Abstract
Today, lancing fingertips or alternative sites for obtaining a blood sample for self-monitoring of blood glucose (SMBG) is a standard procedure for most patients with diabetes. The need for frequent lancing and associated discomfort and pain can be seen as a key hurdle for patients to comply with SMBG regimens. This article provides an overview of the status quo and future of lancing, focusing on key areas for future developments driven by customer and market needs. We also review technical issues and provide a background for possible improvements.
The act of puncturing the skin with a lancet to obtain a blood sample seems to remain the standard procedure for the foreseeable future, because alternate ways of providing a blood sample have not demonstrated overall superiority (e.g., with laser technology). Other methods, which avoid lancing entirely, have also not gained broad market acceptance (e.g., minimally invasive continuous glucose monitoring) or not shown technical viability (e.g., noninvasive glucose monitoring).
In relation to blood glucose (BG) meters and test strips, lancing has been a “stepchild” with regards to commercial attention and development efforts. Nevertheless, significant technological improvements have been made in this field to address key customer needs, including better performance (regarding pain, wound healing, and long-term sensitivity), reduced cost, and higher integration with other components of BG monitoring (e.g., integration of the lancing device with the glucose monitor). From a technical perspective, it is apparent that highly comfortable lancing can be accomplished; however, this still requires fairly advanced and complex devices. New developments are necessary to achieve this level of sophistication and performance with less intricate and costly system designs. Manufacturers' motivation to pursue these developments is compromised by the fact that they might not recoup their development cost on commercial advanced lancing systems through direct profits, but only through its positive influence on adherence and increased more profitable sensor utilization.
We believe that two main driving forces will continue to push the evolution of lancing and sampling technology: (1) the need for maximum lancing comfort and (2) the advent of fully integrated systems, realizing a device in which all steps for SMBG are incorporated, thus providing a “one-step” experience. Rendering lancing a “nonissue” will eliminate a key barrier to adherence with appropriate SMBG regimens. Providing sophisticated lancing devices that allow the highest level of comfort and/or seamless blood sampling is key to improving user acceptance. This may have a greater impact on metabolic control than many of the new and expensive antidiabetic drugs.
Keywords: finger pricking, insulin therapy, lancing, pain, self-monitoring of blood glucose
Introduction
“One of the things I use a lot is a lancing device. Don't we all? Yes, yes we do, but I don't think many people give them that much thought.”1
Millions of people with diabetes lance their fingers many times daily as a starting point for performing self-monitoring of blood glucose (SMBG). Current recommendations suggest that all individuals with type 1 diabetes measure at least 3-4 times/day. For other types with diabetes (e.g., insulin-treated people with type 2 diabetes), differing approaches are recommended, while for individuals with type 2 diabetes and no insulin treatment, recommendations for SMBG are very controversial (i.e., ranging from recommendations for frequent testing to no SMBG testing at all). Self-monitoring of blood glucose is required to adjust the prandial insulin dose based on the current blood glucose (BG) level and carbohydrate intake while employing an intensified insulin regimen. In addition, SMBG allows detection of low BG levels for prevention of hypoglycemia. Over time, SMBG results can be analyzed to detect diurnal patterns in patients' glycemic control, for adjustment of the diabetic treatment regimen.
Unfortunately, the process of frequent blood sampling is inconvenient, fairly painful, potentially costly, and presents long-term issues for finger sensitivity. These issues are major reasons why patients are reluctant to perform SMBG frequently and are often noncompliant with therapy guidelines.2 Reduced BG measurement frequency is highly correlated with suboptimal metabolic control [i.e., high levels of glycated hemoglobin (A1C)],3 which, in turn, is closely associated with the development of diabetes-related complications. Therefore, any reduction of barriers to performing SMBG is highly relevant for patients to help them avoid serious complications of diabetes and is as important to health insurance providers who are concerned about the enormous costs associated with the treatment of these complications.4
Given the history of the SMBG market and its evolutionary step-wise advancement, there exists a major financial incentive for all manufacturers to devote significant resources in introducing a next-generation convenience and performance level of blood glucose meters, along with providing overall system improvements. Market dynamics have changed, however. The advent of new capable competitors in the marketplace and the increasing practice of competitive bidding have accelerated price erosion of glucose test strips and resulted in overall reduction in BG monitoring system profit margins. This has put significant constraints also on the development and introduction of new lancing systems. In order to recoup the investment and gain sufficient market share, these lancing systems will have to be both cost competitive and technologically advanced.
Moreover, one has to put glucose monitoring system advancements into perspective with the entire area of user compliance and the emergence of related disease management programs. Also, new ways of company-user interaction have to be considered when assessing the value of new concepts. It is crucial to understand the interrelationship of these factors to realistically value the benefit of technology-focused system advancements.
Ultimately, the glucose meter market has been and will likely continue on a step-wise evolutionary path. New lancing systems have to strive for a balance of innovation, quality, cost, and risk. Any development project of a future high-performance lancing device, which sets out to capture significant market share, has to accept these realities and offer a design that allows highest performance with at-par or better instrument and disposable cost. The perceived higher value and improved consumer experience with a higher performance factor will unlikely justify any substantial “direct to customer” cost burden.
One can differentiate the views of various stakeholders in the glucose monitoring scheme:
Patients
For patients, SMBG is likely the most uncomfortable part of diabetes therapy, as it is associated with a series of unpleasant issues. For example, pain related to lancing is often much higher than that endured from insulin injections. Frequent lancing of finger tips with suboptimal devices over the years may result in the development of significant scarring and sensitivity loss at the fingertips (which still remain pain receptive). All these effects clearly represent an additional loss of quality of life in addition to the burden of a life-long chronic disease.
Health Care Provider
The health care provider (HCP) is generally aware of the importance of SMBG as part of the overall therapeutic regimen. The HCP wants the patient to comply with a SMBG regimen so that the patient can make appropriate day-to-day (meal-to-meal) therapeutic decisions and avoid severe hypoglycemic crises. This enables the HCP, at the office visit or phone/Internet visit, to make informed decisions about therapy adjustments. The HCP is interested in making sound therapeutic decisions based on frequent SMBG results, though they frequently give little attention to the issue of lancing.
Insurers/Payers
Therapy compliance is a key concern of insurers; however, all cost components linked with a chronic disease, such as diabetes, are under severe scrutiny. Despite the proven fact that frequent SMBG is associated with better metabolic control and a lower rate of long-term complications, insurers often have to focus on short-term cost control.3 Often, this leads to their declining reimbursement for extended or advanced monitoring processes [e.g., continuous glucose monitoring (CGM)]. Insurers started debating the usefulness of SMBG for non-insulin-using patients with type 2 diabetes. The cost pressure has also led them to frequently use competitive bidding, pushing glucose monitoring devices into price wars, as seen in consumer product markets. Through all these changes, the ability for companies to introduce novel, technologically advanced systems—which require a higher price point—has become severely constrained.
Diagnostic Companies
For some major companies, the lancing market represents up to 5-7% of the revenue of the entire glucose monitoring market (>$10 billion in 2010). Investments in advanced lancing systems by major companies are primarily seen in connection to their potential positive impact on companion glucose monitoring systems. Nevertheless, offering advanced lancing systems promises good financial return on its own merit. Market leaders are actually capturing solid revenues, market positioning, and positive customer perception by offering leading lancing systems. A new aspect for investment in advanced lancing systems will play out as companies develop fully integrated glucose monitoring systems (i.e., integration of the lancing device with the glucose meter), where high-performing lancing devices are a must, to capture the benefit of low-volume glucose sensors (blood volume requirement < 0.1 μ1, see following discussion) and realize one-step BG measurement with possible painless sampling. Given the reimbursement landscape, companies often have to go “at risk” and subsidize first-generation systems with the hope of recouping their investment with cost-improved future product generations.
