Abstract
Aims
This study investigated the effects of pioglitazone (PIO), ramipril (RAM), or their combination (PIRA) on low-grade inflammation in nondiabetic hypertensive patients with increased cardiovascular risk.
Methods and Results
Patients enrolled in this placebo-controlled, double-blind, randomized, parallel trial (72 male, 77 female, aged 60 ± 9 years, body mass index 30.4 ± 4.7 kg/m2, duration of hypertension 9 ± 8 years) were treated with either 30/45 mg PIO (dose titration), 2.5/5 mg RAM, or their combination for 12 weeks. A reduction in high-sensitivity C-reactive protein was observed with PIO (−0.89 ± 1.98 mg/liter; -25%) and PIRA (−0.49 ± 2.11 mg/liter; -16%), while an increase was seen with RAM (0.58 ± 2.13 mg/liter; +20%, p < .05 vs PIO and PIRA). The 24-hour blood pressure profile showed a small increase with both monotherapies but a decrease with PIRA (p < .05 vs PIO). Improvements in biomarkers of chronic systemic inflammation and insulin resistance (IR) were observed in the PIO and PIRA arms only [PIO/RAM/PIRA: homeostasis model of assessment of IR: -0.78 ± 1.39 (−29%)/0.15 ± 1.03 (+5%)/ -1.44 ± 2.83 (−40%); adiponectin: 8.51 ± 5.91 (+104%)/ 0.09 ± 2.63 (+1%)/ 8.86 ± 6.37 mg/liter (+107%); matrix metallo-proteinase-9: -48 ± 127 (−12%)/-1 ± 224 (0%)/-60 ± 210 ng/ml (−13%), p < .05 for RAM vs PIO or PIRA in all cases].
Conclusions
Our 3-month study in nondiabetic hypertensive patients showed a decrease in biomarkers of IR and chronic systemic inflammation with the PIO monotherapy and the PIRA combination only, which may help to explain some findings in other cardiovascular outcome trials.
Keywords: cardiovascular pharmacology, clinical trial, hypertension, inflammation, pioglitazone
Introduction
Insulin resistance (IR) has been identified as a driving force for metabolic syndrome, impaired glucose tolerance, and diabetes mellitus,1 and has been considered as a treatment target in published guidelines.2 In concert with β-cell dysfunction and visceral adipogenesis, endothelial and metabolic IR support a chronic systemic inflammation of the vasculature, which impairs the prognosis of the disease and contributes to the overall increased macro-vascular risk of the affected patients.3 In cases of vascular IR, insulin binding to the endothelial receptor does not enhance the vasoprotective endothelial nitric oxide synthase (eNOS) activity but rather activates the mitogen-activated protein kinase (MAPK) pathway resulting in cell proliferation and atherosclerosis.4
The increased insulin demands and the compensating increased insulin secretion by the β-cells support visceral lipid tissue growth when sufficient caloric uptake occurs. This in turn impairs IR and induces activation of monocytes/macrophages by means of a complex adipokine secretion pattern, including but not limited to multiple proinflammatory cytokines such as interleukin-6, tumor necrosis factor-α, and interferon-γ.5,6 Further adipokines contributing to the well-known clinical symptoms of hypertension and dyslipidemia are angiotensin and free fatty acids.7,8 These mechanisms can be prevalent prior to development of hyperglycemia, which occurs when pancreatic compensation mechanisms fail, and needs to be understood as a symptom rather than as a cause of the disorder. Glucotoxicity, however, adds another contributing factor to the increasingly persistent vascular damage.9,10
In this context, patients with normoglycemic vascular IR and chronic systemic inflammation—also referred to as cardiodiabetes—have a particularly critical situation. The underlying metabolic disorder is not visible by measures of routine diagnostics, and treatment guidelines do not reflect the need for an effective IR therapy, e.g., by a peroxisome proliferator-activated receptor-γ (PPARγ) stimulation.
The importance of metabolic and vascular IR for the pathophysiology of the disease is underscored by the findings of a significantly delayed progression of patients with impaired glucose tolerance to overt diabetes when treated with pioglitazone in the Actos Now for Prevention of Diabetes (ACT NOW) study11 or rosiglitazone in the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial.12 Even regression to normal glucose results was seen in a substantial number of patients in both trials. It has also been observed that drugs inhibiting the renin-angiotensin system (RAS) may prevent progression to diabetes in patients with cardiovascular disease or hypertension.13 In the DREAM trial,14 the angiotensin-converting enzyme (ACE) inhibitor ramipril had no significant effect on diabetes progression but significantly increased the number of patients with regression to normoglycemia.
In another study, we demonstrated that nondiabetic patients with vascular IR and dyslipidemia treated with pioglitazone therapy experience an overall reduction in the chronic systemic inflammation, which was significantly more pronounced than with an alternative simvastatin therapy.15 In this study, we investigated the effect of pioglitazone, ramipril, and their combination on chronic systemic inflammation in nondiabetic patients with hypertension.
Patients and Methods
This prospective, double-blind, randomized, parallel, multicenter trial was performed in accordance with all applicable ethical and regulatory standards as set forth by the Declaration of Helsinki, the Guidelines for Good Clinical Practice, and local German clinical trial regulations. The study was approved by the responsible ethics committee, and patients signed informed consent prior to any study procedure. Patients could be included if they presented with the following criteria: arterial hypertension, age 30–75 years, stable treatment with an ACE inhibitor for at least 12 weeks, and a high-sensitivity C-reactive protein (hs-CRP) value of ≥1.0 mg/liter and <10.0 mg/liter. Exclusion criteria were type 1 or type 2 diabetes mellitus, other chronic inflammatory diseases causing elevated CRP values, uncontrolled hypertension (>180/100 mm Hg), persistent systolic hypotension (<90 mm Hg), drug or alcohol abuse, progressive fatal disease, pregnancy or breastfeeding, and significant cardiovascular, respiratory, hepatic, renal, or hematological disease.
