Abstract
Objective:
An increasingly aged, overweight, and sedentary population has resulted in elevated risk of cardiovascular disease (CVD). The escalating incidence of diabetes and other chronic illnesses, deficits in health care budgets, and physician shortages, especially in rural communities, have prompted investigations of feasible solutions. The Diabetes and Technology for Increased Activity (DaTA) study was designed to test the effectiveness of a lifestyle intervention driven by self-monitoring of blood glucose (BG), blood pressure (BP), physical activity (PA), and weight to positively impact CVD risk factors in a medically underserviced rural population with a high incidence of metabolic syndrome (MS).
Research Design and Methods:
Conducted in a community-based research setting, this single-center open feasibility study used smart phones to transmit BP, BG, pedometer, weight, heart rate, and activity measurements to a database. Technology allowed participants to interface with the clinical team and self-monitor their personal health indicators.
Results
Twenty-four participants aged 30 to 71 years completed the 8-week intervention. Participants had significant improvement in clinic (p = .046) and self-monitored diastolic BP (p = .001), body mass index (p = .002), and total cholesterol (p = .009), and steps per day. Daily PA increased as well as participants' interest in and willingness to make lifestyle changes that impact health outcomes.
Conclusions
The DaTA study demonstrated that self-monitoring of the risk factors for MS and increased PA improved the participant's CVD risk profile. Considering the 8-week time period of this intervention, results are encouraging. This lifestyle intervention, which uses education and technology as tools, confirms the utility of remote health monitoring.
Keywords: diabetes, lifestyle management, remote monitoring, rural primary care, technology
Introduction
An increasingly aged, overweight, and sedentary population has resulted in elevated risk of cardiovascular disease (CVD). Cardiovascular (CV) risk factors include hypertension (HT), dysglycemia (elevated fasting glucose and insulin resistance), dyslipidemia [elevated trigylcerides and low-density lipoprotein (LDL) cholesterol and decreased high-density lipoprotein (HDL) cholesterol], and obesity (central adiposity).1 These risk factors are the hallmark of metabolic syndrome (MS), more often occur together than in isolation,1 and have been shown to directly increase atherosclerotic CVD.2 In 2001, Adult Treatment Panel III guidelines called specific attention to the importance of targeting CV risk factors of MS as a method of CVD risk reduction therapy.3 Current Canadian Diabetes Association clinical guidelines state that there is evidence to support an aggressive approach to identify those individuals with MS.4 A study demonstrated the important connection between precursors for development of diabetes, MS, and increased risk for cardiovascular complications (CVCs).5 A retrospective analysis of the prevalence and treatment of HT and dyslipidemia, in Southwestern Ontario, Canada, identified that treatment patterns were not in alignment with current guidelines and that treatment levels are low and recommended control levels even lower.5 Furthermore, the increasing incidence of diabetes and other chronic illnesses in rural communities, deficits in health care budgets, and physician shortages have prompted researchers and policy makers to investigate feasible solutions.
Health complications of diabetes, including hyperglycemia, are associated with an increased risk of developing macro-vascular and microvascular complications (i.e., myocardial infarction and stroke, neuropathy, nephropathy, and retinopathy).6,7 The U.K. Prospective Diabetes Study8,9 and the Diabetes Control and Complications Trial10 demonstrated that progression of these complications can be prevented through improved blood glycemic control. Importantly, these studies found that increased physician contact had a positive effect and improved patient glycemic control. Research has demonstrated the efficacy of exercise and nutrition counseling to decrease the incidence of type 2 diabetes in a MS population.11–13 However, despite this positive evidence that diabetes and MS are preventable with relatively simple interventions such as lifestyle management adoption, implementation of change has been disappointing. The Step Test and Exercise Prescription (STEP) program positively enhanced lifestyle changes by including comprehensive exercise counseling with goal setting and a written exercise prescription.14 The STEP test as an intervention in a primary care setting elicited significant improvements in waist circumference, diastolic blood pressure (DBP) and systolic blood pressure (SBP), fasting blood glucose (BG), weight, body mass index (BMI), resting heart rate (HR), and total and LDL cholesterol levels in MS patients.2 However, it is not clear if dietary changes or changes in physical activity (PA) were the driving force behind these positive results.