In summary, lancing does not have a front row seat in SMBG. It is mostly undervalued, and investments are more focused toward improvement of glucometer performance and (unnecessary) reduction of blood volume. The academic world reflects this situation: there are no symposia; few, if any, presentations about lancing at scientific meetings; and a very small number of publications reporting scientific studies about lancing. A literature search in PUBMED with the search terms self-monitoring of blood glucose and pain resulted in only 14 hits. A few head-to-head studies have been performed comparing different lancing devices, showing considerable differences in pain.5–9 Interestingly, no systematic review about lancing has ever been published, which is in sharp contrast to the considerable number of reviews about SMBG in general.
However, it is of note that, on the Internet, the topic of lancing is regularly discussed by patients themselves on a number of sites, for example, www.diabetesmine.com/2008/01/alternative-pri.html (visited November 11, 2010). In the United Kingdom, the Purchasing and Supply Agency of the National Health System also provides information about available lancing systems (www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON2025400?useSecondary=&showpage=2; visited November 11, 2010).
In the Diabetes Forecast Resources Guide 2009, 51 different lancing devices manufactured by 16 different companies are listed (forecast.diabetes.orglfiles/images/LancetsChartREVISED.pdf; visited November 1, 2010). This suggests that lancing devices provide an interesting market opportunity for companies, either as standalone devices or as components of monitoring systems. Despite this attractiveness, there is very little public knowledge about the technology and general performance characteristics of these devices or their development paths. Interestingly, in contrast to glucose meters, the number of lancing-device-related articles and/or direct advertisements in diabetes journals is relatively small, despite the substantial technical know-how that has been accumulated inside the diagnostics companies over the years. Trade secrets, which are hard to protect, are not made available to the public and—more importantly—to competitors (e.g., information about lancing performance with respect to lancet geometry, skin physiology, nerve structure, skin anatomy, and texture properties). In addition, companies are understandably not willing to share potential improvements, advertise research direction, or future product strategies.
This review provides an overview of publicly available knowledge of key aspects of lancing technology and future opportunities for improvement.
The Field of Lancing
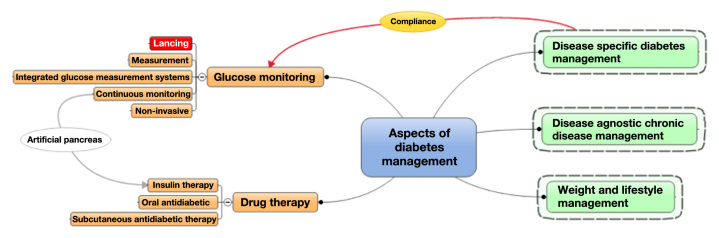
Trying to predict and assess the future of lancing requires an understanding of the priority of customer needs and how many and what classes of different lancing systems are anticipated. In addition, lancing-specific customer needs have to be seen as part of an entire glucose measurement system, as well as viewed in light of overall developments in diabetes and chronic disease management, all trying to improve compliance. Reviewing lancing systems and possible future developments has to keep high therapy compliance as a guideline and ultimate goal. Figure 1 depicts this situation and shows the main aspects of diabetes management and lancing as part of the overall glucose monitoring scheme.
Figure 1.
Landscape of diabetes management and the position of lancing.
Disease management efforts, on their own, play an important role in a patient's compliance with recommended therapy regimens and may help individuals accept or endure technical and inconvenience hurdles (e.g., lancing pain). Sometimes, however, improvements in compliance can be accomplished more easily and efficiently with disease-management-based behavior modifications. Therefore, any company considering an investment in improving therapy compliance through technology advancement should consider an investment in disease management as a real alternative to avoid going overboard with advanced technical developments. Nevertheless, lancing plays a “gate keeper” role in the overall glucose monitoring cascade. One can argue that, today, it represents the key barrier to patients' compliance with their prescribed SMBG regimen. Eliminating this barrier has a lot of merit and could significantly benefit today's diabetes management.
Our discussion of the “future of lancing” is structured along three aspects:
Review of customer needs as prioritized performance criteria in light of
Technical opportunities and limitations, while accounting for
Product system aspects (e.g., cost and reimbursement).
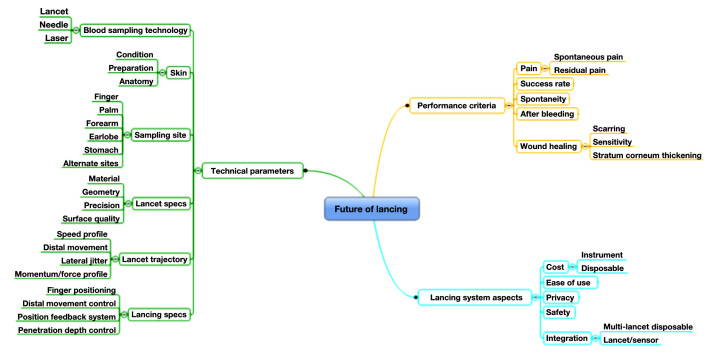
Figure 2 provides an overview of key determining factors within these three areas.
Figure 2.
Future of lancing: overview of determining factors in three key areas.
Customer Needs
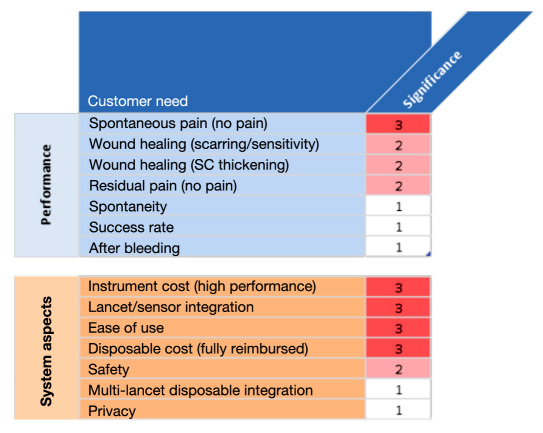
In order to determine what technical improvements and investments are needed, one has to prioritize unmet customer needs and consider their market significance and interdependence (e.g., price versus performance). Unfortunately, no publicly available studies exist that would allow such an objective assessment. Figure 3 provides the subjective view of the authors about the market significance of various customer needs and therefore has to be interpreted with caution when delineating future directions.
Figure 3.
Priority list of customer needs with respect to (i) performance and (ii) system aspects of lancing devices. Scale from 3 (high) to 1 (low). SC, subcutaneous.
Pain and wound healing are top-priority performance aspects. Full reimbursement along with uncompromised ease of use are top system requirements. When prioritizing these aspects, one has to differentiate current lancing device concepts from future fully integrated systems, where sampling and sensor function are integrated into a one-step measurement process (see following discussion). Such systems have great potential, despite the technical difficulties and required major development efforts.
Current Practice
The current practice of lancing and its perceived hurdles are important aspects for projecting future systems. Key questions are as follows:
How do patients practice lancing on a day-to-day basis (and why)?
Why do we lance fingertips (despite their high sensitivity)?
What blood sample volume do we need?
How do we reduce pain?
