Abstract
Objectives
Remote monitoring technologies are ideally suited for rural communities with limited access to health care. In an 8-week pilot study, we examined the feasibility of implementing and conducting a technology-intensive intervention in an underserviced rural setting. Our goal was to test the utility of self-monitoring technologies, physical activity, and education as tools to manage health indicators for the development of the cardiovascular complications (CVCs) of type 2 diabetes.
Research Design and Methods
The Diabetes and Technology for Increased Activity study was an open single-center study conducted in a community-based research setting. All 24 participants were provided with a Blackberry™ Smartphone, blood pressure monitor, glucometer, and pedometer. Smartphones transmitted measurements and survey results to the database, interfaced participants with the clinical team, and allowed for self-monitoring.
Results
Outcomes were improved body composition, improved markers of CVC risk factors, increased daily exercise, and interest in or awareness of lifestyle changes that impact health outcomes. Participants had excellent compliance for measurements, as self-monitoring provided a sense of security that improved from week 4 to week 8.
Conclusions
Our team gained substantial insight into the operational requirements of technology-facilitated health care, including redefined hours of service; data reporting, management, and access protocols; and the utility of real-time clinical measures by remote monitoring. We developed an understanding of knowledge translation strategies as well as successful motivational and educational tools. Importantly, remote monitoring technology was found to be feasible and accepted in a rural setting.
Keywords: diabetes, lifestyle management, remote monitoring, rural primary care, technology
Introduction
Remote monitoring technologies are thought to be ideally suited for rural communities with limited access to health care. In Southwestern Ontario, where health profiles in rural settings differ from urban centers, there is an increased prevalence of hypertension (HT) and dyslipidemia compared with the remainder of Ontario and Canada, and these morbidities are undertreated in this region.1 June 2010 Statistics Canada Health Profile data2 show that rural communities, specifically Huron County in Southwestern Ontario, have a population with more obesity, diabetes, and elevated blood pressure (BP) than nearby urban centers (London, Ontario). According to the 2010 Ontario Ministry of Health and Long-Term Care list of areas designated as underserviced for general and family practitioners, Huron County has a 27.5% vacancy (number of positions for general/family practitioners) compared with 0% vacancy in London and 20.2% vacancy province-wide.3 Therefore, this population was thought to be ideal for testing the feasibility and impact of remote wireless monitoring because of their heightened risk of cardiovascular (CV) morbidity and mortality, limited access to health care, and poorly controlled risk factors.
Since 2001, Adult Treatment Panel III4 guidelines have called specific attention to the importance of targeting the CV risk factors of the metabolic syndrome (MS) as a method of cardiovascular disease (CVD) risk reduction therapy. The Canadian Diabetes Association clinical guidelines state that there is evidence to support an aggressive approach to identify those individuals with MS.5 However, the definition of MS has been a subject of debate. In 2009, the International Diabetes Federation Task Force determined MS to be a cluster of complex, interrelated risk factors for diabetes and CVD.6 Diagnosis of MS requires three of five risk factors that include elevated BP, dyslipidemia (elevated triglycerides and low high-density lipoprotein cholesterol), elevated fasting blood glucose (BG) and central obesity. A literature review demonstrated that the multifactorial origin of MS necessitates strategies for reducing CV risk that include lifestyle changes as well as pharmacologic interventions for diabetes, HT, and dyslipidemia.7 Lifestyle modifications such as increased physical activity (PA) and dietary changes were shown to have a positive impact that improved MS and reduced the risk of developing diabetes by 39% to 58%.
A systematic review of health technologies for monitoring and managing type 1 and type 2 diabetes mellitus determined that there are gaps in current understanding of evidence for effectiveness of self-monitoring devices and technologies.8 This review stated that evaluations of adherence and compliance with self-monitoring devices would be beneficial if included in future CVD studies. Self-monitoring may, in fact, enhance patient motivation to use technology for risk factor management as well as provide health care providers with reliable real-time clinical information. Therefore, we determined that there was a need to examine the feasibility and utility of self-monitoring devices for assessment of cardiovascular complications (CVC) of type 2 diabetes risk factors.
