Abstract
The importance of biomechanics in glucose sensor function has been largely overlooked. This article is the first part of a two-part review in which we look beyond commonly recognized chemical biocompatibility to explore the biomechanics of the sensor–tissue interface as an important aspect of continuous glucose sensor biocompatibility. Part I provides a theoretical framework to describe how biomechanical factors such as motion and pressure (typically micromotion and micropressure) give rise to interfacial stresses, which affect tissue physiology around a sensor and, in turn, impact sensor performance. Three main contributors to sensor motion and pressure are explored: applied forces, sensor design, and subject/patient considerations. We describe how acute forces can temporarily impact sensor signal and how chronic forces can alter the foreign body response and inflammation around an implanted sensor, and thus impact sensor performance. The importance of sensor design (e.g., size, shape, modulus, texture) and specific implant location on the tissue response are also explored. In Part II: Examples and Application (a sister publication), examples from the literature are reviewed, and the application of biomechanical concepts to sensor design are described. We believe that adding biomechanical strategies to the arsenal of material compositions, surface modifications, drug elution, and other chemical strategies will lead to improvements in sensor biocompatibility and performance.
Keywords: biocompatibility, biomechanics, foreign body response, glucose sensor, micromotion, pressure
Introduction
It is well established that tight regulation of glucose reduces the risk of diabetes-related complications1 and that availability of continuous monitoring data to patients improves outcomes.2,3 However, despite 40+ years of research and development, there does not exist an accurate, long-term continuous glucose sensor to aid patients in maintaining euglycemia or to make the much-needed artificial pancreas a reality. The state-of-the-art is the percutaneous (PerQ) electrochemical sensor that continually monitors the glucose levels in the subcutaneous (SubQ) interstitium. These devices, while FDA-approved for patient use, are only indicated as adjunctive to self-monitoring blood glucose finger stick data. To realize the full potential of continuous glucose monitoring (CGM) and to improve patient adoption of these technologies, glucose sensors will need improved accuracy and ease of use. The accuracy limitations are not inherent in the sensors, as demonstrated by good in vitro sensor performance.4–6 However, in the in vivo environment, sensor signals generally decline over time in an erratic, unpredictable fashion.4,7–9 The primary culprit is the complex and dynamic foreign body response (FBR), which includes inflammation, biofouling, fibrosis, receding microvasculature, and a barrage of free radicals and degradative enzymes at the sensor–tissue interface.10–12
Historical Perspective
For years, the major focus of sensor development was to apply traditional engineering approaches, with little understanding of the biological interactions. Electronics were hermetically sealed, size exclusion membranes were added to control analyte fluxes and enzymes were stabilized. The realization that sensor surface chemistries direct the cellular interactions that dictate tissue response, which in turn dictate overall functionality of the sensor, led to extensive surface modification efforts. Various surface chemistries, coatings, and exterior membranes were developed to reduce protein fouling at the sensor surface.13 Additionally an understanding emerged that surface microarchitecture independent of surface chemistry affects tissue response14 and subsequently sensor functionality.15–18 Application of coatings with specific pore sizes and microarchitechtural details have been shown to induce angiogenesis surrounding implanted sensors,4,19,20 but so far, a porous or textured sensor coating has not led to practical clinical use. Efforts to overcome the deleterious effects of the FBR have focused on the use of active surface coatings21 including the use of drugs to reduce inflammation and induce angiogenesis,22–38 enzymatic scavengers,39 gene transfer to induce neovascu-larization,40 and stem cell attachment.41 Few of these strategies have yielded significant improvements, and in some cases, have resulted in poorer sensor performance.4,20 All of these studies involving both passive and active coatings have increased our understanding of the sensor–tissue interface, but there remain major gaps in our understanding of how to control and stabilize the biological environment surrounding the sensor to enable long-term CGM.
In light of these confounding results, we review one less-explored contributing factor to in vivo performance of glucose sensors: the biomechanics of the sensor–tissue interface. The presence of an implanted sensor imposes chronic mechanical disruption to tissue, and in response, the tissue remodels to accommodate the new loading conditions.42 The way in which tissue remodels around sensors affects sensor function.15 Furthermore, on a short time scale (quicker than tissue remodeling), forces acting on tissue surrounding the sensor may alter key physiological parameters such as blood flow and cell metabolism around the sensor. Also, rubbing or irritation may disrupt cell membranes releasing tissue factor and other proinflammatory agents. Variable stresses and loading conditions give rise to motion and pressure (typically micromotion and micropressure) and impact implanted sensor functionality. We propose that the surface chemistry view of sensor biocompatibility is only a portion of the story, and that mechanical forces imposed at the sensor–tissue interface also have a critical impact on the tissue reaction surrounding the sensor. Therefore, establishing a theoretical framework of how mechanical forces act on the sensor and surrounding tissue is warranted.