How Do Patients Practice Lancing on a Day-to-Day Basis (and Why)?
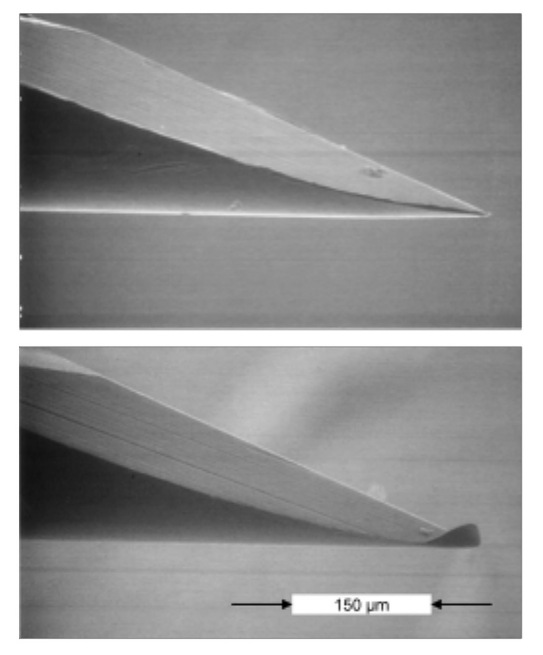
Lancing discomfort, for example, spontaneous pain (i.e., pain that is felt immediately when lancing), residual pain (i.e., lingering pain that is felt for minutes or even hours after the lancing event), and wound healing, is increased when patients use the same lancet multiple times, as the sharpness of the lancet diminishes. Actually, the issue is not so much the sharpness of the lancet (edges of facets) but the bending of the tip of the lancet (Figure 4).
Figure 4.
Native and used lancet after a single skin penetration.
A bent lancet tip affects spontaneous pain differently than residual pain and wound healing. When the lancet is bent during its inbound trajectory and retrieved in a jittery or nonstraight line, the bent tip “roughens” the wound channel, which causes (i) residual pain, (ii) bad wound closure (including after bleeding), and (iii) compromised wound healing (e.g., scarring). The amount of jitter in the motion of a retrieving lancet varies considerably among different lancing devices, which explains why the negative effects of the lancet tip bending (which always occurs) varies according to the lancing device being used. Therefore, residual pain is low for systems with straight outbound lancet trajectories. This means that, with some devices, the lancet can be used more often before it hurts (i.e., residual pain), which is of particular importance for frequent testers who may be hesitant to change lancets several times a day.
Pain associated with lancing also fosters a negative perception of diabetes and its therapy in general. Lancing discomfort often contributes to situational depression as a psychological side effect and often adds to a general therapy resistance, particularly in children, who are hard to convince to perform SMBG several times a day. This resistance also adds stress to the family situation, as it puts parents and care givers in a conflict situation as they try to avoid pain for their loved one but yet enforce the necessary and painful therapy regimen. Interestingly, the fairly harmless procedure of a finger stick appears to drive the negative perception of diabetes as much or even more than the essentially pain free but potentially far more risky subcutaneous injection of insulin (i.e., overdosing or underdosing of insulin associated with severe health risks).
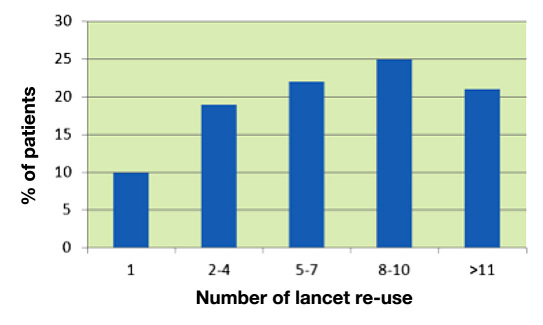
There are only few published data from surveys reporting how often patients with diabetes use a given lancet in daily life. In one survey, which was more focused on the measurement steps of SMBG, only 10% of the patients reported replacing the lancet with every use10 (Figure 5).
Figure 5.
Self-reported frequency of use of a given lancet by patients with diabetes (1, used once).
The cited survey provides the following reasons why patients did not change their lancets more often:
Lancets do not become blunt (64%)
Lancets do not have to be sterile (57%)
Ease of use (45%)
Forgot to change lancet (38%)
Cost (31%)
Performance of this survey was supported by one of the major diagnostic companies. Data from another survey performed in Germany, again sponsored by a manufacturer of lancing devices, also indicates that many patients rarely change their lancets and do so in irregular intervals. For example, patients waited approximately 2 to 3 weeks—which is equivalent to approximately 50-100 measurements, considering 3-5 measurements/day—before changing their lancet.11 However, no mention was made as to which actual lancing device was used, which, as explained earlier, is important when drawing conclusions. These surveys face a potential selection bias, because well-motivated patients are more likely to participate. It may well be that lancet re-use is even more prevalent than suggested by these surveys.
It would be interesting to see results of more surveys on the reality of lancing, also from different countries. Factors other than pain and wound healing may play a more dominant role in countries where lancing systems and disposables are not reimbursed (e.g., Australia). Such surveys could also address how much patients learn and remember about lancet re-use during their initial diabetes training, if they participate at all. To our knowledge, the depth and breadth of diabetes training often faces time and cost constraints, at least in the United States, whereby lancing-related aspects of education and training rarely receive sufficient attention despite their major impact on overall compliance. Better training and in-depth understanding would result in better lancing habits and likely improve quality of life, even without any technical improvement in the lancing system. It would be helpful if patients were actively involved in the selection of their first lancing device. Unfortunately, they usually start their diabetes monitoring life with the lancing device that comes along with the glucose meter, which may not offer the best performance. Where appropriate, using a better device from the start would go a long way.
Another important aspect of lancing in which patients should receive training is proper adjustment of the lancet penetration depth. Today, many devices allow such an adjustment even though their precision is often poor. Patients need to realize and appreciate how much they can reduce lancing discomfort by correctly adjusting depth settings for different lancing sites (i.e., fingers). They need to find the optimal balance between depth setting, sufficient blood sample, and success rate.
Some modern lancing devices, such as Roche's Accu-Chek Multiclix,® contain a cartridge or drum with a number of lancets. This is an important aspect for many patients (not just elderly patients) who usually avoid replacing conventional lancing devices simply because it is often cumbersome and requires good dexterity and vision.12 These devices also have the psychological advantage that individuals do not have to handle or see the individual lancet,13 which addresses general lancing anxiety and needle (lancet/sharp) phobia. Despite the higher cost, using a fresh lancet for each measurement offers clear advantages: it improves wound quality, reduces pain, and avoids possible infections and even (rare) finger sepsis.11,14
Clearly, of key importance for patients is how well they were instructed to perform lancing on a day-to-day basis. Most often, such instructions were performed preferably by certified diabetes educators (CDEs) and not by the treating physician. Training provided by the CDE should not be underestimated; this training is an excellent opportunity to optimize the lancing process with the currently available products. The CDE can identify and train patients to use the best lancet device for their individual needs by assessing dexterity, cognition, learning style, coordination, skin type/sensitivities, and visual acuity. In addition, patients tend to be less timid in asking for clarifications from a CDE versus the physician. The following aspects should be addressed by the CDE during training:
Evaluation and collaborative decision making of lancet and assistive lancing device;
Device function, including assessment of penetration required; and
Troubleshooting.