A number of studies have examined the use of remote BG monitoring via cell phone to manage diabetes. Some research found that glycated hemoglobin is decreased,9–11 while one study found no change.12 For patients with diabetes and uncontrolled HT, BP monitoring with a Bluetooth™-enabled home BP monitor and cell phone resulted in improved BP control.13 The Internet and cell phones used to assist with diabetes self-care in a clinic population,9 nurse short message service (SMS) and Internet for management of BG,10 and a Web-based SMS protocol for BG management11 were well accepted by study subjects. Compliance was relatively good with older subjects and those who had diabetes for a longer time.11 For those employed in a technical occupation or for those in the information technology field, more readings were transmitted than in other subject groups.12
Systematic reviews of health behavior change in smoking cessation and diabetes self-management with mobile telephone SMS14 and self-monitoring devices for management of individuals with type 1 and type 2 diabetes8 concurred that the greatest weakness of published studies was that, while they monitored clinical outcomes, they failed to effectively assess the targeted behavior. Remote monitoring of behavior change concurrent with BG and BP would allow immediate data assessment by practitioners and the ability to provide more appropriate feedback to patients. Moreover, remote self-monitoring has not previously been investigated as a preventative measure in patients with MS. There remain gaps in the knowledge pertaining to the use of Bluetooth-compatible technologies and equipment that include understanding of the level of adoption of the technologies by the patients being studied, the requirements for providers of data management and analysis, and the application by physicians providing care—the ultimate consumers of the data collected. Additionally, there is little knowledge about the impact of real-time consultation on the patient-physician relationship with remote monitoring.
Therefore, the primary objective of the Diabetes and Technology for Increased Activity (DaTA) study was to test the feasibility of a technology-intensive intervention in a rural setting. Secondary objectives included testing self-monitoring technology utility and PA and education as tools to manage health indicators for development of CVCs of type 2 diabetes.
Research Design and Methods
Study Design
The DaTA study was an open single-center pilot study hosted in a rural, community-based research setting. This study was conducted in compliance with the Declaration of Helsinki with ethics approval from Institutional Review Board Services (RP-2008) and the University of Western Ontario Research Ethics Board. A Southwestern Ontario community was selected, as it had a medically underserviced population and a newly opened rural research center (Gateway) that served as the study center. Study participants were recruited through social marketing strategies, including advertisements in local newspapers; posters in family medical clinics, pharmacies, and community bulletin boards; and word-of-mouth recommendations. Interested potential candidates contacted the study coordinator and responded to screening questions. Only those candidates who met all inclusion and exclusion criteria and screening requirements and provided written informed consent were enrolled.
A team of academic and industry researchers were assembled, including a principal investigator (PI), a technology implementation and systems administration specialist (TSS), experts in technical and database applications (Healthanywhere, IgeaCare, Inc.), and a clinical team (CT) consisting of a study coordinator, a certified kinesiologist, graduate students, consultants, physicians, and nurses dedicated to this study.
