Abstract
Cytokinins are phytohormones that are involved in various regulatory processes throughout plant development, but they are also produced by pathogens and known to modulate plant immunity. A novel transgenic approach enabling autoregulated cytokinin synthesis in response to pathogen infection showed that cytokinins mediate enhanced resistance against the virulent hemibiotrophic pathogen Pseudomonas syringae pv tabaci. This was confirmed by two additional independent transgenic approaches to increase endogenous cytokinin production and by exogenous supply of adenine- and phenylurea-derived cytokinins. The cytokinin-mediated resistance strongly correlated with an increased level of bactericidal activities and up-regulated synthesis of the two major antimicrobial phytoalexins in tobacco (Nicotiana tabacum), scopoletin and capsidiol. The key role of these phytoalexins in the underlying mechanism was functionally proven by the finding that scopoletin and capsidiol substitute in planta for the cytokinin signal: phytoalexin pretreatment increased resistance against P. syringae. In contrast to a cytokinin defense mechanism in Arabidopsis (Arabidopsis thaliana) based on salicylic acid-dependent transcriptional control, the cytokinin-mediated resistance in tobacco is essentially independent from salicylic acid and differs in pathogen specificity. It is also independent of jasmonate levels, reactive oxygen species, and high sugar resistance. The novel function of cytokinins in the primary defense response of solanaceous plant species is rather mediated through a high phytoalexin-pathogen ratio in the early phase of infection, which efficiently restricts pathogen growth. The implications of this mechanism for the coevolution of host plants and cytokinin-producing pathogens and the practical application in agriculture are discussed.
Phytohormones affect various aspects of growth and differentiation in higher plants and are involved in both biotic and abiotic interactions. Among the plant hormones, ethylene (ET), salicylic acid (SA), and jasmonate (JA) are known for differentially regulating defense responses against biotrophic and necrotrophic pathogens and are considered as the immunity hormones (Bari and Jones, 2009; Tsuda and Katagiri, 2010). In contrast, only recently was the involvement of other hormones in plant-pathogen interactions recognized. In particular, a cross talk of abscisic acid, auxin, and GA3 signaling with the SA-JA-ET backbone of the defense signaling network has been elucidated in Arabidopsis (Arabidopsis thaliana; Grant and Jones, 2009; Pieterse et al., 2009).
The aminopurine-derived cytokinins (CKs) regulate many physiological and developmental processes in higher plants, including cell division, leaf senescence, nutrient mobilization, apical dominance, and seed germination (Sakakibara, 2006). CKs are also produced by a range of various microbial pathogens, including Pseudomonas syringae (Akiyoshi et al., 1987; Morris et al., 1991) and leaf-mining insects (Engelbrecht, 1968), causing the formation of green islands, galls, growth abnormalities, and manipulation of primary carbon metabolism (Balibrea Lara et al., 2004). For several pathogens, it was shown that this CK production is essential for infection (Crespi et al., 1994; Hwang et al., 2010). Based on the induction of sink metabolism by CKs (Ehness and Roitsch, 1997; Walters et al., 2008), it has been suggested that the host physiology is altered in response to CKs to allow the pathogen maximum access to nutrients early in (hemi)biotrophic interactions (Walters et al., 2008). In contrast, the role of plant-derived CKs in defense responses against pathogens that are unable to produce CKs is largely unknown. Elevated CK levels were shown to lead to resistance against different viruses (Sano et al., 1996; Pogany et al., 2004), suppression of the hypersensitive response (HR; Barna et al., 2008), and the induction of SA in the wounding response (Sano et al., 1996). Only recently, CKs were shown to promote resistance in the model pathosystem Arabidopsis-P. syringae pv tomato DC3000 via TGACG (TGA) MOTIF-BINDING PROTEIN3/NONEXPRESSOR OF PR PROTEINS1 (TGA3/NPR1)-dependent SA signaling (Choi et al., 2010).
The role of CKs in defense responses has been addressed by exogenous CK supply or by different functional approaches involving the ectopic expression of CK biosynthetic genes. However, interpretation of the available literature is complicated by the choice of promoters used for the transgenic approaches: (1) application of constitutive promoters to drive the expression of the bacterial isopentenyl transferase (ipt) gene dramatically alters growth, development, and the metabolic state of the host plant and thus indirectly affects the interaction (Sakakibara, 2006); (2) application of inducible promoters requires stimuli also known to interfere with defense responses such as light (Chandra-Shekara et al., 2006; Griebel and Zeier, 2008) and wounding (Sano et al., 1996). To overcome these limitations, we addressed the role of endogenous CK levels in pathogen responses by expression of the ipt gene from the Agrobacterium tumefaciens T-DNA, encoding the rate-limiting enzyme in CK biosynthesis, driven by the synthetic pathogen-inducible 4xJERE promoter (Rushton et al., 2002) for autoregulated CK production in response to infection by the hemibiotrophic bacterium P. syringae pv tabaci (Pst). Local pathogen-dependent increased CK levels at the infection site greatly enhanced resistance against Pst in tobacco (Nicotiana tabacum), which was further substantiated by two alternative independent transgenic approaches to modulate endogenous CK biosynthesis and two different ways of exogenous CK application. This CK-induced immunity in tobacco is essentially independent from SA signaling and unrelated to SA levels and also does not confer resistance against necrotrophic fungi, which is in contrast to the SA-dependent, transcriptionally regulated, CK-mediated resistance mechanism in Arabidopsis directed against P. syringae pv tomato (Choi et al., 2010). The CK-induced resistance in tobacco is predominantly mediated by induction of the major antimicrobial phytoalexins of tobacco, scopoletin and capsidiol, resulting in a high phytoalexin-pathogen ratio in the early phase of infection, which efficiently restricts pathogen growth.
RESULTS
Induction of Endogenous CK Biosynthesis in Tobacco Leaves Enhances Resistance against the Virulent Pst
The hemibiotrophic bacterium Pst is infectious for various tobacco cultivars and visible as chlorotic leaf spots that turn into dark brown to black necrosis. To address the role of CKs in the pathosystem tobacco-Pst, we engineered a novel autoregulated system for the pathogen-dependent induction of endogenous CK levels in plants. The synthetic pathogen-inducible 4xJERE promoter (Rushton et al., 2002) was used to drive the expression of the bacterial ipt gene from the A. tumefaciens T-DNA, encoding isopentenyl transferase that catalyzes the rate-limiting step in CK biosynthesis (4xJERE:ipt). This promoter comprises four repetitions of a 24-bp element and was shown to be jasmonate and elicitor responsive and strongly induced by various pathogens and wounding (Rushton et al., 2002). The following experimental scheme was carried out for local pathogen-induced CK production in tobacco leaves (Fig. 1A): cv W38 leaves were preinfiltrated with A. tumefaciens strain LBA4404 harboring either the 4xJERE:ipt construct or an empty vector. After 24 h, the infiltrated spots were subjected to a second infiltration either with PstT (see “Materials and Methods”) or with MgCl2 as a mock infection. RNA gel-blot analyses revealed a pathogen-dependent induction of the ipt transgene (Fig. 1B). Higher CK concentrations in the corresponding ipt-expressing regions corroborated functional transgenic expression (Supplemental Table S1).
Figure 1.
Accumulation of CKs by overexpressing ipt causes resistance against PstT. A, Experimental scheme for transient 4xJERE:ipt expression in tobacco leaves followed by PstT infection. Tobacco leaves were preinfiltrated with A. tumefaciens containing [At(ipt); blue line] or without [At(0); black line] the 4xJERE:ipt construct followed by a second infiltration with either PstT (Pst; red line) or a mock infiltration with MgCl2 (gray line) after 24 h. Numbers refer to time points of sampling for CK determination (Supplemental Table S1), JA and SA (Supplemental Table S2), free sugar (Supplemental Tables S3 and S4), antimicrobial activities (Table II), or phytoalexins (Table III). B, RNA-blot analysis of tobacco tissue transiently transformed with 4xJERE:ipt. A 28S rRNA loading control is shown at the bottom. C, Effect of transient 4xJERE:ipt expression and subsequent PstT infection on symptom development and green island formation. Tobacco leaves were preinfiltrated with A. tumefaciens with (ipt) or without (0) the 4xJERE:ipt construct followed by a second infiltration of PstT (Pst) or mock infiltration (0). The infiltrated areas are marked by circles. Photographs were taken at the indicated times (dpi). D, Effect of senescence-induced ipt expression in SAG12:ipt plants and subsequent PstT (red) infection on symptom development (left) in comparison with the wild type (right). E, Effect of tetracycline-induced ipt expression in TET:ipt plants and subsequent PstT infection on symptom development. Leaves of the transgenic TET:ipt tobacco line were preinfiltrated with tetracycline (TET; yellow) or water (0; gray) followed by a second infiltration with PstT (Pst; red). F, Effect of transient 4xJERE:ipt expression on the proliferation of PstT. Tobacco leaves were preinfiltrated with A. tumefaciens with (ipt) or without (0) the 4xJERE:ipt construct followed by a second infiltration with PstT. Bacteria were reisolated from leaf discs, plated, and quantified. Mean values ± sd of three independent replicates are shown.
In ipt-expressing regions challenged with PstT, pathogen resistance was markedly enhanced. Even 10 d post infection (dpi), only very mild symptoms developed in infiltrated regions (Fig. 1C), while control infiltrations (empty vector) showed disease symptoms already 2 dpi after infection, and in many cases the complete infiltrated tissue became necrotic (Fig. 1C). When leaves entered senescence, typically after a period of 3 to 4 weeks, the control leaf halves turned completely necrotic, whereas their ipt-expressing counterparts retained chlorophyll and were clearly visible as green islands, further supporting the stimulation of CK biosynthesis by the 4xJERE:ipt construct (Fig. 1C). Therefore, induction of ipt expression at the site of infection is highly effective in preventing disease development.