Percutaneous versus Fully Implantable Sensors
Before a discussion of mechanical forces, it is worth distinguishing first between PerQ and fully implantable sensors because they can vary greatly in their size, insertion/implantation procedures, operational life, and most importantly for this review, experience different mechanical forces once implanted in tissue. Percutaneous sensors that are commercially available are needle-type electrochemical sensors that penetrate the outer layers of skin. Sensing elements reside in the SubQ compartment where they are exposed to interstitial fluid from which they sense glucose. The electrochemical signal is electrically transduced to the externally worn transmitter that adheres to the outer layers of the skin. These indwelling devices are easily inserted and removed by the patient and are currently the only type of FDA-approved continuous glucose monitors.
Fully implantable sensors represent the class of sensors that are surgically implanted or injected into the body. They have not been marketed and most implantable sensors in development are intended for use in the SubQ space. Peritoneal and other implantation sites besides SubQ tissue are not considered in this review. SubQ sensors may vary greatly in size and componentry. Fully implantable SubQ sensors are typically much larger than the indwelling portion of PerQ sensors because they must incorporate all sensing, power, and telemetry components. They tend to be on the scale of several centi-meters32,43–47 although advancements in nanotechnology and microelectronics are enabling the development of miniaturized versions.48,49 Important to note is the surgical implantation of SubQ sensors and the resulting settling period, which is the time it takes for tissue response to settle or stabilize after sensor implantation.46 The larger the sensor, the more tissue damage occurs during implantation. Gilligan and Updike reported a settling period from days to months after implantation of their sensors in a SubQ pocket created through blunt dissection through a 1–3-cm incision.43,44,46,50 However, once stabilized, SubQ sensors have been reported to have long-term sensing capabilities of several months and even beyond a year.32,43,45,47,51 In our discussion of biomechanical influences on sensors performance, we describe various forces to which sensors may be exposed, including differential forces caused by PerQ versus SubQ sensor designs.
Wound Healing and Foreign Body Response
The very act of sensor implantation causes tissue injury and induces the wound healing response through a series of complex events.52,53 The continued presence of the sensor disrupts the normal course of wound healing and elicits a FBR.11,13 Immune cells attempt to engulf and digest the foreign body (sensor). A cascade of degradative substances (e.g., superoxides and free radicals) may be released in an attempt to break down the foreign body. If the foreign object is not small enough to be engulfed by macrophages or other phagocytozing cells (<5–20 μm), the body's natural response is to form foreign body giant cells and isolate the implanted device in a fibrous capsule to minimize its interaction with surrounding tissue.11,13,54,55
There is evidence to suggest the fibrotic capsule plays a role in mechanically stabilizing the foreign body. For example, Hori and colleagues found a positive correlation between the migration distances of implants and increased capsule thickness.56 The thicker capsule was attributed to the constant friction between migrating implants and tissue. Numerous others have also reported that surface-textured implants (e.g., pillars, porous surfaces) exhibit a significantly thinner and less dense fibrous capsule.56–60 The greatly increased surface area of these porous and pillared materials serves to reduce interfacial stresses at the tissue–implant interface by allowing the stresses to distribute over a much greater surface area.58 Therefore, it is hypothesized that the body does not attempt to provide as much mechanical stabilization when forces are distributed, so capsules are thinner and less dense. PerQ devices are exposed to greater biomechanical forces compared to fully implantable SubQ devices. It has been shown that capsule thickness decreases over time for SubQ implants but remains constant for PerQ devices,61 thus supporting the notion that encapsulation is the body's attempt to mechanical stabilize implants. Finally, collagen, which is the primary constituent of the fibrous capsule, varies in type and amount depending on the mechanical stimuli or load on the tissue.42 Here, we discuss mechanical forces and tissue–sensor biomechanics as important aspects of biocompatibility ultimately affecting the degree of the FBR to the sensor and its resultant performance.