Study Methods
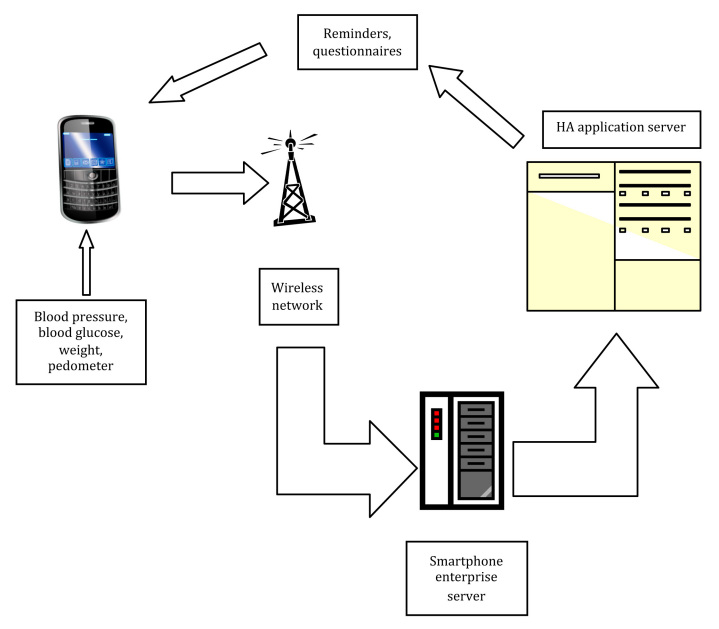
Enrolled study participants attended three clinic appointments at week 0 (visit 1), week 4 (visit 2), and week 8 (visit 3). At visit 1, participants were provided with a Smartphone (BlackBerry™ Curve 8300), a Bluetooth-enabled BP monitor (A & D Medical #UA-767PBT) and glucometer (Lifescan One Touch Ultra2™ with Polymap wireless adaptor PWR-08-03), and a pedometer (Omron # HJ-150). Smartphones transmitted data and survey responses to the database, interfaced participants with the CT and TSS, and allowed them to monitor their personal health indicators. All Bluetooth-compatible devices were paired to a unique Smartphone. At visit 1, the CT trained each participant on techniques necessary to take proper clinical measurements, to transmit clinical data, and to understand outputs. When study participants measured their BP, heart rate (HR), and BG, the reading was sent automatically to their Smartphone via the Bluetooth connection. The Smartphone then transmitted the readings through a wireless network to Healthanywhere servers (Figure 1), a secure repository for all participant electronic health measurements. Following each new measurement, the participant profile was updated on the server. The server controlled access to participant data and recorded access history. To view and monitor participant health data, only authorized study personnel with security-level specific identifiers and passwords through a secure portal had access. Security hierarchy for data access was predetermined and dictated by the clinical protocol based on the need for the team member to respond to alarms or alerts. The PI had access to all data and profiles, including personal information for identification and contact. If required, wirelessly transmitted health monitoring measurements could be viewed by the PI within seconds of receipt at the data center via the health monitoring database.
Figure 1.
Schematic of self-monitoring technologies utilized and data flow for the DaTA study.
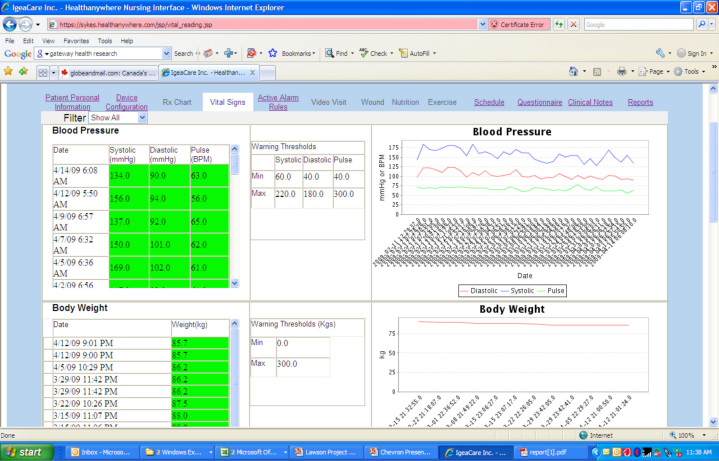
Predetermined thresholds and safety parameters for MS and CVD risk factor variables measured were programmed into the database, with alarms for readings outside threshold limits triggering an email sent directly to the PI. Participants were contacted by the PI or the CT based on the significance of the alarm. The TSS provided technical troubleshooting and monitoring for devices, data portal, database, and alerts. Alerts for technical issues such as missed or late readings were triggered by the database and sent to the TSS. The TSS triaged alerts by contacting the study participant directly or via email or by contacting and involving the PI and/or CT, if required. Participants used the study-dedicated Smartphone to contact the CT by email only for equipment failures, e.g., technical questions or responses to alerts. Healthanywhere staff could not access participant identifiers and therefore managed application and database problems as directed by the PI and TSS. Through a secure administrative portal, the TSS accessed the database to establish CT member access and passwords and study participant access and managed surveys and survey menus. The CT accessed all clinical information for the study participants and reacted to alarms or alerts as directed by the PI and TSS. The CT logged in through a secure portal and contacted the participant if required. Study participants monitored only personal data and progress by signing into a secure portal on the Smartphone to view their results. Participants were able to view logs of their readings as well as graphical outputs of their measurements (Figure 2). They communicated directly with the CT for scheduling and exercise or lifestyle queries. Reminders and PA “staging” questionnaires were initiated by the TSS or CT and sent to the participant's Smartphone via a remote prompt from the monitoring database.
