basipetal auxin transport. This assay was also successfully applied to dark-grown tomato hypocotyls and similar results were obtained, except that PAT in etiolated tomato seedlings was about twice as much as in etiolated Arabidopsis seedlings (Fig. 1C).
PAT in Etiolated Seedlings Was Increased by Light
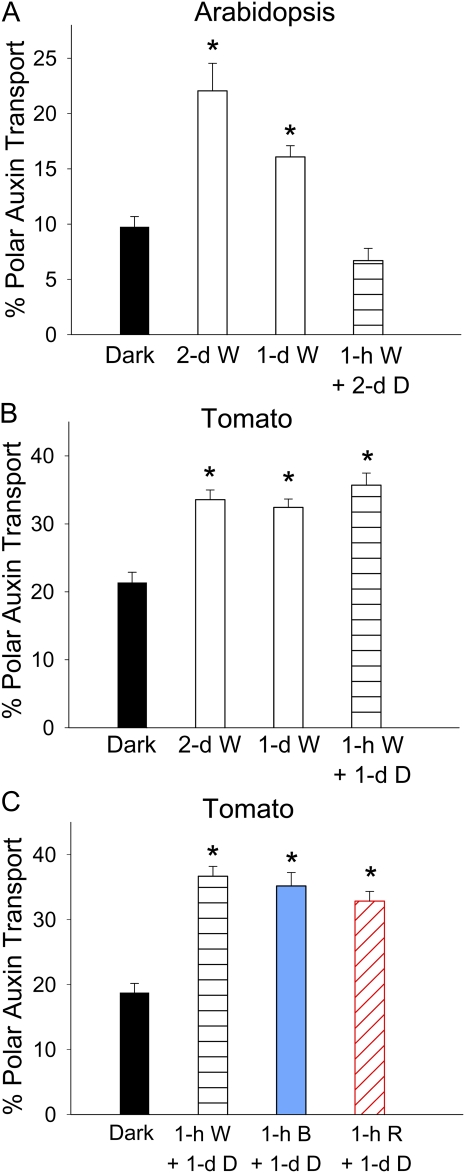
To explore the light effect on PAT in etiolated seedlings, Arabidopsis plants were kept in the dark for 4 d after sowing and tomato plants were kept in the dark for 3 d after germination before light exposure. Seedlings were then exposed to continuous white (W) light for 2 d, while control plants were kept in the dark for the same length of time. As shown in Figure 2, A and B, 2 d of continuous W light doubled the PAT in Arabidopsis hypocotyls and increased PAT in tomato hypocotyls by about 60%. When the W light exposure period was shortened to 1 d, the promotion of PAT in Arabidopsis still occurred but was reduced relative to a 2-d exposure, while tomato showed the same amount of PAT promotion as with 2 d of W. We further shortened the light exposure period to 1 h and then returned plants to the dark for an additional 1 d (tomato) or 2 d (Arabidopsis) to allow the same time period for biochemical processes to occur as in the long light exposure treatments, and we found that the light-induced PAT promotion completely disappeared in Arabidopsis, but it still occurred at the same level as after 1- or 2-d light exposure treatment in tomato (Fig. 2B). Because a short light exposure period minimized the effects of deetiolation and photosynthesis, and thus would allow analyses of a variety of photobiological features, and because the size of tomato seedlings was more amenable to physical manipulation and analytical analysis of IAA, we continued our research using tomato as the primary system.
Figure 2.
Light increased PAT in etiolated Arabidopsis and tomato seedlings. Percentage PAT equals percentage basipetal auxin transport minus the percentage acropetal auxin transport. Asterisks indicate significant changes. A, PAT in Arabidopsis hypocotyls was significantly increased by 2 d or 1 d of continuous 80 μmol m−2 s−1 W light (P < 0.0005, n = 10, Student’s t test) but not by 1 h of W light followed by 2 d of darkness (D). B, PAT in tomato hypocotyls was significantly increased by 2 d or 1 d of continuous 21 μmol m−2 s−1 W light and by 1 h of W light followed by 1 d of darkness (P < 0.0001, n = 10, Student’s t test). C, PAT in tomato hypocotyls was significantly increased by 1 h of 21 μmol m−2 s−1 W light or by 1 h of 4 μmol m−2 s−1 B or R light followed by 1 d of darkness (P < 0.0001, n = 10, Student’s t test). [See online article for color version of this figure.]
Promotion of PAT in Tomato Was Sensitive to B and R Light
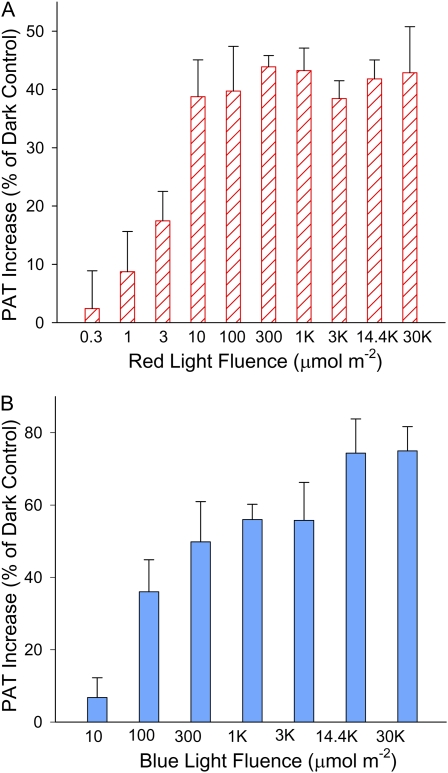
Because W light includes a large range of wavelengths, we analyzed whether light quality affected the promotion of PAT in etiolated tomato seedlings. As shown in Figure 2C, when W light was replaced by either R light or B light, a 1-h light exposure followed by 1 d of darkness increased PAT to a similar level, suggesting that R or B light alone was sufficient to trigger the response. We then varied the fluence of R or B light to determine the minimum fluence followed by 1 d of darkness that was required to promote PAT. When R light was applied, as shown in Figure 3A, a significant PAT promotion was found when the fluence was as low as 3 μmol m−2 (P < 0.05), and the maximal promotion was achieved with 10 μmol m−2 R light, corresponding to a duration of 5 s. As shown in Figure 3B, when the fluence of B light was reduced to 100 μmol m−2, a significant PAT promotion was found (P < 0.05). The promotion became more pronounced when higher fluences were applied and reached the maximal level when the B light fluence was 14,400 μmol m−2 (equivalent to 1 h of B in the previous experiment). These results suggested that the promotion of PAT in tomato hypocotyls was very sensitive to the amount of B and R light and was more sensitive to R light than to B light.
Figure 3.
Increase of PAT in etiolated tomato hypocotyls in response to different B and R light fluences followed by 1 d of darkness. The increase in PAT is shown as percentage of the dark control. A, Increase of PAT in etiolated tomato seedlings when different R light fluences were applied. The increase was significant when R light fluence was equal to or higher than 3 μmol m−2 (P < 0.05, n = 8, Student’s t test). B, Increase of PAT in etiolated tomato seedlings when different B light fluences were applied. The increase was significant when B light fluence was equal to or higher than 100 μmol m−2 (P < 0.05, n = 8, Student’s t test). [See online article for color version of this figure.]
Phytochrome Involvement in the Promotion of PAT
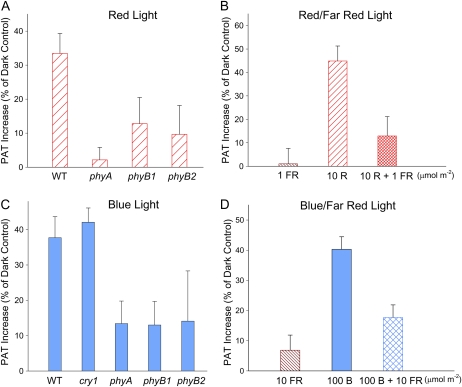
Phytochromes absorb and respond to both R and FR light and, to a lesser extent, B light as well (Pratt and Briggs, 1966; Cordonnier and Pratt, 1982; Weller et al., 2001). Because the promotion of PAT was more sensitive to R light than B light, it is likely that both the B and R light responses were mediated by phytochromes. To test this hypothesis, we analyzed the promotion of PAT in available tomato photoreceptor mutants, including cry1, phyA, phyB1, and phyB2 (Van Tuinen et al., 1995a, 1995b; Weller et al., 2001), and the wild-type plants of the same background. When plants were treated with 10 μmol m−2 R light followed by 1 d of darkness (Fig. 4A), PAT in wild-type tomato seedlings was significantly increased compared with the corresponding dark controls, and the increase was significantly reduced in phyA, phyB1, or phyB2 mutants. After being treated with 3,000 μmol m−2 B light followed by 1 d of darkness, PAT in wild-type and cry1 tomato seedlings was also significantly increased compared with the corresponding dark controls (P < 0.0005; Fig. 4C), suggesting that cryptochrome was not involved in the promotion of PAT induced by B light. On the other hand, B light exposure did not increase PAT significantly in phyA, phyB1, or phyB2 mutants (P > 0.2), suggesting that phytochrome was involved in this B light response.