Since it has been shown that A. tumefaciens may interfere with symptom development after P. syringae infection (Rico et al., 2010), we employed two additional transgenic approaches, which confer ectopic elevation of endogenous CK levels independent of prior A. tumefaciens infiltration, to confirm the specificity of the observed CK effect. First, we used SAG12:ipt transgenic tobacco plants expressing the ipt gene under the control of a senescence-induced promoter (Gan and Amasino, 1995). Detached transgenic leaves were subjected to a heat shock to induce senescence and thus to stimulate CK biosynthesis, which were subsequently infiltrated with PstT. In such leaves, visible symptoms always developed much later and were clearly less pronounced than in the corresponding wild-type leaves (Fig. 1D). Second, we studied the development of disease symptoms in TET:ipt transgenic tobacco plants expressing ipt under the control of a chemically inducible, tetracycline-dependent promoter (Redig et al., 1996). Transgenic TET:ipt leaves were subjected to local preinfiltration with tetracycline to induce ipt expression or a corresponding mock infiltration, which were subsequently infiltrated with PstT. Disease symptoms appeared 2 dpi only at mock-treated sites but not at the sites of tetracycline-mediated CK biosynthesis. In tetracycline-treated spots, weak symptoms could be detected only 7 dpi, whereas mock-induced sites were already highly necrotic (Fig. 1E).
To determine the influence of altered CK levels on PstT proliferation, we reisolated bacteria from wild-type tobacco leaves following PstT infection, according to Figure 1A. As shown in Figure 1F, the number of bacteria started to increase strongly 12 h post infection (hpi) in the control incubation, whereas they remained low in samples with elevated CK levels. Therefore, the reduced disease development correlated both with the induction of CK biosynthesis and restricted pathogen growth. Thus, three independent transgenic approaches involving the ectopic induction of ipt expression in leaves by different mechanisms clearly demonstrated that the defense response is mediated by induced CK biosynthesis.
Exogenous Supply of CKs Also Enhances Resistance of Tobacco against Pst
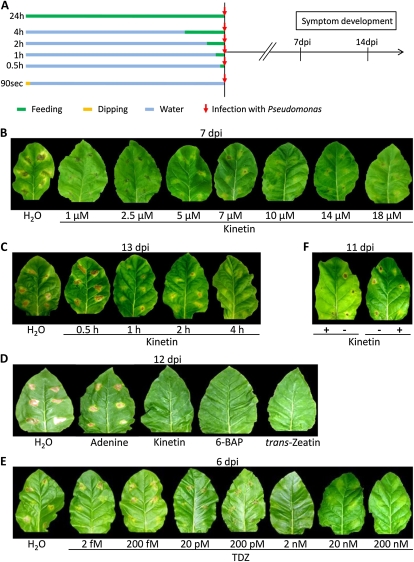
In addition to the three transgenic approaches, we tested the effect of feeding varying concentrations (1–18 μm) of kinetin, which is a naturally occurring adenine-derived CK (Ge et al., 2005), to detached leaves via petioles for 24 h, followed by infection with PstT, according to Figure 2A. Kinetin application enhanced the resistance against PstT infection in 86% of all treated leaves (275 out of 319; Fig. 2B) and in 95% of the leaves treated with 10 μm kinetin (144 out of 151), which was superior in preventing pathogen proliferation. Both lower and higher doses were less effective, with higher kinetin concentrations (14 and 18 μm) causing wilting. This is in agreement with the known CK effect on stomata opening (Meidner, 1967), which apparently increased host sensitivity toward the pathogen, interfering with the positive kinetin effect on pathogen resistance. These data demonstrate that exogenous CK feeding for 24 h enhanced resistance in a dose-dependent manner.
Figure 2.
Exogenously supplied CKs enhance resistance against PstT. A, Experimental scheme of exogenous applications of CKs by petiole feeding (green bars) and dipping (yellow bar) prior to infection with PstT (red arrows). The petioles from both CK-treated and control leaves were kept in water for symptom development. B, Effect of feeding varying kinetin concentrations (1–18 μm) 24 h prior to PstT infection on symptom development at 7 dpi. C, Effect of short-pulse feeding (0.5–4 h) with 10 μm kinetin prior to PstT infection on symptom development at 13 dpi. D, Comparison of efficiency to induce resistance (12 dpi) against PstT for different adenine-derived CKs applied in 10 μm concentration at 24 h prior to infection. BAP, 6-Benzyladenine. E, Dose-dependent (2 fm to 200 nm) ability of phenylurea-derived CK TDZ to induce resistance against PstT (6 dpi) by petiole feeding. F, Induction of resistance against PstT (11 dpi) by dipping leaf halves for 60 s (left) and 90 s (right) in 140 μm kinetin 24 h before infection. Leaves were infiltrated with PstT at three sites per leaf half (B–F).
Next, we analyzed whether shorter pulses of kinetin pretreatment are sufficient. Kinetin (10 μm) was fed to detached leaves for 0.5, 1, 2, and 4 h, followed by infection with PstT, according to Figure 2A. Supplying a kinetin pulse for 4 h was already sufficient to decrease symptom development in 75% of the infected leaves (18 out of 24; Fig. 2C). Shorter pretreatments were less effective but still led to about 25% of leaves with reduced symptoms (Fig. 2C).
To assess whether the time point of CK treatment relative to the time of infection is critical, the effect of feeding kinetin 24 h prior to infection according to the standard protocol (Fig. 2A) was compared with delayed CK pulses: feeding simultaneously with the time of infection, or 24 and 48 h thereafter, according to Figure 3A. Figure 3B shows that CK feeding simultaneously with the infection (0 hpi) was as efficient as the 24-h CK pretreatment (24 h before infection), with 77% (23 out of 30) of the infected leaves showing decreased symptom development. A delayed CK application was still effective, although it resulted in a time-dependent lower degree of resistance. The number of resistant leaves dropped to 67% (20 out of 30; 24 hpi) and 55% (16 out of 29; 48 hpi).
Figure 3.
Exogenously supplied CKs mediate resistance before as well as after PstT infection. A, Experimental scheme of varying starting times of the 24-h kinetin pulse (10 μm) relative to infection with PstT (24 h before infection [hbi] to 48 hpi). B, Effect of varying starting times of the 24-h kinetin pulse (10 μm) relative to infection with PstT (24 hbi to 48 hpi) on symptom development at 5 dpi. C, Experimental scheme for analysis of the continuance of CK-induced resistance with infections at different time points after CK feeding. D, CK-induced resistance effect (12 dpi) in leaves infected with PstT at the end of CK application (0 dpc) and several days after CK pulse (2–7 dpc) in comparison with the water control. Leaves were infiltrated at three sites with PstT (left) and 10 mm MgCl2 (right; B and D).
For possible practical applications, it is important to determine how long the protection of a single CK treatment lasts. Therefore, we fed leaves with 10 μm kinetin for 24 h and infected them either immediately thereafter according to the standard protocol or 2, 4, and 7 d after the end of the kinetin pulse, according to Figure 3C. Figure 3D shows that the degree of protection gradually decreased upon delayed PstT infection following CK pretreatment. Compared with the standard protocol (0 d past cytokinin [dpc]), 91% of the infected leaves (21 out of 23) showed reduced symptom development when the infection took place 2 d (2 dpc) after the end of the CK treatment, whereas 57% of the leaves (13 out of 23) still showed reduced symptom development when leaves were infected even 4 d (4 dpc) and 7 d (13 out of 23; 7 dpc) after the kinetin pulse. Thus, a single kinetin pulse is sufficient to confer at least a limited resistance for 1 complete week.
The effect of kinetin was compared with other adenine-type CKs (Fig. 2D) to investigate the specificity of the type of CK in mediating the increased resistance. Both transzeatin and 6-benzyladenine were as efficient as kinetin, each resulting in 97% resistant leaves, whereas control incubations with adenine were not effective. Phenylurea-derived compounds such as thidiazuron (TDZ) are also known to exhibit CK activity (Bruce and Zwar, 1966), and 84% of all TDZ-treated leaves (96 out of 114) displayed highly reduced symptoms compared with control incubations. Figure 2E shows that concentrations as low as 2 nm TDZ were sufficient to induce immunity and that a concentration of 200 nm TDZ induced resistance in virtually all treated leaves (96%; 24 out of 25). Therefore, TDZ proved to be even more potent than the adenine-type CKs.
To address the possible transfer of the identified CK-mediated resistance mechanism to crop species via application methods that are feasible in agriculture and do not require transgenic plants, we tested whether the resistance can be induced by supplying CKs via the leaf surface. Kinetin was supplied by dipping one leaf half into a kinetin solution (140 μm; Mothes, 1960) for only 60 to 90 s, whereas the second leaf half was left untreated as a control. This alternative way of kinetin application reduced symptom development in 81% of the dipped leaf halves (21 out of 28) compared with nontreated halves (Fig. 2F). Although kinetin supply via the leaf surface is not as efficient as petiole feeding of detached leaves, a highly elevated level of resistance could be obtained, demonstrating a high potential for practical application. Likewise, spraying 140 μm kinetin on single detached leaves resulted in resistance against PstT.
To gain insight into the specificity of the CK-mediated resistance mechanism, the effect of CKs on additional pathosystems was tested. According to Figure 2A, leaves were fed with kinetin for 24 h and infected with the avirulent pathogen P. syringae pv phaseolicola (Psp). No effect of the CK pretreatment on HR development was observed (Supplemental Fig. S1A). Likewise, the number of Psp bacteria present in the tissue was not affected. In addition, the spread of the necrotrophic fungus Botrytis cinerea was strongly enhanced in CK-pretreated leaves, evident from the formation of extended necrotic areas (Supplemental Fig. S1B), whereas susceptibility toward Sclerotinia sclerotiorum was not affected by the elevated CK levels (Supplemental Fig. S1C). Thus, in contrast to the CK-mediated resistance against the hemibiotrophic bacterium PstT, elevated CK levels do not enhance resistance against the two tested necrotrophic fungi. In the case of B. cinerea, CK treatment rather had the opposite effect and even enhanced the susceptibility of tobacco.