Figure 2.
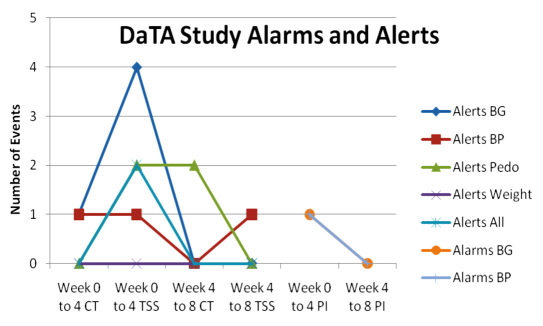
Numbers of alarms and alerts generated by the Healthanywhere database that were sent to the PI, TSS, and CT for follow-up and notification of the study participant. SBP, systolic blood pressure; DBP, diastolic blood pressure; pedometer, steps; weight, manual weight on home or clinic scale; all, BG, BP, pedometer, and weight.
Participant self-measurement data transmission included two times daily BG [fasting morning and nonfasting before sleep (sent automatically from the glucometer)], steps walked per day (pedometer readings were entered week systolic and diastolic BP and HR upon waking in the morning (sent automatically from the sphygmo-manometer). Body weight was measured and recorded once per week, at home (scale not calibrated) or at Gateway (clinic scale), and submitted by manual entry into the Smartphone. Participant assessment of technology usability and satisfaction and adherence and self-assessment questionnaires regarding PA, wellbeing, and health management were collected at clinic visits. Reporting of final data was generated by Healthany where and password-
protected and then forwarded to the study statistician with an access identifier and password that was provided in a separate email.
Database and Data Security
Real-time data were transmitted from the Smartphone to the server and database via secure Internet protocol. Personal identifiers were not stored in the Smartphone. All communication of data between participant devices and the server were encrypted using secure sockets layer (SSL) certificates before storage. Data transfers between the Smartphones and the server were encrypted and only accessible using the Verisign certified HT TPS session that employs SSL with a 128-bit encryption across all channels for the Smartphone to server (key pair) and server to database. All data were encrypted end-to-end according to the latest encryption standards and were authenticated and checked for integrity to ensure that data were sent from an authorized source and had not been tampered with. All clinical variables were transmitted as numbers only, were not accompanied by identifiable information, and were linked to participant data once on the protected server.
Access to data was limited to authorized researchers with valid user identification and passwords. A unique username and password was required to log into the database and to ensure that only authorized persons viewed participant information. If a Smartphone was lost or needed to be replaced, safeguards (secure link) were reprogrammed by changing the participant log in and password to ensure personal health information was accessed by authorized personnel only. Healthanywhere accounts were also controlled and protected remotely so that, if a device was lost, accounts were deactivated to prevent unauthorized access to participant health information.
Data Plans
Basic data plans of 4 MB per month were enabled for 2 months only. Plans included roaming to allow study participants to travel within North America. For participants who traveled outside of the data coverage area, all measures were stored at the time of measurement and automatically forwarded to the server, via Smartphone, upon reentry into data coverage areas.