Scope
This review (Part I—Theoretical Framework) explores biomechanical factors that affect percutaneous and fully implantable glucose sensors. Here, we focus on soft tissue biomechanics, particularly SubQ tissue, as this is the compartment most typical for sensor placement. Not discussed is the growing body of single cell biomechanics literature that attempts to relate forces on various cell types to regulation of cell function and structure, including cytoskeletal remodeling, cytokine release, proliferation, adhesion, and cell spreading.62–79 For example, Ingber and colleagues80,81 have described the transduction of force from the extracellular matrix to the cytoskeleton via transmembrane integrin receptors and assert that mechanical forces are critical cellular and developmental regulators. Although single cell bio-mechanics literature is certainly relevant, it is beyond the scope of this article, which takes a more macrocellular, tissue-level view. The sister paper (Part II—Examples and Applications) reviews specific examples from the literature that relate biomechanics to sensor performance and explores application of biomechanical concepts to sensor design.82
Biomechanics of the Sensor–Tissue Interface
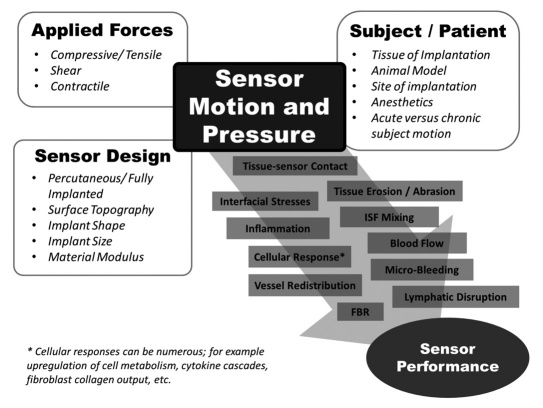
The effects of mechanical stress at the tissue–implant interface have been recognized in the biomaterial literature.42,83,84 We found the reviews of Hilborn (2007) and Sanders (1997) to be particularly helpful in our exploration of soft tissue biomechanics; however, trans-mission of both external and internal forces that generate motion and/or pressure at the sensor–tissue interface is a relatively unexplored and underappreciated aspect of sensor biocompatibility. These forces may be large and cause short-term modulation of sensor signal or be small in magnitude, cause no pain, and go completely unnoticed by the patient but still have an effect on the tissue environment surrounding the sensor and hence affect the sensor signal in the long term. A review of biomechanical forces affecting sensor performance suggests that even low magnitude forces such as micro-motion can affect wound healing and FBR. Figure 1 illustrates some of the contributors to motion and pressure at the implant–tissue interface and groups them into three main categories that are explored in this review: (1) applied forces, (2) sensor design, and (3) subject/species considerations.
Figure 1.
Applied forces, sensor design, and subject/patient consideration all contribute to sensor motion and pressure. Motion and pressure can affect sensor performance in a variety of ways. These biomechanical effects impact the tissue environment adjacent to the sensor in addition to chemical, electrical, magnetic, optical, or other possible effectors of the tissue response.
Applied Forces (Generation and Transmission)
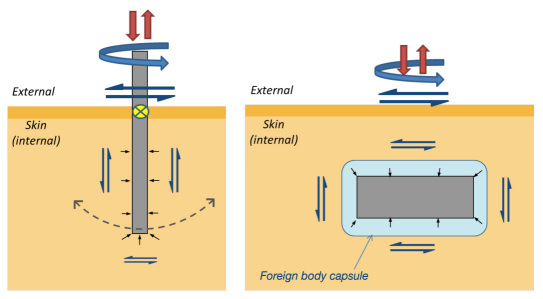
Motion and pressure arise from the application of various forces. These forces may act directly on the device, as in the case of a person or animal scratching or rubbing up against external sensor components, or indirectly, as occurs in the case of running and walking. Even breathing and pulsing of blood vessels have been shown to create cyclical sensor micromotion artifacts (with brain electrodes).85 Although the forces that must be endured are likely more severe in animal models compared to humans, there are clearly many forces pertinent to marketed sensors during wear (e.g., rubbing of the beltline or other clothing, sleeping directly on the sensors, etc.). The magnitude, duration and transmission of each type of force can be quite different. Figure 2 describes some of the relevant forces affecting glucose sensor performance, including normal (compressive and tensile), shear, and contractile forces.
Figure 2.