The experiments involving exogenous CK application provide independent and complementing experimental support that CKs in tobacco mediate resistance specifically toward PstT in a dose-dependent manner, with the possibility for easy transfer of this technology to crop species.
The CK-Mediated Resistance Mechanism in Tobacco Is Independent of SA Signaling, JA Levels, Reactive Oxygen Species, High Sugar Resistance, and Extracellular Invertase Function
It has been established that SA signaling is generally important for immunity against biotrophs or hemibiotrophs such as Pst, while JA signaling generally is relevant for defense responses directed against necrotrophs, although there are exceptions (Tsuda and Katagiri, 2010). Therefore, we determined SA levels following PstT infection according to Figure 1A for transient expression of the 4xJERE:ipt construct and according to Figure 2A for CK feeding. The finding that SA levels were not specifically affected by the various treatments (Table I; Supplemental Table S2) indicates that the CK-mediated resistance is unrelated to SA levels. Furthermore, leaves of a transgenic tobacco line (cv Samsun NN) expressing the PATHOGENESIS-RELATED GENE1a (PR1a):GUS reporter construct (Grüner et al., 2003), a widely used SA marker, were fed with 10 or 40 μm kinetin for 24 h, according to Figure 2A. Only feeding with 40 μm kinetin led to weak local induction of the PR1a promoter, as evident by scattered GUS staining (Supplemental Fig. S2A). Likewise, PR1a mRNA levels were weakly affected by CK but strongly by PstT infection (Supplemental Fig. S2B). However, the presence of elevated CK levels resulted in synergistic PstT induction of the PR1a mRNA. Semiquantitative reverse transcription (RT)-PCR expression analysis (Supplemental Table S7; Supplemental Materials and Methods S1) was performed to investigate additional markers for SA-JA-ET signaling and secondary metabolite biosynthesis. This showed that although ENHANCED DISEASE SUSCEPTIBILITY1 expression was clearly transiently induced directly following CK treatment (24h K/0), the NPR1 gene downstream in the SA pathway was not affected. Furthermore, the PR1 genes are slightly induced by CK treatment but considerably stronger in combination with Pst infection (K+Ps/0), corroborating the northern expression data (Supplemental Fig. S2B). Thus, the additional marker analysis supports our conclusion that CK-mediated resistance is essentially independent from SA signaling. To further address whether CK-mediated resistance functions through the regulation of SA levels, we tested the effect of CK feeding on PstT infection in a transgenic tobacco line expressing the nahG gene encoding the SA-degrading salicylate hydroxylase from Pseudomonas putida. Indeed, the SA levels are strongly reduced in the nahG transgenic plant compared with the wild type (Supplemental Fig. S4). Our results corroborated earlier findings that the nahG transgenic plants are more susceptible to infection by Pst (Delaney et al., 1994). However, the CK treatment was still able to confer an increased resistance against Pst (Fig. 4A; Supplemental Fig. S3) independent of SA levels (Supplemental Fig. S4, A and B), and accordingly, phytoalexin production was stimulated to a similar degree (ratio between kinetin feeding and control) as in the wild type. Thus, different lines of experimental evidence show that the CK-mediated resistance is essentially independent from SA signaling and unrelated to SA levels.
Table I. SA and JA levels are not specifically affected by CK feeding.
According to the experimental scheme shown in Figure 2A, samples were obtained at different time points (h) from tobacco leaves pretreated with kinetin (Kin) or water (0) followed by infiltration with PstT (Pst) or a mock infiltration (0). Sample = sampling time-first infiltration-second infiltration. Mean values of two technical replicates ± sd from three biological replicates are shown. For JA determination, the levels for one biological replicate were below the level of detection in most individual samples, and this replicate was omitted from the analysis. * Significantly different after CK feeding from the control treatment (P < 0.05); + significantly different after Pst infection from the control treatment (P < 0.05).
| Sample | SA | JA |
| ng g−1 fresh wt | ||
| 0-0-0 | 25.3 ± 5.8 | 20.0 ± 11.9 |
| 24-0-0 | 53.1 ± 24.5 | 29.5 ± 6.7 |
| 24-Kin-0 | 70.9 ± 10.6 | 17.5 ± 9.5 |
| 36-0-0 | 109.4 ± 24.3 | 20.7 ± 8.4 |
| 36-Kin-0 | 275.6 ± 13.3* | 33.6 ± 16.3 |
| 36-0-Pst | 440.1 ± 283.2+ | 26.2 ± 12.2 |
| 36-Kin-Pst | 1,032.5 ± 610.7+ | 38.5 ± 20.3 |
| 48-0-0 | 65.7 ± 31.5 | 36.9 ± 11.6 |
| 48-Kin-0 | 185.1 ± 127.1 | 40.3 ± 21.2 |
| 48-0-Pst | 807.0 ± 315.0+ | 58.2 ± 43.4 |
| 48-Kin-Pst | 980.2 ± 103.6+ | 54.7 ± 25.6 |
Figure 4.
CKs induce resistance independent of SA, JA, ROS, and extracellular invertase activity. A, Effect of a 24-h kinetin application (10 μm) before PstT infection on symptom development in nahG-overexpressing tobacco leaves in comparison with the control at 8 dpi. Leaves were infiltrated at two sites with PstT (left) and 10 mm MgCl2 (right). B, Effect of transient 4xJERE:ipt expression and PstT infection on H2O2 formation. Tobacco leaves were preinfiltrated with A. tumefaciens with (ipt) or without (0) the 4xJERE:ipt construct followed by a second infiltration with PstT (Pst). Leaves were stained with DAB to visualize H2O2 formation at 24 h after preinfiltration of A. tumefaciens (0 hpi; left), 24 h after infiltration of PstT (24 hpi; middle), and at the time of symptom development 168 h after PstT infiltration (168 hpi; right). C, Effect of CIN1 expression in TET:CIN1 tobacco leaves induced by a 24-h feeding of 1 mg L−1 TET in comparison with a 24-h application of 10 μm kinetin prior to infection and water control treatment on symptom development at 10 dpi. Leaves were infiltrated with PstT at two sites per leaf half.
JA is typically not involved in defense responses against Pst but is involved in mediating wounding-specific defense responses. Since the infiltration procedures for transient expression of the 4xJERE:ipt construct and PstT infection could potentially result in mechanical wounding of the tissue, we addressed whether JA levels are affected. Supplemental Table S2 and Table I show that JA levels were low and not specifically affected in any of the treatments. The expression analysis for CORONATINE INSENSITIVE1 and ETHYLENE INSENSITIVE2 (Supplemental Table S7) not only confirmed that the CK-mediated resistance is independent from JA but also showed independence from ET signaling. Thus, neither the elevated CK levels nor our experimental approach interfered with the synthesis of the JA defense signal.
Reactive oxygen species (ROS) also play an important role in stress responses. In host plants, they cause strengthening of the cell wall, may kill intruders, and act as signaling molecules to activate defense pathways (Torres et al., 2006). No difference in the low level of hydrogen peroxide (H2O2) accumulation visualized by diaminobenzidine (DAB) staining could be detected after infiltration of A. tumefaciens (4xJERE:ipt) or the corresponding empty vector strain, ruling out an effect of A. tumefaciens on ROS status (Fig. 4B). After infiltration of PstT, no differences in DAB staining were visible up to 24 hpi and thus prior to symptom development. In contrast, a relatively high amount of H2O2 production was detected in non-ipt-expressing areas displaying severe necrosis 168 hpi compared with areas of increased ipt levels. Thus, the CK-mediated resistance is not caused by prior accumulation of ROS. Rather, increased ROS levels were the consequence of pathogen spread and concomitantly killed host cells.
Since a mechanism of high sugar resistance (Horsfall and Dimond, 1957) and sugar-mediated defense gene induction (Herbers et al., 1996; Ehness et al., 1997) have been described, we also addressed the role of carbohydrates in CK-mediated resistance. The levels of the major soluble carbohydrates (Glc, Fru, Suc) and starch varied significantly in different experiments but were not specifically altered in leaves with increased CK levels in the presence of PstT (Supplemental Tables S3 and S4) and did not correlate with the CK-mediated resistance. Extracellular invertase, a key Suc-cleaving sink enzyme required to supply carbohydrates via an apoplasmic pathway (Roitsch and González, 2004), has been shown to be required for defense responses against various pathogens (Berger et al., 2007; Bonfig et al., 2010) and to be induced by CKs in various species, including tobacco (Ehness and Roitsch, 1997; Balibrea Lara et al., 2004). Therefore, we tested whether a CK-induced extracellular invertase is involved in the CK-mediated resistance against PstT, employing the tetracycline-inducible promoter system (Balibrea Lara et al., 2004). Figure 4C shows that induction of the extracellular invertase CIN1 from Chenopodium rubrum by either local infiltration of tetracycline or feeding of tetracycline via leaf petioles did not affect subsequent PstT infection. However, when CK levels were elevated in leaves of these transgenic plants, by either feeding of kinetin via the petioles or local 4xJERE:ipt expression, resistance was strongly enhanced. These results demonstrate that the CK-mediated resistance is not directly or indirectly related to an elevated sugar status and is independent of the metabolic function of extracellular invertase.