Results
All 24 study participants had a minimum of two risk factors for MS (18 female and 6 male; 56.6 ± 8.9 years) and completed the 8-week intervention. Six participants had type 2 diabetes, and 1 had a diagnosis of prediabetes. Outcomes were weight loss, improvement in clinical markers for MS, increased daily exercise, and interest in or awareness of lifestyle changes that impact health outcomes. Importantly, there was an improved willingness for participants to make these lifestyle changes. Clinical outcomes are reported elsewhere.15
During this 8-week study, two alarms were generated by two participants determined to have previously undiagnosed conditions. On day 10 of the study, a participant submitted an elevated BG of 20.9 mmol/liter, and on day 13, another participant submitted a systolic BP of 195 mm Hg, which was lower than the alarm threshold but detected by the CT when monitoring the database. In response to both alarms, the PI was immediately notified electronically and the participants were referred to local clinical resources for corrective action and monitoring. As a result of this protocol and subsequent clinical investigations, new diagnoses were confirmed for type 2 diabetes and HT.
The database was regularly monitored by the CT and TSS to identify participants who were not completing their readings. When missed readings were noted, participants were contacted by email through the Smartphone or by home phone for follow-up. Over the 8-week time frame, there were 14 alerts, with 10 (71%) alerts addressed by the TSS and 4 (29%) by the CT. As shown in Figure 3, the most common alerts for weeks 0 to 4 were for missed BG (n = 4), with a single participant causing multiple alerts due to misunderstanding of measurement times. Blood pressure measurements were taken instead of BG in error by a participant, and an alert was generated for BP issues because of missed readings. Missed readings for all parameters occurred only twice, and reminders to measure were sent by the TSS. Pedometer readings were manually submitted and therefore were the next most frequent alert triggers. From weeks 0 to 4, two alerts for missed pedometer readings prompted reminders by the TSS, and for weeks 4 to 8, two alerts prompted reminders by the CT. The numbers of alerts requiring action decreased from week 0 to 4 (n = 11) to weeks 4 to 8 (n = 3). Duplicate readings were submitted in error by participants with more duplicate readings found in the first week of the study than at week 8. Duplicate submissions were most frequent for pedometer steps due to manual entry, followed by BG, where operator confidence that the reading was sent to the database was an issue. Timing of measurements for some participants posed problems, with shift work causing values to be submitted outside the expected time frame.
Figure 3.
Graphical output of participant self-monitored data as viewed by the participant, PI, CT, and TSS.
Compliance and Technology Burden
The technology experience survey demonstrated that participants were somewhat technology naïve upon enrollment into the study. Only four of the participants had previously used a Smartphone, personal digital assistant, or similar device (rarely n = 2 and frequently n = 2). Some participants had previous experience with BP monitors (n = 13) and glucometers (n = 7). Despite low levels of previous experience with technology, participants quickly became comfortable with the use of the equipment provided. When surveyed at week 4, managing the technology did not interfere with activities of daily living, required minimal effort (n = 16, 66.7%), did not take too much time in a day (n = 14, 58.3%), and required less than 20 minutes per day to use the equipment provided, for all but one subject. The impact on daily living improved from visit 1 to visit 3.
Compliance data (presented as percentage of readings completed) are as follows: 96.79 ± 4.01% of all readings were completed with 97.34 ± 4.75% compliance for morning BG, 97.77 ± 4.70% for BG prior to sleep, 94.97 ± 14.10% for BP, 96.75 ± 3.70% for pedometer steps, and 91.78 ± 11.35% for weight measurements. For 4 of 24 participants (16.67%), all measures were completed, with 12 others missing less than five readings. Missed readings were due to shift work, technology failure, and participant's forgetfulness.
The technology experience survey demonstrated that remote monitoring improved the participants' overall sense of wellbeing at visits 2 and 3 (Table 1). The self-monitoring program provided a sense of security to the study participants that improved from week 4 to week 8. Participants had an overall willingness to invest their time in health self-monitoring and increase their levels of exercise. At the conclusion of the study, most participants attended a meeting in which a database-generated health report was provided to each participant. These reports were a valuable tool for communication with their primary care physician and were used to initiate dialogue with other community health care providers, including their pharmacist, dietician, exercise trainer, and nurse practitioner. For these participants with limited access to a primary care physician and other health care resources, these study tools gave them a heightened awareness of the impact of lifestyle modification recommendations on their health as well as immediate feedback regarding changes in activities of daily living such as diet and exercise.
