Abstract
Insulin pump therapy [continuous subcutaneous insulin infusion (CSII)] requires regular change of infusion sets every 2-3 days in order to minimize the risk of skin irritations or other adverse events. This has been discussed to be a potential burden to the environment. The purpose of this analysis was to perform an environmental assessment of insulin pump infusion sets based on loss of resources occurring during incineration of the discarded products and by means of a lifecycle concept used to weight a material in relation to its rareness on earth and its consumption. In addition to five infusion sets (Inset30, InsetII, Comfort, Quick-set, and Cleo), a patch pump (Omnipod) was also included in this analysis. The annual loss in waste of the so called “person reserve” of 3 days of catheter use was compared with daily consumption of a cup of coffee in a disposable paper cup and to a soft drink in an aluminum can. The weight-based loss in resources through waste for the infusion sets (except for Cleo) corresponded to 70-200% of the loss of resources for a coffee cup (Cleo, 320%; Omnipod, 1,821,600%) and to 1-3% of the loss from an aluminum soft drink can (Cleo, 5%; Omnipod, 31,200%). The loss or resources by use of infusion sets used in insulin pump therapy appears to be low and is similar to the burden induced by the uptake of one cup of coffee per day. The loss or resources with regular CSII is considerably lower than the loss or resources induced by patch pumps.
Keywords: continuous subcutaneous insulin infusion, insulin infusion sets, environment, loss of resources, waste
Introduction
Since 1980, the potential environmental impact of pharmaceuticals and personal care products has received growing attention and has become (next to safety and efficacy) a major point of consideration in development of new products.1 The major issues associated with the origins and occurrence of chemicals in surface, subsurface, and drinking waters have been captured in a number of reviews, books, and proceedings.2–5 Nowadays, medicinal product companies are required to analyze the potential consequences and loss of resources by product disposal as waste in the environment and submit these evaluation reports as part of the documents for regulatory approval. Current regulations of both the U.S. Food and Drug Administration and the European Medicinal Agency on Good Manufacturing Practice and effluent emission (use and disposal) and on manufacturing effluent discharge and emission require evaluation of contained manufacture, use, and disposal of pharmaceuticals with the goal of minimizing the release of pharmaceutical chemicals into the environment.6 In this respect, physicochemical and biological aspects of polymeric materials represent vital areas in reliable, safe, and efficacious functioning of controlled drug-delivery devices. In the case of infusion systems, potential biological problems include incompatibility of polymers and their degradation products with the physiological environment, adverse metabolic consequences of degradation products, and occlusion of drug conduits (catheters) with thrombi and/or drugs, (e.g., insulin aggregates).7 One way to perform this type of environmental analysis is to calculate the loss of natural resources through the use and incineration of the products. It is considered that the resources lost represent a burden to the environment when deposited in waste instead of being recycled.
Insulin therapy in patients with diabetes mellitus requires daily performance of numerous blood glucose readings and use of injection/infusion devices with regular change of disposable components, and is believed to be associated with a substantial generation of waste, e.g., blood glucose test strips, needles, catheters and cartridges. Continuous subcutaneous insulin infusion (CSII) by means of insulin pump devices is considered to be the most optimal method to achieve near-normal blood glucose levels in patients with type 1 diabetes.8,9 However, CSII especially requires frequent change of insulin infusion sets and tubings. We have shown in a pilot study that a regular change of infusion sets every 2-3 days may be required in order to avoid infusion-site events or other technical problems, such as skin irritations, infections, tubing occlusions, or loosening of the adhesive at the infusion site.10 In another laboratory investigation, Kerr and colleagues11 demonstrated with all three short-acting insulin analogs that early catheter occlusions (within 72 h) are rare and independent of the choice of insulin analog. The authors also concluded that, for patients using insulin pump therapy, the importance of catheter change within 72 h should be emphasized irrespective of the insulin used.
The purpose of this investigation and analysis was to perform an environmental assessment of insulin pump infusion sets based on the loss of resources occurring during incineration of the discarded products and by means of a lifecycle concept used to weight a material in relation to how rare it is and its consumption.
Methods
The following infusion sets and patch pumps were included in the analysis: Inset30, InsetII, and Comfort Short (all Convatec, Lejre, Denmark); Quick-Set (Medtronic Diabetes, Northridge, CA); and Cleo (Smiths Medical, Ashford, UK). We also investigated the first tubing-free insulin infusion system, Omnipod (Insulet, Bedford, MA). For this analysis, it was assumed that infusion sets and the Omnipod device were changed every 3 days. After determination of their composition, the theoretical resource consumption of 1 year of use of each of the devices was calculated and compared with the resource consumption of the daily uptake of a disposable cup of coffee and an aluminum soft drink can.