Clearly, patients should not only be instructed but also supervised while practicing lancing to learn about:
Skin preparation,
Site selection,
Tips to obtain adequate blood sample,
Tips to reduce discomfort, and
Alternative sites—when and how.
Patients should receive written instruction for reinforcement, and there should always be a follow-up patient visit to assess lancing technique as well as any potential difficulties.
Why Do We Lance Fingertips (Despite Their High Sensitivity)?
The fingertips are the area of the human body with the highest density of three types of nerve receptors (touch, pressure, and pain), all having different stimulation and threshold values. One can actually stimulate one type of receptor and leave the other dormant. Everybody has probably experienced this: cutting one's finger with a piece of glass, which was only barely perceptible (touch sensation), did not cause a pressure sensation and, surprisingly, did not cause any immediate/spontaneous pain. This example actually has important practical relevance when designing high-performance lancing devices. The closer a lancing device can mimic this “cutting with a piece of glass” situation, the less painful it is (as long as it stays within thresholds of important parameters such as penetration, depth, lancet trajectory, and others).
However, most lancing devices have yet to reach this ideal and thereby cause spontaneous and residual pain—at times, to a great degree. Nevertheless, patients primarily lance their fingertips to collect capillary blood samples mainly because there is a good blood supply at the fingertips.2,15,16 Capillary blood can be derived reliably from the upper dermal plexus of the skin, with a typical lancing depth of less than 2000 μm. These plexuses get in-flow from capillaries and arterioles via arteriovenous shunts. The papillary capillary density is 50-70/mm2 at the fingertip, corresponding to a capillary-to-capillary distance of 120-140 μm (mesh size). In comparison, the density at the calf is only 20-40/mm2>, corresponding to a mesh size of 160-220 μm. The arteriovenous shunts are numerous in nonhairy skin (e.g., finger, palm, earlobe) and nearly absent in hairy skin (e.g., arm, leg, abdomen).
Obtaining a blood sample of sufficient volume to perform a successful BG measurement requires the lancet to cut through the upper skin layers to a depth that opens a sufficient number of small blood vessels. The capillary and/or venous blood pressure will then drive the blood outward through the wound channel. However, this spontaneous blood flow will occur only if the local blood pressure is sufficient to force the wound channel open.
Patients prefer lancing the fingertips because it is convenient (i.e., the finger aids blood transfer to the end of the test strip of the glucometer) and provides a high success rate. Success rate is defined as the percentage of lancing trials that yield a sufficient amount of blood with the first lancing attempt and minimize the need for annoying relancing. Today's lancing devices vary, but it is fair to assume that most of them achieve a success rate at the fingertips greater than 90%. Success is also affected by other “system” factors, including ambient temperature, finger temperature, overall circulation, and others. It is important to emphasize the most ideal lancing site on the fingertip when taking into account both adequacy of blood volume and pain. Namely, lancing should be on the lateral aspects of the fingertip, not in the “pad.” The vascular arcades are much denser on the sides of the finger whereas the nerve endings are much denser in the pad.
What Size Blood Drop Do We Need?
The blood volume required by glucometers for a reliable BG measurement has been drastically reduced since the 1980s from approximately 20-30 μl to 0.3 μl or less.17 However, handling blood volumes less than 1 to 2 μl on a daily routine basis appears to be impractical for many—if not most—users, likely due to impaired vision and dexterity.
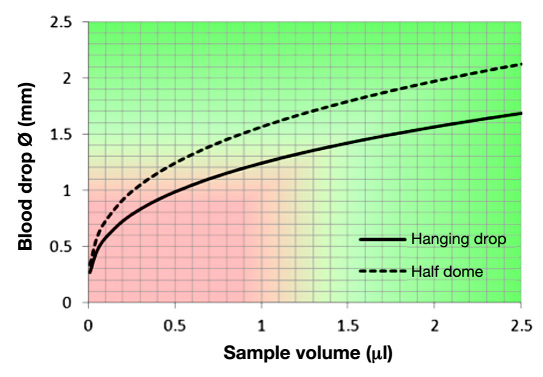
Figure 6 shows the relationship between blood drop volume and its diameter; a 1 μl blood drop has a diameter of 1.24-1.56 mm, depending on hematocrit, surface tension, and finger positioning. A 0.5 μl drop has a diameter of 0.98-1.24 mm. The blood entry channel of a typical test strip of modern glucometers has an approximate 0.3-0.5 mm width; the user has to position the blood drop on his/her finger to this opening. This provides a 1:2-1:3 alignment safety ratio when considering the apparent blood drop diameter. If the user positions the test strip outside of this “capillary pull” window, the sample can get “caught” between the front of the test strip and the skin's surface, which often leads to smearing of blood and wasting of the sample, and will require relancing of the fingertip.
Figure 6.
Relationship between the diameter of the blood drop and its volume.
For this reason, any reduction of blood volume requirement below this “user handling threshold” of 1-2 μl is only an academic advantage. Most users cannot really benefit from the highly advertised “super low volume” feature of modern test strips. Therefore, with regard to required blood volume, current sensor development has reached a level of technical sophistication that is beyond practical utility. Therefore, further developments should focus on improving lancing performance.
The situation changes entirely if the system itself “handles” the blood transfer, which will be the case with fully integrated SMBG measurement systems. Several types of these integrated systems are already in development at all four major companies and some small companies. Early steps in this direction have been commercialized with mixed results. Abbott SofTact was taken off the market in late 2006; Roche Compact II® uses a “semi-integration” approach with a test-strip and lancet “side-by-side” configuration; AccuChek Mobile® uses a tape containing 50 tests and an attached lancet device; Accu-Chek FastClix Mobile® offers six lancets in a drum and is detachable for lance at alternate sides. Other approaches have been announced in the patent literature: Kumetrix (US 5,801,057), LifeScan (US 03/0212423), Roche (US 6,572,566), Vyteris (US 04/0064068), Pelikan Technologies (US 2003/0199893), iSense (US 04/0138541), and Rosedale Medical (now Intuity; US 6,540,675).
Another important aspect of the current mismatch of test strip sophistication (i.e., required blood volume) and practical handling issues is excess blood volume, which is no longer “consumed” by super low volume sensors. This extra blood must be handled and cleaned by the user. This is not only annoying; it also raises a hygiene concern.
In addition, the reproducibility of generating a blood sample of sufficient volume is critical. The technical difficulty of reproducibly obtaining ever smaller amounts of blood rises nonlinearly. Generating blood samples with exact amounts in small volumes less than 0.5 μl taxes the ability of current systems, mainly with regard to penetration depth control. Current lancing systems do not have means for in situ depth control, which accounts for variations in tissue properties. The only exception is the Pelikan Sun®, which is discussed later. Therefore, attempts to generate smaller volumes repeatedly will lead to unacceptably low success rates because lancing will be imprecise at ever shallower depths because there is no lancing depth control. As stated earlier, the overall success rate of current lancing systems is approximately 90-95%, which is already borderline low. Any worsening of this value, even if accompanied by improvements in pain, wound healing, and other parameters, will not be acceptable.
How to Reduce Pain?