Health monitoring technologies, including BG, blood pressure (BP), and HR monitors as well as pedometers (devices to promote PA), in tandem with clinically prescribed exercise, may encourage lifestyle modifications through patient self-management. In fact, remote monitoring technologies have been shown to reduce BG15–17 and BP18 and increase PA.19 Remote monitoring studies to date have failed to effectively monitor both intended behavior change, such as diet and PA, and risk factor modification, such as BP and BG. Moreover, it is not known if it is feasible to employ health monitoring technologies, particularly in a rural setting. Therefore, the objective of the Diabetes and Technology for Increased Activity (DaTA) study was to test the effectiveness of a lifestyle intervention driven by self-monitoring of BG, BP, PA, and weight to positively impact CV risk factors in a medically underserviced rural population with a high incidence of MS.
Research Design and Methods
Study Design and Participant Recruitment
Hosted in a community-based research setting, the DaTA study was an open single-center feasibility study conducted between November 2009 and May 2010. Research was conducted according to the Declaration of Helsinki with ethics approval from Institutional Review Board Services (Aurora, Ontario, Canada) and the University of Western Ontario Research Ethics Board. An academic-industry research team consisted of: (1) a principal investigator (PI); (2) a technology implementation and systems adminis-tration specialist (TSS; Sykes Technical Assistance Corp.); (3) technical and database applications experts (Healthanywhere, IgeaCare Inc.); and (4) a clinical team (CT; study coordinator, certified kinesiologist, graduate students, consultants, physicians, and nurses dedicated to this study).
Participants were recruited with social marketing techniques, including newspaper advertisements and posters placed in family medical clinics, pharmacies, and community bulletin boards, as well as by word-of-mouth recommendations. Interested potential candidates contacted the study coordinator to confirm eligibility. Only candidates who met all inclusion and exclusion criteria and provided written informed consent were enrolled. Inclusion criteria were a minimum of two risk factors for MS, including high waist circumference or obesity, elevated SBP and DBP (SBP ≥130 mm HG or DBP ≥85 mm HG), fasting plasma BG >6.1 mmol/liter, or impaired glucose tolerance and fasting lipid levels (triglycerides >1.7 mmol/ liter, HDL cholesterol; males <1.04 mmol/liter, females <1.29 mmol/liter).
Study Protocol
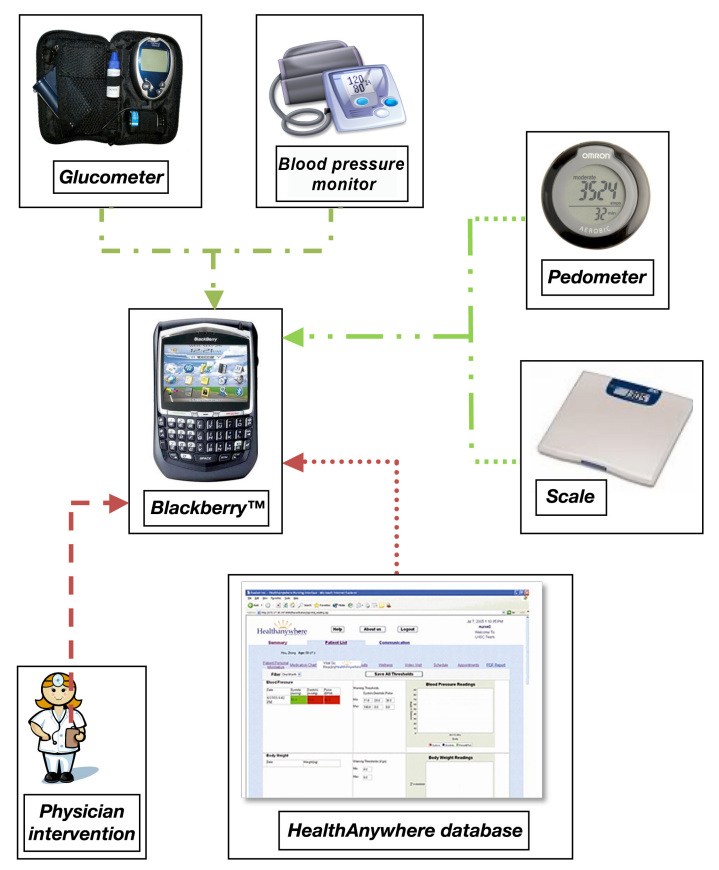
At week 0 (visit 1), study participants were provided with: (1) a Smartphone (BlackBerry™ Curve 8300); (2) a Bluetooth™-enabled BP monitor (A & D Medical #UA-767PBT); (3) a glucometer (Lifescan One Touch Ultra2™ with Polymap wireless adaptor PWR-08-03); and (4) a pedometer (Omron # HJ-150; Figure 1). All Bluetooth-compatible devices were paired with a unique Smartphone. Dedicated to the study, Smartphones transmitted self-monitoring measurements to the database and allowed participants to interface with the CT and TSS, as needed, as well as view graphical outputs of their personal health indicators. At the first visit, participants were trained by the CT how to take proper clinical measurements and by the CT or TSS on the technologies provided. The Smartphone was available for 24 h support for technology and health issues.
