Abstract
Objective
We conducted a systematic review and meta-analysis to assess the efficacy of continuous glucose monitoring (CGM) in improving glycemic control and reducing hypoglycemia compared to self-monitored blood glucose (SMBG).
Methods
We searched MEDLINE, EMBASE, Cochrane Central, Web of Science, and Scopus for randomized trials of adults and children with type 1 or type 2 diabetes mellitus (T1DM or T2DM). Pairs of reviewers independently selected studies, assessed methodological quality, and extracted data. Meta-analytic estimates of treatment effects were generated using a random-effects model.
Results
Nineteen trials were eligible and provided data for meta-analysis. Overall, CGM was associated with a significant reduction in mean hemoglobin A1c [HbA1c; weighted mean difference (WMD) of -0.27% (95% confidence interval [CI] -0.44 to -0.10)]. This was true for adults with T1DM as well as T2DM [WMD -0.50% (95% CI -0.69 to -0.30) and -0.70 (95% CI, -1.14 to -0.27), respectively]. No significant effect was noted in children and adolescents. There was no significant difference in HbA1c reduction between studies of real-time versus non-realtime devices (WMD -0.22%, 95% CI, -0.59 to 0.15 versus -0.30%, 95% CI, -0.49 to -0.10; p for interaction 0.71). The quality of evidence was moderate due to imprecision, suggesting increased risk for bias. Data for the incidence of severe or nocturnal hypoglycemia were sparse and imprecise. In studies that reported patient satisfaction, users felt confident about the device and gave positive reviews.
Conclusion
Continuous glucose monitoring seems to help improve glycemic control in adults with T1DM and T2DM. The effect on hypoglycemia incidence is imprecise and unclear. Larger trials with longer follow-up are needed to assess the efficacy of CGM in reducing patient-important complications without significantly increasing the burden of care for patients with diabetes.
Keywords: biosensing techniques, blood glucose self-monitoring, diabetes mellitus
Introduction
Intensive insulin therapies for diabetes mellitus requiring frequent self-monitoring of blood glucose often fail to reduce glycosylated hemoglobin (HbA1c) to target levels, resulting in continued high prevalence of microvascular and macrovascular complications.1,2 Limitations of these regimens include increased hypo-glycemic episodes, occult postprandial hyperglycemia or nocturnal hypoglycemia, and variable adherence related to pain or inconvenience associated with SMBG. In addition, patients may also be fearful of experiencing hypoglycemia.
Advances in continuous glucose monitoring (CGM) have allowed progression from providing retrospective data only to ongoing real-time assessment of interstitial fluid glucose concentrations. Newer alarm functions present in current real-time personal use CGM devices can alert the patient to trends such as rapid decreases in glucose levels, with the goal being prevention of severe hypoglycemia.3 These supplementary data may allow for more precise insulin administration to improve glycemic control, minimize symptomatic excursions from euglycemia, and limit the emotional impact and possible morbidity associated with such variations.
However, results of previous studies attempting to prove efficacy of these devices have been mixed. Most of these studies have been small and restricted to limited subsets of people with diabetes. Two prior systematic reviews completed on this topic failed to demonstrate significant reductions in HbA1c with use of CGM.4,5 Since the time of publication of these systematic reviews, there have been additional randomized controlled trials (RCTs) performed, including the largest CGM trial to date (322 patients).3
As new CGM technologies are invasive and expensive and provide large volumes of data to patients, these devices could have unintended consequences in relation to patient treatment satisfaction, emotional well-being, and health care resource utilization. To synthesize evidence, we undertook a systematic review and meta-analysis of RCTs to evaluate the efficacy and safety of CGM compared to SMBG with regards to improving glycemic control and reducing hypoglycemia in both adult and pediatric populations with type 1 and type 2 diabetes mellitus (T1DM and T2DM).
Methods
The report of this protocol-driven systematic review follows recommendations by the Cochrane collaboration6 and is reported following the standards set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.7
Eligibility Criteria
We included RCTs of adults and children with T1DM or T2DM in the outpatient setting. Literature search did not discriminate between different types of CGM; both real-time and non-real-time CGM devices were included. The control groups needed to utilize SMBG. We included RCTs regardless of their publication status, language, size, or primary objective. We excluded trials that did not have sufficient follow-up (8 weeks), were conducted in inpatient settings, had different methods of insulin delivery between the intervention and comparison arms, and primarily studied pregnant subjects.
Study Identification
Expert reference librarian Patricia J. Erwin designed and conducted the electronic search strategy with input from study investigators with expertise in conducting systematic reviews. To identify eligible studies, we searched electronic databases (MEDLINE, EMBASE, Cochrane CENTRAL, Web of Science, and Scopus) from 1996 to November 15, 2010. A combination of medical subject headings and text words were utilized. The detailed search strategy is available from the corresponding author.
Assessment of Study Eligibility
Pairs of reviewers independently screened all abstracts and titles and selected potentially eligible studies for full-text assessment. The process was repeated in duplicate for full-text review. Disagreements were resolved by consensus or arbitration. Kappa for study selection was 0.80.