Lancing pain is determined by several factors: depth of penetration, speed of penetration, overall lancet trajectory, lancet geometry, skin surface, skin fixation,5 and anatomic location on the finger tip. Considerable progress with regards to lancet quality has been achieved by most manufacturers i.e., better precision of holding specifications, tip quality, effective polishing, and coating for smooth gliding motion into the skin. However, in the late 1990s, it became clear that it is not the lancet specifications alone (e.g., diameter or gauge size, geometry) that determines lancing pain.7,8,15,16,18–20 Nevertheless, companies continue to advertise reduced diameter as the main factor for pain reduction, despite the fact that reducing lancet diameter (within a range of several gauge sizes) is not necessarily advantageous. The diameter of the lancet must have a certain size in order to cut a sufficient number of blood vessels, yielding a sufficient amount of blood.19 If the lancet is too thin, it has to travel deeper into the skin to realize its full effective diameter at the depth of the capillary bed, while its tip reaches deeper into the nerve layers and consequently induces more pain. In this context, it is important to note that at least three major gauge size systems exist, which are used for referencing wire diameter (see Table 1). In the United States, the American wire gauge system is primarily used. However, in Europe and Japan, other specifications can be found. In other words, if there is no clear indication of which gauge size system is being used, comparison studies and statements about the gauge size of lancets (www.powerwerx.com/wiregauge.asp) can be misleading. It would be beneficial for all companies to use the internationally binding/used International System of Units (ISU) system to report lancet diameter in ‘mm’.
Table 1.
Measurements in Millimeters of the Three Major Gauge Size Systems (American, British, and Standard)
| Gauge | AWG | BWG | SWG |
|---|---|---|---|
| 21 | 0.72390 | 0.78740 | 0.81280 |
| 22 | 0.64262 | 0.71120 | 0.71120 |
| 23 | 0. 57404 | 0.63500 | 0. 60960 |
| 24 | 0.51054 | 0.58420 | 0.55880 |
| 25 | 0.45466 | 0.50800 | 0.50800 |
| 26 | 0.40386 | 0.45720 | 0.45720 |
| 27 | 0.36068 | 0.40640 | 0.41656 |
| 28 | 0.32004 | 0.34290 | 0.37592 |
| 29 | 0.28702 | 0.33020 | 0.34544 |
| 30 | 0.25400 | 0.30480 | 0.31496 |
| 31 | 0.22606 | 0.25400 | 0.29464 |
| 32 | 0.20320 | 0.22860 | 0.27432 |
| 33 | 0.18034 | 0.20320 | 0.25400 |
| 34 | 0.16002 | 0.17780 | 0.23368 |
| 35 | 0.14224 | 0.12700 | 0.21336 |
| 36 | 0.12700 | 0.10160 | 0.19304 |
AWG, American wire gauge; BWG, British wire gauge; SWG, Standard wire gauge.
Additional key factors determining lancing comfort (i.e., reduced spontaneous and residual pain, wound healing, and reduced scarring) are smoothness of lancet motion (including a “soft” stop at the return point of the trajectory) and actual penetration depth.2,15,16 Major improvements have already been accomplished by careful control of these lancet movement characteristics, which will be discussed later.
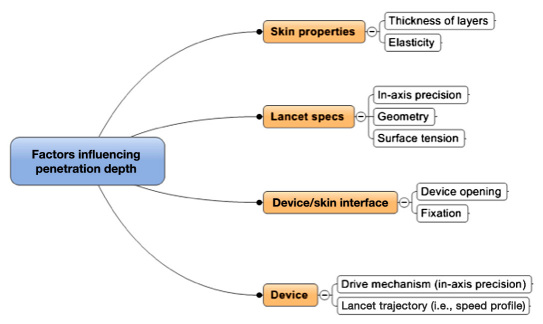
At least four types of factors determine accuracy and precision of lancing with respect to the actual penetration depth and accurate reach of the desired capillary layer (and not beyond; Figure 7). These factors, along with their cumulative variances, determine overall precision and, less importantly, the accuracy of the realized penetration depth. Ideally, the precision should be better (less) than ±100 μm given the anatomical thickness of the capillary bed. Significantly overshooting the capillary bed (+200 will result in extra pain and nerve stimulation, while undershooting will result in an insufficient blood sample.
Figure 7.
Key factors determining precision of penetration depth.
The skin layer with the highest variance in thickness is the stratum corneum, which differs not only from person to person, but also from finger to finger and across the fingertip itself. It can vary more than a few hundred micrometers from central (not recommended for lancing) to lateral (preferred) areas.20 The stratum corneum thickness ranges from <50 μm (e.g., infants) to >1000 μm (e.g., musicians, blue collar adults) and can vary within a person by more than 500 μm across various fingers and sites. The subcutaneous thickness also depends on the age of the patient.21 Therefore, when optimal comfort and high success rate are the goals, penetration depth must be adjusted not only individually,22 but also across fingers (see earlier discussion with respect to training patients).
However, current lancing devices (with the exception of the electronic lancing device Pelikan Sun, Pelikan Technologies, Inc., Palo Alto, CA) are unable to achieve optimal penetration depth precision within a few hundred micrometers. Design and manufacture of mechanical devices that can achieve good precision is particularly difficult because skin properties, such as skin elasticity and hydration, vary during the day and require respective in situ lancing information, which these devices do not gather (yet).
Attempts to Reduce/Avoid the Pain of Lancing
One attempt to reduce lancing pain was developed in the late 1990s by using so-called “alternate sites” for lancing [alternate site testing (AST)].2 Lancing the skin at the abdomen, arm, thigh, or palm of the hand—all sites with much lower density of pain receptors—gained some popularity for a while.23 The proclaimed and intensely advertised advantage of AST is reduced pain when sampling. However, this has not been the case in reality and, to our knowledge, AST has much lower “success rates” than sampling on fingertips. It is also associated with risk of blood stains on the skin/clothes due to incomplete wound closure and prolonged bleeding from these lancing sites. Another drawback is the difficulty in performing this procedure in public without drawing too much attention, a privacy issue. Additionally, it turned out that results of SMBG performed with blood samples obtained from these sites do not match capillary BG levels measured in samples collected at the fingertips when BG levels are changing rapidly.24 Interestingly, no review has ever been published summarizing the numerous studies performed about AST and this so-called AST phenomenon.
Another attempt to sample blood with reduced pain are devices that use a laser, which “burns” holes into skin by high-energy temperature-induced tissue sublimation.2 “Shooting” the skin with a laser creates an audible “bang,” a small cloud of smoke, and an odor of burned flesh. In addition, the generated cylindrical cavities do not heal well because of the elimination of skin tissue. Examples of these devices are ISOTECH's Laser Doctor® (www.abimed.org.br/associadoskotra.pdf) and Personal Lasette™ from Cell Robotics International, which is offered on the Internet (Figure 8; www.medicalproducts.en.ecplaza.net/catalog.asp?CatalogID=696711, visited November 11, 2010). However, these devices are relatively bulky and expensive.
Figure 8.
Devices that use a laser to lance the skin.
Neither of these two alternative attempts (AST, laser) appears to be advantageous, nor have they gained any substantial market traction. Lancing at the fingertip is the current standard for SMBG and will likely remain so for capillary blood sampling.
Projecting the Future
Which Lancing Devices Are the “Best” and Why?
It is important to understand the various types of lancing systems and consider their pros and cons. An uncontrolled inbound trajectory, either mechanical or electronic, can be painful and may even create a wound without yielding any blood. This dry hole situation is the most irritating for the user, as even milking (i.e., pressing the wound surrounding the tissue to express blood) will not yield blood and a second lancing, or more, will become necessary. Not controlling the outbound or withdrawal of the lancet will require milking to obtain a blood sample and may result in the formation of microhematomas or bruising at the wound site that slows the wound-healing process. As a side note, we like to mention that, while excessive milking will compromise tissue integrity and may lead to prolonged wound healing, there is no evidence in the literature that it will generate free-flowing interstitial fluid, which would consequently dilute the blood sample and possibly influence the measurement result.