Figure 4.
R and B light-induced increases of PAT in etiolated tomato seedlings were mediated by phytochrome. A, The increase in PAT in wild-type (WT) tomato seedlings by 10 μmol m−2 R light exposure was reduced or abolished in phyA, phyB1, or phyB2 tomato seedlings. B, FR light (1 μmol m−2) applied immediately after 10 μmol m−2 R light reversed the increase in PAT induced by 10 μmol m−2 R light (P < 0.05, n = 10, Student’s t test). C, B light (3,000 μmol m−2) exposure significantly increased PAT in wild-type and cry1 tomato seedlings but not in phyA, phyB1, or phyB2 tomato seedlings (P > 0.2, n = 8, Student’s t test). D, FR light (10 μmol m−2) applied immediately after 100 μmol m−2 B light reversed the increase in PAT induced by 100 μmol m−2 B light (P < 0.05, n = 10, Student’s t test). FR light alone (10 μmol m−2) did not show significant effects on PAT (P > 0.3, Student’s t test). In A and C, all tomato plants were in the cv MoneyMaker background. Data shown are increased PAT as a percentage of dark controls in corresponding genotypes. [See online article for color version of this figure.]
To further evaluate the phytochrome dependence of the light-induced promotion of PAT, we tested if the light response was FR light reversible, a distinguishing property of many phytochrome responses (Casal et al., 1998). As shown in Figure 4B, 10 μmol m−2 R light followed by 1 d of darkness induced a significant increase in PAT, and this increase was significantly reversed by 1 μmol m−2 FR light applied immediately after the R light exposure (P < 0.05) and thus was not different from the dark control. The FR treatment alone had no significant effect. Similarly, the promotion of PAT induced by 100 μmol m−2 B light was significantly reversed by 10 μmol m−2 FR light applied immediately after B light (Fig. 4D). These results support the conclusion that the PAT promotion induced by R or B light exposure in tomato seedlings was mediated by phytochrome photoreceptors.
Promotion of PAT by Light Required Time to Develop
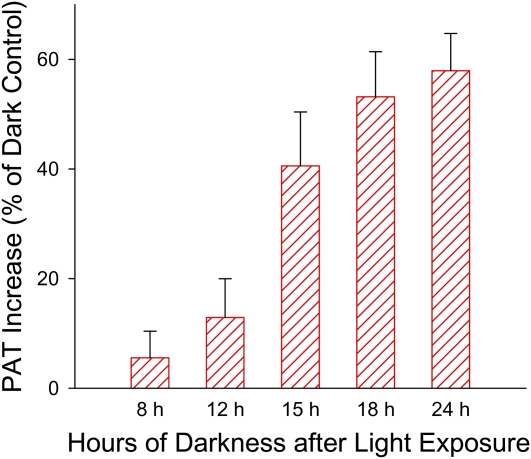
We found in a preliminary experiment that the promotion of PAT in tomato seedlings occurred 1 d after the light exposure but not 2 h after the light exposure, so we asked what was the minimum lag time following the light exposure that was required for plants to develop the response. Therefore, we analyzed PAT in seedlings at different time points following the light exposure along with their corresponding dark controls. We found that PAT was significantly increased after 15 h in darkness following the R light exposure (P < 0.005; Fig. 5). The increase continued and reached the maximal level 18 h after R light exposure. Similar results were also obtained using B light exposure (Supplemental Fig. S1A). These results suggested that a dark period greater than 12 h following the light exposure was required for the PAT promotion to occur.
Figure 5.
Greater than 12 h of darkness after the R light exposure was required for the increase in PAT to occur. When the dark period following 10 μmol m−2 R light exposure equaled or exceeded 15 h, PAT was significantly increased in etiolated tomato seedlings (P < 0.005, n = 10, Student’s t test). A similar result was also obtained using 3,000 μmol m−2 B light exposure (Supplemental Fig. S1A). [See online article for color version of this figure.]
The Velocity of PAT Was Increased by Light
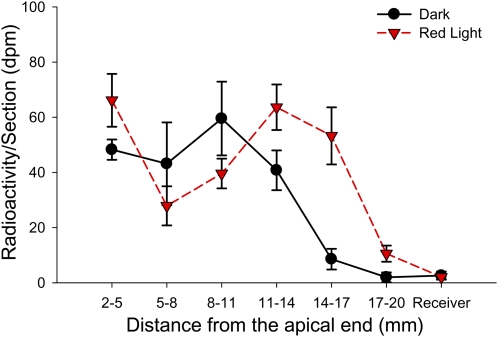
A pulse-chase experiment was carried out to compare the velocity of PAT in tomato seedlings treated with 100 μmol m−2 R light exposure followed by 1 d of darkness and in the dark control plants. The 2-cm excised tomato hypocotyl sections were given a 20-min [3H]IAA pulse followed by unlabeled IAA chase periods. The localization of [3H]IAA in each hypocotyl section was analyzed 1, 2, and 3 h after the start of the chase. The front and the peak of [3H]IAA taken up by hypocotyls moved away from the IAA source over time, and Figure 6 shows the result after a 3-h chase period. In R light-treated seedlings, the front of [3H]IAA movement reached tissue section 17 to 20 mm below the IAA source, and the peak was located in or slightly after tissue section 11 to 14 mm below the IAA source. However, in the dark control plants, the front of [3H]IAA reached tissue section 14 to 17 mm below the IAA source, and the peak was located in tissue section 8 to 11 mm below the IAA source. These results showed that IAA in plants exposed to R light moved faster than in dark control plants and that IAA was transported about 3 mm farther than in the dark control plants during the 3-h transport period. Similar results were also obtained using B light exposure followed by 1 d of darkness (Supplemental Fig. S1B).
Figure 6.
PAT velocity was increased by R light exposure. In tomato seedlings treated with 100 μmol m−2 R light exposure followed by 1 d of darkness, the pulse of [3H]IAA reached the tissue section 17 mm below the IAA source after a 3-h chase period, while the pulse of [3H]IAA reached the tissue section 14 mm below the IAA source in dark control plants (n = 4). A similar result was obtained using B light exposure (Supplemental Fig. S1B). [See online article for color version of this figure.]
The Level of Free IAA Was Increased by Light in Some Tissue Sections
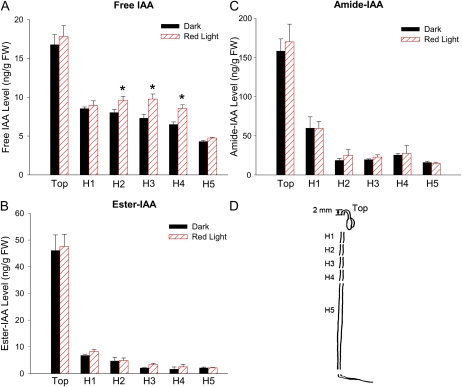
Because altered PAT can be associated with an altered distribution of free IAA among different plant tissues, we quantified the levels of IAA in different tomato tissue sections. In a preliminary experiment, when levels of free IAA were measured in the 6-mm hypocotyl sections used for the PAT assay and in tissues above (meristem, cotyledons, and hook) and below (remaining hypocotyl tissues) the sections, no change in free IAA was found after plants were treated by light followed by 1 d of darkness. However, because the change in IAA levels might be very localized, we measured IAA levels in more discrete tissue sections, as shown in Figure 7D. Consistent with the preliminary result, the level of free IAA was not changed in the top section (consisting of meristem, cotyledons, and hook), H1, and H5 sections, but free IAA was increased 20% to 30% in H2, H3, and H4 sections after 100 μmol m−2 R light followed by 1 d of darkness (P < 0.05; Fig. 7A). We also measured the level of conjugated IAA, including ester-linked IAA and amide-linked IAA, but neither type of IAA conjugate showed significant changes (Fig. 7, B and C), suggesting that the increased free IAA was not due to a change in the rate of release of free IAA from IAA conjugates via hydrolysis.