Data Collection
Working in duplicate, a standardized form was used to extract data on study characteristics: description of participants, age, gender, number of patients involved, type of CGM, duration and frequency of use, and type of SMBG used. We also extracted outcomes of interest: HbA1c, time hyperglycemic and hypoglycemic, incidence of severe hyperglycemia and hypoglycemia, incidence of nocturnal hypoglycemia, emergency medical service utilization for extremes of glycemia, and patient satisfaction of CGM. Reviewers also appraised the methodological quality of eligible RCTs, considering adequacy of allocation concealment, blinding of patients, health care providers, data collectors and outcome assessors, if an RCT was stopped early, and the extent of loss to follow-up (i.e., proportion of patients in whom the investigators were unable to ascertain outcomes). For each of these variables, we used results reported in the original RCTs, thus accepting the authors' definitions of these terms. When needed, we contacted authors of the studies included by email to obtain missing data or confirm data we had extracted.
Statistical Analysis
We estimated the effect of CGM from each study with 95% confidence interval (CI) using the weighted mean difference (WMD) for continuous outcomes and the relative risk (RR) for dichotomous outcomes. The former measure retains the original data units and the latter is a unitless ratio. An event rate ratio was estimated if a study reported hypoglycemic and hyperglycemic events per period of time. The estimates from each study were pooled using a random-effects model8 incorporating the between-study heterogeneity as well as the within-study heterogeneity. The extent of heterogeneity was assessed using the I2 statistic.9 Interaction between subgroups was tested using an interaction test10 and meta-regression. A priori established subgroup analyses were based on the type of CGM (real-time versus non-realtime), loss to follow up, and adherence monitoring. To assess publication bias, we planned to inspect funnel plots visually and test their symmetry statistically using Egger's regression test.11
Results
Search Results
A total of 990 references were screened as abstracts and 150 of them were deemed potentially eligible. Full-text review of these 150 references resulted in exclusion of 131 references (18 were not original research reports, 113 were nonrandomized).
Table 1 describes the characteristics of the 19 trials that enrolled 1801 patients. The baseline HbA1c was at least greater than 7.0% (the vast majority were greater than 8%) in all studies but one,12 in which patients had a mean baseline HbA1c of 6.4%.
Table 1.
Characteristics of Included Studies
| First author, year | Patients | Baseline HbA1c | Mean age | % male | No. CGM | No. control | Type of CGM | CGM duration of use (weeks) | CGM frequency of use during study | SMBG description |
|---|---|---|---|---|---|---|---|---|---|---|
| JDRF-CGMSG,a 200912 | Adults, children, T1DM | 6.4% | 31 | 66 | 67 | 62 | 1. DexCom Seven 2. MiniMed Paradigm Real-Time Insulin Pump and CGMS 3. FreeStyle Navigator | 26 | Daily | Home monitoring done at least four times daily |
| O'Connell, 200915 | Adults, children, T1DM | 7.3% CGM 7.5% control | 23 | 29 | 31 | 31 | Medtronic MiniMed Paradigm REAL-time system | 12 | Willingness to use sensor at 70% of the total study duration | SMBG at least four times daily |
| Raccah, 200916 | Adults, children, T1DM | >9% | 28 | 32 | 55 | 60 | Medtronic MiniMed Paradigm 512/712 | 26 | Required to use glucose sensors at least 70% of the time | Usual SMBG |
| Cooke, 200917 | Adults, T1DM, T2DM | ∼9.0% | 52 | 60 | 102 | 100 | GlucoWatch G2 Biographer or MiniMed | 0.5 | CGM every 6 weeks for first 12 weeks | OneTouch Ultra |
| Cosson, 200918 | Adults, T1DM, T2DM | >9% | 57 (T2DM) 49 (T2DM) | 70 | 14 | 20 | GlucoDay microdialysis system | 12 | 48 h | Patients carried out their usual diet, exercise, medication, and SMBG |
| Yoo, 200819 | Adults, T2DM | 9.1% CGM 8.8% control | 56 | 42 | 32 | 33 | Guardian CGM system | 12 | 3 days/month, total 9 days | Four finger sticks per week at least, analyzed with Accu-Chek |
| Hirsch, 200820 | Adults, children, T1DM | 8.44% | 33 | 44 | 66 | 72 | MiniMed Paradigm 722 System with continuous subcutaneous insulin infusion | 26 | Continuous (approximately 6 days per week) | SMBG combined with Paradigm 715 insulin pump |
| JDRF-CGMSG,a 20083 | Adults, children, T1DM | >7% (mean 7.4%) | 28 | 44 | 165 | 157 | 1. DexCom Seven 2. MiniMed Paradigm Real-Time Insulin Pump and CGMS 3. FreeStyle Navigator | 26 | Daily | Home blood glucose meters provided, home monitoring done at least four times daily |
| Allen, 200821 | Adults, T2DM | 8.6% | 57 | 48 | 27 | 25 | Not specifically listed; MiniMed provided a small equipment grant | 0.5 | Once | Not specified |
| Yates, 200622 | Children, T1DM | 8.2% CGM 7.9% Control | 14 | 36 | 19 | 17 | MiniMed-specifics not reported | 0.5 | 3 days every 3 weeks for a 3 month period | Four to six times daily finger stick testing |
| Lagarde, 200623 | Children, T1DM | 8.4% intervention 8.8% control | 11 | 44 | 18 | 9 | MiniMed- no specifics listed | 0.5 | At 0, 2, and 4 months | Finger stick with BD logic glucometer with once weekly measurement at 2 AM |