Study Objectives and Design
The primary objective of the study was to compare the effects of three therapies (1) pioglitazone (PIO), (2) ramipril (RAM), and (3) combination PIO and RAM (PIRA) on biomarkers of low-grade systemic inflammation and vascular function in patients with increased cardiovascular risk versus the effects of or a combination of both. The primary efficacy variable was the change of the hs-CRP value after 12 weeks of treatment as compared to baseline. Secondary study objectives were the influence of the applied treatments on other laboratory parameters of inflammation and vascular function [macrophage chemoattractant protein-1 (MCP-1), matrix metalloproteinase-9 (MMP-9), P-selectin], measures of glucose metabolism, β-cell function, and IR [fasting glucose, fasting insulin, hemoglobin A1c (HbA1c), intact proinsulin, C-peptide, homeostatic model assessment of insulin sensitivity (HOMA-S), adiponectin], and lipid metabolism [high-density lipoprotein (HDL), low-density lipoprotein (LDL), oxidized low-density lipoprotein (oxyLDL), total cholesterol, triglycerides]. In addition, a 24-hour blood pressure profile was evaluated at baseline and at the end of therapy after 12 weeks. Safety was assessed by documenting the incidence of adverse events, changes in biochemical safety parameters, and drop-out numbers.
After screening, each patient eligible for the study received the next consecutive randomization/patient number from a block of randomization numbers per site. They were randomized to start any of the three treatment arms with a 2-week titration phase followed by 10 weeks of full-dose treatment: PIO: pioglitazone hydrochloride (15/30 mg) + placebo; RAM: ramipril (2.5/5 mg) + placebo; PIRA: pioglitazone hydrochloride (15/30 mg) + ramipril (2.5/5 mg). After 2 weeks, all patients were to be on the highest dose of the respective study drug(s) and no down titration was possible until the end of the observation period. Any existing stable ACE-inhibitor therapy was stopped and replaced by the study drugs on this occasion. At baseline and endpoint, an oral glucose tolerance test (OGTT) was performed to evaluate the diabetes status of the investigated population.
Laboratory Measurements
Blood samples were immediately centrifuged, and plasma and serum samples were kept at -20 °C until laboratory testing. Hs-CRP was measured by turbidimetry and lipids (total cholesterol, LDL, HDL, triglycerides) were measured by means of standard dry chemistry methods (both testings; Olympus System Reagent, Olympus Diagnostica, Hamburg, Germany). HbA1c was determined by means of high performance liquid chromatography (Adams TMA1C HA-8160, Menarini Diagnostics, Florence, Italy). Glucose was measured by using a standard glucose oxidase reference method (Super GL, Ruhrtal Labor Technik, Delecke-Möhnesee, Germany). Fasting serum insulin was determined by means of chemiluminescence (MLT Insulin Assay, Invitron, Monmouth, UK). Intact proinsulin was assessed by enzyme-linked immunosorbent assay (ELISA) (TECOmedical, Heidelberg, Germany). A radioimmunoassay was used to determine total adiponectin levels (Millipore, St. Charles, MO). ELISAs from R&D Systems (Wiesbaden, Germany) were used for the determination of P-selectin, MMP-9, and MCP-1.
Insulin resistance (HOMA-IR) and β-cell function (HOMA-B) were calculated from the fasting insulin and glucose values using homeostasis model assessment analysis [HOMA-IR score = insulin (mU/liter) × glucose (mmol/liter)/22.5,16 with values >2 classified as insulin resistant;17 β-cell function was calculated by the same methodology: HOMA-B = insulin (mU/liter) × 20/glucose (mmol/liter) – 3.5].16
Statistical Analysis
Based on other study results with PIO and RAM, it was calculated that 40 patients per treatment arm would provide 80% power (type II error rate of b = 0.20) to detect an effect size [i.e., the ratio between the difference in means and the common standard deviation (SD)] of 0.634 using a two-group t-test with a type I error rate of a = 0.05 (two-sided). As it was expected that about 16% of the randomized patients would not be available for the full analysis set, a total of 144 patients (i.e., 48 per treatment group) had to be randomized in order to achieve at least 120 evaluable patients for the efficacy analyses.
All analyses with respect to safety and tolerance were performed for all patients treated. All efficacy analyses were carried out for the full analysis set, which consisted of all patients who had a baseline value for hs-CRP <10 mg/liter and at least one postbaseline assessment for hs-CRP ≥10 mg/liter. Patients with hs-CRP values >10 mg/liter were excluded from the analysis, as these values may indicate an unspecific inflammation independent from any chronic systemic vascular inflammation.18 Patients who terminated treatment before the end of week 12 were considered with their individual last value under study medication [last observation carried forward (LOCF) method]. Missing baseline hs-CRP levels were not replaced. The absolute values and percentage changes from baseline were presented after 12 weeks of treatment including titration period. All analyses were performed in an exploratory sense with appropriate parametrical and nonparametrical methods. The hs-CRP and the other efficacy parameters were evaluated using descriptive statistics primarily with adequate methods for continuous or categorical variables (absolute values for each time of documentation and, if appropriate, changes from baseline). In addition, two-sided 95% confidence intervals and two-sided p values for within- and between-group treatment differences were calculated for the absolute change from baseline, considering an analysis of covariance (ANCOVA) model with the fixed effect factors for treatment group and center and with the baseline value as covariate. P values <.05 were considered to be statistically significant.