Table 1.
Technology Experience Survey Resultsa
| Question | Visit 2 | Visit 3 |
|---|---|---|
| 1. Currently use a personal computer (%) | 87.5 | 87.5 |
| 2. Currently use Internet (%) | 70.8 | 79.2 |
| 3. Currently use cell phone (%) | 50 | 58.3 |
| 4. Currently use a personal digital assistant (%) | 20.8 | 16.7 |
| 5. Currently use an MP3 player (%) | 4.2 | 4.2 |
| 6. Currently use a game system (%) | 4.2 | 0 |
| 7. Currently watch television (%) | 58.3 | 66.7 |
| 8. Currently listen to the radio (%) | 75 | 75 |
| 9. How often have you previously used a personal digital assistant, Blackberry, or similar device? | 1.33 ± 0.87 | 1.46 ± 1.06 |
| 10. How often have you previously used a blood pressure cuff at home? | 2.29 ± 1.33 | 2.25 ± 1.29 |
| 11. How often have you previously used a blood glucose monitor at home? | 1.83 ± 1.34 | 1.88 ± 1.33 |
| 12. How often have you previously used a pedometer (step counter) at home? | 1.70 ± 0.95 | 1.54 ± 0.93 |
| 13. I am comfortable using a Blackberry to complete the study. | 3.75 ± 0.44 | 3.58 ± 0.78 |
| 14. I am comfortable using a blood glucose monitor to complete the study. | 3.96 ± 0.20 | 3.79 ± 0.66 |
| 15. I am comfortable using a blood pressure monitor to complete the study. | 3.83 ± 0.48 | 3.67 ± 0.87 |
| 16. I am comfortable using a pedometer to complete the study. | 4.00 ± 0 | 3.83 ± 0.64 |
| 17. I am comfortable using a heart rate monitor to complete the study. | 3.83 ± 0.38 | 3.75 ± 0.68 |
| 18. The display screen on the Blackberry was easy to read. | 3.79 ± 0.41 | 3.63 ± 0.65 |
| 19. The instructions were easy to understand. | 3.71 ± 0.55 | 3.50 ± 0.66 |
| 20. The devices did not cause me any physical discomfort. | 3.54 ± 0.72 | 3.83 ± 0.38 |
| 21. The self-monitoring events were easily scheduled into my daily activities. | 3.54 ± 0.66 | 3.54 ± 0.59 |
| 22. When I had technical problems with the devices, the problem was resolved within 24 h. | 3.60 ± 0.50 | 3.75 ± 0.44 |
| 23. Participation in this self-monitoring program gave me a sense of security. | 3.43 ± 0.51 | 3.58 ± 0.50 |
| 24. Use of the self-monitoring technology helped me adopt new practices that improved my wellbeing. | 3.42 ± 0.72 | 3.83 ± 0.38 |
| 25. Managing the technology used in the DaTA study took too much time in my day. | 1.38 ± 0.58 | 1.25 ± 0.44 |
| 26. Managing the technology used in the DaTA study interfered with other activities of daily living. | 1.46 ± 0.59 | 1.33 ± 0.48 |
| 27. How much time did using the DaTA study technologies require per day? | 1.00 ± 0 | 1.08 ± 0.28 |
Items 1–8 correspond to “Yes” response; items 9–12 correspond to “N” events and standard deviation; items 13–26 correspond to a Likert scale 1–5 where 1 corresponds to “strongly disagree” and 5 corresponds to “strongly agree” with statement; item 27 corresponds to “hours”.
Conclusions
The DaTA study required coordination of a team of researchers and clinicians from urban and rural settings, a rural health clinic, local community resources, industry-based technology providers, and database resources as well as willing and interested study participants. The remote monitoring technologies enabled participants to share in the responsibility for their own health in a medically underserviced area. From the study participant perspective, remote monitoring provided a new understanding of their overall health and physical fitness and motivated them to maintain or increase exercise levels, and feedback encouraged participants to stay focused.