Figure 1.
Transmission of data via manual and Bluetooth-enabled devices. Wireless data transmission from Blackberry to database. Solid line, Bluetooth data entry; dash–dot line, manual data entry; dashed line, automated database responses; dotted line, physician interventions.
The CT provided counseling regarding PA and lifestyle modifications based on American College of Sports Medicine guidelines and tailored to each individual stage of change and fitness level. Exercise was prescribed based on baseline STEP test results, and changes to the exercise regimen were made as required by feedback to the CT through the remote monitoring device, as well as repeat STEP tests at week 4 and 8 study visits.
At the community research center (Gateway), at week 0 (visit 1), week 4 (visit 2), and week 8 (visit 3), participants had a complete physical examination (height, weight, waist circumference, SBP, DBP, and HR), phlebotomy, STEP test, and completed Stages of Change for Physical Activity20 and other questionnaires. Body mass index was calculated and waist circumference was measured as an index of abdominal adiposity. Blood pressure measurement was performed by a health care professional with a sphygmomanometer (BpTRU™). Venipuncture was performed for analyses of BG; hemoglobin A1c (HbA1c); total, LDL, and HDL cholesterol; triglyceride; and C-reactive protein. Level of fitness was measured using the STEP test to assess maximal oxygen uptake, VO2max.14 Each individual exercise program was set at an exercise HR of 70–85% of age-predicted maximal HR. Using SMART (specific, measurable, attainable, realistic, and timely) principles of goal-setting, participants set personal PA goals, including pedometer steps, for the next 4-week period. The recommended goal of 10,000 steps/day, with increments of improvement of 1000 steps/day, was suggested to some less active participants.21 Participants received a stage-matched activity booklet addressing self-efficacy, decisional balance, and stage-appropriate processes of change.
Self-monitoring measurements, wirelessly transmitted to a database via the Bluetooth-enabled Smartphone, included twice daily finger prick BG [fasting morning and nonfasting before sleep (automatically from the glucometer)]; daily number of steps walked (readings from the pedometer entered manually into the Smartphone); three times weekly SBP, DBP, and HR upon waking in the morning (automatically from the BP monitor); and weight once per week at home or at Gateway (manually entered into the Smartphone). Stage of Change for Physical Activity questionnaires were sent to the participants' Smartphone weekly. The Smartphone wirelessly transmitted clinical measurements to Healthanywhere servers. Health monitoring measurements were available to be viewed by the study staff within seconds of receipt at the data center. Study participants could monitor personal readings and progress by signing into a secure portal using their Smartphone to view readings and graphical outputs. Participants were provided with a 2-month data plan that allowed them to contact the CT or TSS by email, as required.