Results
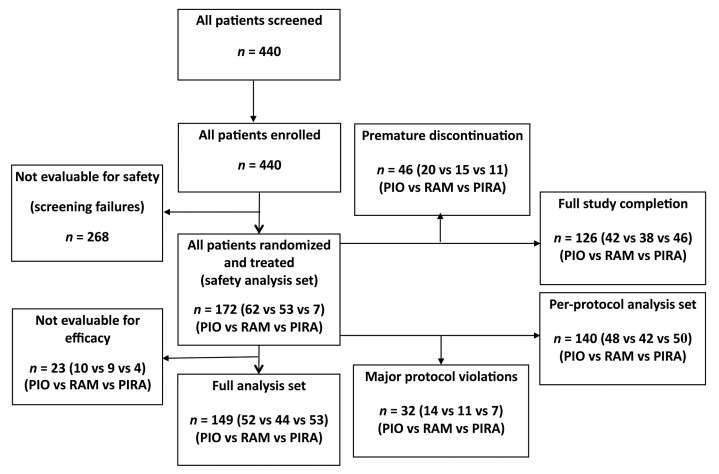
Out of 440 patients screened initially, 172 were randomized and treated (safety analysis set, 62 in PIO monotherapy vs 53 in RAM monotherapy vs 57 in PIRA combination therapy). A total of 46 patients (26.7%; 20 vs 15 vs 11) discontinued the study prematurely. The main reasons for termination were adverse events (n = 22, 8 vs 7 vs 7), uncontrolled hypertension (n = 15; 5 vs 7 vs 3), or patient decision (n = 6, 3 vs 2 vs 1). Patient allocation is shown in Figure 1 and patient characteristics of the full analysis set of 149 patients are provided in Table 1. According to the study documentation, the mean compliance could be calculated between 97.0 and 98.5% for the vast majority of the patients, i.e., nearly all treated patients in this study took two tablets of study medication daily, as planned, for about 12 weeks.
Figure 1.
Patient allocation during the study.
Table 1.
Patient Characteristics at Baseline. Values Are Given as Mean ± SD or as Absolute Percentage
| Parameter | Total | PIO | RAM | PIRA |
|---|---|---|---|---|
| n | 149 | 52 | 44 | 53 |
| Gender (male/female) | 72/77 | 22/30 | 25/19 | 25/28 |
| Age (years) | 60 ± 9 | 60 ± 9 | 61 ± 9 | 60 ± 8 |
| Body mass index (kg/m2) | 30.4 ± 4.7 | 29.9 ± 4.6 | 30.5 ± 4.3 | 30.8 ± 5.1 |
| Duration of hypertension (years) | 9.4 ± 8.4 | 8.9 ± 7.7 | 10.8 ± 10.1 | 8.6 ± 7.4 |
| Smoker | 20 (13.4%) | 6 (11.5%) | 6 (13.6%) | 8 (15.1%) |
| Blood pressure (mm Hg) | ||||
| Systolic | 128 ± 11 | 128 ± 11 | 129 ± 12 | 128 ± 10 |
| Diastolic | 78 ± 8 | 78 ± 9 | 76 ± 8 | 79 ± 7 |
| RAS inhibitors | 100% | 100% | 100% | 100% |
| Ramipril | 42.3% | 36.5% | 40.9% | 49.1% |
| β-blockers | 36.2% | 34.6% | 38.6% | 35.8% |
| Diuretics | 22.8% | 21.2% | 18.2% | 28.3% |
| Calcium channel blockers | 21.5% | 23.1% | 25.0% | 17.0% |
| Centrally acting antihypertensives | 7.4% | 7.7% | 9.1% | 5.7% |
| Antilipidemic treatment | ||||
| Statins | 19.5% | 17.3% | 20.5% | 20.8% |
| Ezetimibe | 2.0% | 0% | 4.5% | 1.9% |
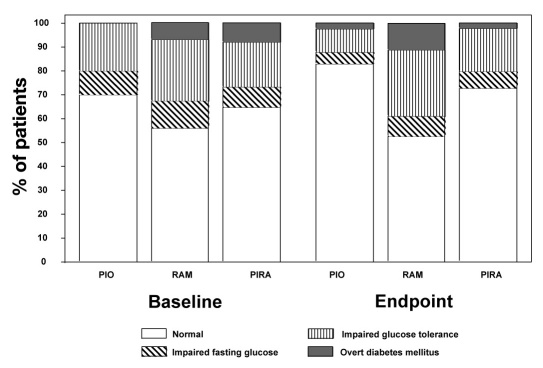
The results of OGTTs performed at baseline and endpoint are provided in Figure 2. Results indicate that the overall majority of the patient cohort was normoglycemic throughout the investigation and had no indication of overt diabetes.
Figure 2.
Classification of patients in the three treatment arms by means of the oral glucose tolerance tests at baseline and endpoint.
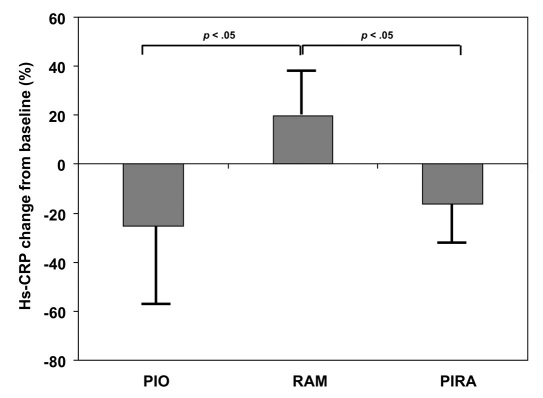
The primary efficacy parameter, mean absolute change of hs-CRP values after 12 weeks of treatment as compared to baseline, was -0.89 ± 1.98 (−0.50) mg/liter for PIO mono-therapy (p = .0237), 0.58 ± 2.13 (0.23) mg/liter for RAM monotherapy (p = .1382), and -0.49 ± 2.11 (−0.60) mg/liter for PIRA therapy (p = .0652). A decrease of hs-CRP was observed in 36/52 (69.2%) PIO monotherapy patients vs 20/44 (45.5%) RAM monotherapy patients vs 35/53 (66.0%) PIRA patients. The changes were significantly different between the groups for PIO vs RAM and PIRA vs RAM (p < .05). The percentage reduction in hs-CRP levels in each treatment group is displayed in Figure 3.