Figure 7.
Light increased the level of free IAA in specific regions of etiolated tomato hypocotyls. Tissue sections that displayed a significant change in IAA level after 100 μmol m−2 R light exposure followed by 1 d of darkness are indicated by asterisks (P < 0.05, n = 4, Student’s t test). A, Level of free IAA in etiolated tomato tissue sections with or without R light exposure. FW, Fresh weight. B, Level of ester-linked IAA (determined following a 1-h 1 n NaOH hydrolysis at room temperature and then subtracting the level of free IAA) in etiolated tomato tissue sections with or without R light exposure. No significant change in response to R light was found in any tissue sections. C, Level of amide-linked IAA (determined following a 3-h 7 n NaOH hydrolysis at 100°C and then subtracting the level of free + ester IAA) in etiolated tomato tissue sections with or without R light exposure. No significant change in response to R light was found in any tissue section. D, Tissue sections analyzed in A to C. Top, Meristem, cotyledons, and the hook region; H1 to H4, 6-mm hypocotyl sections adjacent to each other; H5, the remaining hypocotyl below H4. [See online article for color version of this figure.]
Biosynthesis of IAA in the Top Section Was Increased by Light
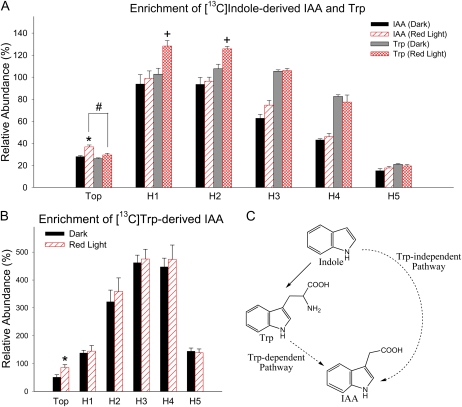
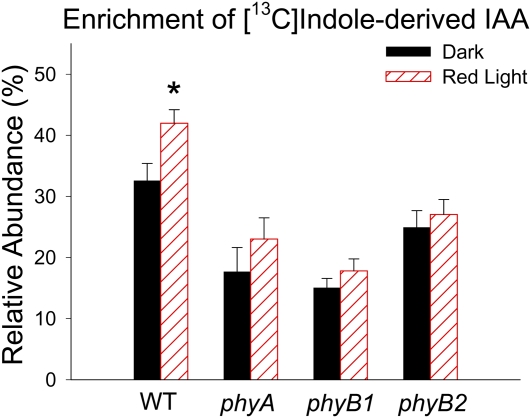
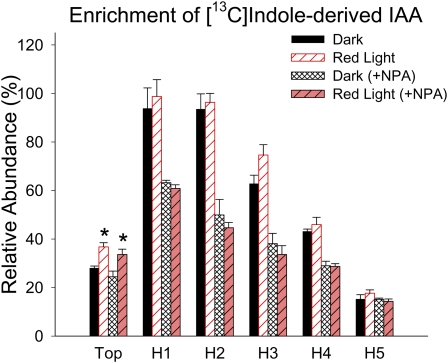
IAA can be synthesized from [13C1]indole directly via the Trp-independent pathway or, following [13C1]indole conversion to Trp, the [13C1]indole-derived Trp can be used in the Trp-dependent pathways. IAA can be synthesized from supplied labeled Trp only via Trp-dependent pathways (Fig. 8C). To understand if IAA biosynthesis was altered by R light followed by 1 d of darkness, we used stable isotope-labeled indole or Trp to feed tomato seedlings, and we analyzed the enrichment of labeled IAA derived from each labeled precursor. We found that 100 μmol m−2 R light followed by 1 d of darkness induced a significant increase of [13C1]indole-derived IAA in the top section (P < 0.05; Fig. 8A; Supplemental Fig. S2A) and a marginally significant increase of [13C1]indole-derived IAA in H3 (P < 0.6; Fig. 8A). This R light-induced increase of [13C1]indole-derived IAA in the top section does not occur in phyA, phyB1, or phyB2 mutants (Fig. 9), suggesting that phytochromes are involved in the regulation of IAA biosynthesis. Importantly, the level of [13C1]indole-derived IAA in the top section after R exposure was significantly higher than the level of [13C1]indole-derived Trp (P < 0.05), while the levels were the same in dark control plants (Fig. 8A); this difference was more obvious when a shorter labeling period was applied (Supplemental Fig. S2B), and these results suggest that TI biosynthesis of IAA was increased by R light and became the predominant path for de novo IAA biosynthesis. Similarly, a significant increase of [13C1]Trp-derived IAA was found in the top section after R light exposure (P < 0.05; Fig. 8B). We also found that the isotopic enrichment in the indole or Trp pool was not different in plants with or without light treatment (Supplemental Fig. S3), supporting the conclusion that the increased amount of labeled IAA was not due to increased availability of labeled precursors but because the biosynthesis of IAA was increased by light. Interestingly, when plants were fed with [13C1]indole and 10 μm NPA simultaneously, labeled IAA was greatly reduced in tissue sections H1, H2, H3, and H4 compared with controls without NPA (Fig. 10), suggesting that IAA synthesized in the top section moved basipetally in the PAT stream while IAA was also synthesized locally in hypocotyl tissues and/or that NPA directly affected IAA biosynthesis as well.
Figure 8.
Light increased the biosynthesis of IAA from labeled precursors in the top section of etiolated tomato seedlings. Tissue sections that show significant change in IAA biosynthesis after 100 μmol m−2 R light exposure followed by 1 d of darkness are indicated by asterisks (P < 0.05, n = 4, Student’s t test). Relative abundance is defined as the ratio of the ion abundance ([m/z 131]/[m/z 130] × 100) corrected for the natural abundance of 13C in the unlabeled IAA. A, Relative enrichment of the unlabeled pools by labeled IAA and Trp synthesized from [13C]indole during a 4-h feeding period. The enrichment of label in Trp in H1 and H2 was significantly increased after R light exposure (+; P < 0.05, n = 4, Student’s t test). In the top section after R light exposure, the enrichment of label in IAA was significantly higher than that of Trp (#; P < 0.05, n = 4, Student’s t test). B, Enrichment of labeled IAA from that synthesized from [13C]Trp during a 4-h feeding period. C, A simplified summary of IAA biosynthetic pathways. The solid arrow represents a single-step process; dashed arrows indicate multiple steps; thus, the pathways are shown in abbreviated form. [See online article for color version of this figure.]
Figure 9.
R light-induced increase in IAA biosynthesis in the top section (meristem, cotyledons, and hook) of tomato seedlings was mediated by phytochrome. IAA biosynthesis was significantly increased after 100 μmol m−2 R light exposure followed by 1 d of darkness in the top section of the wild type (WT [*]; P < 0.05, n = 6, Student’s t test) but not in phyA, phyB1, or phyB2 mutants. In dark-grown seedlings, IAA biosynthesis in phyA and phyB1 was significantly lower than in the wild type (P < 0.01, n = 6, Student’s t test); after R light exposure, IAA biosynthesis in all three mutants was significantly lower than in the wild type (P < 0.001, n = 6, Student’s t test). Relative abundance is defined as described in Figure 8. All tomato plants were in the cv MoneyMaker background, and only the top section as described in Figure 7D was used for analysis. [See online article for color version of this figure.]
Figure 10.
The effect of NPA on IAA biosynthesis from labeled precursors. Enrichment of label in IAA from that synthesized from [13C]indole during a 4-h feeding period in the presence and absence of NPA is shown. Relative abundance is defined as described in Figure 8. When 10 μm NPA was supplied along with [13C]indole, the level of labeled IAA in H1, H2, H3, and H4 sections was significantly reduced compared with controls without NPA (P < 0.01, n = 4, Student’s t test), as predicted from inhibiting PAT. However, the significant labeling in H5 confirms the localized biosynthesis of IAA in this tissue. R light increased the enrichment of [13C]indole-derived IAA in the top section in the presence and absence of NPA (*; P < 0.05, n = 4, Student’s t test). [See online article for color version of this figure.]