| Deiss, 200624 | Adults, children, T1DM | Arm 1 9.5% Arm 2 9.6% Control 9.7% | 27 | 44 | 54 | 54 | MiniMed, Guardian RT | 12 | Biweekly (over 3 days every second week) | Not reported |
| Deiss, 200613 | Children, T1DM | Arm A 7.8% Arm B8.4% | 11 | 53 | 15 | 15 | MiniMed | 0.5 | Twice (once blinded, once unblinded) | Accu-Chek with finger sticks at least five times daily |
| Chase, 200525 | Children, T1DM | 8.0% | 13 | 54 | 99 | 101 | GlucoWatch G2 Biographer | 24 | Four sensors for first week, at least two per week after and weekly use overnight | OneTouch UltraSmart |
| Tanenberg, 200426 | Adults, T1DM, T2DM | 9.0% | 44 | 41 | 62 | 66 | MiniMed | 0.5 | 3 days week 1, 3 days during week 3 | OneTouch fast take glucose monitor; finger stick at least four times daily |
| Ludvigsson, 200314 | Children, T1DM | 7.7% CGM, 7.75% control | 13 | Not provided | Total 27 (crossover study) | Total 27 (crossover study) | Medtronic MiniMed | 12 | 3 days every 2 weeks | SMBG at least two times in a day and seven times in a day once a week |
| Chase, 200327 | Children, T1DM | 8.9% CGM 8.6% control | 12 | 53 | 20 | 20 | GlucoWatch Biographer | 12 | Four times per week | Four times daily with Precision Xtra meter |
| Chico, 200328 | Adults, T1DM | 8.3% T2DM CGM 8.0% T2DM control 7.4% T2DM CGM | 39 | 47 | 40 | 35 | MiniMed | 0.5 | Once for single 3-day period | Four to eight times daily finger stick |
| Chase, 200129 | Children, T1DM | 10% CGM 9.0% control | 13 | 55 | 5 | 6 | MiniMed | 4 | Anytime within the 30-day period | Accu-Chek complete meter |
JDRF-CGMSG, Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group.
As expected, mean age in studies enrolling people with T1DM was younger compared to those with T2DM. Duration and frequency of CGM use was quite variable. Type and frequency of SMBG, although often not well reported, lacked similarity in general between the studies. Two included studies had crossover study design.13,14
The methodological quality of the trials (Table 2) was fair, with only 2 in 19 definitively reporting allocation concealment, a feature that protects the randomization by concealing the allocation sequence from the investigator enrolling patients. The range of loss to follow-up was 0% to 19%.
Table 2.
Quality of the Included Studies
| First author, year | Allocation concealment | Probable study imbalances at baseline | % lost to follow-up | Adherence monitoring |
|---|---|---|---|---|
| JDRF-CGMSG,a200912 | Probably no | No | 2 | Adherence to CGM use was high in all ages although slightly decreased over time (78% versus 67%) for use for at least 6 days/week |
| O'Connell, 200915 | Probably yes | No | 11 | Bases on data uploaded to the Web-based care management program; median time using the sensor was 63% of the total period |
| Raccah, 200916 | Probably no | No | 17 | Lower compliance among adolescents |
| Cooke, 200917 | Probably no | No | 19 | Monitored use of CGM which declined during study |
| Cosson, 200918 | Yes | No | 12 | None or unclear |
| Yoo, 200819 | Yes | No | 12 | None or unclear |
| Hirsch, 200820 | Probably no | No | 6 | Calculated sensor compliance for each subject on CGM |
| JDRF-CGMSG,a20083 | Probably no | No | 2 | Monitored use of sensors among all age groups as well as hours per week of glucose readings recorded; adherence to CGM use was higher in adults than in children and adolescents |
| Allen, 200821 | Probably no | No | 11.5 | CGM data were used to counsel intervention group on glycemic control and physical activity; however, authors do not report compliance data or report how many sets of incomplete CGM data were received |
| Yates, 200622 | Probably yes | No | 0 | Reported “standard compliance problems” in CGM population; average 4.4 finger stick measurements in control group per day |
| Lagarde, 200623 | Probably yes | Intervention group was younger than controls | 0 | Obtained data regarding average number days with valid glucose profiles, and calculated mean days per participants; at all study visits, collected CGM data were sufficient to make management decisions |
| Deiss, 200624 | Probably no | No | 6 | Performed |
| Deiss, 200613 | Probably no | Baseline HbA1c was lower in arm A than in arm B | 17 | CGM data analyzed for completeness and reported |
| Chase, 200525 | Probably no | No | 0.5 | Monitored downloads of sensor data weekly |
| Tanenberg, 200426 | Probably yes | No | 15 | Excluded patients from analysis with incomplete week 12 data download |
| Ludvigsson, 200314 | Probably no | No | 4 | Performed |
| Chase, 200327 | Probably no | No | 5 | Patients, on average, used the Biographers 3.5 times each week during the intervention phase (first 3 months) |
| Chico, 200328 | Probably no | No | Not reported | Downloaded data after 3-day CGM period; however, number noncompliant not reported |
| Chase, 200129 | Probably no | HbA1c in intervention group was higher | 0 | Followed mean number of glucose readings and mean number of hours per glucose sensor, which assessed patient compliance |
JDRF-CGMSG, Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group.