CKs Induce the Synthesis of Bactericidal Activities
Since the CK-mediated resistance strongly interfered with growth of the infiltrated bacteria (Fig. 1E), we analyzed the possible involvement of antimicrobial activities in this resistance mechanism. Bioautography experiments with extracts derived from experiments involving transient expression of the 4xJERE:ipt construct according to Figure 1A failed to detect any antimicrobial activity in the CK-stimulated tissues, indicating the absence of any antimicrobial peptides or proteins active against PstT. Next, disc diffusion assays were performed to address the possible involvement of low-Mr antimicrobial compounds in the CK-induced immunity. Table II demonstrates that extracts derived from samples with elevated CK levels contained antimicrobial activities that are highly active against PstT. In addition, the growth of three other gram-negative bacteria tested, Escherichia coli, Pseudomonas aeruginosa, and Psp, was affected, although to different degrees. In contrast, growth of the two tested gram-positive bacteria, Bacillus subtilis and Staphylococcus aureus, and of the yeast Candida maltosa was only slightly inhibited. Growth of the two necrotrophic fungi S. sclerotiorum and B. cinerea was not restricted, and growth of the latter even seemed to be promoted, which is in agreement with the finding that the induced antimicrobial activities are inactive against the necrotrophic fungi tested (Supplemental Fig. S1, B and C). Control incubations ruled out that CK had an inherent antimicrobial activity in disc diffusion assays or when directly applied to cultures of PstT, demonstrating that the antimicrobial activities are specifically induced in response to the elevated CK levels.
Table II. Disc diffusion assay for antimicrobial activities.
According to the experimental scheme shown in Figure 1A, samples were obtained at different time points (h) from tobacco leaves preinfiltrated with A. tumefaciens with (ipt) or without (0) the 4xJERE:ipt construct followed by infiltration of PstT (Pst) or a mock infiltration (0). Sample = sampling time-first infiltration-second infiltration. d.o., Disc overgrown. Values are means of two independent experiments.
| Diameter of Inhibition Zone |
||||||||||
| Sample No. According to Figure 1A | Sample | Gram-Positive Bacteria |
Gram-Negative Bacteria | Fungi |
||||||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | Psp | Pst | C. maltosa | B. cinerea | S. sclerotiorum | ||
| mm | ||||||||||
| 1 | 0-0-0 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 2 | 24-ipt-0 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | d.o. | 0 |
| 3 | 24-0-0 | 1 | 1 | 0 | 2 | 1 | 2 | 1 | 0 | 0 |
| 8 | 48-ipt-Pst | 3 | 3 | 7 | 4 | 8 | 9 | 3 | d.o. | 0 |
| 9 | 48-0-Pst | 2 | 2 | 1 | 2 | 3 | 3 | 2 | 0 | 0 |
CK-Induced Phytoalexin Production Causes Pathogen Resistance
Phytoalexins and glucosinolate compounds were shown to be essential in pathogen resistance (Ren et al., 2008; Bednarek et al., 2009; Lu et al., 2009). Since the sesquiterpene capsidiol and the hydroxycoumarin scopoletin are the two prominent antimicrobial compounds in Solanaceae (Brooks et al., 1986; Ahl Goy et al., 1993), we tested by quantitative instrumental analyses whether the levels of these phytoalexins were influenced by the CK status of the plant following transient expression of the 4xJERE:ipt construct, according to Figure 1A. Scopoletin and capsidiol levels were 6.4- and 4.6-fold higher, respectively, in ipt-expressing samples compared with the corresponding controls (Table III). Therefore, the CK-induced biosynthesis for the two major phytoalexins of tobacco, the sesquiterpene capsidiol and the hydroxycoumarin scopoletin, is in agreement with the CK-dependent increase in bactericidal activities.
Table III. Scopoletin and capsidiol production is stimulated by CK.
According to the experimental scheme shown in Figure 1A, samples were obtained at different time points (h) from tobacco leaves preinfiltrated with A. tumefaciens with (ipt) or without (0) the 4xJERE:ipt construct followed by infiltration of PstT (Pst) or a mock infiltration (0). Sample = sampling time-first infiltration-second infiltration. N.D., Not determined. Mean values of two technical replicates ± sd from one biological sample are shown.
| Sample No. According to Figure 1A | Sample | Scopoletin | Ratio ipt:0 | Capsidiol | Ratio ipt:0 |
| nmol g−1 fresh wt | nmol g−1 fresh wt | ||||
| 1 | 0-0-0 | 3.1 ± 1.21 | 4.3 ± 0.98 | ||
| 2 | 24-ipt-0 | 7.4 ± 2.94 | 1.10 | N.D. | N.D. |
| 3 | 24-0-0 | 6.7 ± 3.64 | N.D. | ||
| 4 | 30-ipt-Pst | 11.3 ± 4.33 | 1.20 | N.D. | N.D. |
| 5 | 30-0-Pst | 9.4 ± 4.85 | N.D. | ||
| 6 | 36-ipt-Pst | 24.3 ± 1.56 | 1.90 | N.D. | N.D. |
| 7 | 36-0-Pst | 12.8 ± 1.56 | N.D. | ||
| 8 | 48-ipt-Pst | 78.8 ± 10.28 | 6.40 | 74.5 ± 6.04 | 4.63 |
| 9 | 48-0-Pst | 12.3 ± 1.85 | 16.1 ± 2.66 |
Next, we performed time-course analyses of phytoalexin production in relation to bacterial growth in CK feeding experiments, according to Figure 2A, to rule out an influence of prior A. tumefaciens infiltration. CK feeding followed by PstT infection resulted in higher scopoletin levels compared with the control in the early phase of infection (Supplemental Table S5). Consequently, the higher scopoletin levels preceded the increased bacterial growth (Fig. 5A), and at the onset of bacterial growth (12 hpi), the phytoalexin-bacteria ratio was 10-fold higher than that in control infections (Supplemental Fig. S5). Concomitantly, bacterial growth was restricted and even abolished in the late phase of the infection (48–96 hpi) compared with the control treatment (Fig. 5, A and B; Supplemental Tables S5 and S6). Although in control treatments, the absolute scopoletin production following PstT infection reached very high levels in the late phase of infection (48–96 hpi), exceeding the maximum scopoletin levels in CK-pretreated leaves by almost 8-fold (Supplemental Table S5), the increased scopoletin production occurred only after the onset of bacterial growth (Fig. 5B). This resulted in a low phytoalexin-bacteria ratio throughout the infection phase (Supplemental Fig. S5); concomitantly, bacterial growth was unrestricted (Fig. 5B; Supplemental Table S6). Thus, two independent experimental approaches showed that the CK-mediated resistance against PstT strongly correlated with increased phytoalexin biosynthesis.
Figure 5.
CK-induced phytoalexin production causes pathogen resistance. A, Experimental data for time-course analysis of scopoletin production (blue line with circles; nmol g−1 fresh weight [fw]) in relation to bacterial growth (red line with squares; cfu mL−1) in PstT-infected leaves pretreated with CKs. Note the different scale for the scopoletin levels compared with B. B, Experimental data for scopoletin production and bacterial growth in PstT-infected leaves (control). C, Model for CK-mediated phytoalexin production based on A. CK feeding mediates increased phytoalexin levels, resulting in a high phytoalexin-bacteria ratio, which restricts bacterial growth in the early infection phase. In the late infection phase, bacterial growth is abolished, probably due to additional defense mechanisms triggered by the bacterial infection. D, Model for pathogen-induced (control) phytoalexin production based on B. Phytoalexin production is triggered by the bacterial infection, but the increase occurs after the onset of bacterial growth. This results in a low phytoalexin-bacteria ratio throughout the infection phase and consequently unrestricted bacterial growth. E, RNA-blot analyses of tobacco tissue transiently transformed with 4xJERE:ipt for expression levels of EAS, C4H, and TOGT. A 28S rRNA loading control is shown at the bottom. F, Phytoalexin infiltration confers pathogen resistance, as evident by reduced symptom development 6 dpi. W38 leaves were infiltrated with different concentrations of capsidiol and scopoletin (200–400 μm; yellow) or controls (gray) simultaneous with PstT bacteria (107 cfu mL−1; red).
To investigate the regulatory mechanism involved in the CK-mediated increase of scopoletin and capsidiol levels, the mRNA levels for key enzymes involved in the synthesis or remobilization of both phytoalexins were determined by RNA gel blots. Figure 5E shows that the expression of 5-epi-aristolochene-synthase (EAS), catalyzing a rate-limiting step in capsidiol biosynthesis, and cinnamic acid 4-hydroxylase (C4H), catalyzing the irreversible conversion of cinnamic acid into p-coumaric acid that is ultimately converted into scopoletin, were induced in response to induction of the ipt gene, which was corroborated with semiquantitative RT-PCR (Supplemental Table S7; Supplemental Materials and Methods S1). In contrast, the induction of phenylpropanoid-glucosyltransferase (TOGT), mediating the reversible conversion of the storage form scopolin into scopoletin, was delayed and much less pronounced, indicating that CKs primarily affect synthesis rather than remobilization. Thus, elevated CK levels resulted in increased expression for key enzymes involved in phytoalexin synthesis prior to pathogen growth, eventually leading to enhanced scopoletin and capsidiol levels. Secondary metabolite production is not generally increased by elevated CK levels, since PHENYLALANINE AMMONIA LYASE1 expression was not significantly affected, whereas FLAVONOL SYNTHASE1 expression (flavonoid synthesis) was even repressed (Supplemental Table S7).
Because of the strong correlation between increased phytoalexin levels and CK-mediated pathogen resistance, we analyzed whether CK-induced pathogen resistance is mediated through scopoletin and capsidiol. Loss-of-function analysis in tobacco requires transgenic knockdown regulation of scopoletin and capsidiol biosynthesis, which is limited by incomplete knowledge of the genes involved in the final biochemical conversion, alternative biosynthesis routes, and the strength of the knockdown regulation. Alternatively, manipulation of key enzymes in phytoalexin biosynthesis would affect a large range of phenolic compounds, resulting in negative side effects. Therefore, we chose a gain-of-function approach by analyzing the resistance against PstT infection following prior application of the major phytoalexins scopoletin and capsidiol. Based on the scopoletin and capsidiol concentrations determined in W38 leaves following local 4xJERE:ipt expression in response to PstT infection (Table III), we infiltrated wild-type W38 tobacco leaves with various concentrations of scopoletin and capsidiol simultaneous with PstT. The phytoalexin infiltrations conferred reduced symptom development (6 dpi) in 69% of the leaves treated with both scopoletin and capsidiol (172 out of 251), independent of the infiltrated concentrations (Fig. 5F), as evident by reduced bacterial growth in the treated leaves (Supplemental Table S8). The treatment with phytoalexins did not affect hormone levels in the infiltrated regions, which shows that the late increase in SA levels following CK feeding (Supplemental Fig. S4C) is brought about by CKs rather than a secondary effect of increased phytoalexin levels. Similar to the CK-mediated resistance (Supplemental Fig. S1C), treatment with scopoletin and capsidiol did not reduce symptom development by B. cinerea (Supplemental Fig. S6). This showed that phytoalexin infiltration at concentrations present in response to elevated CK levels substituted in planta for the CK signal and mimicked the CK-mediated pathogen resistance. Thus, the CK-induced resistance is predominantly mediated by increased production of antimicrobial phytoalexins.