DISCUSSION
A goal of this study was to understand the effects of light on the transport, biosynthesis, and distribution of IAA in etiolated seedlings. It was reported previously that auxin transport in dark-grown Arabidopsis seedlings was not reduced by NPA (Rashotte et al., 2003). Here, we found that 10% of the IAA taken up by dark-grown Arabidopsis hypocotyls was transported basipetally and that transport was reduced by 50% when NPA was applied to the basal end of the sections (Fig. 1C). This suggested that, similar to other etiolated dicotyledonous plants such as cucumber and tomato, PAT occurs in dark-grown Arabidopsis hypocotyls, and the lack of measured NPA inhibition of PAT in Rashotte et al. (2003) was likely due to the sensitivity limit of the transport assay used. Our results are consistent with those of Nagashima et al. (2008), who showed that NPA inhibited the elongation of 3-d dark-grown Arabidopsis seedlings, but the inhibition was not as great in 5-d dark-grown seedlings. Because hypocotyl growth approached a maximum in 5-d dark-grown etiolated Arabidopsis, probably due to the exhaustion of seed reserves (Gardner et al., 2009), it seemed that NPA-treated dark-grown seedlings could still grow at a lower rate and finally reach their maximal lengths; this may explain why Jensen et al. (1998) found no effect of NPA on the elongation of 7-d-old Arabidopsis seedlings. The slow growth of dark-grown seedlings treated with NPA could be a contribution of the local biosynthesis of IAA (discussed below), but local IAA biosynthesis alone was not sufficient to support the growth of light-grown seedlings, which had a higher level of PAT (Fig. 2A). Therefore, it would appear that auxin transport is required for hypocotyl elongation in both dark-grown and light-grown Arabidopsis seedlings, but it limits the growth of light-grown seedlings to a greater extent.
The promotion of PAT by light seems to be regulated differently in Arabidopsis and tomato seedlings. In tomato, the promotion could be achieved using light fluences in the range of 1 to 1,000 μmol m−2, which fits the definition of a low-fluence response (LFR; Neff et al., 2000), and this response was further confirmed by the R/FR reversibility test. However, the promotion in Arabidopsis required a much longer period of light exposure and also a higher fluence rate; thus, effects such as greening and active photosynthesis could also play an important role in this promotion. LFR has often been considered to be controlled by phyB (Shinomura et al., 1996; Casal et al., 1998), while LFR controlled by phyA has also been reported (Long and Iino, 2001; Stowe-Evans et al., 2001; Shen et al., 2009). We found that the promotion of PAT induced by low-fluence R light was greatly reduced in phyA, phyB1, and phyB2 tomato mutants (Fig. 4A), providing an additional example of LFR mediated by both phyA and phyB. B light-induced PAT promotion was also reduced in these phytochrome mutants (Fig. 4C), which is consistent with the role of phytochromes in B light perception in tomato (Weller et al., 2001). This response was not reduced in a cry1 mutant, and PAT was significantly increased in the cry1 mutant after B exposure (Supplemental Fig. S4), suggesting a negative role of cryptochrome in PAT, as reported previously (Zeng et al., 2010). In the absence of tomato mutants defective in phototropin functions, we could not test for effects of phototropins on the B light-induced PAT promotion, but we found that a FR light pulse following the B light pulse reversed the B light effects (Fig. 4D), suggesting that this B light response was mostly, if not entirely, mediated by phytochromes.
The low-fluence-induced PAT promotion did not occur immediately after light exposure but required a dark period of over 12 h to develop (Fig. 5). This means that the effect of the light pulse persisted over a long period of time, and a series of downstream changes triggered by the light signal, such as changes in the expression and localization of PAT transporters and facilitators, were required to increase PAT. Keuskamp et al. (2010) showed regulation of PIN3 by phytochrome signaling in a shade-avoidance response, and Ding et al. (2011) showed that unilateral W light (which induced phototropic curvature) polarized PIN3 localization to the inner side of hypocotyl endodermis cells (facing the vasculature). These studies suggest that R light could induce polarly localized PIN3, which restricts IAA to the basipetal transport stream in the stele (Petrásek and Friml, 2009), and thus this could increase the basipetal transport of IAA. Interestingly, we found that a brief light pulse increased PAT velocity (Fig. 6), which was also reported in cucumber seedlings after a long period of R light treatment (Shinkle et al., 1998). Although a mechanism for regulating the auxin transport rate in hypocotyls has not been established as yet, light was shown to change the intracellular distribution of PIN2 in Arabidopsis roots (Laxmi et al., 2008). Thus, light may increase the PAT rate by promoting the plasma membrane localization of auxin transporters and facilitators as well as by increasing the turnover rate of these proteins.
PAT is usually considered as a means of controlling the distribution of free IAA, so the transport direction may imply the relative level of free IAA. Thus, when PAT was promoted in the hypocotyl segment by a light pulse, we expected the level of free IAA to be increased in lower regions of the hypocotyl and decreased in the top section. Our free IAA measurement was partially consistent with this expectation, which showed that the free IAA level was significantly increased in H2, H3, and H4 segments (Fig. 7A), but this difference was not significant when all tissues below H1 were pooled together, suggesting that the significant increase in free IAA was localized. On the other hand, the free IAA levels in H1 and in the top section above H1 were not changed by the light pulse, suggesting that transporting IAA away from these tissue sections did not necessarily decrease the level of free IAA. This result was not surprising given that Jones et al. (2005) found no change in the distribution of free IAA in the upper and lower regions of inflorescence stems of the Arabidopsis pin1-1 mutant, where PAT was dramatically decreased (Okada et al., 1991). Furthermore, this result could be explained when IAA biosynthesis was taken into consideration (discussed below). Indeed, the lack of light effects on free IAA levels in these tissues was a result of the combined effects of increased IAA biosynthesis and increased PAT, and transporting newly synthesized IAA basipetally to lower regions of the hypocotyls maintained a constant level of free IAA in the top section.
We used both [13C]indole and [13C]Trp to label the seedlings in order to explore if light had different effects on the TI and TD IAA biosynthetic pathways. The rationale for this approach is that [13C]Trp forms IAA only via TD pathways while [13C]indole forms IAA directly via the TI pathway or, alternatively, can be converted to Trp and subsequently used by the TD pathways. Because the level of [13C]Trp-derived IAA was increased in the top section, it can be concluded that TD IAA biosynthesis was increased by R light (Fig. 8B). In addition, the enrichment of [13C]indole-derived [13C]IAA in the top section in dark control plants was equal to the [13C]indole-derived [13C]Trp, so the [13C]IAA in dark tissue could all have been derived from [13C]Trp via TD pathways; thus, the TD pathways could be the predominant path for de novo IAA biosynthesis in dark-grown plants. On the other hand, in the case of R light-exposed plants, an increased enrichment of [13C]indole-derived [13C]IAA was noted, while the enrichment of [13C]indole-derived [13C]Trp in the same samples was unaffected. This resulted in the enrichment of [13C]IAA exceeding that of [13C]Trp (Fig. 8A). Therefore, it can be concluded that the TI IAA biosynthesis was also increased by R light, and that the TI IAA biosynthetic pathway was the predominant path for de novo IAA biosynthesis in the top section after R light exposure. Moreover, when the enrichment of [13C]indole-derived [13C]IAA was analyzed in the top section of phyA, phyB1, and phyB2 mutants, we found that IAA biosynthesis in these tissues did not change in response to low-fluence R light (Fig. 9), suggesting that the R light effect on IAA biosynthesis is under the control of phytochrome. Additionally, IAA biosynthesis in these phytochrome mutants was lower than in the wild type both in the presence and absence of R light exposure (Fig. 9). Thus, in addition to the red1/cyp83b1 mutant (Hoecker et al., 2004) and the shade-avoidance response (Tao et al., 2008), our work provides an additional link between phytochrome signaling and auxin biosynthesis, confirming that auxin biosynthesis plays an important role in phytochrome-regulated photomorphogenesis.