Schematic of applied forces acting on in vivo sensors. Examples of both external and internal forces generating motion and pressure on sensors relative to surrounding tissue for (a) percutaneous and (b) subcutaneous devices. Normal forces (red) include compressive and tensile forces that can arise from pushing or pulling on the external portion of the PerQ lead or device housing, or, in the case of a fully implanted SubQ sensor, an external force can be transmitted through the tissue to the implant. Common even in well-designed animal studies are attempts at scratching the sensor site and tugging the sensor leads, while human subjects are likely to experience tensile forces arising from snagging clothing and compressive forces from restrictive clothing and lying down on the sensor. Shear forces (blue) that act on PerQ and SubQ sensors include both transverse and torsional loadings. Shear stresses along the length of the sensor can also arise from pulling on the PerQ sensor. These forces may be acute (e.g., brushing across sensor while getting dressed) or repeated (e.g., walking, running). Capsule or wound contracture (short black) include wound contraction as the trauma of implantation is repaired. Contractile forces also arise from the constrictive fibrosis and contraction of the long-term foreign body capsule surrounding the device. The attachment of a PerQ device to the skin is represented by the yellow circle filled with an x and can serve as a fulcrum about which the implanted portion of a stiff sensor will rotate (dotted line) in response to an external shear force.
Normal Forces (Compressive and Tensile)
The most apparent sources of device motion are external forces that act directly on the sensor itself. Tensile forces are expected more in certain implantation sites and in some animal models (e.g., tethered leads to rodents)86 and compressive forces have been reported in large animal studies (e.g., in dogs, direct pressure over the skin led to a marked reduction in sensor output).44 In human and animal use, the sleeping position may put direct compressive forces on the sensor, particularly if sensors are located over bony protuberances. Cases of anomalous sensor readings caused by compressive forces and specifically due to lying directly on the sensors are presented in Part II.82
Shear Forces
Shear conditions may arise in tissue surrounding sensors due to normal physiologic activities that can vary widely in shear rates (0.01 to 1 s-1)87 and frequencies (e.g., 0.1 Hz during walking and 12 Hz during running).88 The magnitude of shear forces on sensors in SubQ tissue has not, to our knowledge, been well characterized. Holt and colleagues submit that even at low magnitudes, shear loading is a highly destructive modality especially under the chronic conditions of an implanted sensor.88 These chronic shear forces in surrounding tissue give rise to micromotion of the sensor relative to surrounding tissue, and persistent interfacial stress has been related to the thickness of the foreign body capsule and the failure of PerQ devices.59,89 Furthermore, continued local trauma arising from micro- motion is evidenced by the necrosis of interfacial cells42,90 despite traditional biocompatibility metrics of the materials (e.g., ISO 10993), and by the presence of microhemorrhages and edema in tissue surrounding implanted sensors.32,91,92 Even after the initial implantation wound has completely healed, the force of the sensor against tissue seems to initiate bleeding in tissue around the sensor in some cases. Dr. Uli Klueh presented this information at the Diabetes Technology Meeting held in Bethesda, Maryland, November 11–13, 2010. In discussions with CGM users, one patient noted predictable bleeding at the site of the sensor during jogging. In this case, movement of the sensor relative to tissue appears to rewound the implantation site, causing microhemorrhages.
Contractile Forces
Quite different from the oscillatory shear stresses that give rise to micromotion are the inward normal, contractile forces. Contraction models (human fibroblasts cultured in three-dimensional collagen matrices), exhibited tension forces from 0.1 to 1 grams of force per million cells (approximately 1–10 nN/cell).93 Stresses in this range (1–10 nN/cell contact) can cause remodeling of cell–cell contacts, cell extracellular matrix (ECM), and ECM.42 Capsular contracture arising from constriction of the foreign body capsule has been reported to be strong enough to bend and fracture subcutaneously implanted polystyrene disks94 and rupture breast implants.95,96 Capsular contraction may not be relevant to available PerQ sensors but will become more relevant to sensor applications as the lifetime of fully implantable sensors is extended beyond 1-week implantation time.
Sensor Design Considerations
Various sensor design features are also important contribu- tors to the way motion and pressure propagate to tissue surrounding the sensor and vice versa. The sensor size, shape, and material properties will affect the relative motion, intensity, and concentration of forces at the interface.