After visually evaluating funnel plots and statistically testing their symmetry, we could not find evidence for publication bias; however, the number of included studies is quite small, making these methods highly underpowered. In fact, publication bias is quite likely present considering the small size of most of the studies.30
Glycemic Control
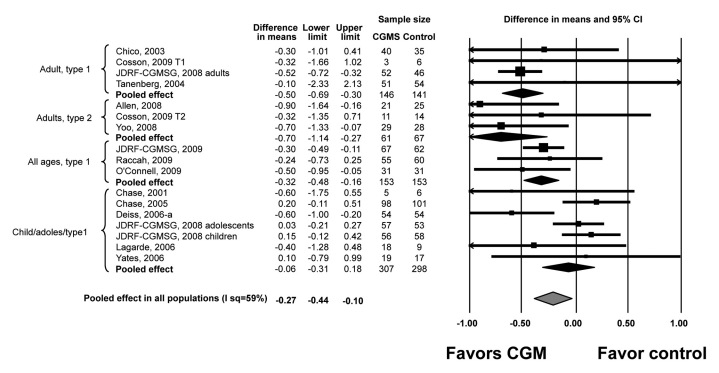
Meta-analyses of the trials included showed that, compared with SMBG, CGM is associated with a significant reduction in mean HbA1c in adults with T1DM and T2DM [WMD of -0.50% (95% CI, -0.69 to -0.30) and -0.70 (95% CI, -1.14 to -0.27), respectively]. No significant effect was noted in children and adolescents. The quality of supporting evidence is considered moderate (i.e., at high risk for bias) due to imprecision (wide CIs). Inconsistency across studies assessed by the I2 statistic was significant in studies of children and adolescents (55%) and low in studies of adults with T2DM (0%) and T2DM (0%) diabetes (Figure 1).
Figure 1.
Random effect meta-analysis of WMD of HbA1c. Upper and lower limits are limits of 95% CI. JDRF-CGMSG, Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group.
Subgroup analysis (Table 3) and meta-regression demonstrate no effect of type of CGM used (real-time versus non-real-time). Studies with potentially lower quality (higher loss to follow-up, no adherence monitoring) are associated with exaggerated treatment effect (benefit).
Table 3.
Subgroup Analysesa
| Subgroup | Number of Patients CGM/control | WMD | LL | UL | P value for interaction | I2 |
|---|---|---|---|---|---|---|
| Type of CGM | ||||||
| Non-real-time devices | 252/247 | -0.22 | -0.59 | 0.15 | 0.71 | 37% |
| Real-time devices | 415/412 | -0.30 | -0.49 | -0.10 | 66% | |
| Loss to follow-up | ||||||
| <10% | 399/379 | -0.17 | -0.44 | 0.09 | 0.04 | 74% |
| >10% | 132/138 | -0.62 | -0.95 | -0.30 | 0% | |
| Adherence monitored | ||||||
| No | 54/54 | -0.60 | -1.00 | -0.20 | 0.13 | Not available |
| Yes | 477/463 | -0.25 | -0.49 | -0.01 | 68% | |
I2 incalculable when number of studies <3. All analyses used random effect model.
LL, lower limit of 95% CI; UL, upper limit;
Adverse Effects
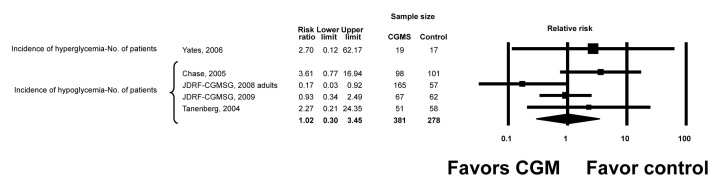
No significant adverse effects related to the device were reported in any of the trials. Data on the incidence of severe hypoglycemia, hyperglycemia, nocturnal hypoglycemia, or severe hyperglycemia were very sparse, poorly reported, and imprecise. The RR for hypoglycemia (based on number of patients suffering at least one episode of hypoglycemia as the unit of analysis) was 1.02 (95% CI, 0.3 to 3.45). Using the number of events as the unit of analysis, the rate ratio for hypoglycemia was 3.50 (95% CI, 1.07 to 11.44) and for hyperglycemia 1.42 (95% CI, 0.26 to 7.82) (Figures 2 and 3). The definitions and conclusions regarding hypoglycemia are presented for individual studies in Table 4. Hypoglycemia was the primary end point of one study12 in which patients had good glycemic control at baseline.
Figure 2.
Incidence of hyperglycemia and hypoglycemia. JDRF-CGMSG, Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group.
Figure 3.
Rate ratios of hyperglycemia and hypoglycemia. JDRF-CGMSG, Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group.
Table 4.