Predetermined thresholds for MS and CVD risk factor variables were programmed into the database, with alarms for readings outside threshold limits immediately triggering an email directly to the PI's dedicated Smartphone. Real-time alerts for technical issues such as late or missed readings were triggered by the database and notices sent to the TSS, which prompted follow-up with the participant. Complete details about technical aspects of the study, including data access and security, are reported elsewhere.22
All study data were analyzed with SPSS version 17, and t-tests were used to assess significant changes in the variables measured. A paired t-test was used to compare results at week 0 and week 8. All results are expressed as a mean plus or minus one standard deviation with a p value of < .05 determining statistical significance. Any self-monitoring measurements transmitted between the hours of midnight and 5:00 am were considered as data from the previous calendar day. Missing data were handled using the mean substitution approach.
Results
Twenty-six potential candidates were screened. Six participants had a diagnosis of type 2 diabetes and one of pre-diabetes. Of the 25 participants who met all inclusion criteria and provided informed consent, 1 withdrew shortly after enrollment because of hospitalization for respiratory infection not related to the study. Twenty-four sedentary participants (18 female and 6 male) between the ages of 30 and 71 years (mean age of 56.6 ± 8.9 years), completed the 8-week intervention. Thirteen participants had never smoked, and 11 participants were former smokers.
No serious adverse events were reported. At day 10 of the study, a participant transmitted an elevated BG (20.9 mmol/liter) that triggered an alarm to the PI. The participant was immediately contacted by the PI and referred to a local family physician for corrective action and monitoring. A new diagnosis of type 2 diabetes was confirmed as a result of this protocol. An unexplained, markedly elevated BP was noted by the CT when monitoring the database, and the study participant was referred to a primary health care provider. A new diagnosis of HT was made. Adherence to self-monitoring protocols was high. Participant compliance and satisfaction are reported elsewhere.21
This intervention improved some clinical markers of MS and CVD risk factors (Table 1), increased daily exercise, and most notably increased interest in and willingness to make lifestyle changes that impact health outcomes. For clinic assessments, at baseline, BMI was 33.1 ± 2.4 kg/m2 compared with 32.7 ± 4.3 kg/m2 at week 8 (p = .03) and waist circumference significantly changed from week 0 to week 8 (p = .002). These changes occurred without a statistical change in weight. There was no significant change in fasting clinic or self-monitored BG (p = .221 and p = .264, respectively). Hemoglobin A1c did not change from week 0 to week 8 (p = .22).
Table 1.