We also noticed that the predominance of TI IAA biosynthesis after R light exposure was even more evident when a shorter labeling period was analyzed (Supplemental Fig. S2B). This result suggests the possibility that one could fail to see the differential labeling if too long a labeling period was used and the Trp pool became saturated by [13C]Trp derived from [13C]indole. Changes in the use of TI and TD IAA biosynthetic pathways were reported previously as a result of plant organ development and as a response to stress (Ljung et al., 2001b; Epstein et al., 2002; Rapparini et al., 2002; Sztein et al., 2002), indicating that the different IAA biosynthetic pathways play distinct roles under different developmental and environmental conditions. Additionally, our data showed that the biosynthesis of [13C]Trp from [13C]indole was also increased by R light exposure in sections H1 and H2 but not in any other tissue section (Fig. 8A). This change could potentially be hidden if the whole seedling was used for analysis, as was done for microarray analyses (where both R and FR exposure decrease Trp synthase-β At1g01010 gene expression in Arabidopsis; http://bbc.botany.utoronto.ca/efp/cgi-bin/efpWeb.cgi). The change in Trp biosynthesis was not associated with changes in TD IAA biosynthesis in these tissue sections (Fig. 8B), suggesting that it is a consequence or trigger for photomorphogenic changes other than IAA biosynthesis. While, to our knowledge, phytochrome regulation of Trp synthase-β activity has not been reported, phytochrome is known to regulate the expression, translation, and thus activity of a plethora of other plastid-localized enzymes (Christopher, 2003).
It is worth pointing out that the amount of [13C]IAA derived from [13C]Trp or [13C]indole was different in different tissue sections, and one might conclude that the utilization of these precursors for IAA biosynthesis was different in different tissues. However, the enrichment of [13C]Trp was not uniform among the tissue sections (Supplemental Fig. S2B), and the relative abundance corresponded to that of [13C]Trp-derived IAA, suggesting that the amount of [13C]Trp-derived IAA was associated with the availability of [13C]Trp in the tissue. However, the enrichment of [13C]indole was uniform in all tissue sections (Supplemental Fig. S3A), so the abundance of [13C]indole-derived IAA in each tissue section represented IAA biosynthesis activity. Therefore, compared with Trp, indole is a better precursor for comparative study of IAA biosynthesis across different tissues, not only because it covers both TD and TI biosynthetic pathways but also because it labels the endogenous pool uniformly.
The role of localized IAA biosynthesis in different regions of the hypocotyl was further explored using NPA to disrupt PAT. When seedlings were treated with NPA to inhibit PAT (Fig. 10), the enrichment of newly synthesized labeled IAA in the H1, H2, H3, and H4 segments was greatly reduced, and the slight increase of labeled IAA by the light pulse was also abolished in these segments but not in the top section, suggesting that light increased IAA biosynthesis only in the top section and thus the increased amount of free IAA in the H2, H3, and H4 segments resulted from increased IAA biosynthesis in the top section. Because IAA has been found to promote the plasma membrane localization of PIN proteins and therefore increase IAA’s own efflux from cells (Paciorek et al., 2005), the increased IAA biosynthesis at the top section may be the driving force for increased PAT triggered by light exposure. Although it was expected that the level of labeled IAA at the top section might be increased by NPA due to disrupted PAT, it was actually unchanged, suggesting a feedback regulation of IAA biosynthesis by IAA itself, which has also been reported in other systems (Ribnicky et al., 1996; Ljung et al., 2001a). The level of labeled IAA in the major portion (H5) of the hypocotyls was not changed by NPA during the 4-h feeding period, suggesting that the enrichment of labeled IAA in this tissue section was primarily due to local biosynthesis of IAA at a rate that was lower than that found in the upper part of the shoot. In fact, the enrichment of labeled IAA in the H5 tissue section in untreated plants could be detected in a 2-h feeding period (Supplemental Fig. S2A), and it would be impossible for IAA synthesized at the top section to reach this region within a 2-h period, because the transport rate in hypocotyls was at most 6 mm h−1 (calculated from Fig. 5). Therefore, the top section of the seedling was not the only tissue for de novo IAA biosynthesis, and local biosynthesis of IAA might also contribute to hypocotyl growth. Although the hypocotyl tissues were capable of synthesizing IAA, the top section contained the majority of the IAA conjugates (Fig. 7, B and C), which could serve as IAA precursors that slowly release free IAA (Nowacki and Bandurski, 1980; Chisnell and Bandurski, 1988; Bialek and Cohen, 1992; Ljung et al., 2001b). Thus, PAT plays an important role in supplying free IAA to lower parts of seedlings by basipetal transport of newly synthesized and conjugate-derived IAA in the top section.
The increase of free IAA in a region of tomato hypocotyls after a R light pulse was consistent with what Behringer and Davies (1992) reported in pea. However, in this work, the increase in free IAA was not associated with a differential hypocotyl elongation rate, and the growth of hypocotyls in plants that were exposed to R light followed by 1 d of darkness was not different from dark control plants (Supplemental Fig. S5). Thus, instead of regulating hypocotyl elongation, it is likely that the redistribution of free IAA triggered other developmental changes, such as promoting vascular development in the stem tissues (Mattsson et al., 1999; Aloni, 2010). Alternatively, the altered PAT might be a way to tightly regulate the level of free IAA in the top section, where growth was most active, or to supply free IAA in root tissues to initiate lateral root formation and promote root development (Bhalerao et al., 2002; Ljung et al., 2005; Salisbury et al., 2007). Although we have found that a single very brief R light pulse was sufficient to alter auxin biosynthesis and transport in etiolated tomato seedlings, no dramatic change in hypocotyl elongation was observed in this LFR (Supplemental Fig. S5). However, it was previously shown that hypocotyl elongation was decreased when dark-grown tomato seedlings were exposed to R light pulses with hourly repeats (Van Tuinen et al., 1995a), which was also defined as a LFR (Casal et al., 1998), and the R light effect was reversible by FR light pulses following each R light pulse (Van Tuinen et al., 1995a). The mechanism related to the requirement of repeated R light pulses for the inhibition of hypocotyl elongation during the deetiolation process remains to be further studied.
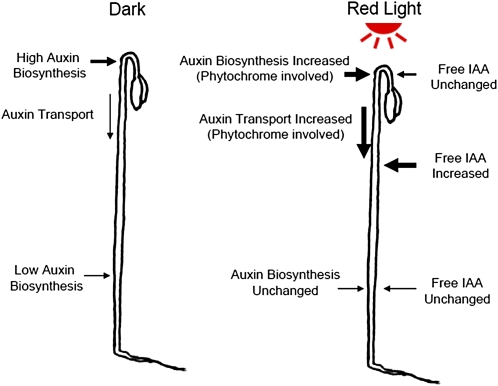
In summary (Fig. 11), we have found that in the absence of light, IAA is synthesized in all regions in the shoot, with more synthesized at the top section, while PAT also occurs to transport IAA produced at the top section to lower regions in the hypocotyl, maintaining free IAA gradients across the shoot with maxima at the top section. When etiolated seedlings are exposed to low-fluence R light, IAA biosynthesis at the top section is increased and PAT in the adjacent hypocotyl tissue is also increased to transport more IAA to the basal part of the seedling, leading to an unchanged level of free IAA in the top section and an increased level of free IAA in the lower regions of the hypocotyl.
Figure 11.
A summary of the low-fluence R light effect on the transport and biosynthesis of IAA. In dark-grown seedlings, IAA biosynthesis is high at the top of the hypocotyl and is low in the lower part of the hypocotyl. IAA synthesized in the top section moves basipetally in the PAT stream to supply free IAA to lower hypocotyl regions. When seedlings were exposed to R light, IAA biosynthesis at the top section increased, and IAA transport in the adjacent hypocotyl region also increased to move newly synthesized IAA to the lower hypocotyl regions, which maintained a constant free IAA level at the top section and increased free IAA in the lower portion of the hypocotyl. [See online article for color version of this figure.]
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Tomato (Solanum lycopersicum ‘Ailsa Craig’) seeds were purchased from Thompson and Morgan Wholesale and were grown to increase seed quantity in an isolated field in Hugo, Minnesota, during the summer of 2007. Seeds of cv MoneyMaker were purchased from Gourmet Seed International. Monogenic mutants (in a background of cv MoneyMaker) phyA, phyB1, phyB2, and cry1 were from the Tomato Genetics Resource Center at the University of California and were grown for seed increase in a greenhouse at 27°C with 16 h of light per day. Individual plants were grown in 5-gallon pots, watered daily, and fertilized with slow-release fertilizer (Osmocote Plus; Scotts-Sierra Horticultural Products). All seeds were imbibed under running tap water overnight, surface sterilized by incubation in 0.1% Micro 90 (International Products) and 10% commercial bleach (0.6% sodium hypochlorite) for 10 min with constant slow shaking, and then washed 10 times with sterile water. Seeds were sown onto Murashige and Skoog medium (4.33 g L−1 Murashige and Skoog salts [MSP01-50LT; Caisson Laboratories]) in 0.8% (w/v) Phytoblend agar (PTP01-500GM; Caisson Laboratories), pH 5.7, in Magenta GA-7 Plant Culture Boxes (765200; bio-WORLD [http://www.bio-world.com/index.php]) that were capped by Magenta G-7 boxes with couplers (765201; bio-WORLD). Boxes were placed in a physiological dark room at 24°C, with only brief exposure to a dim green safelight as needed for manipulations. Seeds germinated on day 3 after sowing were allowed to grow in the dark for 3 d before being used for light treatments or other experiments.