Individual components of each infusion set were identified on the technical drawings, physically identified for each of the devices, and weighed by means of a high-precision balance. Metal needles were weighed by cutting a length of the needle, weighing the cut-off part, measuring its length, and then calculating the total weight of the needle based on its original length. For Omnipod, materials were identified using material analyses (polymer type in plastic materials: gravity separation in water, incineration test, solubility in solvents; ferrous materials: magnetic tests; brass components: X-ray analysis for composition; batteries: mean value of the composition as provided by the manufacturer).12
Based on the identification of the infusion device components and their material, materials were divided into three categories: metals, plastics, and paper/wood. The material content in the products were compared with the material content of a disposable coffee cup with a volume of 473 ml and a U.S. aluminum soft drink can with a volume of 350 ml.
Mathematical Calculations and Baseline Assumptions
Person reserves is a lifecycle concept used to evaluate material in relation to its prevalence on earth and its consumption. Person reserves are expressed in mPr/kg (i.e., parts per thousand of person reserves per kilogram of pure material), which is an accepted unit for resource content in products. Renewable materials, e.g., paper and wood, have an mPr of 0, as the resource is not limited. For metals such as gold, silver, or iron, their available quantity on earth is limited and thus have a high mPr/kg value (gold, 87,000 mPr/kg; silver, 7500 mPr/kg; iron, 0.22 mPr/kg). The mPr/kg numbers for various components of the investigated products were taken from two Danish registries.13,14
According to the manufacturers, all infusion sets are discarded and incinerated after use. In this process, a significant part of the energy content of plastics is recovered, but the plastics are not recycled. The proportion of metallic components of the products is so small that no large-scale recovery of metals from the incineration residue was estimated. Therefore, a metal recycling rate of 0% from incineration residue was assumed in this study. For the aluminum soft drink can, a recycling rate of 52% was included in the analyses.15 If materials are recycled at the end of their lifecycle, the loss of resources in the form of person reserves is reduced. For instance, if 1 kg of copper is used in a product (with an mPr of copper of 23 mPr/kg), and 90% copper is recovered during the recycling process, the total consumption of the product is 23 × (100 - 90) / 100 = 2.3 mPr of copper during the product's lifetime.
Environmental assessment was made based on loss of resources occurring during incineration of discarded products based on loss in person reserves on an annual basis.13,14 This describes the amount of materials that are lost in the incineration process that can no longer be recycled and reused, which represents the ultimate loss of materials. This method links the rareness of the material to the level of consumptions, and the content of precious materials will thus be weighted higher than the content of plastics. We estimated a use of 122 infusion devices per year (365 coffee cups and 365 soft drink cans).
Results
Material compositions of the investigated infusion devices are provided in Table 1. It is assumed that all infusion sets and the Omnipod device are discarded after 3 days. For comparison purposes, loss of resources for consumption of one soft drink can per day and one typical disposable cup coffee cup per day is also shown. It can be seen that the loss of resources per infusion set is small with the exception of the Cleo set and the Omnipod. Metallic content is significantly higher in the Cleo product than in the other infusion sets. Metallic content of the Omnipod is much higher than in any of the infusion sets, as this product contains printed circuit boards, batteries, and various other metallic components. This explains why loss of resources with Omnipod is 18,000 times higher than for a disposable cup of coffee and 312 times higher than for a soft drink can.
Table 1.