There are three classes of lancing technologies and actuation methods:
Linear motion actuation (mechanical)
Cam actuation (mechanical)
Electronic actuation
Linear Motion Actuation
Conventional mechanical lancing devices use a linear motion actuation. The drive mechanism is frequently constructed with a pair of springs. The user compresses the springs when cocking and then releases the mechanism when pushing a firing button. The first spring releases the lancet into the skin; the second withdraws the lancet back into the protective housing of the actuator.
The lancet path is not precisely controlled along both the “normal” (i.e., forward direction of lancet) and “orthogonal” (i.e., perpendicular to main motion vector) of the trajectory. This results in a series of issues:
inexact end point of motion (variance in pain and success rate);
hard stop (“bang”) at the deepest point of penetration (i.e., return point), which is the main cause for spontaneous pain;
jittery path, resulting in a rough wound channel (residual pain);
uncontrolled outbound speed (slow and delayed wound closure and after bleeding);
overall motion “wobble” (residual pain, long-lasting trauma, and scarring); and
bouncing of mechanism (leading to unintended relancings).
The abruptness with which the lancet comes to a stop in the skin at maximum depth, before it starts its outbound motion and returning to its starting position, is an inherent issue of this design. With the lancet at its deepest point of penetration, the greatest amount of force is applied to the skin. The drive mechanism simply bounces off the end of the device like a ball bounces back from the floor. The lancet, coming to an abrupt stop at the end point of its inbound motion, sends a shockwave into the skin, causing many pain receptors in the vicinity of the lancet to fire, even though they are not directly struck. This amplifies spontaneous pain substantially.
Cam Actuation (Mechanical)
Devices with cam-actuation design somewhat avoid “hard stopping” of the lancet. A cam mechanism is usually spring driven and generally offers a better guided actuation. The trajectory of the lancet is tightly controlled through a guided path of the lancet holder via a pin riding in a cam. The cam mechanism allows for a predetermined speed profile with a softer return and distinct speed control for the lancet outbound trajectory. However, the outbound speed reduction is limited due to the mechanical nature of the cam. This mechanism also effectively avoids a bounce back of the lancet into the skin when the mechanism reaches its motion end point. In addition, the mechanical oscillation (or jitter/wobble) of the lance path in both directions is reduced when fired in air. However, when lancing into skin, the cam shaft, respectively, the “riding lancet holder,” will transmit any mechanical wobble of the drive mechanism (e.g., due to uneven or rough cam slots) directly into the tissue because of its “forced motion profile.” This effect potentially causes more harm through its forced wobble than a free-flowing (jittery) ballistic device, which is substantially dampened when traveling in skin. The first two commercial devices of this kind were developed by Roche Diagnostics (Mannheim, Germany): Accu-Chek Softclix®, a single lancet device introduced in 1992, and Accu-Chek Multiclix, a multilancing device containing six lancets in a drum, introduced in 2004.
Electronic Actuation
The third technology provides complete control of the actuation process through an electronically controlled drive mechanism. This technology uses a miniaturized electronic motor (e.g., voice coil, solenoid) coupled with a very accurate position sensor, moving the lancet into and out of the skin with precisely controlled motion and velocity. A commercial device that uses this technology was the Pelikan Sun, which was introduced in 2006 by Pelikan Technologies, Inc. (www.pelikantechnologies.com). Following rapid entry, the device decelerates the lancet to an exact, preset depth to return smoothly, without jitter, and relatively slowly. This allows quick wound closure and avoids long-term trauma. With this device, the force required to penetrate the lancet into the skin is controlled while the lancet is progressing. The benefit of tightly controlling the lancet actuation “profile” is a reproducible painless lancing that yields a sufficient and consistent blood sample for testing (also see the white paper at http://www.pelikantechnologies.com/PTITechnology.htm).
Costs and Reimbursement
Pelikan Sun was able to raise the interest level in the lancing aspect of SMBG in general. In particular, children with diabetes highly appreciated the low pain and “soft lancing” overall (as expressed and discussed in various diabetes-related blogs). “We've had the Pelikan for about 3 months now and rarely use anything else. Everyone says they are ‘used to’ their current lancing device, but it is amazing what a relief it is that finger pricking does not hurt. There is a world of difference between ‘virtually painless’ like most devices, and ‘really, truly, actually painless’ like the Pelikan. The greatly reduced scarring is an excellent bonus.” (http://www.diabetesmine.com/2008/04/the-pelikan-cha.html; visited on January 5, 2011).
This situation is a good example of how development and commercialization of a new technological approach is very complex. In addition, reimbursement issues affect the market launch of new lancing devices in most countries.
One big advantage of mechanical lancing devices is that they can be manufactured at much lower costs than any electronic device. Currently, the best performing devices on the market are cam actuation devices, which pay particular attention to controlling the lancet movement and result in lowest pain and best wound healing.6,25 This requires that the lancet is guided in a straight trajectory by a fairly advanced mechanical design. However, compared to the electronic drive mechanism, all available mechanical devices still induce more pain and wound rupture. The big question is how much room for improvement mechanical systems can offer while staying below apparent cost thresholds.
In defense of the payers, industry has generated little to no robust comparative data to demonstrate to payers or HCPs that these advances are truly beneficial and/or cost-effective. This is true of SMBG in non-insulin-using type 2 diabetes and many other aspects of device development in general (i.e., pumps for type 2 patients). We would like to encourage the industry to spend more on clinical data generation beyond technical device development and marketing activities, such as costly television commercials.
A fundamental issue with lancing devices is that no clinical trials have been performed to date that demonstrate that investing in a lancing device with reduced pain and better wound healing generates definite and substantial cost savings. At the same time, this issue is the key to convince insurers to reimburse such a product. It must be demonstrated that lancing devices with reduced pain and better wound healing lead to improved clinical and patient-reported outcomes and are cost-effective.
As a first step, these studies should focus on lancing comfort in the context of the glucose meter being used and its respective blood volume requirement. Discomfort (e.g., pain, wound rupture) while using the novel lancing device should be compared to that of a well-characterized “standard” device. Such a head-to-head comparison should be performed with a single-blinded study design and employ an adequate measure to evaluate pain, lancing comfort, and overall success rate.6,7,16 Most studies performed have been financed by manufacturers of the tested lancing device; published outcomes of such studies tend to favor the manufacturer's device(s).6,7,21 Also, these studies should be registered at www.clinicaltrials.gov to ensure publication of the results.
As a second step, a critical and independent evaluation of the long-term benefits of such novel lancing devices is needed to obtain high acceptance by regulatory authorities, payers, and the scientific community. Pursuing such a long-term study, which would require a considerable sample size and a study period of at least one year, is quite expensive and often out of reach for small companies. Questions are, what are relevant endpoints of such a study: improvement in A1C, reduction in the frequency of hypoglycemic events, or improvement in quality of life? How will these be measured? It will be important to demonstrate that use of a novel superior lancing device leads to higher SMBG frequency with improved metabolic control and a better quality of life. Such a study would also need to show that all these improvements lead to tangible short- and mid-term cost savings through more indirect effects such as general acceptance of therapy, reduced hurdles to compliant behavior patterns, and better overall quality of life, all driven by substantially reducing the hurdle of a painful and stigmatizing lancing experience. The likelihood of this ever happening, regrettably, is close to zero.