Clinical Measurements Conducted at Gateway for Assessment of Impact of the 8-Week Intervention
| Clinic measurements | n | Mean week 0 | ±Standard deviation | Mean week 8 | ±Standard deviation | t-test | Mean difference ± standard deviation | 95% confidence interval, lower | 95% confidence interval, upper |
|---|---|---|---|---|---|---|---|---|---|
| Waist circumference (cm) | 24 | 111.54 | 81.04 | 107.68 | 135.47 | 0.002a | -3.86 ± 5.43 | -6.16 | -1.57 |
| BMI kg/m2 | 24 | 33.14 | 19.01 | 32.67 | 18.76 | 0.03a | -0.465 ± 0.987 | -0.88 | -0.05 |
| Glucose (mmol/liter) | 24 | 6.03 | 5.74 | 5.52 | 1.22 | 0.221 | -0.508 ± 1.981 | -1.345 | 0.328 |
| HbA1c (%) | 16 | 0.060 | 0.000 | 0.059 | 0.000 | 0.182 | -0.001 ± 0.003 | -0.002 | 0.000 |
| SBP (mm Hg) | 24 | 141 | 95 | 139 | 351 | 0.475 | -2.125 ± 14.342 | -8.181 | 3.931 |
| DBP (mm Hg) | 24 | 85 | 71 | 80 | 80 | 0.046a | -4.458 ± 10.37 | -8.836 | -0.081 |
| Total cholesterol (mmol/liter) | 24 | 5.48 | 1.61 | 5.19 | 1.23 | 0.009a | -0.295 ± 0.508 | -0.510 | -0.080 |
| LDL cholesterol (mmol/liter) | 24 | 3.14 | 2.37 | 3.13 | 1.15 | 0.983 | -0.04 ± 0.189 | -0.396 | 0.388 |
| HDL cholesterol (mmol/liter) | 24 | 1.34 | 0.11 | 1.35 | 0.16 | 0.655 | 0.013 ± 0.029 | -0.048 | 0.074 |
| Triglycerides (mmol/liter) | 24 | 1.80 | 1.76 | 1.53 | 0.55 | 0.153 | -0.273 ± 0.905 | -0.655 | 0.109 |
| C-reactive protein (mmol/liter) | 24 | 3.98 | 3.49 | 0.284 | -0.493 ± 2.198 | -1.420 | 0.435 | ||
| Training HR (bpm) | 24 | 118 | 63 | 124 | 85 | <0.000a | 5.958 ± 6.182 | 3.348 | 8.569 |
| VO2max (ml/kg/min) | 24 | 29.54 | 31.39 | 34.68 | 49.24 | <0.000a | 5.139 ± 4.911 | 7.213 | 3.066 |
| Self-monitoring measurements | |||||||||
| SBP (mm Hg) | 24 | 136 | 15 | 133 | 18 | 0.165 | 3.875 ± 13.224 | -1.709 | 9.459 |
| DBP (mm Hg) | 24 | 88 | 10 | 84 | 9 | 0.001a | 4.375 ± 5.640 | 1.993 | 6.757 |
| HR (bpm) | 24 | 70 | 12 | 67 | 13 | 0.008a | 2.91 ± 6.8 | 0.0046 | 5.82 |
| Glucose am (mmol/liter) | 24 | 6.71 | 2.40 | 6.30 | 1.20 | 0.264 | 1.731 ± 0.353 | -0.327 | 1.135 |
| Glucose pm (mmol/liter) | 24 | 7.48 | 2.80 | 6.93 | 1.54 | 0.213 | 0.550 ± 2.105 | -0.339 | 1.439 |
| Pedometer (steps/day) | 24 | 5671 | 1989 | 6757 | 2454 | 0.003a | -1085 ± 1613 | -1767 | -404 |
| Weight (kg) | 21 | 92.74 | 13.97 | 92.12 | 13.77 | 0.312 | 0.620 ± 2.942 | -0.622 | 1.863 |
Significant statistical difference for values from week 0 to week 8.
For BP measurements (clinic), mean SBP did not change significantly from week 0 to week 8 (p = .475); however, mean DBP decreased significantly from 85 ± 71 mm Hg at baseline to 80 ± 80 mm Hg at week 8 (p = .046). For self-monitored measurements, only DBP decreased significantly from week 0 to week 8 (p = .001).
Total cholesterol decreased significantly from 5.48 ± 1.61 mmol/liter (week 0) to 5.19 ± 1.23 mmol/liter (week 8; p = .009). There were no significant changes in LDL or HDL cholesterol and triglycerides or C-reactive protein. Pedometer steps improved significantly (p = .003) from 5671 steps/day (week 0) to 6757 steps/day (week 8). Physical activity changed significantly from week 0 to week 8 (p < .001) as shown by the improved training HR. Some participants achieved the 10,000 step/day goal, with most improving from sedentary to low active step count ranges. VO2max increased from 29.54 ± 31.39 to 34.68 ± 49.24 ml/kg/min (p < .001).