All 24 participants enrolled voluntarily completed the study with few technical issues. Compliance with technology was higher in the DaTA study than in other studies. In a 3-month intervention, compliance was only 72%,9 and only 2 of 15 patients submitted all BG and pedometer readings in another 3-month pilot study.11 Discrepancies in compliance may be due to a longer intervention period in these studies9,11 and broader age range11 compared with our study. In fact, we speculate that the good compliance and motivation demonstrated by our participants may be due to an older adult population that may not be generalized to all age groups. Their dedication to the study may, in part, be because of fewer commitments to caregiving and employment and greater interest in disease prevention and may possibly be due to the DaTA study's shorter time period. A 4-month study examining home BP monitoring found that weekly compliance was high throughout but had a tendency to decrease in the last 4 weeks of the study.13 We found that study participants with the least compliance were shift workers or those with family/caregiver commitments.
Other research has shown that one of the greatest barriers to the use of technology is the time commitment by physicians to monitor the database.13 Our study required less physician intervention, as triage of clinically important measurements was conducted by the database, thus reducing and limiting burden on the PI and the CT. Participants also had access to their personal data throughout the study that provided self-reflection, goal setting, and self-motivation that served as positive and negative support.
Pedometers have gained popularity as a tool for increasing PA in sedentary individuals. Our pedometer data are presented elsewhere.15 Participants thought that the pedometer gave them a realistic impression of their PA, and that it motivated them to integrate more walking into their daily routines. They felt more successful at increasing PA when they were accountable to the technology and CT compared with before-study enrollment and following discharge from the program.
Upon completion of the study, clinical result printouts (aggregate positive and negative results) were given to participants for consultation with their primary care physician. This report was useful, as it provided the physician with an overview of clinical trends. Limitations of this study include the short time frame that may not have allowed for measurement compliance to diminish, the older age of the study population that may have enhanced compliance and motivation, and, importantly, the costs for the technologies, data, and database access and availability of the resources of the TSS and CT. All participants agreed that they would continue to use the self-monitoring technology if it were publicly available, and most were willing to pay up to $20 (CDN) per month for the service. Additionally, the technology experience survey was developed specifically for this study and lacks the methodological rigor of a validated questionnaire. Nonetheless, this study was designed to determine feasibility, and the questionnaire provided important information for consideration in the design of a more rigorous clinical trial.
In conclusion, we have shown that self-managed remote technologies can increase the reach of existing primary health care resources as well as evidence-based lifestyle management of diabetes risk factors, particularly in underserviced rural areas. Importantly, this complex data collection protocol was easily managed by the research team and had minimal impact on health care resources and clinical time. The devices were found to be easy to use, had low impact on user lifestyle and time, and were accepted as a tool to change health and PA. For those in an underserviced area, these interventional tools not only increased awareness of lifestyle modifications and overall physical health that positively impacted clinical markers of CVC risk factors and precursors of MS, but identified, in real time, abnormal clinical manifestations and accelerated care for two individuals. Our findings demonstrate an overall willingness by participants to change and adopt change, to invest their time in remote health monitoring, and to increase their levels of PA. The database-generated health reports proved to be valuable two-way tools for communication between participants and their primary care physician and health care providers. The education and coaching components resulted in increased awareness of MS and diabetes risk factors and allowed focused communication with their health care providers with specific clinical data. This approach may address real or perceived barriers to preventative lifestyle strategies in current clinical practice, including provider time, expertise, and follow-up for patients at risk.