Weight-Based Loss of Resources (Breakdown of Content before Incineration) from Products by Material Type
| Product | Number of units consumed after three days | Recycling (%) | Metals (g) | Plastics (g) | Paper/wood (g) |
|---|---|---|---|---|---|
| Inset 30 | 1 | 0 | 0.05 | 22.14 | 1.49 |
| InsetII | 1 | 0 | 0.02 | 23.62 | 0.09 |
| Comfort | 1 | 0 | 0.06 | 8.11 | 0.89 |
| Quick-set | 1 | 0 | 0.02 | 11.32 | 0.87 |
| Cleo | 1 | 0 | 2.10 | 27.13 | 1.00 |
| Omnipod | 1 | 0 | 11.83 | 30.31 | 1.06 |
| Coffee cup | 3 | 0 | 0 | 12.09 | 44.80 |
| Soft drink can | 3 | 52 | 18.82 | 0 | 0 |
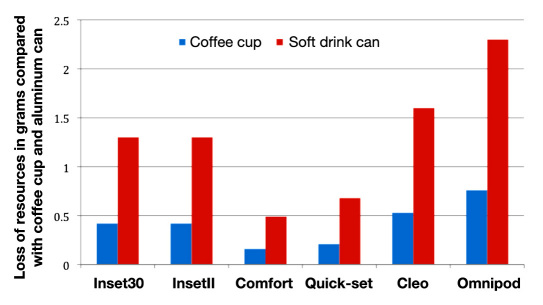
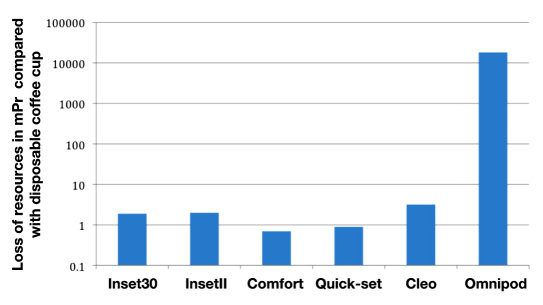
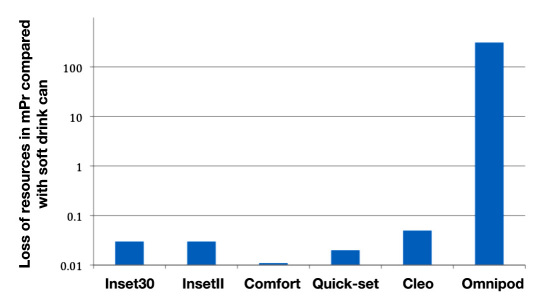
Weight-based loss of resources with waste for the infusion sets compared with the loss of resources for a typical disposable coffee cup and for a soft drink can is shown in Figure 1. While Inset30, InsetII, Comfort, and Quick-set correspond to 0.15-0.4 times the loss of resources, Cleo corresponds to 0.5 times and Omnipod to 0.75 times the loss of resources for a typical coffee cup. In comparison with the soft drink can, the Convatec systems as well as Quick-set correspond to 0.5-1.3 times the loss of resources (Cleo, 1.6 times; Omnipod, 2.3 times). Annual loss of resources in waste from infusion sets via waste for incineration in comparison with a disposable coffee cup and a soft drink can are shown in Figures 2 and 3.
Figure 1.
Weight-based loss of resources with waste for single infusion sets compared with the loss of resources for a single typical disposable coffee cup and for a single soft drink can (based on material content of the products).
Figure 2.
Annual loss of resources in mPr/kg in comparison with daily consumption of a typical disposable cup of coffee (based on material content of the products).
Figure 3.
Annual loss of resources in mPr/kg in comparison with daily consumption of a soft drink aluminum can (based on material content of the products).
Discussion
Current Good Manufacturing Practice and effluent emission (use and disposal) regulations of the U.S. Food and Drug Administration, manufacturing effluent discharge and emission regulations of the U.S. Environmental Protection Agency, and corresponding regulations and directives in the European Union require contained manufacture, use, and disposal of pharmaceuticals and medicinal products with the goal of minimizing the release of chemicals into the environment. In this context, insulin therapy, and in particular CSII, are considered to lead to higher amounts of ecological waste than other antidiabetic interventions. While blood glucose measurement requirements are similar for intensive insulin therapy with pen injectors or insulin pumps, the regular change of infusion sets every 2-3 days to avoid skin reactions10 represents an additional environmental impact when performing insulin pump therapy.
The environmental assessment in this investigation revealed that, for insulin pump infusion sets with the recommended exchange of infusion sets every 2-3 days (except Cleo), environmental loss of resources is similar to the loss occurring through consumption of one disposable cup of coffee per day and much lower than having one soft drink in an aluminum can per day. One person consuming one soft drink or one beer in a can only every 3 days has a similar impact on the environment than 11 insulin pump patients using one infusion set each in the same time period.
The main loss in infusion sets (except for Cleo) originates from plastics, whereas the loss from Cleo is higher due to the metallic content. Based on the efficacy of the recycling process at the incineration plants, the relatively large spring of Cleo may in some case be separated together with metal for recycling. Even in this case, however, overall loss of resources for Cleo is still higher than with the other infusion sets because of the higher plastic content.