DISCUSSION
This study demonstrates that CKs induce resistance in tobacco against the virulent hemibiotrophic bacterial pathogen Pst, which is predominantly mediated by increased synthesis of the two major antimicrobial phytoalexins, scopoletin and capsidiol, resulting in a high phytoalexin-pathogen ratio in the early phase of infection, efficiently restricting pathogen growth. This CK-mediated resistance was substantiated by three functionally independent transgenic approaches: a novel pathogen-inducible expression system to induce endogenous CK biosynthesis and two different ways of exogenously supplying CKs. The use of the synthetic 4xJERE promoter (Rushton et al., 2002) for autoregulated synthesis of CKs in response to pathogen infection proved to be a valuable tool to dissect the primary role of CKs in defense from indirect effects related to the developmental stage and/or the metabolic status. The high percentage of leaves with increased resistance following CK treatment among at least 1,500 individual replicate leaves, reflecting more than 6,200 infection sites analyzed, demonstrates the reproducibility and robustness of the observed CK-mediated pathogen resistance in our study despite the varying greenhouse conditions. Importantly, this resistance mechanism was effective even when initial defense reactions of the host plant failed. The protection was restricted to tissues with elevated CK levels, revealed by the various local treatments on a single leaf, ruling out a systemic effect on other parts of the plant. This study extends the well-established role of CKs in regulating various aspects of plant growth and development to triggering the primary plant defense response through increased phytoalexin production.
CKs were recently shown to also induce resistance in the virulent interaction of Arabidopsis with P. syringe pv tomato (Choi et al., 2010). However, distinct species-specific differences from tobacco are apparent, both in the underlying regulatory mechanism and the specificity of the immunity. In Arabidopsis, the CK-mediated resistance is a solely transcriptional control mechanism that interferes with SA-dependent and most likely also JA-dependent signaling pathways to enhance resistance against bacterial and also fungal pathogens (Choi et al., 2010). This demonstrates that in Arabidopsis, in addition to auxin, abscisic acid, and GAs, also CKs cross-communicate with the central ET-SA-JA signal transduction pathways in immunity. In contrast, in tobacco, the CK-mediated resistance is induced also in SA-depleted (nahG-overexpressing) plants, whereas CKs cannot rescue the pathogen susceptibility in nahG transgenic Arabidopsis plants. In addition, the CK-mediated resistance in tobacco is independent from SA and JA levels, although the synergistic effect of CKs on pathogen-dependent PR1a expression is still conserved, and this resistance is rather mediated through an increased antimicrobial phytoalexin production. Another striking difference is that in tobacco, CKs conferred resistance only against the bacterial pathogen Pst but not against the two necrotrophic fungi tested, B. cinerea and S. sclerotiorum, whereas in Arabidopsis, the resistance was enhanced against bacterial as well as necrotrophic fungal pathogens.
CK-Induced Phytoalexin Production Causes Pathogen Resistance in Tobacco
The two main phytoalexins known from tobacco and other solanaceous species are the sesquiterpene capsidiol and the hydroxycoumarin scopoletin (Brooks et al., 1986; Ahl Goy et al., 1993). Their antimicrobial activity against various pathogens is well established, and they accumulate to high levels in infected plants (Dropkin et al., 1969; Ward et al., 1974; Kim et al., 2000; El Oirdi et al., 2010). Scopoletin and capsidiol are often restricted to diseased tissues (Guedes et al., 1982) or a ring of apparently healthy cells around lesions (Chong et al., 2002). The determined specificity of the antimicrobial activities induced by CKs is in agreement with the specificity of scopoletin and capsidiol against microbial pathogens. It was shown that capsidiol levels were not affected in SA-overproducing tobacco (Nugroho et al., 2002). Furthermore, the promoter of the tobacco EAS4 gene, central for capsidiol synthesis (Bohlmann et al., 2002), strongly responded to pathogen or elicitor treatment but not to SA, JA, or H2O2 (Yin et al., 1997). This is in agreement with our observation that the CK-mediated resistance is independent from these stress signaling compounds and with our conclusion that it is predominantly mediated by the observed elevated levels of capsidiol and scopoletin. Indeed, CK feeding still induced scopoletin production in nahG transgenic plants at similar ratios compared with wild-type plants. Interestingly, in Arabidopsis, phytoalexins were shown to be essential for resistance against fungal pathogens, while SA signaling was not involved in either resistance or phytoalexin production (Ferrari et al., 2007; Lu et al., 2009). Thus, the involvement of phytoalexins in the host range specificity exemplifies another species-specific difference between Arabidopsis and tobacco. Only two previous studies addressed the effect of CK feeding on phytoalexin production. In one case, scopoletin levels in tobacco tissues treated with CK dramatically decreased while scopolin, the presumed storage form, increased significantly (Skoog and Montaldi, 1961). This discrepancy with our findings may originate from the fact that an artificial tissue culture system was used by those authors. In the other case, the levels of phenolic compounds, including the scopoletin precursor p-coumaric acid, were increased in CK-overproducing ipt transgenic tobacco plants, whereas SA levels were reduced (Schnablová et al., 2006).
In this work, we focused on the two major phytoalexins of tobacco. The combined scopoletin and capsidiol infiltration conferred stronger pathogen resistance compared with either single treatment, which shows that the bactericidal effect of these phytoalexins is either additive or synergistic. Nevertheless, the increased pathogen resistance resulting from the combined scopoletin and capsidiol infiltration was not as prominent as that mediated by increased CK levels. At least 13 additional phytoalexins have been described for Nicotiana species (Tanaka and Fujimori, 1985; Nugroho and Verpoorte, 2002), and also in other plant species, many different phytoalexins are produced following pathogen infection (Bednarek et al., 2005; Pedras et al., 2008). Therefore, it is possible that the two most prominent phytoalexins, capsidiol and scopoletin, account for just a part of the antimicrobial activity mediated by increased CK levels in tobacco leaves and that several phytoalexins act in concert to ward off pathogens. Furthermore, an effect of the subcellular phytoalexin compartmentalization on its antimicrobial activity cannot be ruled out (Rogers et al., 1996). Due to the experimental approach, the phytoalexin infiltration resulted in increased extracellular phytoalexin levels. Considering the time lag until the onset of bacterial growth (12 hpi), it is possible that the infiltrated phytoalexins remaining in the extracellular space are modified, reducing their antimicrobial activity. In addition, diffusion, active transport, and metabolism can take place, reducing the level of active phytoalexins following infiltration. Indeed, scopoletin infiltration and recovery experiments revealed a substantial reduction of the infiltrated scopoletin amounts to physiological levels, which efficiently inhibited bacterial proliferation. On the other hand, high phytoalexin levels can confer cell death (Rogers et al., 1996; Kim et al., 2005), limiting the concentration range that could be used in the phytoalexin infiltration experiment. Considering these limitations, it is remarkable that exogenously supplied scopoletin and capsidiol substituted for the CK signal and mimicked the CK-mediated pathogen resistance, thereby functionally identifying in planta scopoletin and capsidiol as the main antimicrobial phytoalexins in tobacco. Thus, the CK-induced pathogen resistance is predominantly mediated by increased antimicrobial phytoalexin production.
Time-course analysis of scopoletin production and bacterial growth in CK feeding experiments showed that the CK-mediated scopoletin production, strengthened by pathogen-triggered scopoletin production, resulted in an increased phytoalexin-pathogen ratio in the early phase of infection, which efficiently restricted pathogen growth (Fig. 5, A and C; Supplemental Table S6). Following pathogen infection, a complex signaling network comprising many interacting defense pathways is activated in plants (Sato et al., 2010). Since the bacterial growth initially continued in the presence of increased scopoletin levels after CK feeding (Fig. 5A), either certain phytoalexin threshold levels are required for bactericidal activities or other defense mechanisms participate in the complete abolishment of bacterial growth in the late infection phase. Probably, these additional defense mechanisms are induced following pathogen infection and can only function properly when pathogen growth is already restricted, as observed for the CK-mediated immunity. The weaker pathogen resistance observed following phytoalexin treatment and in CK-treated nahG transgenics, and the late increase in SA levels with respect to the increased phytoalexin levels following CK treatment in wild-type plants (Table I; Supplemental Fig. S4C), suggest that SA-dependent pathogen responses act as an additional defense mechanism to eliminate the pathogens weakened by the bactericidal phytoalexins. In contrast, in control treatments, the pathogen-triggered scopoletin production reached very high levels, exceeding that in CK-pretreated leaves by almost 8-fold. However, this increase occurred only after the onset of bacterial growth (Fig. 5, B and D), resulting in a low phytoalexin-pathogen ratio throughout the infection phase (Supplemental Fig. S5). Therefore, the scopoletin production occurred too late to restrict pathogen growth (Fig. 5, B and D).