PerQ versus SubQ
The first design consideration is simply the type of sensor, an indwelling (PerQ) versus a fully implanted (SubQ) sensor. As described in the literature, the FBR to a PerQ implant is significantly different compared to a fully implanted device.19,20,61 A PerQ device is subjected to direct external forces as well as the transmission of forces from various layers of skin. Data exhibiting the persistence of a foreign body capsule61 support the notion that tissue remodels to adapt to the mechanical loading imparted by the implanted device.42,84 Observed presence of fibrin was hypothesized to be the result of PerQ sensor movement,97 which likely caused inflammation and/or direct microvascular damage, thereby increasing vascular permeability.
To minimize motion, various nonsensor PerQ devices (e.g., peritoneal dialysis ports) have been anchored to surrounding tissue. This has been achieved through first implanting meshes or porous flanges under the skin.98–100 Once healed in place, the PerQ portion is inserted through the supporting mesh or flange. These approaches may require secondary surgery for device placement, and the new wound created during PerQ device injection could affect sensor performance if this long-term PerQ technique were applied to sensors.
Beyond the conduit for potential infection posed by PerQ sensors, the lifetime of PerQ devices may be limited by the micromotion and pressure exerted on the exterior of the device; forces propagate along the sensor and impact the cells and tissues at the sensor interface, thereby impacting sensor performance in an uncontrolled way. Conversely, a fully implanted SubQ sensor of (equivalent size and shape as the implanted PerQ portion) will experience less direct force, which likely leads to a relatively more stable sensing environment.20,101 Certainly, researchers have shown considerably longer experimental data sets extending several months32,43,44,46,47 and even beyond a year51 with fully implanted sensors. However, one of the concerns with SubQ sensors is the longer settling period43,44,46,50 required before the sensors track glucose, although this settling period is likely to be reduced as sensor miniaturization48,49 enables microinjections and minimizes tissue damage.
Surface Topography
Surface microarchitecture (including microtopography, pillars, and porous coatings) is an area of abundant biomaterial research14,58,60,102,103 and has been applied to glucose sensors.4,19,20,43,50,104 It is known that materials of the exact same chemical composition can exhibit vastly different FBR depending on the surface micro-architecture.14,17,102,103 These findings serve as direct evidence of biomechanical cues contributing (in addition to surface chemistry) to the tissue response. Ridges, fibers, or pores on the exterior of textured materials serve to increase surface area, and thus the force per unit area observed by an implant can be greatly reduced. Macrophages are not able to flatten on the surface of materials with certain pore or fiber size, and this likely changes their expression pattern to trigger an altered FBR. Picha and Drake found that surface pillars reduced interfacial shear by improving tissue integration and resulted in reduced fibrosis, improved vascularity, and improved overall tissue response (Figure 3).58 Similarly, porous coatings have been shown to improve tissue integration and FBR.14,17,102 One caveat to the use of porous coatings for PerQ soft-tissue sensors is that some researchers suggest that the tissue integration could actually be detrimental, as external forces on the sensor are transmitted to the region occupied by the coating and beyond, a phenomenon that has been described as tearing the device from its implantation bed.19,89
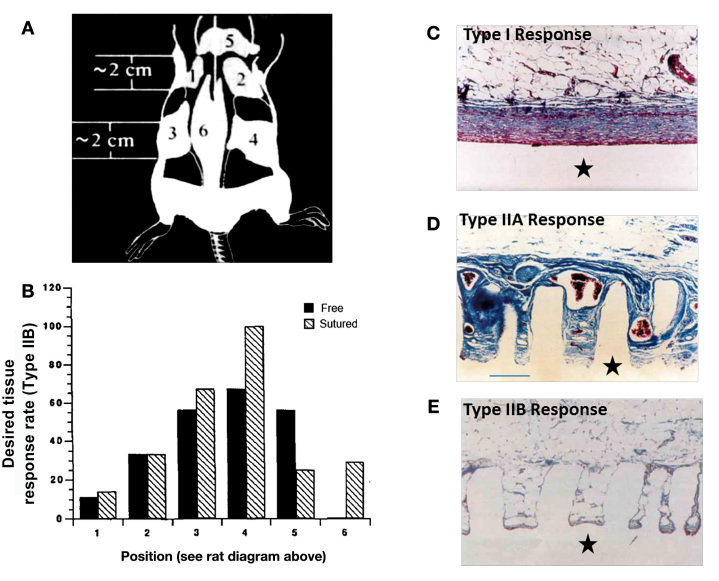
Figure 3.