For Omnipod, loss of resources is very high (18,000 times higher than for a disposable cup of coffee and 312 times higher than for a soft drink can) because of the content of rare metals such as silver, copper, tin, and gold in the batteries as well as the printed circuit board. A direct comparison between pump catheters and the Omnipod based on our methodology has some weaknesses, which need to be considered before drawing conclusions. The Omnipod is a fully operating therapeutic system. A fair comparison of loss of resources between Omnipod and insulin pump therapy would also need to consider the loss of resources by the insulin pump cartridges and by the regular pump, which is exchanged every 4 years. These data were not available to us when we performed the analysis. However, given the magnitude of the observed differences, the estimated loss of resources through these pump components would certainly not change the overall conclusions from our analysis. As given in the Methods section, this calculation assumes that materials contained in the Omnipod are not recycled. If the electronic components and batteries were recycled, it is expected that loss of resources could be reduced by approximately 90%. A rough estimate reveals that the batteries contribute by 91.1% to the overall loss of resources of the Omnipod device (printed circuit board, 8.7%; zinc components, 0.14%; brass components, 0.07%), which was approximately 10,000 times higher for the Omnipod than for infusion sets. In any case, our calculations have been made on a conservative basis by using only the lowest possible content of precious metals in the batteries as provided by the manufacturer's specifications16 in this analysis.
In conclusion, the environmental burden of infusion sets used in insulin pump therapy appears to be very low and is similar to the burden induced by the uptake of one cup of coffee per day. The burden with regular CSII is much lower than the burden induced by patch pumps.
Glossary
Abbreviations
- (CSII)
continuous subcutaneous insulin infusion
Funding
This work was supported by a grant from Unomedical A/S, Lejre, Denmark.
Disclosures
Dr. Pfützner has received consultant and speaker fees, travel support, and research grants from Unomedical. Dr. Thomas Forst has received research grants from Unomedical.
References
- 1.Daughton CG. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. II. Drug disposal, waste reduction, and future directions. Environ Health Perspect. 2003;111(5):775–785. doi: 10.1289/ehp.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daughton CG. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. I. Rationale for and avenues toward a green pharmacy. Environ Health Perspect. 2003;111(5):757–774. doi: 10.1289/ehp.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daughton CG, Ternes TA. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect. 1999;107(Suppl 6):907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daughton CG. Environmental stewardship and drugs as pollutants. Lancet. 2002;360(9339):1035–1036. doi: 10.1016/S0140-6736(02)11176-7. [DOI] [PubMed] [Google Scholar]
- 5.Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett. 2002;131(1-2):5–17. doi: 10.1016/s0378-4274(02)00041-3. [DOI] [PubMed] [Google Scholar]
- 6.Velagaleti R, Burns PK, Gill M, Prothro J. Impact of current good manufacturing practices and emission regulations and guidances on the discharge of pharmaceutical chemicals into the environment from manufacturing, use, and disposal. Environ Health Perspect. 2002;110(3):213–220. doi: 10.1289/ehp.02110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruck SD, Mueller EP. Materials and biological aspects of synthetic polymers in controlled drug release systems: problems and challenges. Crit Rev Ther Drug Carrier Syst. 1988;5(3):171–187. [PubMed] [Google Scholar]
- 8.Pfützner A, Berger S, Spinas GA. [Current value of continuous subcutaneous insulin infusion (CSII) with insulin pumps in the therapy of Diabetes mellitus] Swiss Med Wkly. 2000;130:1954–1961. [Google Scholar]
- 9.Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care. 2003;26(4):1079–1087. doi: 10.2337/diacare.26.4.1079. [DOI] [PubMed] [Google Scholar]
- 10.Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol. 2010;4(4):976–982. doi: 10.1177/193229681000400429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr D, Morton J, Whately-Smith C, Everett J, Begley JP. Laboratory-based non-clinical comparison of occlusion rates using three rapid-acting insulin analogs in continuous subcutaneous insulin infusion catheters using low flow rates. J Diabetes Sci Technol. 2008;2(3):450–455. doi: 10.1177/193229680800200314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krause A, Lange A, Ezrin M. Chemical and instrumental methods. Munich: Hanser; 1983. Plastics analysis guide. [Google Scholar]
- 13.Pommerer K, Malmgern-Hansen B, Olesen S. Ressourceeffektivitet-forslagtildefinition samt praktiskeeksemplerpåanvendelseafbegrebet, Milijøprojekt No. 1053, 2005.
- 14. Milyøstyrelsen, Håndbogimiljøvurderingafproducter, Miljønyt No. 58, 2001.
- 15. Reuters. Alcoa sets goal to raise North American aluminum beverage can recycling rate from 52% to 75% by 2015. http://www.reuters.com/article/pressRelease/idUS211970+22-Jan-2008+BW20080122. Accessed June 23, 2011.
- 16. Varta Material Data Sheet for Silver Oxide Micro Batteries.