The Role of CKs in Plant Stress Resistance
The plant hormone CK is emerging as a new important player in stress response signaling, and in addition to its role in pathogen resistance, it is also important in promoting the tolerance toward abiotic stress conditions (Tran et al., 2007; Rivero et al., 2009; Ghanem et al., 2011). In agreement with our model on the CK-mediated defense mechanism in tobacco, several bacteria have been described that exert positive effects on plants by promoting plant growth, controlling diseases (Compant et al., 2005; Wu et al., 2009), and/or increasing tolerance to abiotic stress (Mayak et al., 2004). Corresponding to the plant growth promotion by such biocontrol bacteria, also the production and secretion of different phytohormones has been shown (Boiero et al., 2007; Wu et al., 2009), including notably CKs (García de Salamone et al., 2001; Ortíz-Castro et al., 2008; Abo-Elyousr and Mohamed, 2009; Wu et al., 2009). The CK production by the plant growth-promoting microorganisms as well as CK signaling in plants were shown to be essential for the beneficial effect on plant growth (Arkhipova et al., 2005; López-Bucio et al., 2007; Ortíz-Castro et al., 2008). So far, results from studies that linked CKs with either resistance or susceptibility to herbivores and pathogens were mostly inconclusive (Dropkin et al., 1969; Beckman and Ingram, 1994; Choi et al., 2010). In our study, this beneficial effect of CKs is linked to natural infection conditions, and we show that CKs mediate pathogen resistance by increasing phytoalexin production in tobacco.
Infection of crop species by fungal and bacterial pathogens results in dramatic losses in harvest yield, which are usually fought against with pesticides. With respect to their negative impact on human health and increased resistance of microbial pathogens against commercially available pesticides, the practical application of the CK-mediated resistance could complement the protection of various crop species to increase harvest yield and contribute to food safety. Possible applications include transgenic plants harboring a pathogen-inducible ipt gene (4xJERE:ipt), spraying of CKs, or the application of CK-producing biocontrol microorganisms. We routinely observed the CK-mediated pathogen resistance under many independent greenhouse experiments, which are subject to varying growth conditions, and under stable growth conditions in controlled growth chambers. This shows that this novel pathogen resistance mechanism is robust, an important requirement for practical agronomical application. Our data demonstrate that a single, not yet optimized, CK treatment is sufficient to provide at least a limited protection against pathogen infection for a minimum of 1 week, supporting the possible agronomical practical application.
The species-specific differences in the CK-mediated defense mechanism between Arabidopsis (Choi et al., 2010) and tobacco (this study) show that the evolution of this defense mechanism probably occurred independently in several plant species in response to pathogens that make use of the natural role of CKs in the host plant to facilitate infection. This arms race evolution started when the function of CKs in regulating growth and sink-source relationships in the host plant was hijacked by plant pathogens that either produce CKs or manipulate CK production in the infected plant to alter the host physiology, and it resembles the zigzag model for the plant immune system (Jones and Dangl, 2006) in pathogen-associated molecular patterns-triggered immunity and effector-triggered immunity (Tsuda and Katagiri, 2010). As a consequence, CK-mediated defense mechanisms coevolved in plants to counter the infection by such pathogens. Finally, agonistic microorganisms with plant growth-promoting characteristics evolved the ability to produce CKs to enhance the overall plant defense capacity or prime the host plants for a faster defense response. A lack in the plant’s ability to respond to infection via the HR, which would stop the progress of biotrophic pathogens, could result in uncontrolled spread of bacteria. Interestingly, despite the absence of the HR, the elevated CK levels induced bactericidal activities and restricted bacterial growth. During pathogen defense, the plant needs to balance two apparently conflicting demands: efficiently abolish the pathogen infection and limit the negative impacts of immune responses on tissue integrity, photosynthetic capacity, and plant primary metabolism (Sato et al., 2010). Thus, the CK-mediated pathogen resistance could have evolved as an important immunity mechanism that limits negative effects on plant fitness during pathogen defense responses.
MATERIALS AND METHODS
Plant Growth Conditions
All tobacco (Nicotiana tabacum) plants used in this study were grown under greenhouse conditions at 20°C to 24°C achieved by additional heating or cooling during cold or warm outside temperatures, respectively, and a 16-h/8-h day/night cycle by supplemented lighting (Envirolite 200-W 6400K fluorescent lamps).
Cloning of the 4xJERE:ipt Construct
The ipt fragment was PCR amplified from the pUC19-IPT plasmid (provided by T. Schmülling) with the primers IPTfwd and IPTrev (Supplemental Table S9), adding restriction sites for XhoI and SacI. The PCR fragment was cloned into a pGEM-T Easy cloning vector (Promega) to obtain pGEM-T Easy-ipt. To place the ipt gene under the control of the 4xJERE promoter (Rushton et al., 2002), the GUS fragment from the pBT10-GUS vector (Sprenger-Haussels and Weisshaar, 2000) was replaced by ipt from pGEM-T Easy-ipt using XhoI and SacI to create the pBT10-4xJERE:ipt plasmid. The 4xJERE:ipt expression cassette was subsequently cloned into the pCambia1380 vector (Cambia) using the restriction enzymes BgIII and HindIII, creating the vector pCam1380-4xJERE:ipt (4xJERE:ipt).
Pathogen Experiments
The virulent hemibiotrophic pathogen strain Pseudomonas syringae pv tabaci was transformed with plasmid pMP4655 (Bloemberg et al., 2000) to confer tetracycline resistance (PstT), required for experiments involving tetracycline-inducible plant gene expression, and all P. syringae infections were performed with this PstT strain for comparison. An exponentially growing culture of PstT containing 20 mg L−1 tetracycline was harvested and adjusted to 106 to 107 colony-forming units (cfu) mL−1 in 10 mm MgCl2. Approximately 100 μL of the suspension was infiltrated into the abaxial side of leaves with a needleless syringe. Determination of bacterial growth was carried out as described before (Griebel and Zeier, 2008). The avirulent strain P. syringae pv phaseolicola was grown accordingly in cultures containing 50 mg L−1 rifampicin and resuspended in 10 mm MgCl2 after harvesting. For the fungal infection experiments, fully developed leaves from three different varieties (cv Xanthi, Xanthi nc, and W38) were cut with a razor blade and immediately placed into water, 10 μm kinetin, or 40 μm kinetin. After 24 h, they were inoculated either by infiltration with Botrytis cinerea spore suspension (approximately 105 spores mL−1) or by placing a 50-μL drop of spore suspension onto the abaxial side of the leaf. For the latter method, spores were preincubated in 3 g L−1 Gamborg’s B5 medium (in 10 mm KH2PO4 buffer, pH 7) supplemented with 25 mm Glc for 2 h to stimulate germination. Leaves were placed in sealed petri dishes with moist sterile filter paper to ensure high humidity and stored under ambient light conditions.
From each plant, four leaves of similar developmental stage were used for the different treatments, and the replicates from each treatment were randomly distributed among the individual plants from one experimental series. For each treatment, at least three individual experimental series were performed. Likewise, for the detached-leaf experiments (petiole feeding), four leaves from each plant were taken and randomly distributed for the different treatments. Although the overall symptom development and final disease phenotype at the end of the infection phase were always similar and the changes in susceptibility are evident throughout symptom development following Pst infection, the speed of symptom development varied for each experimental series. The symptom development following PstT infection was binned into seven classes (0 = no symptoms; 0.5 = reduced chlorosis [up to 75%]; 1 = more than 75% chlorosis; 2 = 10% or less necrosis; 3 = 10%–50% necrosis; 4 = 51%–75% necrosis; 5 = 76%–100% necrosis) reflecting different degrees of visible pathogen response, which were subsequently used to determine the effects of the different treatments compared with control plants.
Determination of Bacterial Growth in Leaf Tissue
From PstT-infiltrated areas, leaf discs were punched out using a cork borer (0.4 cm diameter). Each leaf disc was homogenized in 1 mL of sterile 10 mm MgCl2. One hundred-microliter serial dilutions were plated in three replicates on Luria-Bertani (LB) medium supplemented with 20 mg L−1 tetracycline. Colony formation was determined after 36 to 48 h of incubation at 28°C. The statistical significance of differences between treatments was analyzed using the unpaired Student’s t test.
Induction of the ipt Gene in Plants
Before leaf infiltration, the 4xJERE:ipt strain (pCam1380-4xJERE:ipt in Agrobacterium tumefaciens strain LBA 4404) was precultured in 5 mL of LB liquid medium and subsequently diluted 1:100 in 50 mL of LB liquid medium containing 50 mg L−1 kanamycin, 200 mm MES (pH 5.5), and 40 μm acetosyringone for 24 h each. Cells were harvested at an optical density at 600 nm of 0.8, resuspended in 10 mm MgCl2, 200 mm MES (pH 5.5), and 200 μm acetosyringone, and kept at room temperature for 2 h in darkness. For transient ipt expression in tobacco plants before pathogen infection, approximately 350 μL of the suspension was infiltrated into marked regions on one-half of 9- to 10-week-old cv W38 leaves. For all controls, the A. tumefaciens strain LBA 4404 harboring an empty vector (control) was infiltrated into defined areas on the other half of the same leaf. After 24 h, all pretreated regions were inoculated with PstT. Induction of the SAG12:ipt construct by heat treatment of detached leaves and of the TET:ipt construct by chlortetracycline was carried out as described previously (Balibrea Lara et al., 2004).
Exogenous Application of CKs
For petiole feeding, 7- to 10-week-old leaves from wild-type cv SR1 plants were placed in water (control) or in aqueous solutions of either kinetin or the indicated CK (Duchefa). For all feeding experiments, the leaves were immediately transferred to water after infiltration of PstT, and symptom development was continuously observed for up to 14 d. Alternatively, leaves were dipped for 60 or 90 s into kinetin solution containing 0.005% Silwet l-77 (Lehle Seeds) and kept with petioles in water 24 h before infection and afterward.
Exogenous Application of Phytoalexins
Stock solutions of capsidiol (20 mm in ethanol) and scopoletin (50 mm in methanol) were diluted to 100 μm in water. Approximately 100 μL of scopoletin, capsidiol, or scopoletin + capsidiol was infiltrated in 9- to 10-week-old cv W38 leaves 1 to 2 h before infiltration with PstT (107 cfu mL−1). Control treatments contained the corresponding ethanol and/or methanol concentration. In total, three biological replicate experiments were performed, yielding similar results, and the infiltration spots were randomized to exclude positional effects on symptom development, which was continuously observed for up to 10 d.