Figure 3.
Percentage change in mean hs-CRP from baseline to endpoint in the three treatment arms.
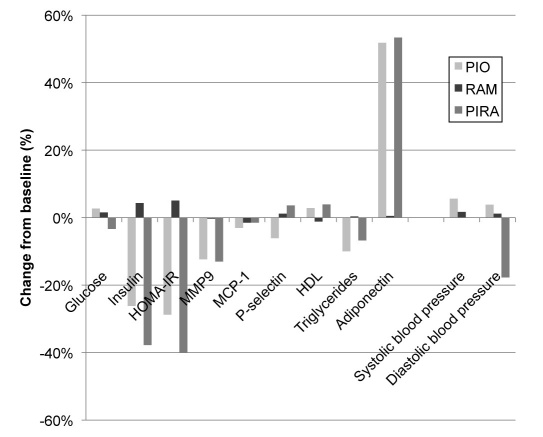
Also, comprehensive changes were seen in many of the secondary efficacy parameters. The baseline and endpoint values of these observation parameters are provided in Table 2 and the percentage changes from baseline in Figure 4. Insulin resistance as assessed by the HOMA-IR score improved only with PIO monotherapy and with PIRA combination therapy, whereas an impairment was seen with RAM monotherapy (p < .001 vs the two other groups). A comparable pattern of changes from baseline that partly reached statistical significance between the groups was also seen for MMP-9, (PIO vs RAM: p < .05), triglycerides (PIO vs RAM: p < .05), HDL-cholesterol, (RAM vs PIRA: p = .0573), LDL-cholesterol (not significant), and adiponectin (PIO or PIRA vs RAM: p < .001). There were differences among the groups with regard to glucose metabolism in several patients. Therefore, these analyses were repeated exclusively with patients showing no glucose deterioration during the entire study. They were similar to those obtained with the entire study population for all observation parameters, but some differences were not statistically significant because of the smaller sample size.
Table 2.
Primary and Secondary Efficacy Parameters at Baseline and Endpoint for the Entire Study Population (n = 149; Values Are Given as Mean ± SD)
| Parameter | Weeks | PIO; n = 52 | RAM; n = 44 | PIRA; n = 53 |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| hs-CRP (mg/liter) | 0 | 3.54 ± 2.54 | 2.90 ± 2.26 | 2.98 ± 2.15 |
| 12 | 2.65 ± 2.02a,d | 3.47 ± 2.62 | 2.50 ± 1.98a | |
| HbA1c (%) | 0 | 5.4 ± 0.3 | 5.6 ± 0.4 | 5.5 ± 0.4 |
| 12 | 5.4 ± 0.3 | 5.6 ± 0.6 | 5.4 ± 0.3 | |
| HOMA-IR (mU × mmol/liter2) | 0 | 2.71 ± 1.75 | 2.98 ± 1.45 | 3.61 ± 3.10 |
| 12 | 1.93 ± 1.37a | 3.13 ± 1.61d | 2.17 ± 1.65a | |
| HOMA-B (mU/mmol) | 0 | 111 ± 83 | 109 ± 48 | 142 ± 122 |
| 12 | 86 ± 57a,d | 114 ± 62d | 99 ± 69a,d | |
| Fasting insulin (µU/ml) | 0 | 10.7 ± 6.6 | 11.6 ± 5.1 | 14.3 ± 12.2 |
| 12 | 7.9 ± 5.4a | 12.0 ± 5.9d | 8.9 ± 6.5a | |
| Fasting glucose (mg/dl) | 0 | 100 ± 11 | 103 ± 12 | 101 ± 11 |
| 12 | 97 ± 8b | 105 ± 13d | 98 ± 11a | |
| 2-hour OGTT glucose (mg/dl) | 0 | 119 ± 33 | 132 ± 41 | 123 ± 34 |
| 12 | 111 ± 41 | 130 ± 43 | 111 ± 31 | |
| Triglycerides (mg/dl) | 0 | 136 ± 69 | 135 ± 65 | 132 ± 75 |
| 12 | 122 ± 79b | 135 ± 69 | 123 ± 51 | |
| Total cholesterol (mg/dl) | 0 | 189 ± 30 | 183 ± 35 | 194 ± 34 |
| 12 | 189 ± 31 | 184 ± 32 | 197 ± 37 | |
| LDL cholesterol (mg/dl) | 0 | 138 ± 29 | 137 ± 31 | 144 ± 40 |
| 12 | 135 ± 30 | 104 ± 34 | 145 ± 38 | |
| HDL cholesterol (mg/dl) | 0 | 53 ± 12 | 51 ± 11 | 52 ± 11 |
| 12 | 54 ± 12 | 50 ± 10d | 54 ± 12 | |
| MCP-1 (pg/ml) | 0 | 457 ± 103 | 514 ± 127 | 436 ± 129 |
| 12 | 443 ± 106 | 507 ± 122 | 429 ± 105 | |
| MMP-9 (ng/ml) | 0 | 388 ± 180 | 432 ± 201 | 461 ± 242 |
| 12 | 340 ± 141b | 430 ± 237 | 401 ± 215 | |
| P-selectin (ng/ml) | 0 | 143 ± 52 | 139 ± 62 | 135 ± 48 |
| 12 | 135 ± 51 | 140 ± 56 | 140 ± 57 | |
| Adiponectin (mg/l) | 0 | 8.2 ± 5.0 | 8.7 ± 6.5 | 8.3 ± 5.2 |
| 12 | 16.8 ± 8.2a,d | 8.8 ± 5.8d | 17.1 ± 8.2a,d | |
| 24-hour systolic blood pressure (mm Hg) | 0 | 128 ± 11 | 129 ± 12 | 128 ± 10 |
| 12 | 135 ± 12c,d | 131 ± 13 | 128 ± 11c,d | |
| 24-hour diastolic blood pressure (mm Hg) | 0 | 78 ± 9 | 76 ± 8 | 79 ± 7 |
| 12 | 81 ± 9c,d | 77 ± 8 | 77 ± 7c,d | |
| 24-hour heart rate (beats/min) | 0 | 70 ± 9 | 70 ± 9 | 70 ± 11 |
| 12 | 71 ± 9 | 69 ± 10 | 73 ± 9 | |
| Nocturnal systolic blood pressure decrease (mm Hg) | 0 | 12.1 ± 6.9 | 12.0 ± 6.8 | 11.8 ± 6.8 |
| 12 | 12.6 ± 6.6 | 11.2 ± 5.4 | 11.2 ± 5.3 | |
| Nocturnal diastolic blood pressure decrease (mm Hg) | 0 | 14.1 ± 8.5 | 14.4 ± 8.3 | 14.2 ± 7.6 |
| 12 | 13.8 ± 7.2 | 15.0 ± 6.4 | 15.0 ± 6.4 | |
p < .05 for PIO vs RAM and RAM vs PIRA
p < .05 for PIO vs RAM
p < .05 for PIO vs PIRA
p < .05 vs baseline
Figure 4.
Percentage changes of major secondary efficacy parameters from baseline to endpoint in three treatment arms.