The Future of Lancing Devices
Projecting the future of lancing devices requires a vision of the glucose monitoring market as a whole and must differentiate the various lancing and blood-sampling applications and related customer needs. There are four market/product segments for lancing, each of which requires a different set of technology sophistication and product deliveries and presents different cost and reimbursement hurdles:
Standard devices,
Devices for less frequent use (e.g., for calibration purposes with CGM),
No pain devices (e.g., for special customer groups like children), and
Lancing/sampling technology for integrated devices.
Standard Devices
Standard lancing devices and the lancets per se have improved since the 1990s and benefited from research leading to increased technical sophistication of front runner products such as SoftClix. The standard lancing device market has a volume of $500 million+ annual revenue. Most of the lancing devices are copackaged with companion glucose meter products and are rarely sold on their own. Lancet sales provide the bulk revenue for this market.
To reduce costs, most body designs of the lancet itself are standard and allow usage across lancing devices from different manufacturers, except for the Roche Softclix system and multilancet cartridge systems. As described earlier, lancing comfort (e.g., pain, wound healing) relies on a few key factors, which are linked to quality of the lancing device and the lancet itself. The result of pairing a good lancing device with low-quality lancets and vice versa diminishes the benefit of distinct device/lancet combinations of certain manufacturers and often leads to unpredictable and frustrating experiences for the user. This is a problem that is rarely discussed in public and is only amplified when users assume that their “high end” lancing device determines their lancing experience and shop for lowest cost lancets.
Pain generation is at least partly understood, and the underlying principles need to be integrated into a device that is cost competitive. This market segment asks for maximum reimbursement and very low co-pay (if any). A good idea and related technology are not sufficient to make a successful product in this segment. Often, low-quality third-party lancets enter the market and advertise performance features that the lancet in itself cannot deliver reliably—if at all.
Because of its size, this market segment influences the overall speed of evolution. Production cost and cost/price pressure limit options for a more aggressive evolutionary path. Any new technology in lancing/sampling will try to capture part of this segment (at least eventually) and therefore has to forgo expensive and elusive technology solutions.
Devices for Less Frequent Use (e.g., for Calibration Purposes)
Since 2000, CGM systems have been on the market, and several companies such as ABBOTT, Roche, DexCom, Medtronic, SMSI, and Vista BioSciences continue with their efforts to replace a major portion of the conventional SMBG market with new systems. One of the hopes with the invention of CGM systems was to eliminate the need for lancing altogether. However, to guarantee acceptable measurement accuracy, regulatory agencies require all current CGM systems to utilize initial calibration and regular recalibrations. For these calibrations, conventional SMBG measurements are mandatory. Usage of CGM as a novel monitoring option reduces the SMBG test frequency per day but cannot avoid the need for finger lancing entirely. It is hoped that further progress in the development of indwelling or novel implantable CGM technologies (www.s4ms.com/index.htm) will lead to CGM systems that do not require recalibrations. Such a development, which could make SMBG and thereby lancing obsolete, is eagerly anticipated by all patients and HCPs but is very unlikely to occur by 2020.
No Pain Devices (e.g., for Special Customer Groups Like Children)
High-performance lancing systems face less cost burden in the market segments for children, pain-sensitive adults, and needle (lancet/sharp)-phobic individuals. Here, high-cost systems can be marketed to some degree. Unfortunately, the market size of these segments does not allow recovery of substantial development cost nor justify massive marketing and advertisement campaigns. High-quality systems offered at low cost usually evolve as second and third generations of new systems, which require sufficient production volume to reach competitive price points. It is a shame, but until first generations of high-end technology systems can be offered at low cost, this segment will likely remain underserved.
Lancing/Sampling Technology for Integrated Devices
A relatively new direction, where advanced lancing is required, came with the advent of integrated glucose monitoring devices, combining all steps of SMBG within one device into a more-or-less “one-step” procedure. These devices are placed on the skin and perform the lancing step on demand. They automatically sample and measure the glucose level in the blood sample, avoiding any step wherein the user handles blood. Several of such complex fully integrated machines are in the final stages of development and being prepared for market release. Two examples are Mendor and the POGO® system of Intuity Medical, www.mendor.com and www.presspogo.com. Also, Roche Diagnostics followed an evolutionary path with stepwise integration of multisensor and multilancet cartridges, which led to the drum/drum “sidekick” integration in ACCU-CHEK Compact Plus, www.accu-chek.com/us.
The lancing and blood sampling step for fully integrated glucose monitoring systems require ultimate technological sophistication, where seamless blood sampling at the highest success rate (>95%) is a priority. This requires extreme control of actual penetration depth and spontaneous blood flow. The key functional areas influencing system performance are precise lancing and device/skin interface. High spontaneity, control of sufficient blood volume, and seamless blood transfer are the most important performance factors for integrated systems, all determined by lancet trajectory and finger positioning.
Lancing comfort (e.g., pain, wound healing) can be seen as a confounding factor and a differentiating performance requirement when designing an integrated system. A psychological benefit of such systems is the virtual disappearance of lancets as users will no longer see nor handle the individual lancet.
Summary and Conclusions
None of the minimally invasive or noninvasive CGM alternatives on the market or in development can replace SMBG right away. The need for SMBG, and therefore lancing, will not become obsolete, even if CGM systems that do not require calibration are developed and become commercially available. Continuous glucose monitoring will likely remain a smaller segment play and capture approximately 10-15% of the market, primarily due to cost and convenience reasons.
Lancing will be with us for the foreseeable future. It has come of age and should no longer be seen as the “stepchild” of glucose monitoring. Advanced blood sampling has evolved as an important aspect for monitoring system acceptance and overall compliance.
Specifically on the future of lancing, it is apparent that pain-free lancing can be accomplished, in principle, with a sophisticated electronic device. Further development of highly integrated and less costly mechanical system designs and lower cost manufacturing technologies will be required to reach this level of sophistication. The costs of SMBG are a high burden for health care systems. Although some patients may be willing and capable of paying a premium for lancing devices with markedly improved comfort, such as with CGM systems, maintaining the economic viability of such devices would be difficult without adequate reimbursement.
Currently, there are no breakthrough technologies on the horizon that allow sophisticated lancing at a reasonable cost. We encourage companies to continue with systematic research and development while highlighting the need for appropriate broader clinical studies.
We believe that two key driving forces behind the evolution of lancing and sampling technology are as follows:
The need for maximum lancing comfort (including no pain, best wound healing) and
The advent of fully integrated systems.
In summary, rendering lancing a “nonissue” is of high importance to eliminate the barrier to better adherence to prescribed therapy regimens. This might have a greater impact on metabolic control than many of the new and expensive antidiabetic drugs that currently receive reimbursement. There is a sarcastic saying that diabetes is a “sticky” disease. However, in reality, there is a significant need to reduce the discomfort associated with lancing, which would, in turn, be important/relevant to the diabetes therapy/regimen in general.
Acknowledgments
We thank a number of colleagues for reading the manuscript critically and providing important insights into lancing devices: Dr. Guido Freckmann, Dr. M. Essenpreis, Dr. Ortrud Quarder, Dr. Volker Lodwig, Dr. Lars Krinelke, Dr. F. Schaebsdau, Dr. Larry Hirsch, and Dr. Juan Frias.