Even in the same compartment (SubQ), tissue response varies with site of implantation. (A) Solid and microtextured (100-μm-diameter pillars by 500 μm in height, spaced 200 μm center to center) silicone disks (⋆) were implanted into rat SubQ tissue in six different locations as illustrated. (B) The most favorable, Type IIB, response rate is plotted versus implantation site for free and sutured micropillared implants (4 weeks). A 100% type IIB response is observed for the sutured implant in the fatty tissue bed of site 4. No type IIB responses were noted for free implants in position 6, which is consistent with the authors' supposition that both microtexturing and reduced motion are as more likely to lead to a type IIB response. Trichrome stained, 100x. (C) A Type I response as measured for a flat (nontextured) implant, encapsulated by a highly aligned fibrous capsule. (D) A Type IIA response: dense collagen and large blood vessels (~100–200 μm) found in and around pillared surface material. (E) The optimal, Type IIB response with little to no capsule and capillaries in intimate contact with the pillar surface (too small to be imaged in this diagram). The varying tissue responses observed in (D) and (E) are dependent on the SubQ implantation site (B) as well as fixation to reduce motion. Figures adapted from Picha and colleages.58
Shape
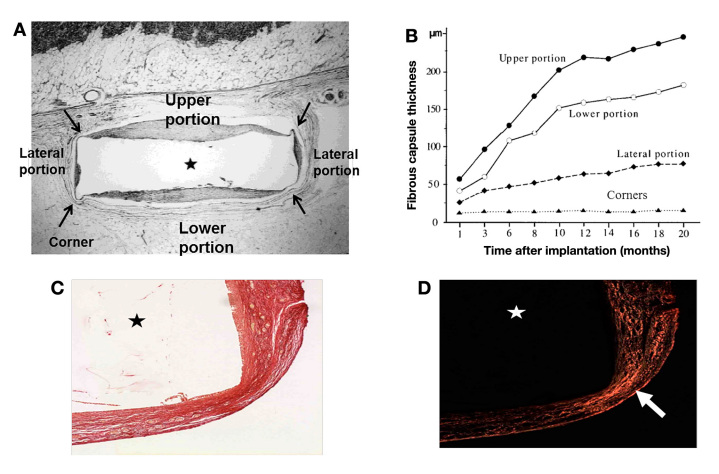
The shape of sensors also greatly impacts the interfacial forces. Matlaga and colleagues105 demonstrated the altered response to implants of different cross-sectional shapes and found that acute angles initiate a different tissue response. The regions of higher stress concentration at sharp angles elicit a denser encapsulation response (i.e., collagen density) compared to broad angles or flat edges. From the authors' personal experience, dense fibrous tissue found at the corners of rectangular-shaped implants is often purposefully neglected in FBR implant evaluations because it differs substantially from the reaction around the majority of the implant surface. However, from the perspective of sensor development, a complete understanding of the FBR may be necessary to help choose the optimal placement of electrodes or sensing components. Li and colleagues demonstrate that capsule thickness varies with time and position surrounding a rectangular-shaped SubQ implant (Figure 4).106 Their work underscores the importance of not only the location of capsule measurements (corners versus nonangled surfaces) but also the implant orientation within the SubQ space.
Figure 4.
The foreign body capsule surrounding an implant varies (thickness and density) with position on the surface of the implant and time of residence in the body. (A) Foreign body capsule surrounding a hydroxyapatite disk (⋆) implanted subcutaneously into the interscapular region of rats. (B) Capsule thickness increases with time in all positions except for the corners (e.g., upper and lower capsule thicknesses increased 200% in the first 10 months and 20% per month thereafter, and lateral portion increased ~200% between months 1 and 20. Based on this data, it can be expected that sensing elements facing outward toward the skin versus inward will experience differential FBR. Furthermore, the plot clearly shows the time-dependent nature of the foreign body capsule.
In a different study, similarly shaped, pHEMA hydrogels (⋆) were implanted for four weeks in mice. (C) The implant region is visualized with picrosirius red stain under a light microscope. (D) Collagen density is estimated using circularly polarized light analysis of picrosirius red stained sections (4x magnification). Collagen density appears highest (brightest) in the regions of highest interfacial stress, including the corners (white arrow denotes high collagen density at one corner). Figures A and B adapted from Li and colleagues.106 Figures C and D are unpublished data courtesy of Eric Sussman, University of Washington.