Changes in the 24-hour blood pressure profile revealed increases in mean systolic blood pressure and mean diastolic blood pressure in the PIO and RAM groups, whereas a slight decrease occurred in the PIRA treatment group. These within-group differences were statistically significant for the PIO and PIRA groups. Furthermore, the between-group difference for PIO monotherapy vs PIRA combination therapy was statistically significant for both mean systolic (p = .0048) and mean diastolic (p = .0026) blood pressures. The remaining within- and between-group comparisons did not show clinically relevant or statistically significant differences. There were no major differences within and/or between the groups regarding the dynamics of nocturnal blood pressure changes.
Both drugs were well tolerated. Body weight only increased to a minor extent (+0.5 kg) with PIO in both groups (PIO and PIRA) and remained stable with RAM. There were no significant differences between the groups at baseline or endpoint. Adverse events were documented in 134/172 (77.9%; 54 in PIO vs 39 in RAM vs 41 in PIRA) patients, showing 359 (147 vs 99 vs 113) single events classified as treatment adverse events. Most frequently reported events were nasopharyngitis in 30/172 patients (17.4%; 9 vs 9 vs 12), headache in 24 (14.0%; 12 vs 3 vs 9), hypertension in 17 (9.9%; 7 vs 5 vs 5), peripheral edema in 15 (8.7%; 10 vs 2 vs 3), arteriosclerosis (symptoms of peripheral occlusive artery disease or angina) in 11 (6.4%; 3 vs 5 vs 3), upper abdominal pain in 9 (5.2%; 4 vs 3 vs 2), and hypertensive crisis in 7 patients (4.1%; 2 vs 4 vs 1).
The events were predominantly classified to be nonserious and mild or moderate in nature (344/359; 141 vs 98 vs 105). In 7/172 patients (4.1%; 3 vs 1 vs 3), a total number of 15 (6 vs 1 vs 8) events were classified as serious adverse events (SAE), mainly due to hospitalization. All SAEs were described as isolated episodes except for hypertensive crisis (2 patients). The SAEs were allocated to injuries in 3 patients (2 vs 0 vs 1), to musculoskeletal and vascular disorders in 2 patients each (0 vs 1 vs 1 and 1 vs 0 vs 1, respectively), and to gastrointestinal, general, and nervous system disorders in 1 patient each (all PIRA). In 2 patients (RAM and PIRA), the documented SAEs (arthralgia and malaise/loss of consciousness/subdural hematoma, respectively) were rated as possibly related to study drug administration. There were no cases of coughing reported as adverse events in the study.
Discussion
Metabolic syndrome is associated with an increase in cardiovascular complications. Disturbances in insulin efficacy and insulin secretion are major features of metabolic syndrome and might precede the development of diabetes mellitus by decades. Investigations have highlighted the link between disturbances in insulin physiology and subsequent mechanisms of atherosclerosis. Insulin resistance is an early feature of increasing visceral adipose tissue and is directly associated with the activation of a couple of atherogenic pathways. Independent from metabolic IR, vascular IR may have deleterious effects, including a further increase in chronic systemic inflammation and the activation of the MAPK pathway, accelerating the atherogenic process.4
These circumstances may explain the glucose-independent antiinflammatory effects of PPARγ-activation by PIO or RAM, which have been demonstrated in numerous clinical trials targeting all kinds of clinical and biochemical surrogate markers of cardiovascular risk, including carotid intima media thickness,19–21 plaque volume,22 hs-CRP,23–26 and MMP-927. In the PROactive study,28–30 these effects resulted in significant and clinically meaning-ful reductions in several secondary outcome measures such as macrovascular death, reinfarction, and restroke. In our opinion, these results support the use of PIO for treatment of systemic vascular inflammation early on in the development of cardiometabolic syndrome with all its different phenotypes and clinical pictures, including patients without overt diabetes.