Conclusions
This novel program was based on a wireless platform where participants, their lifestyle team, and health care professionals (physician, nurse) had remote, real-time access to their markers of CVCs of diabetes and the ability to modify their personal lifestyle prescription. This study demonstrated the utility of self-monitoring of the risk factors for MS and resulted in improved PA and CVD risk profile. Results are very encouraging considering the short, 8-week time period for this intervention. Participant compliance and willingness to complete the activity requirements were positive outcomes. Our findings support findings from a review of clinical benefits of pedometers to increase PA.19 We also demonstrated a significant change in DBP, BMI, total cholesterol, and steps per day. Notably, there was little change in SBP (−3 mm Hg) compared with a significant decrease of 3.8 mm Hg in the review.19 However, the impact of a decrease by 3 mm Hg is important, as a 2 mm Hg reduction in SBP translates into a 10% reduction in stroke mortality and a 7% reduction in mortality from vascular causes in a middle-aged population.23 Diastolic blood pressure was significantly decreased when measured at home and in the clinic, and changes were greater than published data from pedometer trials (−5 and -4 mm Hg, respectively, versus -0.3 mm Hg).19 Our findings may be attributed to participant use of an exercise prescription in tandem with their pedometer. The technology-supported intervention may have contributed to an increased interest in overall health, thus informing other lifestyle changes such as dietary improvement, which were not monitored. Our findings are supported by a short-term (4-month) remote BP monitoring study that found -10 and -4 mm Hg changes in SBP and DBP, respectively.18 Improved vascular function may be the mechanism responsible for the reduction in BP. Aizawa and colleagues24 found a significant increase in distensibility in patients with MS following 8 weeks of physician-prescribed exercise and Mediterranean diet.
Studies have reported either significant decreases15–17 or no change25 in HbA1c following remote BG monitoring interventions with cellular phones, but changes in home-monitored BG were not reported in these studies. Exercise is known to help control BG in insulin resistance and diabetes, and intracellular mechanisms have been investigated. A study examined the effects of a 4-week therapeutic lifestyle modification in rural women with MS26 and showed that the intervention group had significant reductions in body weight, waist circumference, triglyceride, BG, SBP, and LDL and increased HDL. These results differ from ours and may be due to racial differences (Korean versus Caucasian) or differences in intervention delivery. Information booklets were provided to all participants at the onset of the trial, and the intervention group attended education and exercise sessions three times per week for 2 hours each time. The intervention focused on both nutrition and exercise. In the DaTA study, we provided basic exercise prescription, but the participants were left to exercise on their own, and dietary advice was not given. Tjønna and associates27 noted greater improvements in MS in a group of patients completing high-intensity interval training compared with a continuous moderate exercise group, thus reinforcing that higher intensities are better for reducing CV risk. Our participants were prescribed a target HR of 70–85% (moderate intensity) of predicted maximum from the STEP test. Greater improvements may have been elicited from higher intensity activities, but as our participants were in an unsupervised setting, moderate intensity was chosen for safety.
Although only some DaTA study participants met the goal of 10,000 steps/day, this increase in PA (mean of 2239 steps/day) was sufficient to improve markers of MS. However, the population sample remained in a low-active exercise class. Furthermore, DaTA study participants may not reflect a typical response of MS patients because volunteers are generally more motivated than nonvolunteers. Nonetheless, the intervention helped participants increase PA successfully in a short time frame. Individual exercise prescriptions were tailored to include other activities as well as walking, with several participants involving physical activities that are not conducive to pedometer monitoring including cycling, swimming, and resistance training exercise. In retrospect, an accelerometer and/or exercise journal may have more effectively captured nonwalking exercise. The results suggest that the intervention may be helpful in preventing stage regression (relapse). It has been proven that short-term exercise interventions have higher adherence rates than long-term interventions, thus the results of this pilot study may not be applicable to a longer-term intervention.