Description of Hypoglycemia Outcomesa
| First author, year | Study excludes patients with severe hypoglycemia | Study conclusions | Asymptomatic threshold (in mg/dl) | Definition of hypoglycemia |
|---|---|---|---|---|
| JDRF-CGMSG, 200912 | No | No difference in time spent in hypoglycemia or hypoglycemic excursions | ≤70 or ≤50 | Required assistance from another person to administer carbohydrate, glucagon, or other resuscitative actions |
| O'Connell, 200915 | Yes | No episodes of sever hypoglycemia reported | ≤70 | Severe hypoglycemia: resulting in seizures or coma or requiring third party assistance or the use of glucagon or intravenous glucose for recovery |
| Raccah, 200916 | No | No difference in time spent in hypoglycemia or hypoglycemic excursions | <70 | |
| Cooke, 200917 | No | CGM significantly increased hypoglycemic events (reported as RR and percentage of total readings in the hypoglycemic range) | <63 | |
| Cosson, 200918 | No | No difference in time spent in hypoglycemia or hypoglycemic excursions | <70 | |
| Yoo, 200819 | No | CGM mildly increased (nonsignificant) the time spent in the hypoglycemic range (<60 mg/dl); there were no reports of clinically symptomatic hypoglycemic events | <60 | |
| Hirsch, 200820 | No | CGS significantly increased time under hypoglycemia AUC; CGS significantly increased mean number of hypoglycemic episodes per patient per day within the CGM arm (change from baseline) but difference between CGM and controls was nonsignificant; CGM significantly reduced number of severe hypoglycemic events compared with controls | <70 | Resulting in seizure or coma, requiring hospitalization or intravenous glucose or glucagon, or required assistance from another person |
| JDRF-CGMSG, 20083 | No | No difference in time spent in hypoglycemia or hypoglycemic excursions | ≤70 or ≤50 | Required assistance from another person to administer carbohydrate, glucagon, or other resuscitative actions |
| Allen, 200819 | Hypoglycemia not specifically excluded, but no patients on insulin were included | Hypoglycemia outcomes not reported | ||
| Yates, 200620 | Not specific comment; those poorly compliant or HbA1c >10% excluded | There were no cases of hypoglycemia causing coma or seizures; CGM increased (nonsignificantly) percentage of monitoring time with hypoglycemia and increased (nonsignificant) percentage of night spent in hypoglycemic range | <70 | Mild, self treated; severe, coma or seizures |
| Lagarde, 200623 | Patients with “acute metabolic decompensation” were excluded; they list diabetic ketoacidosis as an exclusion, do not mention hypoglycemia | CGM increased (nonsignificant) minor hypoglycemic episodes, mean daily time <70 and AUC less <70; no severe hypoglycemic events in either group | <70 | Central nervous system symptoms consistent with hypoglycemia with inability to self-treat along with capillary blood glucose <50 or reversal of symptoms after glucose intake or glucagon administration |
| Deiss, 200624 | No | Asymptomatic hypoglycemia episodes not reported; severe hypoglycemia, one episode each per group. | Alert set at 50-80 | Resulting in seizure or coma, requiring hospitalization or intravenous glucose or glucagon, or required assistance from another person |
| Deiss, 200613 | Not excluded | Hypoglycemic indicators (AUC, event rate per month) comparable between two groups | Below 60 (AUC below this values counted as “time hypoglycemic”) | |
| Chase, 200525 | Not reported | CGM increased (nonsignificant) severe events per patient and total. | Two values <60 with no intermediate values >70; discrete episodes separated by 30 min | Seizure or loss of consciousness |
| Tanenberg, 200426 | Included | CGM significantly reduced mean duration of hypoglycemia episodes; CGM (nonsignificantly) decreased number of hypoglycemic events/ patient/day and (nonsignificantly) decreased number of nocturnal hypoglycemic events per patient per day; one CGM patient with two severe hypoglycemic events, and one control patient with one severe hypoglycemic event | <60; end of hypoglycemic event defined as the absence of hypoglycemic sensor readings ≥30 min | Less than or equal to 60 from 10 pm to 6 am |
| Ludvigsson, 200314 | No | No difference in low subcutaneous glucose levels between the two study arms | <54 | Not defined |
| Chase, 200327 | Excluded; none had severe hypoglycemia in previous 6 months, but many had had a seizure in past secondary to hypoglycemia | CGM increased hypoglycemia detection (significantly) compared with the control group; the greatest relative increase in detection of hypoglycemia was at night in CGM patients wearing the device; data are represented as event rates/100 patient hours | Blood or biographer glucose <70; only one event/h counted of low readings | |
| Chico, 200328 | Not reported | CGM increased (significantly) detection of unrecognized hypoglycemia (reported as number of patients with detected episode); majority of asymptomatic events nocturnal | <60 | |
| Chase, 200129 | Not reported | CGM significantly increased hypoglycemic episodes detected per participant during the first month; CGM effective at detecting asymptomatic nocturnal hypoglycemia; no severe events in either group. | >60 | Seizure, loss of consciousness, or requiring help from another |
JDRF-CGMSG, Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; AUC, area under the curve
Other Patient-Important Outcomes
Patient satisfaction and feasibility were reported in seven trials; these outcomes are reported in Table 5 qualitatively, because meta-analysis of these outcomes was not feasible due to heterogeneity of outcome assessment and reporting.15,17,19,25,27-29 Studies used acceptance questionnaires relating to specific features of the study arm treatment system. Results generally were favorable to the CGM. Study participants rated CGM as being superior to their previous monitoring system and recommended its use to others. Quality-of-life measures did not change with the use of CGM.31 Some concerns were raised about the technical functioning of the devices (mainly GlucoWatch). Some participants in the studies stopped wearing the continuous glucose sensors because of inconvenience, problems sleeping, bathing, and difficulty taking part in sporting activities.