Clearly, we did not consider PIO to be an antihypertensive drug. The expected improvement in vascular IR and the antiinflammmatory effects of PIO led us to investigate the use of this drug in normoglycemic patients with elevated chronic systemic inflammation and hypertension. In another investigation (PIOstat),15,31 we treated normo-glycemic patients with vascular IR and increased macrovascular risk because of dyslipidemia with PIO, simvastatin, or their combination and were able to demonstrate a significantly more pronounced effect of PIO on biomarkers of cardiovascular risk as compared to simvastatin. A synergistic effect was observed when both drugs were given in combination. In this trial, we used a comparable protocol to investigate the effects of PIO and RAM in nondiabetic patients with increased cardiovascular risk because of hypertension. Again, the treatment groups with PIO showed the best outcome regarding the changes in surrogate biomarkers of cardiovascular and metabolic risk. While RAM in a 5-mg dose alone did not significantly influence the macrovascular or metabolic risk profile, addition of the PPARγ agonist PIO altered the outcome after 3 months in the same direction as PIO monotherapy alone. There were some differences regarding glycemic control in the investigated patient population but repeating the analysis only with patients showing no glycemic deterioration (e.g., impaired glucose tolerance or overt diabetes) during the entire observation period resulted in similar changes of the observation parameters compared to those seen in the total study cohort. In the Heart Outcomes Prevention Evaluation (HOPE) study,13 RAM significantly reduced the rates of death, myocardial infarction, and stroke in a broad range of high-risk patients with and without diabetes after 5 years of therapy. However, in the HOPE study, RAM was used at a concentration of 10 mg/day as compared to the 5 mg/day investigated in our trial. In HOPE,32 RAM treatment also appeared to have a delaying effect on the progression of impaired glucose tolerance to overt type 2 diabetes mellitus. However, when RAM was tested and compared over 4 years with rosiglitazone in the DREAM trial with a 2×2 facultative study design, none of these effects could be confirmed in a patient population with impaired glucose tolerance.14 On the other hand, both rosiglitazone and PIO have demonstrated an impressive efficacy in delaying diabetes progression and even driving regression to normoglycemia in a considerable number of patients with impaired glucose tolerance in the DREAM study12 and the ACT NOW trial11, respectively. Based on the findings of our trial, which demonstrated no improvement of cardiovascular and metabolic risk markers by RAM, it can be speculated that the findings in the placebo-controlled HOPE study can be attributed to the higher RAM dose with better hypertension control. In addition, the groups were not entirely comparable and the antihypertensive effects of RAM may be more important for risk reduction in patients with a more advanced cardiovascular disease and hypertension than in a population with a moderate risk profile such as in this study.
There was a slight increase of blood pressure with PIO and stable blood pressure with RAM monotherapy in this trial, as RAM is an antihypertensive drug and PIO has consistently shown antihypertensive effects in many other trials.33–36 However, a replacement of the stable antihypertensive pretreatment with ACE inhibitors by the respective study medication took place at the time of randomization. There was no wash-out period of the previous ACE inhibitor, as hypertension control was not the primary objective of this trial; we wanted to avoid any hypertensive situation because of a potentially delayed onset of antihypertensive effects with PIO. Hypertension is clearly not the primary indication for use of PIO, and RAM may not have been dosed high enough to further improve blood pressure. This explains the findings on hypertension control with both drugs when given as monotherapy. The combination of PIO and RAM, however, was able to maintain and improve the hypertension control in comparison to the two mono-therapy arms, most likely because of synergistic effects on blood pressure.
About a third of all patients with essential hypertension reveal an impaired circadian pattern of blood pressure. This phenomenon, called non-dipping (i.e., a lack of normal nocturnal fall in blood pressure), is related to a higher incidence of end-organ damage such as left ventricular hypertrophy and may worsen the prognosis of hypertensive subjects.37,38 PIO has been described to reverse this situation.33,35 Although not reaching the level of statistical significance, this was also indicated by an improvement in the nocturnal decrease in systolic blood pressure in our present trial. However, there was no significant difference regarding the number of patients affected by these changes (e.g., switching from dipper to nondipper or vice versa) between the groups in this study.
Our 12-week treatment with PIO in nondiabetic hypertensive patients with increased cardiovascular risk and activated inflammation clearly showed a decrease of more than 15% in mean hs-CRP within the studied period for both PIO monotherapy and PIRA combination therapy groups. In terms of safety, the study did not reveal any potentially new or unexpected signs or symptoms allocated to the study drugs in comparison to the known range of thiazolidinedione- and/or ACE-inhibitor-specific adverse reactions. Observations such as hypotension, peripheral edema, headache, dizziness, and gastrointestinal problems are consistent with the expected adverse event profiles of the drugs used. In contrast, findings such as anxiety, nervousness, hyperhidrosis, diabetes, and general indisposition can be rated as usual for a clinical trial considering a patient collective with an increased cardiovascular risk.
Both groups with PIO offer clearly positive results in terms of several established clinical and laboratory markers for cardiovascular diseases and risk factors. Obvious beneficial influences in terms of pleiotropic effects were observed for the parameters of inflammation and vascular function (hs-CRP, MCP-1, MMP-9), glucose tolerance/insulin sensitivity (glucose, insulin, adiponectin, HOMA-S, HOMA-B), and lipid metabolism (HDL, LDL, triglycerides, adiponectin). Many of the observed effects were statistically significant versus RAM monotherapy for the primary pooled-center analysis on hs-CRP, as well as for the per-protocol evaluation.