Improvements in VO2max suggest that participant PA increased during the study. VO2max has been shown to improve with weight loss alone; however, weight loss was not accomplished in our study participants. Therefore, the improvements in aerobic fitness are likely attributed to increased PA. Participants were prescribed a target HR of 70–85% of their maximum; however, this value was not captured, and therefore we cannot be sure that they achieved this target.
The DaTA study was conducted as a feasibility study; therefore, a control group was excluded. This limitation does not allow us to conclude that the interventions alone were responsible for the improvement in MS risk factors and clinical outcomes.
Our objective was to assess utility of the intervention to change health outcome measures of the precursors of MS. This pilot study demonstrated that, in a short time frame, a lifestyle intervention using the STEP protocol, education, and, importantly, interactive monitoring driven by technology is effective in a rural setting. Our findings support evidence that remote self-managed monitoring technologies can effectively impact risk factors of MS and can be used where access to health care is limited. Further validation with a rigorous clinical trial process is required. This study confirms the willingness by participants to change and to invest their time in remote health monitoring measurements and increase their levels of exercise that, in turn, will prevent development of CVCs of type 2 diabetes. Furthermore, database-generated health reports proved to be a valuable two-way tool for communication between the patients and their primary care physician or other health care providers (dieticians, pharmacists). For those without a care provider, the interventions employed provided an awareness of the impact of lifestyle modification recommendations as well as their overall physical health, which may not otherwise be available to them.
In conclusion, this feasibility study supports the foundation for a large-scale randomized clinical trial. We have shown that it is possible for those at risk for MS to monitor BP, BG, PA, HR, and weight remotely and to respond to extraordinary changes with confidence at arm's length from their primary health care provider. Moreover, in an underserved rural setting, health care can be supplemented with a remote monitoring system. Through the technologies, the participants are integrated into the process of personal health care and become responsible for healthy living choices, therefore favorably impacting their CVCs of diabetes.
Glossary
Abbreviations
- (BG)
blood glucose
- (BMI)
body mass index
- (BP)
blood pressure
- (CT)
clinical team
- (CV)
cardiovascular
- (CVC)
cardiovascular complication
- (CVD)
cardiovascular disease
- (DaTA)
Diabetes and Technology for Increased Activity
- (DBP)
diastolic blood pressure
- (HbA1c)
hemoglobin A1c
- (HDL)
high-density lipoprotein
- (HR)
heart rate
- (HT)
hypertension
- (LDL)
low-density lipoprotein
- (PA)
physical activity
- (PI)
principal investigator
- (SBP)
systolic blood pressure
- (STEP)
Step Test and Exercise Prescription
- (TSS)
technology implementation and systems administration specialist
Funding
Financial support for this research was provided by the Canadian Institutes of Health Research/Canadian Diabetes Association/ Heart and Stroke Foundation for the Team Canada and Finland (2007–2012) Grant 83029.
Disclosures
Robyn Fulkerson is program director of Sykes Assistance Services Corporation, London, Ontario, Canada, and provided in-kind time for technology implementation and systems administration, data plans (Bell Canada), technology devices, as well as free access to the Healthanywhere database facilities.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa K, Shoemaker JK, Overend TJ, Petrella RJ. Metabolic syndrome, endothelial function and lifestyle modification. Diab Vasc Dis Res. 2009;6(3):181–189. doi: 10.1177/1479164109336375. [DOI] [PubMed] [Google Scholar]
- 3.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 4.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2008;32(Suppl 1):S1–201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Petrella RJ, Merikle E. A retrospective analysis of the prevalence and treatment of hypertension and dyslipidemia in Southwestern Ontario, Canada. Clin Ther. 2008;30(6):1145–1154. doi: 10.1016/j.clinthera.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S33–50. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 7.Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R. Monitoring blood glucose control in diabetes mellitus: a systematic review. Health Technol Assess. 2000;4(12):i–iv. 1–93. [PubMed] [Google Scholar]
- 8.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications of type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125(2):177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 11.National Diabetes Information Clearinghouse. Diabetes Prevention Program. 2008 NIH Publication No. 09-5099. http://www.diabetes.niddk.nih.gov/dm/pubs/preventionprogram/. Accessed July 7, 2010.