Determination of Antimicrobial Activities
Total antimicrobial activity was tested using a paper-disc diffusion assay (Murray et al., 1995). The presence of antimicrobial proteins was determined by bioautography (Vigers et al., 1991).
Instrumental Analyses
CKs (Novák et al., 2008), soluble sugars (Bonfig et al., 2006), scopoletin (Thoma et al., 2003), and SA and JA (Thoma et al., 2003) in the infiltrated leaf areas were analyzed according to published procedures. For capsidiol determination, leaf material was frozen and ground in liquid nitrogen. Ground material was mixed three times with 75% ethanol for 1 h and centrifuged at 5,000 rpm for 10 min. Supernatants from all three extractions were combined and dried using a SpeedVac concentrator (Eppendorf). After drying, the residue was dissolved in 10 mL of methanol (Roth) for further analysis. The analysis of capsidiol was done by gas chromatography-mass spectrometry using a Trace Ultra gas chromatograph (Thermo Fisher Scientific) and a Zebron ZB-5-capillary column (30 m × 0.25 mm × 0.25 μm; Phenomenex) with helium as carrier gas at a constant gas flow of 1.0 mL min−1. Injector temperature was 250°C, and samples were injected under splitless conditions. Transfer line temperature was 300°C. For mass spectrometry, a Trace DSQ (Thermo Electron) was used with an ion source temperature of 200°C and ionization at 70 eV (electron ionization mode). Data were acquired under the control of the Finnigan Xcalibur version 1.4 software package (Thermo Electron). Both analyses were repeated three times. The statistical significance of differences between treatments was analyzed using the unpaired Student’s t test.
Histochemical Determinations
The distribution of GUS activity and H2O2 in leaves was determined according to published procedures (Grüner et al., 2003; Barna et al., 2008).
Northern Blotting
For total RNA isolation, 100 mg of leaf material was ground in liquid nitrogen and mixed with 1 mL of Total RNA Isolation Reagent (Thermo Fisher Scientific). After chloroform extraction, the RNA was precipitated with isopropanol. The RNA pellet was washed in 3 m LiCl (to remove polysaccharides) and subsequently dissolved in RNase-free water. For gel electrophoresis, the RNA was separated on 1.2% (v/v) denaturing (formamide) agarose gels. The resulting RNA was transferred to nitrocellulose membranes (Macherey-Nagel) in 20× SSC, which were fixed by baking the membranes for 2 h at 80°C. The DNA probes to detect expression for the respective genes were labeled with [α-32P]dATP with the HexaLabel DNA Labeling Kit (MBI-Fermentas). The primers used to amplify the DNA probes from cDNA generated from tobacco total RNA are shown in Supplemental Table S9.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. CKs do not affect the avirulent interaction with Psp or resistance against necrotrophic fungi.
Supplemental Figure S2. PR1a expression is induced by CKs and PstT infection.
Supplemental Figure S3. CKs induce resistance independent of SA.
Supplemental Figure S4. SA levels are depleted in nahG transgenic plants and not induced following CK treatment.
Supplemental Figure S5. CKs induce a high phytoalexin-bacteria ratio early in the infection phase.
Supplemental Figure S6. Application of capsidiol and scopoletin does not restrict the proliferation of B. cinerea.
Supplemental Table S1. CK levels are increased following 4xJERE:ipt expression.
Supplemental Table S2. SA and JA levels are not specifically affected by ipt expression
Supplemental Table S3. Free sugar levels are not specifically affected by CK, low sugar levels.
Supplemental Table S4. Free sugar levels are not specifically affected by CK, high sugar levels.
Supplemental Table S5. Scopoletin production is stimulated by CK feeding.
Supplemental Table S6. Proliferation of PstT is reduced by CK feeding.
Supplemental Table S7. Semiquantitative RT-PCR expression analysis.
Supplemental Table S8. Proliferation of PstT is reduced by combined scopoletin and capsidiol feeding.
Supplemental Table S9. Primers used in this study.
Acknowledgments
We thank T. Müller and E. Wirth for the determination of sugar concentrations, G. Gresser for help with scopoletin and capsidiol determination, K. Remele and P. Krbez for help with the instrumental analysis, T. Schmülling for providing the pUC19-IPT plasmid, and S. Berger for stimulating discussions.
References
- Abo-Elyousr K, Mohamed H. (2009) Biological control of Fusarium wilt in tomato by plant growth-promoting yeasts and rhizobacteria hormones produced by Azospirillum brasilense for resistance. Plant Pathol J 25: 199–204 [Google Scholar]
- Ahl Goy P, Signer H, Reist R, Aichholz R, Blum W, Schmidt E, Kessmann H. (1993) Accumulation of scopoletin is associated with the high disease resistance of the hybrid Nicotiana glutinosa × Nicotiana debneyi. Planta 191: 200–206 [Google Scholar]
- Akiyoshi DE, Regier DA, Gordon MP. (1987) Cytokinin production by Agrobacterium and Pseudomonas spp. J Bacteriol 169: 4242–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkhipova T, Veselov S, Melentiev A, Martynenko E, Kudoyarova G. (2005) Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272: 201–209 [Google Scholar]
- Balibrea Lara ME, Gonzalez Garcia MC, Fatima T, Ehness R, Lee TK, Proels R, Tanner W, Roitsch T. (2004) Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16: 1276–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Jones JD. (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69: 473–488 [DOI] [PubMed] [Google Scholar]
- Barna B, Smigocki AC, Baker JC. (2008) Transgenic production of cytokinin suppresses bacterially induced hypersensitive response symptoms and increases antioxidative enzyme levels in Nicotiana spp. Phytopathology 98: 1242–1247 [DOI] [PubMed] [Google Scholar]
- Beckman K, Ingram D. (1994) The inhibition of the hypersensitive response of potato tuber tissues by cytokinins: similarities between senescence and plant defence responses. Physiol Mol Plant Pathol 45: 229–246 [Google Scholar]
- Bednarek P, Piślewska-Bednarek M, Svatoš A, Schneider B, Doubský J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, et al. (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323: 101–106 [DOI] [PubMed] [Google Scholar]
- Bednarek P, Schneider B, Svatoš A, Oldham NJ, Hahlbrock K. (2005) Structural complexity, differential response to infection, and tissue specificity of indolic and phenylpropanoid secondary metabolism in Arabidopsis roots. Plant Physiol 138: 1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Sinha AK, Roitsch T. (2007) Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot 58: 4019–4026 [DOI] [PubMed] [Google Scholar]
- Bloemberg GV, Wijfjes AH, Lamers GE, Stuurman N, Lugtenberg BJ. (2000) Simultaneous imaging of Pseudomonas fluorescens WCS365 populations expressing three different autofluorescent proteins in the rhizosphere: new perspectives for studying microbial communities. Mol Plant Microbe Interact 13: 1170–1176 [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Stauber EJ, Krock B, Oldham NJ, Gershenzon J, Baldwin IT. (2002) Gene expression of 5-epi-aristolochene synthase and formation of capsidiol in roots of Nicotiana attenuata and N. sylvestris. Phytochemistry 60: 109–116 [DOI] [PubMed] [Google Scholar]
- Boiero L, Perrig D, Masciarelli O, Penna C, Cassán F, Luna V. (2007) Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl Microbiol Biotechnol 74: 874–880 [DOI] [PubMed] [Google Scholar]
- Bonfig KB, Gabler A, Simon UK, Luschin-Ebengreuth N, Hatz M, Berger S, Muhammad N, Zeier J, Sinha AK, Roitsch T. (2010) Post-translational derepression of invertase activity in source leaves via down-regulation of invertase inhibitor expression is part of the plant defense response. Mol Plant 3: 1037–1048 [DOI] [PubMed] [Google Scholar]
- Bonfig KB, Schreiber U, Gabler A, Roitsch T, Berger S. (2006) Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 225: 1–12 [DOI] [PubMed] [Google Scholar]
- Brooks C, Watson G, Freer I. (1986) Elicitation of capsidiol accumulation in suspended callus cultures of Capsicum annuum. Phytochemistry 25: 1089–1092 [Google Scholar]
- Bruce MI, Zwar JA. (1966) Cytokinin activity of some substituted ureas and thioureas. Proc R Soc Lond B Biol Sci 165: 245–265 [DOI] [PubMed] [Google Scholar]
- Chandra-Shekara AC, Gupte M, Navarre D, Raina S, Raina R, Klessig D, Kachroo P. (2006) Light-dependent hypersensitive response and resistance signaling against Turnip crinkle virus in Arabidopsis. Plant J 45: 320–334 [DOI] [PubMed] [Google Scholar]
- Choi J, Huh SU, Kojima M, Sakakibara H, Paek KH, Hwang I. (2010) The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev Cell 19: 284–295 [DOI] [PubMed] [Google Scholar]
- Chong J, Baltz R, Schmitt C, Beffa R, Fritig B, Saindrenan P. (2002) Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell 14: 1093–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S, Duffy B, Nowak J, Clément C, Barka EA. (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71: 4951–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi M, Vereecke D, Temmerman W, Van Montagu M, Desomer J. (1994) The fas operon of Rhodococcus fascians encodes new genes required for efficient fasciation of host plants. J Bacteriol 176: 2492–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, et al. (1994) A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Dropkin VH, Helgeson JP, Upper CD. (1969) The hypersensitivity reaction of tomatoes resistant to Meloidogyne incognita: reversal by cytokinins. J Nematol 1: 55–61 [PMC free article] [PubMed] [Google Scholar]
- Ehness R, Ecker M, Godt DE, Roitsch T. (1997) Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 9: 1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehness R, Roitsch T. (1997) Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J 11: 539–548 [DOI] [PubMed] [Google Scholar]
- El Oirdi M, Trapani A, Bouarab K. (2010) The nature of tobacco resistance against Botrytis cinerea depends on the infection structures of the pathogen. Environ Microbiol 12: 239–253 [DOI] [PubMed] [Google Scholar]