In our other investigation with normoglycemic patients, vascular IR, and dyslipidemia (PIOstat study),15,31 we demonstrated the importance of IR therapy for reduction of chronic systemic inflammation, which was independent from applied antilipidemic simvastatin therapy. In the PIOstat study, simvastatin lowered hs-CRP by 16% and PIO by 30%. The combination of both showed a synergistic effect with a 44% lowering of hs-CRP. Stronger statins such as atorvastatin or rosuvastatin have shown stronger hs-CRP-lowering effects that reach reductions similar to what we report herein and in other trials for PIO. In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial,39 rosuvastatin lowered hs-CRP by 37% in 12 months, which was associated with substantial reduction in cardiovascular events. In this context, the combination of drugs with antiinflammatory effects via different mechanisms of action may help generate attractive hypotheses for further clinical studies.
In this article, we now confirm these findings also for elevated blood pressure, where PIO also has an additional benefit on hypertension and chronic systemic inflammation in comparison to antihypertensive therapy with RAM alone. If these results are confirmed in further studies, PIO may be a suitable addition to antihypertensive therapy with ACE inhibitors in patients with vascular IR and increased chronic systemic inflam-mation, as shown, e.g., by hs-CRP levels indicating a moderate to high cardiovascular risk (1–10 mg/dl). In any case, our results underscore the benefit of an early IR treatment in this patient population at high risk for macrovascular disease.
Glossary
Abbreviations
- (CURES)
Chennai Urban Rural Epidemiological Study
- (CV)
coefficient of variation
- (CVD)
cardiovascular disease
- (CI)
confidence interval
- (HbA1c)
hemoglobin A1c
- (HDL-C)
high-density lipoprotein cholesterol
- (HOMA-IR)
homeostasis model assessment of insulin resistance
- (hs-CRP)
high-sensitivity C-reactive protein
- (IL-6)
interleukin-6
- (IR)
insulin resistance
- (LDL-C)
low-density lipoprotein cholesterol
- (MS)
metabolic syndrome
- (NCEP)
National Cholesterol Education Program
- (OR)
odds ratio
- (TNF-α)
tumor necrosis factor-α
- (VCAM-1)
vascular cell adhesion molecule-1
References
- 1.Meigs JB. Epidemiology of the metabolic syndrome. Curr Diab Rep. 2003;3(1):73–79. doi: 10.1007/s11892-003-0057-2. [DOI] [PubMed] [Google Scholar]
- 2.German Diabetes Association, Matthaei S, Bierwirth R, Fritsche A, Gallwitz B, Häring HU, Joost HG, Kellerer M, Kloos Ch, Kunt T, Nauck M, Schernthaner G, Siegel E, Thienel F. Medical antihyper-glycaemic treatment of type 2 diabetes mellitus: update of the evidence-based guideline of the German Diabetes Association. Exp Clin Endocrinol Diabetes. 2009;117(9):522–557. doi: 10.1055/s-0029-1239559. [DOI] [PubMed] [Google Scholar]
- 3.Van Gaal LF, Mertens L, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 4.Forst T, Hohberg C, Pfützner A. Cardiovascular effects of disturbed insulin activity in metabolic syndrome and in type 2 diabetes patients. Horm Metab Res. 2009;41(2):123–131. doi: 10.1055/s-0028-1119378. [DOI] [PubMed] [Google Scholar]
- 5.Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120(Suppl 1):S3–S8. doi: 10.1016/j.amjmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep. 2008;10(2):93–98. doi: 10.1007/s11906-008-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanefeld M. Postprandial hyperglycemia: noxious effects on the vessel wall. Int J Clin Pract Suppl. 2002;129:45–50. [PubMed] [Google Scholar]
- 10.Home P. Contribution of basal and post-prandial hyperglycemia to micro- and macrovascular complications in people with type 2 diabetes. Curr Res Med Opin. 2005;21(7):989–998. doi: 10.1185/030079905x49662. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, Mack WJ, Mudaliar S, Ratner RE, Williams K, Stentz FB, Musi N, Reaven PD ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364(12):1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541):1096–1105. doi: 10.1016/S0140-6736(06)69420-8. Erratum Lancet. 2006;368(9549):1770. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inihibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 14.Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, Fodor G, Holman RR DREAM Trial Investigators. The effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355(15):1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 15.Hanefeld M, Marx N, Pfützner A, Baurecht W, Lübben G, Kargiannis E, Stier U, Forst T. Anti-inflammatory effect of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protethe PIOSTAT Study. J Am Coll Cardiol. 2007;49(3):290–297. doi: 10.1016/j.jacc.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet Med. 2000;17(4):299–307. doi: 10.1046/j.1464-5491.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 18.Pfützner A, Forst T. High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus. Diab Technol Ther. 2006;8(1):28–36. doi: 10.1089/dia.2006.8.28. [DOI] [PubMed] [Google Scholar]
- 19.Langenfeld MR, Forst T, Hohberg C, Kann P, Lübben G, Konrad T, Füllert S, Sachara C, Pfützner A. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation. 2005;111(19):2525–2531. doi: 10.1161/01.CIR.0000165072.01672.21. [DOI] [PubMed] [Google Scholar]