Table 5.
Patient Satisfaction, Quality of Life, Feasibility, and Side Effects
| First author, year | Qualitative comments as described by the authors |
|---|---|
| O'Connell, 200915 | One participant in the intervention group admitted to hospital for new-onset depression. Mechanical problems noted in five participants using CGM. Five withdrew in intervention group: burden of recurrent alarms (n = 2), significant skin irritation (2), inability to maintain skin adhesion due to perspiration. |
| Cooke, 200917 | By 18-month follow-up, 80% of the GlucoWatch group and 33% of the CGMS group had stopped using their devices. The most common reasons for stopping use in the GlucoWatch group were skin reactions (49%) followed by difficulties with the devices (10%). In the CGMS group, the main reasons for stopping use were more varied, including inconvenience, problems sleeping, and difficulty taking part in sporting activities. |
| Cosson, 200918 | The only adverse events related to the device over the 68 CGM periods were four (5.8%) cases of uncomplicated implantation-site skin reactions, which required no specific treatment. The tolerability questionnaires found that pain during sensor implantation was nonexistent, mild, moderate, and severe in 54%, 38%, 6%, and 1%, respectively, and the bulkiness of the device was bothersome during usual day-to-day activities in 37%, 24%, 29%, and 10% patients, respectively. |
| Yoo, 200819 | Nearly all felt confident about the device. |
| Chase, 200525 | At the 6-month visit, the reasons for not using the GlucoWatch G2 Biographer included skin irritation (76%), skips too frequently (56%), alarms too frequently (47%), and does not provide accurate readings (33%). Participants were “mildly dissatisfied” with use of the GlucoWatch G2 Biographer and attributed dissatisfaction to the technical functioning of the device than the psychological ramifications of using it. |
| Chase, 20032 | Each participant completed the Fear of Hypoglycemia and the DCCT Quality of Life questionnaires. There were no significant differences in either scale between the control and Biographer groups during the intervention phase of the study. |
| Chico, 200328 | Patients felt confident and satisfied with CGMS use (not quantified). Only five patients had difficulty with system instructions. No skin lesions, although eight patients felt some discomfort. |
| Chase, 20012 | There were no significant differences in results for the Fear of Hypoglycemia or the Quality of Life surveys between the test and control participants at any time. |
Discussion
Main Findings
This systematic review and meta-analysis suggests that currently available personal real-time CGM devices improve glycemic control in adults with T1DM ot T2DM. As is true for any meta-analysis, pooling from primary studies at risk for bias reduces confidence in the results.
Strengths, Limitations, and Comparison with Other Reviews
Our work has several strengths. We asked focused questions, conducted a comprehensive and systematic literature search using explicit eligibility criteria, made reproducible judgments of eligibility and quality, and conducted a protocol-driven analysis.
The other two systematic reviews covering this topic4,5 predominantly included children with T1DM, allowed only Medtronic CGM devices to be included, did not include real-time CGM, and were completed before publication of the largest RCT.3 Our study allowed any brand of CGM and included patients with T1DM and T2DM.
The current body of literature suffers from several shortcomings. Device studies in general are structured to demonstrate a maximal difference in the outcome and will often provide more education, visits, support, feedback, and access to patients in the device group. This can result in a co-intervention effect and bias that exaggerates the treatment effect. This additional support is not well-documented in the published manuscripts and was hard to ascertain and thus remains a concern for the internal validity of the studies and poses a real challenge to applicability and evidence translation. The documentation of hypoglycemia was heterogeneous across studies (e.g., per patient, per event, percentage of time spent in hypoglycemic range, varying definitions of hypoglycemia, and no stratification according to severity of hypoglycemia), limiting the ability to conduct meaningful meta-analysis. Also, CGM technology changes rapidly, making evidence synthesis challenging. In this meta-analysis, the majority of the included trials did not conceal allocation. The inference is also limited by the small number of RCTs in patients who used the currently available CGM devices. There is much heterogeneity in the reporting of hyperglycemia and hypoglycemia, making it difficult to draw collective inferences that are helpful to clinicians. Therefore, the strength of the current evidence is low to moderate.32 Most of the studies included used devices manufactured by one company. Thus there could be differences between the different sensors available regarding insertion device, size precision, lag time that may affect patient satisfaction, and results. Adults included in these studies tended to be fairly young. It is difficult to extrapolate the findings to geriatric patients, and use of this technology may not have the same desirable findings as in younger adults. In the studies included, the number of patients with T2DM is rather low, and thus, meaningful translation of evidence of reducing HbA1c in that population is limited because of sparse data. Many of the GlucoWatch studies were done in children. Children and adolescents may use continuous glucose sensors less frequently. It has been observed that sensor use is related to effect size. For these reasons, the present meta-analysis may not have found a significant improvement in HbA1c in children and adolescents.