Glossary
Abbreviations
- (A1C)
glycated hemoglobin
- (AST)
alternate site testing
- (BG)
blood glucose
- (CDE)
certified diabetes educator
- (CGM)
continuous glucose monitoring
- (HCP)
health care provider
- (SMBG)
self-monitoring of blood glucose
Disclosures
Lutz Heinemann is partner of Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany, and Profil Institute for Clinical Research, Inc., San Diego, CA. He is also an employee of the latter institute. These institutes perform clinical trials in cooperation with many pharmaceutical companies. Lutz Heinemann is not stockholder in any of the companies with which the institutes perform clinical trials. Lutz Heinemann is member of numerous advisory boards and speakers bureaus and has received honoraria from such companies.
Dirk Boecker is president of Toto Consulting, LLC, in Palo Alto, CA. Toto Consulting provides strategy development, portfolio analysis, and in-depth technology assessments in the area of diagnostics, medical devices, and disease management. Dirk Boecker has been president and chief executive officer of Pelikan Technologies, Inc. (2001-2008), a company that developed innovative lancing and BG measurement devices. Dirk Boecker assists several venture capital firms in strategy reviews and technology evaluations.
References
- 1. Don't Fear Diabetes. http://www.dontfeardiabetes.com/2010/06/onetouch-delica-my-first-product-reviewl. Accessed June 12, 2010.
- 2.Yum SI, Roe J. Capillary blood sampling for self-monitoring of blood glucose. Diabetes Technol Ther. 1999;1(1):29–37. doi: 10.1089/152091599317549. [DOI] [PubMed] [Google Scholar]
- 3.Schütt M, Kern W, Krause U, Busch P, Dapp A, Grziwotz R, Mayer I, Rosenbauer J, Wagner C, Zimmermann A, Kerner W, Holl RW, DPV Initiative Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114(7):384–388. doi: 10.1055/s-2006-924152. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann L. Finger pricking and paa never ending story. J Diabetes Sci Technol. 2008;2(5):919–921. doi: 10.1177/193229680800200526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kocher S, Tshiananga JK, Koubek R. Comparison of lancing devices for self-monitoring of blood glucose regarding lancing pain. J Diabetes Sci Technol. 2009;3(5):1136–1143. doi: 10.1177/193229680900300517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westhoff AN, Jendrike N, Haug C, Freckmann G. Schmerzempfinden an Finger und Handballen bei Verwendung verschiedener Blutzuckermesssysteme. Diabetologie Stoffwechsel. 2010;5(Suppl):S41. [Google Scholar]
- 7.Fruhstorfer H, Schmelzeisen-Redeker G, Weiss T. Capillary blood volume and pain intensity depend on lancet penetration. Diabetes Care. 2000;23(4):562–563. doi: 10.2337/diacare.23.4.562. [DOI] [PubMed] [Google Scholar]
- 8.Fruhstorfer H. Capillary blood sampling: the pain of single-use lancing devices. Eur J Pain. 2000;4(3):301–305. doi: 10.1053/eujp.2000.0179. [DOI] [PubMed] [Google Scholar]
- 9.Warunek D, Stankovic AK. Evaluation of lancets for pain perception and capillary blood volume for glucose monitoring. Clin Lab Sci. 2008;21(4):215–218. [PubMed] [Google Scholar]
- 10.Koschinsky T. Blutzuckerselbstmanagement-Report 2006 offenbart Wissens- und Handlungsdefizite. Diabetes Stoffw Herz. 2007;16:185–192. [Google Scholar]
- 11.Schwarz PE, Dominguez OL, Neumann A. Blutzuckerselbst-kontrolle: Komplikationen bei Mehrfachbenutzung steriler Einmallanzetten. Diabetes Aktuell. 2009;7(7):327–330. [Google Scholar]
- 12.Fujisawa T, Ikegami H, Kasayama S, Matsuhisa M, Yamasaki Y, Miyagawa J, Funahashi T, Shimomura I. Age-dependent difference in factors affecting choice of system for self-monitoring of blood glucose. Diabetes Res Clin Pract. 2008;79(1):103–107. doi: 10.1016/j.diabres.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Lekarcyk J, Ghiloni S. Analysis of the comparison of lancing devices for self-monitoring of blood glucose regarding lancing pain. J Diabetes Sci Technol. 2009;3(5):1144–1145. doi: 10.1177/193229680900300518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monami M, Mannucci E, Masotti G. Finger sepsis in two poorly controlled diabetic patients with reuse of lancets. Diabetes Care. 2002;25(6):1103. doi: 10.2337/diacare.25.6.1103. [DOI] [PubMed] [Google Scholar]
- 15.Fruhstorfer H, Müller T, Scheer E. Capillary blood sampling: how much pain is necessary? Part 2: relation between penetration depth and puncture pain. Pract Diabetes Int. 1995;12(4):184–185. [Google Scholar]
- 16.Fruhstorfer H, Lange H. Capillary blood sampling: how much pain is necessary? Part 3: pricking the finger can be less painful. Pract Diabetes Int. 1995;12(6):253–254. [Google Scholar]
- 17.Hönes J, Müller P, Surridge N. The technology behind glucose meters: test strips. Diabetes Technol Ther. 2008;10(Suppl 1):S10–S16. [Google Scholar]
- 18.Fruhstorfer H, Selzer K, Selbman O. Capillary blood sampling: how much pain is necessary? Part 4: comparison of lancets for automatic lancing devices. Pract Diabetes Int. 1996;13(2):58–60. [Google Scholar]
- 19.Fruhstorfer H, Schmelzeisen-Redeker G, Weiss T. Capillary blood sampling: relation between lancet diameter, lancing pain and blood volume. Eur J Pain. 1999;3(3):283–286. doi: 10.1053/eujp.1999.0132. [DOI] [PubMed] [Google Scholar]
- 20.Fruhstorfer H, Abel U, Garthe CD, Knüttel A. Thickness of the stratum corneum of the volar fingertips. Clin Anat. 2000;13(6):429–433. doi: 10.1002/1098-2353(2000)13:6<429::AID-CA6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Pacaud D, Lemay JF, Buithieu M, Yale JF. Blood volumes and pain following capillary punctures in children and adolescents with diabetes. Diabetes Care. 1999;22(9):1592–1594. doi: 10.2337/diacare.22.9.1592. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama T, Kudo H, Sakamoto S, Tanaka A, Mano Y. Painless self-monitoring of blood glucose at finger sites. Exp Clin Endocrinol Diabetes. 2008;116(4):193–197. doi: 10.1055/s-2007-993146. [DOI] [PubMed] [Google Scholar]
- 23.Ito T, Kamoi K, Minagawa S, Kimura K, Kobayashi A. Patient perceptions of different lancing sites for self-monitoring of blood glucose: a comparison of fingertip site with palm site using the OneTouch Ultra Blood Glucose Monitoring System. J Diabetes Sci Technol. 2010;4(4):906–910. doi: 10.1177/193229681000400420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jungheim K, Koschinsky T. Glucose monitoring at the arm: risky delays of hypoglycemia and hyperglycemia detection. Diabetes Care. 2002;25(6):956–960. doi: 10.2337/diacare.25.6.956. [DOI] [PubMed] [Google Scholar]
- 25.Wallace DA, Pynes MK, Pineau M, Shemain A, Ginsberg BH. A randomized, controlled trial assessing perceived pain of lancing with the OneTouch® Delica™ lancing device. Diabetes. 2010;59(Suppl 1):P507. [Google Scholar]