- 12.Eriksson J, Lindström J, Valle T, Aunola S, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Lauhkonen M, Lehto P, Lehtonen A, Louheranta A, Mannelin M, Martikkala V, Rastas M, Sundvall J, Turpeinen A, Viljanen T, Uusitupa M, Tuomilehto J. Prevention of type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia. 1999;42(7):793–801. doi: 10.1007/s001250051229. [DOI] [PubMed] [Google Scholar]
- 13.Petrella RJ, Lattanzio CN, Demeray A, Varallo V, Blore R. Can adoption of regular exercise later in life prevent metabolic risk for cardiovascular disease? Diabetes Care. 2005;28(3):694–701. doi: 10.2337/diacare.28.3.694. [DOI] [PubMed] [Google Scholar]
- 14.Petrella RJ, Koval JJ, Cunningham DA, Paterson DH. Can primary care doctors prescribe exercise to improve fitness? The Step Test Exercise Prescription (STEP) project. Am J Prev Med. 2003;24(4):316–322. doi: 10.1016/s0749-3797(03)00022-9. [DOI] [PubMed] [Google Scholar]
- 15.Faridi Z, Liberti L, Shuval K, Northrup V, Ali A, Katz DL. Evaluating the impact of mobile telephone technology on type 2 diabetic patients' self-management: the NICHE pilot study. J Eval Clin Prac. 2008;14(3):465–469. doi: 10.1111/j.1365-2753.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Kim NC, Ahn SH. Impact of a nurse short message service intervention for patients with diabetes. J Nurs Care Qual. 2006;21(3):266–271. doi: 10.1097/00001786-200607000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Kwon HS, Cho JH, Kim HS, Lee JH, Song BR, Oh JA, Han JH, Kim HS, Cha BY, Lee KW, Son HY, Kang SK, Lee WC, Yoon KH. Development of web-based diabetic patient management system using short message service (SMS) Diabetes Res Clin Pract. 2004;66(Suppl 1):S133–S137. doi: 10.1016/j.diabres.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Logan AG, McIsaac WJ, Tisler A, Irvine MJ, Saunders A, Dunai A, Rizo CA, Feig DS, Hamill M, Trudel M, Cafazzo JA. Mobile phone-based remote patient monitoring system for management of hypertension in diabetic patients. Am J Hypertens. 2007;20(9):942–948. doi: 10.1016/j.amjhyper.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 20.Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63(1):60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 21.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Stuckey M, Fulkerson R, Russell-Minda E, Munoz C, Kleinstiver P, Petrella R. Remote Monitoring Technologies for the Prevention of Metabolic Syndrome: the Diabetes and Technology for increased Activity (DaTA) study. Diabetes Care. doi: 10.1177/193229681100500417. To be published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 24.Aizawa K, Shoemaker JK, Overend TJ, Petrella RJ. Effects of lifestyle modification on central artery stiffness in metabolic syndrome subjects with pre-hypertension and/or pre-diabetes. Diabetes Res Clin Pract. 2009;83(2):249–256. doi: 10.1016/j.diabres.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Vähätalo M, Virtamo HE, Niikari JS, Ronemaa T. Cellular phone transferred self blood glucose monitoring:prerequisites for positive outcome. Prac Diabetes Int. 2004;21(5):192–194. [Google Scholar]
- 26.Oh EG, Hyun SS, Kim SH, Bang SY, Chu SH, Jeon JY, Kang MS. A randomized controlled trial of therapeutic lifestyle modification in rural women with metabolic syndrome: a pilot study. Metabolism. 2008;57(2):255–261. doi: 10.1016/j.metabol.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]