Recommendations for Research and Practice
Future trials should explicitly report the education and support provided to both study arms. Perhaps a standardized score of such support could be provided and validated for all device trials across specialties. Hypoglycemia should be reported uniformly with standard cutoffs and definitions of severity (minor, resolving without treatment, requiring treatment, or requiring third party assistance) and, preferably, results are reported per patient as opposed to per event.33
While we await a clinically applicable closed-loop automated pancreas or a cure for diabetes, real-time CGM seems to assist certain patients with glycemic control. It may prevent glycemic excursions and variability and may prevent life-threatening hypoglycemic events. It is challenging to make clinically important generalizations from these data regarding hypoglycemia. Perhaps CGM prevented symptomatic hypoglycemia by warning patients of trends prior to an adverse event, making the overall incidence low. However, with blood glucose readings being presented to patients every few minutes, subjects utilizing real-time CGM are bound to have more acknowledged asymptomatic readings that fall below any definition of hypoglycemia used compared to SMBG patients. Finally, and aside from hypoglycemic events, CGM may also lower the time spent in hyperglycemic state and reduce glucose variability in general.3,19
The general sense from studies that directly assessed patient satisfaction with CGM18,19 was that, overall, patients were satisfied with the devices and recommended their use to others; however, in some studies, we also saw large declines in the use of CGM devices,3,16 which may underlie part of the rationale as to why certain groups of patients did not seem to benefit, particularly adolescents.3 Also, satisfaction with a device within volunteers and perhaps early technology adopters does not necessarily transfer to satisfaction among patients who have different expectations and interests. There may be some disconnect between directly asking how satisfied a patient is with this particular technology and what causes them to discontinue actively using it. One study adjusted for compliance20 and found that compliance of more than 60% was associated with significant reduction in HbA1c compared to those with lower level of compliance. Thus careful selection of patients who may benefit from this technology—by virtue of their enthusiasm, interest, and skills—and adequate support systems may enhance the results seen in clinical trials and inform health care delivery systems in the future. Social and psychological factors also can affect the use of this technology and can help in choosing appropriate candidate individuals. In a qualitative study, the involvement of significant others, such as a spouse, and presence of adequate coping skills were factors identified to be associated with increased CGM use.34
Guidelines and technologies for interpreting large volumes of CGM data could improve the counseling and management support that follows CGM use. The impact of CGM across patients of different age and diabetes type remains largely uncertain. Optimal frequency and duration of CGM use are unknown. Finally, the cost-effectiveness of CGM is yet to be determined. Preliminary analyses projected that long-term use of CGM is cost-effective among patients with T1DM at the $100,000/quality-adjusted life year threshold; these estimates, however, are very sensitive to the various modeling assumptions and are surrounded by great uncertainty.35
Conclusion
Real-time CGM lowers HbA1c in adult patients with T1DM and T2DM and has no discernible impact in other patients with diabetes. Potential benefits of these technologies need to be considered in light of the burden it places on patients, clinicians, and health systems.
Glossary
Abbreviations
- (CGM)
continuous glucose monitoring
- (CI)
confidence interval
- (HbA1c)
hemoglobin A1c
- (RCT)
randomized controlled trial
- (RR)
relative risk
- (SMBG)
self-monitored blood glucose
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
- (WMD)
weighted mean difference
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet 2011. http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed April 14, 2011.
- 2.DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289(17):2254–2264. doi: 10.1001/jama.289.17.2254. [DOI] [PubMed] [Google Scholar]
- 3.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 4.Golicki DT, Golicka D, Groele L, Pankowska E. Continuous Glucose Monitoring System in children with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetologia. 2008;51(2):233–240. doi: 10.1007/s00125-007-0884-9. [DOI] [PubMed] [Google Scholar]
- 5.Chetty VT, Almulla A, Odueyungbo A, Thabane L. The effect of continuous subcutaneous glucose monitoring (CGMS) versus intermittent whole blood finger-stick glucose monitoring (SBGM) on hemoglobin A1c (HBA1c) levels in Type I diabetic patients: a systematic review. Diabetes Res Clin Pract. 2008;81(1):79–87. doi: 10.1016/j.diabres.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JP, Green S, editors. West Sussex: Wiley-Blackwell; 2008. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borenstein MH, Higgins JP, Rothstein HP. Westchester: Wiley and Sons; 2009. Introduction to meta-analysis (statistics in practice) [Google Scholar]