Glossary
Abbreviations
- (BG)
blood glucose
- (BP)
blood pressure
- (CT)
clinical team
- (CV)
cardiovascular
- (CVC)
cardiovascular complication
- (CVD)
cardiovascular disease
- (DaTA)
Diabetes and Technology for Increased Activity
- (HR)
heart rate
- (HT)
hypertension
- (MS)
metabolic syndrome
- (PA)
physical activity
- (PI)
principal investigator
- (SMS)
short message service
- (SSL)
secure sockets layer
- (TSS)
technology implementation and systems administration specialist
Funding
The authors gratefully acknowledge financial support for this research from the Canadian Institutes of Health Research/Canadian Diabetes Association/ Heart and Stroke Foundation for the Team Canada and Finland (2007-2012) grant 83029.
Disclosures
Robyn Fulkerson is program director of Sykes Assistance Services Corporation, London, Ontario, Canada, and provided in-kind time for technology implementation and systems administration, data plans (Bell Canada), technology devices, as well as free access to Healthanywhere database facilities.
References
- 1.Petrella RJ, Merikle E. A retrospective analysis of the prevalence and treatment of hypertension and dyslipidemia in Southwestern Ontario, Canada. Clin Ther. 2008;30(6):1145–1154. doi: 10.1016/j.clinthera.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Statistics Canada. 2010 health profile. http://www12.statcan.gc.ca. Accessed July 14, 2010. [Google Scholar]
- 3.Ontario Ministry of Health and Long-Term Care. http://www.health.gov.on.ca. Accessed July 9, 2010.
- 4.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3221. [PubMed] [Google Scholar]
- 5.Canadian Diabetes Association Clinical Practice Guidleines. Can J Diabetes. 2008;32(Suppl 1):S1–201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr, International Diabetes Federation Task Force on Epidemiology Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Athero sclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Qiao Q, Gao W, Zhang L, Nyamdorj R, Tuomilehto J. Metabolic syndrome and cardiovascular disease. Ann Clin Biochem. 2007;44(Pt 3):232–263. doi: 10.1258/000456307780480963. [DOI] [PubMed] [Google Scholar]
- 8.Russell-Minda E, Jutai J, Speechley M, Bradley K, Chudyk A, Petrella R. Health technologies for monitoring and managing diabetes: a systematic review. J Diabetes Sci Technol. 2009;3(6):1460–1471. doi: 10.1177/193229680900300628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faridi Z, Liberti L, Shuval K, Northrup V, Ali A, Katz DL. Evaluating the impact of mobile telephone technology on type 2 diabetic patients' self-management: the NICHE pilot study. J Eval Clin Prac. 2008;14(3):465–469. doi: 10.1111/j.1365-2753.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Kim NC, Ahn SH. Impact of a nurse short message service intervention for patients with diabetes. J Nurs Care Qual. 2006;21(3):266–271. doi: 10.1097/00001786-200607000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Kwon HS, Cho JH, Kim HS, Lee JH, Song BR, Oh JA, Han JH, Kim HS, Cha BY, Lee KW, Son HY, Kang SK, Lee WC, Yoon KH. Development of web-based diabetic patient management system using short message service (SMS) Diabetes Res Clin Pract. 2004;66(Suppl 1):S133–S137. doi: 10.1016/j.diabres.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Vähätalo M, Virtamo HE, Niikari JS, Ronemaa T. Cellular phone transferred self blood glucose monitoring: prerequisites for positive outcome. Prac Diabetes Int. 2004;21(5):192–194. [Google Scholar]
- 13.Logan AG, McIsaac WJ, Tisler A, Irvine MJ, Saunders A, Dunai A, Rizo CA, Feig DS, Hamill M, Trudel M, Cafazzo JA. Mobile phone-based remote patient monitoring system for management of hypertension in diabetic patients. Am J Hypertens. 2007;20(9):942–948. doi: 10.1016/j.amjhyper.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36(2):165–173. doi: 10.1016/j.amepre.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 15.Stuckey M, Russell-Minda E, Munoz C, Shoemaker K, Kleinstiver P, Petrella R. Diabetes and technology for increased activity (DaTA) study: results of a remote monitoring intervention for prevention of metabolic syndrome. J Diabetes Sci Technol. 2011;5(4):928–935. doi: 10.1177/193229681100500416. [DOI] [PMC free article] [PubMed] [Google Scholar]