Arabidopsis (Arabidopsis thaliana ecotype Colombia) seeds were surface sterilized in 2.0-mL microcentrifuge tubes with 1 mL of 70% ethanol for 2 min, followed by 1 mL of 30% commercial bleach (to yield 1.8% sodium hypochlorite) for 5 min, and washed with sterile water five times. Seeds were sown on Murashige and Skoog medium, 0.8% (w/v) Phytoblend agar, 1.0% (w/v) Suc (Sigma-Aldrich), pH 5.7, and agar plates were wrapped with Parafilm (Pechiney Plastic Packaging) and kept in a dark room at 24°C for 4 d before seedlings were used for light treatments.
Light Sources and Light Treatments
All the light treatments were done in a physiological dark room at 24°C. R light was provided by two R fluorescent lamps (Sylvania F48T12/2364/HO) filtered through an Encapsulite R tube guard (Lighting Plastics of Minnesota) and a single layer of Roscolux 66 cool blue filter; and FR light was provided by two FR fluorescent lamps (Sylvania F48T12/232/HO) filtered through an Encapsulite FR tube guard (Lighting Plastics of Minnesota) and a single layer of Lee 85 deeper blue filter (Howe et al., 1996). B light was provided by two B fluorescent lamps (Science-Lite Blue-64805), and W light was provided by two or four W fluorescent tubes (Philips F34T12/CW/RS/EW and Philips F48T12/CW/HO). The radiometric spectra of the R, FR, and B light sources are shown in Supplemental Figure S6. Photosynthetically active radiation (total irradiance between 400 and 700 nm) was measured with a LI-250A Light Meter (LI-COR Biosciences). R and FR light fluences were measured with an SKR 110, 660/730-nm sensor (Skye Instruments). Spectral quality was measured with a model SPEC-UV/PAR Spectroradiometer (Apogee Instruments). Specific light intensity was obtained by adjusting the distance between the plants and the light sources or by covering the plants with neutral density plastic filters. When 10 μmol m−2 or lower R light fluence was applied, plants were kept in a steel canister (diameter = 11.5 cm, height = 23.5 cm), and light was allowed to reach the plants only through a neutral density filter on top of the canister. Different light fluences were achieved by adjusting light intensity and the duration of light exposure. Fluence rates varied from 0.07 to 5 μmol m−2 s−1 for R and 2 to 5 μmol m−2 s−1 for B, and durations varied from 2 s to 100 min.
Quantification of PAT in Hypocotyls
The hypocotyl basipetal IAA transport assay was modified from that described previously (Gardner and Sanborn, 1989; Lewis and Muday, 2009). Six millimeters of an etiolated tomato hypocotyl section 2 mm below the hook tip, or 6 mm of an etiolated Arabidopsis hypocotyl section directly below the shoot apex, was placed on an agar plate after excision, and an auxin donor agar block of 1.5% agar (Sigma-Aldrich) containing 0.2 m MES (Sigma-Aldrich) and 10−7 m [3H]IAA (Amersham, GE Healthcare; original specific activity of 25 Ci mmol−1, diluted to 15 Ci mmol−1 by the addition of nonradioactive IAA) was placed in contact with the apical end of the tissue section, while a receiver agar block containing 0.2 m MES (pH 6.5) was placed in contact with the basal end. Receiver blocks containing 0.2 m MES (pH 6.5) and 10 μm NPA (ChemService) were used as the +NPA control, and a second control was used where the orientation of the tissue section was inverted (acropetal control). Two strips of polyethylene film (Saran Original; S.C. Johnson & Sons) were placed between the agar blocks and the support agar on the plates to avoid diffusion of [3H]IAA through the support agar and thus prevent an undesirable increase in background counts. The agar plates were placed vertically with donor blocks down in a chamber with high humidity for 3 h, and each of the hypocotyl sections was then divided into apical and basal halves. The receiver block and each half-section of the hypocotyl were extracted individually in scintillation cocktail (Econo-Safe; Research Products International) overnight, and the radioactivity was determined by liquid scintillation counting (LS 6500; Beckman). All the manipulations before scintillation cocktail extraction were done in a dark room under dim green safelight.
Measurement of Auxin Transport Velocity in Hypocotyls
The setup used for the assay was similar to the PAT assay described above. Two centimeters of a tomato hypocotyl section cut from 2 mm below the hook tip was incubated for 20 min with an auxin donor agar block containing 10−7 m [3H]IAA on its apical end and a receiver agar block on the basal end. Next, the auxin donor agar block was replaced by a new auxin donor agar block containing 10−7 m unlabeled IAA (Sigma) and again placed in the humid chamber. After different incubation time periods, each 2-cm hypocotyl section was cut into 3-mm segments, except that the apical top segment was 2 mm. Each segment of the hypocotyl and the receiver block was incubated individually in scintillation cocktail overnight, and the radioactivity was determined by liquid scintillation counting.
Quantification of Free IAA and Conjugated IAA
Six tomato seedlings were dissected into different tissue sections as shown in Figure 7D, and the six replicates from each tissue section were pooled into one microcentrifuge tube, weighed, frozen in liquid nitrogen, and stored in a −80°C freezer. For every 10 mg of frozen tissue, 70 μL of homogenization buffer (35% 0.2 m imidazole, 65% isopropanol, pH 7) containing 1.4 ng of [13C6]IAA was added, and tissues were homogenized using a Mixer Mill (MM 300; Qiagen) with tungsten carbide beads (3-mm beads for 1.5-mL tubes and 2.3-mm beads for 0.5-mL tubes; Craig Ball Sales).
Micro solid phase extraction (SPE) methods were modified from conventional SPE methods described previously (Ribnicky et al., 1998; Barkawi et al., 2010). Each SPE resin (5 mg of NH2 anion exchange [12213020; Varian] and 20 mg of polymethylmethacrylate epoxide [Macro-Prep; Bio-Rad]) was packed into 200 μL of TopTips (TT2EMT.96; Glygen; http://www.glysci.com/) that were placed over 2-mL microcentrifuge tubes with adaptors (Glygen), and solvents were added and passed through the tips by centrifugation at 3,000g to 6,000g for a few seconds.
After 1 h on ice, 20 μL of the homogenate was diluted with 180 μL of water and the extract was purified by micro SPE. First, the diluted plant homogenate was centrifuged at 10,000g for 10 min, and then the supernatant was purified through two sequential TopTips, first NH2 and then polymethylmethacrylate epoxide, similar but on a smaller scale to that described by Barkawi et al. (2010), using 10% of the specified volumes of each liquid except for the final methanol elution. IAA was eluted twice using 50 μL of methanol, and the eluent was collected in a 250-μL glass insert (CTI-9425; ChromTech). The sample was methylated in the insert by adding ethereal diazomethane, waiting 5 min, and then the solvents were evaporated under a stream of N2 gas and the sample was redissolved in 15 μL of ethyl acetate.
For the rest of the homogenate, 10 μL was hydrolyzed in 1 n NaOH (1 h, room temperature; Baldi et al., 1989) for measurement of free plus ester-linked IAA and 10 μL was hydrolyzed in 7 n NaOH (3 h, 100°C under N2; Bialek and Cohen, 1989) for measurement of total IAA (free plus ester-linked and amide-linked IAA). After hydrolysis, the pH of the hydrolysate was adjusted to 2.7, and the free IAA plus that released from the conjugates was purified from the extracts using TopTips containing 7.5 mg of C18 resin (12213020; Varian), washed twice with 60 μL of water, eluted twice with 50 μL of methanol, collected in 250-μL glass inserts, and methylated as described above.