- 20.Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D'Agostino RB Sr, Perez A, Provost JC, Haffner SM. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 2006;296(21):2572–2581. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- 21.Forst T, Wilhelm B, Pfützner A, Fuchs W, Lehmann U, Schaper F, Weber M, Müller J, Konrad T, Hanefeld M. Investigation of the vascular and pleiotropic effects of atorvastatin and pioglitazone in a population at high cardiovascular risk. Diab Vasc Dis Res. 2008;5(4):298–303. doi: 10.3132/dvdr.2008.043. [DOI] [PubMed] [Google Scholar]
- 22.Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochellière R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299(13):1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 23.Pfützner A, Marx N, Lübben G, Langenfeld M, Walcher D, Konrad T, Forst T. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J Am Coll Cardiol. 2005;45(12):1925–1931. doi: 10.1016/j.jacc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 24.Hamann A, Garcia-Puig J, Paul G, Donaldson J, Stewart M. Comparison of fixed-dose rosiglitazone/metformin combination therapy with sulphonylurea plus metformin in overweight individuals with type 2 diabetes inadequately controlled on metformin alone. Exp Clin Endocrinol Diabetes. 2008;116(1):6–13. doi: 10.1055/s-2007-984441. [DOI] [PubMed] [Google Scholar]
- 25.Karagiannis E, Pfützner A, Forst T, Lübben G, Roth W, Grabellus M, Flannery M, Schöndorf T. The IRIS V study: pioglitazone improves systemic chronic inflammation in patients with type 2 diabetes under daily routine conditions. Diabetes Technol Ther. 2008;10(3):206–212. doi: 10.1089/dia.2008.0244. [DOI] [PubMed] [Google Scholar]
- 26.Kato T, Sawai Y, Kanayama H, Taguchi H, Terabayashi T, Taki F, Yamada K, Yamazaki Y, Hayakawa N, Suzuki A, Oda N, Katada N, Itoh M. Comparative study of low-dose pioglitazone or metformin treatment in Japanese diabetic patients with metabolic syndrome. Exp Clin Endocrinol Diabetes. 2009;117(10):593–599. doi: 10.1055/s-0029-1202792. [DOI] [PubMed] [Google Scholar]
- 27.Forst T, Karagiannis E, Lübben G, Hohberg C, Schöndorf T, Dikta G, Drexler M, Morcos M, Dänschel W, Borchert M, Pfützner A. Pleiotrophic and anti-inflammatory effects of pioglitazone precede the metabolic activity in type 2 diabetic patients with coronary artery disease. Atherosclerosis. 2008;197(1):311–317. doi: 10.1016/j.atherosclerosis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 29.Erdmann E, Dormandy JA, Charbonnel B, Massi-Benedetti M, Moules IK, Skene AM PROactive Investigators. The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study. J Am Coll Cardiol. 2007;49(17):1772–1780. doi: 10.1016/j.jacc.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 30.Wilcox R, Bousser MG, Betteridge DJ, Schernthaner G, Pirags V, Kupfer S, Dormandy J. PROactive Investigators. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke. 2007;38(3):865–873. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- 31.Forst T, Pfützner A, Lübben G, Weber M, Marx N, Karagiannis E, Köhler C, Baurecht W, Hohberg C, Hanefeld M. Effect of simvastatin and/or pioglitazone on insulin resistance, insulin secretion, adiponectin, and proinsulin levels in nondiabetic patients at cardiovascular risk—the PIOSTAT Study. Metabolism. 2007;56(4):491–496. doi: 10.1016/j.metabol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Scheen AJ. Prevention of type 2 diabetes through inhibition of the renin-angiotensin system. Drugs. 2005;64(22):2537–2565. doi: 10.2165/00003495-200464220-00004. [DOI] [PubMed] [Google Scholar]
- 33.Negro R, Dazzi D, Hassan H, Pezzarossa A. Pioglitazone reduces blood pressure in non-dipping diabetic patients. Minerva Endocrinol. 2004;29(1):11–17. [PubMed] [Google Scholar]
- 34.Giles TD, Sander AG. Effects of thiazolidinediones on blood pressure. Curr Hypertens Rep. 2007;9(4):332–337. doi: 10.1007/s11906-007-0060-0. [DOI] [PubMed] [Google Scholar]
- 35.Anan F, Masaki T, Fukunaga N, Teshima Y, Iwao T, Kaneda K, Umeno Y, Okada K, Wakasugi K, Yonemochi H, Eshima N, Saikawa T, Yoshimatsu H. Pioglitazone shift circadian rhythm of blood pressure from non-dipper to dipper type in type 2 diabetes mellitus. Eur J Clin Invest. 2007;37(9):709–714. doi: 10.1111/j.1365-2362.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 36.Schöndorf T, Forst T, Hohberg C, Pahler S, Link C, Roth W, Pfützner A. The IRIS III study: pioglitazone improves metabolic control and blood pressure in patients with type 2 diabetes without increasing body weight. Diab Obes Metab. 2007;9(1):132–133. doi: 10.1111/j.1463-1326.2006.00582.x. [DOI] [PubMed] [Google Scholar]
- 37.Cicconetti P, Donadio C, Pazzaglia MC, D'Ambrosio F, Marigliano V. [Circadian rhythm of blood pressure: non-dipping pattern and cardio-vascular risk][Article in Italian] Recenti Prog Med. 2007;98(7-8):401–406. [PubMed] [Google Scholar]
- 38.Zweiker R, Eber B, Schumacher M, Toplak H, Klein W. “Non-dipping” related to cardiovascular events in essential hypertensive patients. Acta Med Austriaca. 1994;21(3):86–89. [PubMed] [Google Scholar]
- 39.Kones R. Rosuvastatin, inflammation, C-reactive protein, JUPITER, and primary prevention of cardiovascular disease—a perspective. Drug Des Devel Ther. 2010;4:383–413. doi: 10.2147/DDDT.S10812. [DOI] [PMC free article] [PubMed] [Google Scholar]