- 12.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, Buckingham B, Chase P, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Huang ES, Kollman C, Kowalski AJ, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer SA, Wilson DM, Wolpert H, Wysocki T, Xing D. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32(8):1378–1383. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deiss D, Hartmann R, Schmidt J, Kordonouri O. Results of a randomised controlled cross-over trial on the effect of continuous subcutaneous glucose monitoring (CGMS) on glycaemic control in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2006;114(2):63–67. doi: 10.1055/s-2006-923887. [DOI] [PubMed] [Google Scholar]
- 14.Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics. 2003;111(5 Pt 1):933–938. doi: 10.1542/peds.111.5.933. [DOI] [PubMed] [Google Scholar]
- 15.O'Connell MA, Donath S, O'Neal DN, Colman PG, Ambler GR, Jones TW, Davis EA, Cameron FJ. Glycaemic impact of patientled use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250–1257. doi: 10.1007/s00125-009-1365-0. [DOI] [PubMed] [Google Scholar]
- 16.Raccah D, Sulmont V, Reznik Y, Guerci B, Renard E, Hanaire H, Jeandidier N, Nicolino M. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32(12):2245–2250. doi: 10.2337/dc09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke D, Hurel SJ, Casbard A, Steed L, Walker S, Meredith S, Nunn AJ, Manca A, Sculpher M, Barnard M, Kerr D, Weaver JU, Ahlquist J, Newman SP. Randomized controlled trial to assess the impact of continuous glucose monitoring on HbA(1c) in insulin-treated diabetes (MITRE Study) Diabet Med. 2009;26(5):540–547. doi: 10.1111/j.1464-5491.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 18.Cosson E, Hamo-Tchatchouang E, Dufaitre-Patouraux L, Attali JR, Pariès J, Schaepelynck-Bélicar P. Multicentre, randomised, controlled study of the impact of continuous sub-cutaneous glucose monitoring (GlucoDay) on glycaemic control in type 1 and type 2 diabetes patients. Diabetes Metab. 2009;35(4):312–318. doi: 10.1016/j.diabet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Yoo HJ, An HG, Park SY, Ryu OH, Kim HY, Seo JA, Hong EG, Shin DH, Kim YH, Kim SG, Choi KM, Park IB, Yu JM, Baik SH. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82(1):73–79. doi: 10.1016/j.diabres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–383. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 21.Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract. 2008;80(3):371–379. doi: 10.1016/j.diabres.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates K, Hasnat Milton A, Dear K, Ambler G. Continuous glucose monitoring-guided insulin adjustment in children and adolescents on near-physiological insulin regimens: a randomized controlled trial. Diabetes Care. 2006;29(7):1512–1517. doi: 10.2337/dc05-2315. [DOI] [PubMed] [Google Scholar]
- 23.Lagarde WH, Barrows FP, Davenport ML, Kang M, Guess HA, Calikoglu AS. Continuous subcutaneous glucose monitoring in children with type 1 diabetes mellitus: a single-blind, randomized, controlled trial. Pediatr Diabetes. 2006;7(3):159–164. doi: 10.1111/j.1399-543X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 24.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 25.Chase HP, Beck R, Tamborlane W, Buckingham B, Mauras N, Tsalikian E, Wysocki T, Weinzimer S, Kollman C, Ruedy K, Xing D. A randomized multicenter trial comparing the GlucoWatch Biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care. 2005;28(5):1101–1106. doi: 10.2337/diacare.28.5.1101. [DOI] [PubMed] [Google Scholar]
- 26.Tanenberg R, Bode B, Lane W, Levetan C, Mestman J, Harmel AP, Tobian J, Gross T, Mastrototaro J. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526. doi: 10.4065/79.12.1521. [DOI] [PubMed] [Google Scholar]
- 27.Chase HP, Roberts MD, Wightman C, Klingensmith G, Garg SK, Van Wyhe M, Desai S, Harper W, Lopatin M, Bartkowiak M, Tamada J, Eastman RC. Use of the GlucoWatch biographer in children with type 1 diabetes. Pediatrics. 2003;111(4 Pt 1):790–794. doi: 10.1542/peds.111.4.790. [DOI] [PubMed] [Google Scholar]
- 28.Chico A, Vidal-Ríos P, Subirà M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care. 2003;26(4):1153–1157. doi: 10.2337/diacare.26.4.1153. [DOI] [PubMed] [Google Scholar]
- 29.Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, Garg SK. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107(2):222–226. doi: 10.1542/peds.107.2.222. [DOI] [PubMed] [Google Scholar]
- 30.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Beck RW, Lawrence JM, Laffel L, Wysocki T, Xing D, Huang ES, Ives B, Kollman C, Lee J, Ruedy KJ, Tamborlane WV. Quality-of-life measures in children and adults with type 1 diabetes: Juvenile Diabetes Research Foundation Continuous Glucose Monitoring randomized trial. Diabetes Care. 2010;33(10):2175–2177. doi: 10.2337/dc10-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swiglo BA, Murad MH, Schünemann HJ, Kunz R, Vigersky RA, Guyatt GH, Montori VM. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93(3):666–673. doi: 10.1210/jc.2007-1907. [DOI] [PubMed] [Google Scholar]
- 33.Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: hypoglycemia with intensive insulin therapy: a systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab. 2009;94(3):729–740. doi: 10.1210/jc.2008-1415. [DOI] [PubMed] [Google Scholar]
- 34.Ritholz MD, Atakov-Castillo A, Beste M, Beverly EA, Leighton A, Weinger K, Wolpert H. Psychosocial factors associated with use of continuous glucose monitoring. Diabet Med. 2010;27(9):1060–1065. doi: 10.1111/j.1464-5491.2010.03061.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang ES, O'Grady M, Basu A, Winn A, John P, Lee J, Meltzer D, Kollman C, Laffel L, Tamborlane W, Weinzimer S, Wysocki T, Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2010;33(6):1269–1274. doi: 10.2337/dc09-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]