The methylated IAA was analyzed using gas chromatography (GC)-selected reaction monitoring-mass spectrometry (MS) on a Thermo Trace GC Ultra coupled to a TSQ Vantage triple quadrupole MS system (Thermo Scientific). The GC column and separation conditions were as described previously (Barkawi et al., 2010). Compounds eluted from the GC device were ionized in the electron impact mode with electron emission at 70 eV and emission current of 100 μA. The molecular ions (mass-to-charge ratio [m/z] 189 for endogenous IAA and m/z 195 for [13C6]IAA) were selected by the first quadrupole and collided with argon in the second quadrupole using 10 V of collision energy and a 1.5-mTorr collision gas pressure. The quinolinium ions produced from the molecular ions (m/z 130 from m/z 189 and m/z 136 from m/z 195) were selected by the third quadrupole and detected using 0.025-s scan times. Levels of free IAA, free plus ester-linked IAA, and total IAA were quantified by isotope dilution analysis based on the [13C6]IAA internal standard, as described previously (Barkawi et al., 2010).
Stable Isotope Labeling and Isotopic Enrichment Analysis
Tomato seeds were planted on vertical agar plates on a sheet of 20-μm nylon filter screen (146510; Spectrum Laboratories) that had been placed on top of the agar medium. The nylon screen allowed tomato seedlings to grow straight on the surface and prevented root hairs from penetrating into the agar medium. This procedure ensured that a rapid switch of the tomato plants from normal growth agar to growth agar containing stable isotope-labeled compounds could be accomplished. It also minimized the effects of wounding and gravitropic responses during seedling movement. The growth conditions and light treatments were the same as those described above. To start the labeling process, tomato plants were transferred to labeling medium by picking up the filter screen with sterile forceps and transferring the screen and the attached tomato seedlings onto agar medium containing either 0.1 mm [2-13C]indole (CLM-1863; Cambridge Isotope Laboratories) or 0.5 mm l-[13C-indole 2]Trp (CLM-1543; Cambridge Isotope Laboratories). To uniformly label all the tissues, the entire seedlings were attached to the nylon filter. Seedlings fed by [13C1]Trp (four per plate) were covered by a 11-cm × 11-cm piece of KimWipe tissue (Kimberly-Clark) moistened with labeling medium, as this was found to prevent shoots from bending away from the screen and labeling medium, which otherwise was a consequence of asymmetric growth induced by the supplied Trp. The plates were then placed vertically during the labeling period. At the end of the labeling period, the four seedlings were dissected, pooled, and collected as described above. All the manipulations prior to storing the samples in a −80° freezer were done in a physiological dark room under dim green light.
Free IAA was extracted and analyzed similarly to IAA quantification, but the quinolinium ions m/z 130 and 131 produced from the molecular ions m/z 189 and 190, respectively, were monitored for analysis of isotopic enrichment in the free IAA pool. Indole was partitioned from diluted plant homogenate before the anion-exchange resin extraction, using 80 μL of pentane (P399-4; Fisher Scientific), and ions m/z 117 and 118 were monitored in the selected ion monitoring mode for analysis of isotopic enrichment in the indole pool. To analyze the Trp pool, the flow-through of the plant homogenate, after it passed through the anion-exchange resin, was collected. Extraction and GC-MS analysis of Trp were similar to those described by Chen et al. (2010) using methyl chloroformate derivatization, and ions m/z 276 and 130 as well as m/z 277 and 131 were monitored in the selected ion monitoring mode for analysis of isotopic enrichment in the Trp pool. The natural abundance of 13C was determined using unlabeled chemical standards (Sigma) and is corrected for in the calculations and the data reported.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Increase in PAT following B light exposure.
Supplemental Figure S2. Light increased Trp-independent biosynthesis of IAA in the top section of etiolated tomato seedlings.
Supplemental Figure S3. Enrichment of 13C-labeled precursors in tissues that synthesize IAA from these precursors.
Supplemental Figure S4. PAT was significantly increased in cry1 mutant tomato hypocotyls treated with 3,000 μmol m−2 B light exposure followed by 1 d in darkness.
Supplemental Figure S5. R light exposure followed by 1 d in darkness did not alter hypocotyl length.
Supplemental Figure S6. Spectral photon distributions of the R, FR, and B light sources.
Acknowledgments
We thank Dr. Gloria Muday (Wake Forest University) for sharing methods of PAT assay using Arabidopsis hypocotyls. We thank the Tomato Genetics Resource Center (University of California, Davis) for providing the cry1, phyA, phyB1, and phyB2 tomato mutant seeds. We thank Doug Brinkman for assistance with spectral irradiance measurements, Dr. Lana Barkawi and Dr. Wen-Ping Chen for help with analytical techniques, and Ray Nieh, Sarah Braman, and Stacey Noble for helpful technical assistance (all from the University of Minnesota). We also thank Drs. Jane Glazebrook and Adrian Hegeman (University of Minnesota) for critical reading of the manuscript.
References
- Aloni R. (2010) The induction of vascular tissues by auxin. Davies P, , Plant Hormones, Ed 3. Springer, Dordrecht, The Netherlands, pp 485–518 [Google Scholar]
- Baldi BG, Maher BR, Cohen JD. (1989) Hydrolysis of indole-3-acetic acid esters exposed to mild alkaline conditions. Plant Physiol 91: 9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski RS, Schulze A, Cohen JD. (1977) Photo-regulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun 79: 1219–1223 [DOI] [PubMed] [Google Scholar]
- Barkawi LS, Tam Y-Y, Tillman JA, Normanly J, Cohen JD. (2010) A high-throughput method for the quantitative analysis of auxins. Nat Protoc 5: 1609–1618 [DOI] [PubMed] [Google Scholar]
- Barker-Bridges M, Ribnicky D, Cohen J, Jones A. (1998) Red light-regulated growth. II. Changes in the abundance of indoleacetic acid in the maize mesocotyl. Planta 204: 207–211 [Google Scholar]
- Behringer FJ, Davies PJ. (1992) Indole-3-acetic acid levels after phytochrome-mediated changes in the stem elongation rate of dark- and light-grown Pisum seedlings. Planta 188: 85–92 [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29: 325–332 [DOI] [PubMed] [Google Scholar]
- Bialek K, Cohen JD. (1989) Quantitation of indoleacetic acid conjugates in bean seeds by direct tissue hydrolysis. Plant Physiol 90: 398–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K, Cohen JD. (1992) Amide-linked indoleacetic acid conjugates may control levels of indoleacetic acid in germinating seedlings of Phaseolus vulgaris. Plant Physiol 100: 2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Olney MA. (2001) Photoreceptors in plant photomorphogenesis to date: five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol 125: 85–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Sánchez R, Botto J. (1998) Modes of action of phytochromes. J Exp Bot 49: 127–138 [Google Scholar]
- Chen W-P, Yang X-Y, Hegeman AD, Gray WM, Cohen JD. (2010) Microscale analysis of amino acids using gas chromatography-mass spectrometry after methyl chloroformate derivatization. J Chromatogr B Analyt Technol Biomed Life Sci 878: 2199–2208 [DOI] [PubMed] [Google Scholar]
- Chisnell JR, Bandurski RS. (1988) Translocation of radiolabeled indole-3-acetic acid and indole-3-acetyl-myo-inositol from kernel to shoot of Zea mays L. Plant Physiol 86: 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher DA. (2003) Photosensory pathways regulating chloroplast gene expression. Abdel-Mottaleb MSA, Nalwa HS, , Handbook of Photochemistry and Photobiology, Vol 4. American Scientific Publishers, Valencia, CA, pp 249–268 [Google Scholar]
- Cordonnier M-M, Pratt LH. (1982) Immunopurification and initial characterization of dicotyledonous phytochrome. Plant Physiol 69: 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski Ł, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, et al. (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Epstein E, Cohen J, Slovin J. (2002) The biosynthetic pathway for indole-3-acetic acid changes during tomato fruit development. Plant Growth Regul 38: 15–20 [Google Scholar]
- Gardner G, Lin C, Tobin EM, Loehrer H, Brinkman D. (2009) Photobiological properties of the inhibition of etiolated Arabidopsis seedling growth by ultraviolet-B irradiation. Plant Cell Environ 32: 1573–1583 [DOI] [PubMed] [Google Scholar]