- Engelbrecht L. (1968) Cytokinin in den grünen Inseln des Herbstlaubes. Flora 159: 369–374 [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J. (2007) Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol 144: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S, Amasino RM. (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270: 1986–1988 [DOI] [PubMed] [Google Scholar]
- García de Salamone IE, Hynes RK, Nelson LM. (2001) Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can J Microbiol 47: 404–411 [DOI] [PubMed] [Google Scholar]
- Ge L, Yong JW, Goh NK, Chia LS, Tan SN, Ong ES. (2005) Identification of kinetin and kinetin riboside in coconut (Cocos nucifera L.) water using a combined approach of liquid chromatography-tandem mass spectrometry, high performance liquid chromatography and capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci 829: 26–34 [DOI] [PubMed] [Google Scholar]
- Ghanem ME, Albacete A, Smigocki AC, Frébort I, Pospísilová H, Martínez-Andújar C, Acosta M, Sánchez-Bravo J, Lutts S, Dodd IC, et al. (2011) Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 62: 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MR, Jones JD. (2009) Hormone (dis)harmony moulds plant health and disease. Science 324: 750–752 [DOI] [PubMed] [Google Scholar]
- Griebel T, Zeier J. (2008) Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol 147: 790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüner R, Strompen G, Pfitzner AJ, Pfitzner UM. (2003) Salicylic acid and the hypersensitive response initiate distinct signal transduction pathways in tobacco that converge on the as-1-like element of the PR-1a promoter. Eur J Biochem 270: 4876–4886 [DOI] [PubMed] [Google Scholar]
- Guedes M, Kuc J, Hammerschmidt R, Bostock R. (1982) Accumulation of six sesquiterpenoid phytoalexins in tobacco leaves infiltrated with Pseudomonas lachrymans. Phytochemistry 21: 2987–2988 [Google Scholar]
- Herbers K, Meuwly P, Métraux JP, Sonnewald U. (1996) Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett 397: 239–244 [DOI] [PubMed] [Google Scholar]
- Horsfall J, Dimond A. (1957) Interactions of tissue sugar, growth substances and disease susceptibility. Z Pflanzenkr Pflanzenschutz 64: 415–421 [Google Scholar]
- Hwang HH, Wang MH, Lee YL, Tsai YL, Li YH, Yang FJ, Liao YC, Lin SK, Lai EM. (2010) Agrobacterium-produced and exogenous cytokinin-modulated Agrobacterium-mediated plant transformation. Mol Plant Pathol 11: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kim EK, Kwon KB, Shin BC, Seo EA, Lee YR, Kim JS, Park JW, Park BH, Ryu DG. (2005) Scopoletin induces apoptosis in human promyeloleukemic cells, accompanied by activations of nuclear factor kappaB and caspase-3. Life Sci 77: 824–836 [DOI] [PubMed] [Google Scholar]
- Kim Y, Choi D, Yeo W, Kim Y, Chae S, Eun Kyung P, Sang Seock K. (2000) Scopoletin production related to induced resistance of tobacco plants against Tobacco mosaic virus. Plant Pathol J 16: 264–268 [Google Scholar]
- López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodríguez R, Macías-Rodríguez LI, Valencia-Cantero E. (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant Microbe Interact 20: 207–217 [DOI] [PubMed] [Google Scholar]
- Lu H, Salimian S, Gamelin E, Wang G, Fedorowski J, LaCourse W, Greenberg JT. (2009) Genetic analysis of acd6-1 reveals complex defense networks and leads to identification of novel defense genes in Arabidopsis. Plant J 58: 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S, Tirosh T, Glick BR. (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42: 565–572 [DOI] [PubMed] [Google Scholar]
- Meidner H. (1967) The effect of kinetin on stomatal opening and the rate of intake of carbon dioxide in mature primary leaves of barley. J Exp Bot 56: 556–561 [Google Scholar]
- Morris RO, Jameson PE, Laloue M, Morris JW. (1991) Rapid identification of cytokinins by an immunological method. Plant Physiol 95: 1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes K. (1960) Über das Altern der Blätter und die Möglichkeit ihrer Wiederverjüngung. Naturwissenschaften 47: 337–351 [Google Scholar]
- Murray P, Baron E, Pfaller M, Tenover F, Yolke R, (1995) Manual of Clinical Microbiology. ASM Press, Washington [Google Scholar]
- Novák O, Hauserová E, Amakorová P, Doležal K, Strnad M. (2008) Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 69: 2214–2224 [DOI] [PubMed] [Google Scholar]
- Nugroho L, Peltenburg-Looman A, de Vos H, Verberne M, Verpoorte R. (2002) Nicotine and related alkaloids accumulation in constitutive salicylic acid producing tobacco plants. Plant Sci 162: 575–581 [Google Scholar]
- Nugroho L, Verpoorte R. (2002) Secondary metabolism in tobacco. Plant Cell Tissue Organ Cult 68: 105–125 [Google Scholar]
- Ortíz-Castro R, Valencia-Cantero E, López-Bucio J. (2008) Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav 3: 263–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedras MS, Zheng QA, Gadagi RS, Rimmer SR. (2008) Phytoalexins and polar metabolites from the oilseeds canola and rapeseed: differential metabolic responses to the biotroph Albugo candida and to abiotic stress. Phytochemistry 69: 894–910 [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC. (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5: 308–316 [DOI] [PubMed] [Google Scholar]
- Pogany M, Koehl J, Heiser I, Elstner E, Barna B. (2004) Juvenility of tobacco induced by cytokinin gene introduction decreases susceptibility to Tobacco necrosis virus and confers tolerance to oxidative stress. Physiol Mol Plant Pathol 65: 39–47 [Google Scholar]
- Redig P, Schmulling T, Van Onckelen H. (1996) Analysis of cytokinin metabolism in ipt transgenic tobacco by liquid chromatography-tandem mass spectrometry. Plant Physiol 112: 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang S. (2008) A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 105: 5638–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico A, Bennett MH, Forcat S, Huang WE, Preston GM. (2010) Agroinfiltration reduces ABA levels and suppresses Pseudomonas syringae-elicited salicylic acid production in Nicotiana tabacum. PLoS ONE 5: e8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Shulaev V, Blumwald E. (2009) Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol 150: 1530–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Glazebrook J, Ausubel FM. (1996) Mode of action of the Arabidopsis thaliana phytoalexin camalexin and its role in Arabidopsis-pathogen interactions. Mol Plant Microbe Interact 9: 748–757 [DOI] [PubMed] [Google Scholar]
- Roitsch T, González MC. (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9: 606–613 [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Reinstädler A, Lipka V, Lippok B, Somssich IE. (2002) Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen- and wound-induced signaling. Plant Cell 14: 749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H. (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57: 431–449 [DOI] [PubMed] [Google Scholar]
- Sano H, Seo S, Koizumi N, Niki T, Iwamura H, Ohashi Y. (1996) Regulation by cytokinins of endogenous levels of jasmonic and salicylic acids in mechanically wounded tobacco plants. Plant Cell Physiol 37: 762–769 [Google Scholar]
- Sato M, Tsuda K, Wang L, Coller J, Watanabe Y, Glazebrook J, Katagiri F. (2010) Network modeling reveals prevalent negative regulatory relationships between signaling sectors in Arabidopsis immune signaling. PLoS Pathog 6: e1001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnablová R, Synková H, Vicánková A, Burketová L, Eder J, Cvikrová M. (2006) Transgenic ipt tobacco overproducing cytokinins overaccumulates phenolic compounds during in vitro growth. Plant Physiol Biochem 44: 526–534 [DOI] [PubMed] [Google Scholar]
- Skoog F, Montaldi E. (1961) Auxin-kinetin interaction regulating the scopoletin and scopolin levels in tobacco tissue cultures. Proc Natl Acad Sci USA 47: 36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger-Haussels M, Weisshaar B. (2000) Transactivation properties of parsley proline-rich bZIP transcription factors. Plant J 22: 1–8 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Fujimori T. (1985) Accumulation of phytuberin and phytuberol in tobacco callus inoculated with Pseudomonas solanacearum or Pseudomonas syringae pv. tabaci. Phytochemistry 24: 1193–1195 [Google Scholar]
- Thoma I, Loeffler C, Sinha AK, Gupta M, Krischke M, Steffan B, Roitsch T, Mueller MJ. (2003) Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J 34: 363–375 [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL. (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LS, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K. (2007) Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA 104: 20623–20628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Katagiri F. (2010) Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol 13: 459–465 [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Roberts WK, Selitrennikoff CP. (1991) A new family of plant antifungal proteins. Mol Plant Microbe Interact 4: 315–323 [DOI] [PubMed] [Google Scholar]
- Walters DR, McRoberts N, Fitt BD. (2008) Are green islands red herrings? Significance of green islands in plant interactions with pathogens and pests. Biol Rev Camb Philos Soc 83: 79–102 [DOI] [PubMed] [Google Scholar]
- Ward E, Unwin C, Stoessl A. (1974) Postinfectional inhibitors from plants. XIII. Fungitoxicity of the phytoalexin, capsidiol, and related sesquiterpenes. Can J Bot 52: 2481–2488 [Google Scholar]
- Wu CH, Bernard SM, Andersen GL, Chen W. (2009) Developing microbe-plant interactions for applications in plant-growth promotion and disease control, production of useful compounds, remediation and carbon sequestration. Microb Biotechnol 2: 428–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Mei L, Newman J, Back K, Chappell J. (1997) Regulation of sesquiterpene cyclase gene expression: characterization of an elicitor- and pathogen-inducible promoter. Plant Physiol 115: 437–451 [DOI] [PMC free article] [PubMed] [Google Scholar]