- Gardner G, Sanborn JR. (1989) Aryl-substituted α-aminooxycarboxylic acids: a new class of auxin transport inhibitors. Plant Physiol 90: 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Östin A, Sandberg G, Romano CP, Estelle M. (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95: 7197–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker U, Toledo-Ortiz G, Bender J, Quail PH. (2004) The photomorphogenesis-related mutant red1 is defective in CYP83B1, a red light-induced gene encoding a cytochrome P450 required for normal auxin homeostasis. Planta 219: 195–200 [DOI] [PubMed] [Google Scholar]
- Howe G, Gardner G, Hackett W, Furnier G. (1996) Phytochrome control of short-day-induced bud set in black cottonwood. Physiol Plant 97: 95–103 [Google Scholar]
- Iino M. (1982a) Action of red light on indole-3-acetic-acid status and growth in coleoptiles of etiolated maize seedlings. Planta 156: 21–32 [DOI] [PubMed] [Google Scholar]
- Iino M. (1982b) Inhibitory action of red light on the growth of the maize mesocotyl: evaluation of the auxin hypothesis. Planta 156: 388–395 [DOI] [PubMed] [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M. (1998) Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol 116: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Cochran DS, Lamerson PM, Evans ML, Cohen JD. (1991) Red light-regulated growth. I. Changes in the abundance of indoleacetic acid and a 22-kilodalton auxin-binding protein in the maize mesocotyl. Plant Physiol 97: 352–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Demeo JS, Davies NW, Noonan SE, Ross JJ. (2005) Stems of the Arabidopsis pin1-1 mutant are not deficient in free indole-3-acetic acid. Planta 222: 530–534 [DOI] [PubMed] [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LA, Peeters AJ, Pierik R. (2010) Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA 107: 22740–22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Kamiya Y, Iino M. (1995) Biosynthesis of indole-3-acetic acid from L-tryptophan in coleoptile tips of maize (Zea mays L.). Plant Cell Physiol 36: 1503–1510 [Google Scholar]
- Kraepiel Y, Miginiac E. (1997) Photomorphogenesis and phytohormones. Plant Cell Environ 20: 807–812 [Google Scholar]
- Laxmi A, Pan J, Morsy M, Chen R. (2008) Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS One 3: e1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Muday GK. (2009) Measurement of auxin transport in Arabidopsis thaliana. Nat Protoc 4: 437–451 [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. (2001a) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28: 465–474 [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G. (2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17: 1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Ostin A, Lioussanne L, Sandberg G. (2001b) Developmental regulation of indole-3-acetic acid turnover in Scots pine seedlings. Plant Physiol 125: 464–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Iino M. (2001) Light-dependent osmoregulation in pea stem protoplasts: photoreceptors, tissue specificity, ion relationships, and physiological implications. Plant Physiol 125: 1854–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. (1999) Responses of plant vascular systems to auxin transport inhibition. Development 126: 2979–2991 [DOI] [PubMed] [Google Scholar]
- Nagashima A, Uehara Y, Sakai T. (2008) The ABC subfamily B auxin transporter AtABCB19 is involved in the inhibitory effects of N-1-naphthyphthalamic acid on the phototropic and gravitropic responses of Arabidopsis hypocotyls. Plant Cell Physiol 49: 1250–1255 [DOI] [PubMed] [Google Scholar]
- Neff MM, Fankhauser C, Chory J. (2000) Light: an indicator of time and place. Genes Dev 14: 257–271 [PubMed] [Google Scholar]
- Nishimura T, Mori Y, Furukawa T, Kadota A, Koshiba T. (2006) Red light causes a reduction in IAA levels at the apical tip by inhibiting de novo biosynthesis from tryptophan in maize coleoptiles. Planta 224: 1427–1435 [DOI] [PubMed] [Google Scholar]
- Normanly J, Slovin J, Cohen J. (2005) Auxin biosynthesis and metabolism. Davies PJ, , Plant Hormones: Biosynthess, Signal Transduction, Action! Ed 3. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 36–62 [Google Scholar]
- Nowacki J, Bandurski RS. (1980) Myo-inositol esters of indole-3-acetic acid as seed auxin precursors of Zea mays L. Plant Physiol 65: 422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Zazímalová E, Ruthardt N, Petrásek J, Stierhof Y-D, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, et al. (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Petrásek J, Friml J. (2009) Auxin transport routes in plant development. Development 136: 2675–2688 [DOI] [PubMed] [Google Scholar]
- Pratt LH, Briggs WR. (1966) Photochemical and nonphotochemical reactions of phytochrome in vivo. Plant Physiol 41: 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapparini F, Tam YY, Cohen JD, Slovin JP. (2002) Indole-3-acetic acid metabolism in Lemna gibba undergoes dynamic changes in response to growth temperature. Plant Physiol 128: 1410–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Poupart J, Waddell CS, Muday GK. (2003) Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiol 133: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribnicky DM, Cooke TJ, Cohen JD. (1998) A microtechnique for the analysis of free and conjugated indole-3-acetic acid in milligram amounts of plant tissue using a benchtop gas chromatograph-mass spectrometer. Planta 204: 1–7 [DOI] [PubMed] [Google Scholar]
- Ribnicky DM, Ilic N, Cohen JD, Cooke TJ. (1996) The effect of exogenous auxins on endogenous indole-3-acetic acid metabolism: implications for somatic embryogenesis in carrot. Plant Physiol 112: 549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury FJ, Hall A, Grierson CS, Halliday KJ. (2007) Phytochrome coordinates Arabidopsis shoot and root development. Plant J 50: 429–438 [DOI] [PubMed] [Google Scholar]
- Seidel C, Walz A, Park S, Cohen JD, Ludwig-Müller J. (2006) Indole-3-acetic acid protein conjugates: novel players in auxin homeostasis. Plant Biol (Stuttg) 8: 340–345 [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhou Z, Feng S, Li J, Tan-Wilson A, Qu L-J, Wang H, Deng X-W. (2009) Phytochrome A mediates rapid red light-induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response. Plant Cell 21: 494–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Uemura M, Tsurumi S, Rahman A. (2009) Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms. Plant Cell 21: 3823–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle JR, Kadakia R, Jones AM. (1998) Dim-red-light-induced increase in polar auxin transport in cucumber seedlings. I. Development of altered capacity, velocity, and response to inhibitors. Plant Physiol 116: 1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. (1996) Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA 93: 8129–8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I. (1999) Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126: 4235–4245 [DOI] [PubMed] [Google Scholar]
- Stowe-Evans EL, Luesse DR, Liscum E. (2001) The enhancement of phototropin-induced phototropic curvature in Arabidopsis occurs via a photoreversible phytochrome A-dependent modulation of auxin responsiveness. Plant Physiol 126: 826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons GM, Reid JB. (2003) Hormone levels and response during de-etiolation in pea. Planta 216: 422–431 [DOI] [PubMed] [Google Scholar]
- Sztein E, Ilić N, Cohen J, Cooke T. (2002) Indole-3-acetic acid biosynthesis in isolated axes from germinating bean seeds: the effect of wounding on the biosynthetic pathway. Plant Growth Regul 36: 201–207 [Google Scholar]
- Tao Y, Ferrer J-L, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tuinen A, Kerckhoffs L, Nagatani A, Kendrick RE, Koornneef M. (1995a) A temporarily red light-insensitive mutant of tomato lacks a light-stable, B-like phytochrome. Plant Physiol 108: 939–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tuinen A, Kerckhoffs LH, Nagatani A, Kendrick RE, Koornneef M. (1995b) Far-red light-insensitive, phytochrome A-deficient mutants of tomato. Mol Gen Genet 246: 133–141 [DOI] [PubMed] [Google Scholar]
- Weller JL, Perrotta G, Schreuder ME, van Tuinen A, Koornneef M, Giuliano G, Kendrick RE. (2001) Genetic dissection of blue-light sensing in tomato using mutants deficient in cryptochrome 1 and phytochromes A, B1 and B2. Plant J 25: 427–440 [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelená E. (2000a) The effect of light on metabolism of IAA in maize seedlings. Plant Growth Regul 30: 23–29 [Google Scholar]
- Zelená E. (2000b) The effect of light on IAA metabolism in different parts of maize seedlings in correlation with their growth. Plant Growth Regul 32: 239–243 [Google Scholar]
- Zeng J, Wang Q, Lin J, Deng K, Zhao X, Tang D, Liu X. (2010) Arabidopsis cryptochrome-1 restrains lateral root growth by inhibiting auxin transport. J Plant Physiol 167: 670–673 [DOI] [PubMed] [Google Scholar]