Size
The smaller the sensor, the less tissue disruption created by the implantation procedure and by the continued presence of the sensor. Kvist and colleagues107 demonstrated that using smaller insertion needles (compared 14, 18, and 21 gauge) significantly reduced insertion wound trauma (measured hemorrhage, inflammatory cells, and giant cells on day 3). In addition to reducing the short-term inflammatory response to the insertion wound, it is likely that miniaturization of sensors through advancements in nanotechnology and microelectromechanical systems will improve the long-term FBR.108 In fact, it has already been shown that small-diameter implants exhibit less FBR and in some cases, surprisingly, appear to elude the host response all together (e.g., fibers <6 μm).109 Ward and colleagues60 demonstrated that implants of varying thickness elicit different responses and that thinner implants exhibit thinner capsules. However, the orientation of the implant is critical and the thinnest dimension should be placed parallel to the surface of the skin to minimize extracellular matrix distortion in the vertical direction.110 Sanders and colleagues110 postulate that implant height (dimension perpendicular to the skin) is important because thicker implants substantially separate adjacent collagen fibers and create low-pressure void regions that require filling with new matrix. The presence of capsule was shown to be less dependent on the length of the implant (dimension parallel to skin), than implant height (dimension perpendicular to skin). The authors explain this observation is likely due to collagen fibers orienting principally in planes parallel with the skin surface, so there is less collagen disruption with sensor displacement parallel to the skin.110
Implant size was the likely contributor to the vastly different FBR observed around implants with the same microtopography and surface chemistry but very different thicknesses.59,95,103,111 Campbell and von Recum found little fibrosis surrounding paper-thin, micro-textured implants of polyvinyl chloride/polyacrylonitride (PVC/PAN) and silicone-coated PVC/PAN. The authors concluded that a defined surface topography of 1 to 2 microns allowed direct fibroblast attachment and minimized connective tissue response. However, the paper-thin dimension was likely an underappreciated contributor to the reduced fibrosis they observed because the results were not replicated. In a similar study by den Braber111 using thicker (1.45–2 mm) implants of the same material, there were no significant differences in capsule thickness for smooth and microgrooved silicone implants.
Another important aspect of sensor size is the settling period or time it takes for the sensor signal to stabilize after implantation. SubQ sensors that are small enough to be injected via syringe and reduce the wound created by surgical implantation are expected to settle more quickly. Available commercial PerQ sensors (approximately 200–500 μm in diameter) have a 2-hour wait time before sensor use, presumably to allow the immediate tissue response to settle (and also possibly for sensor wetting). Experimentally injected thin percutaneous fibers (100 μm) have been shown to have settling periods of only 3–6 minutes under anesthetized conditions.112 Movement or normal activities would likely extend the settling time.
Material Modulus
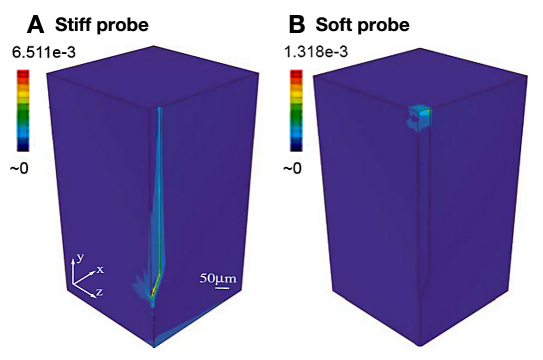
Another sensor design challenge is the mismatch in mechanical properties of the device and skin, as this incongruity results in stress concentrations at the inter-face.42,88,94,113,114 Sanders and colleagues113 demonstrated that polyurethane implants, which had moduli two to three orders of magnitude lower than other plastics tested [polyester, polyethylene, poly(L-lactic acid)], exhibited significantly less foreign body capsule as compared to the more rigid materials. Similarly, a computational model of a brain electrode corroborated stress concentrations at the interface arising from modulus mismatch. Subbaroyan and colleagues114 demonstrated significant differences in strain profiles and the shift in the high strain location for electrodes of different stiffness (Figure 5). Furthermore, the pressure exerted by the stiff electrode on surrounding tissue results in compression, expansion, and possibly tearing of the tissue,114 and there exists a direct relationship between the electrode modulus and interfacial strain, which is hypothesized to contribute to the inflammatory response.115 Therefore, to the extent that sensor electrode properties can be manipulated, the use of softer materials with material properties similar to the implantation tissue could reduce interfacial strain and improve sensor biocompatibility.
Figure 5.
Finite element models demonstrate force transmission to the surrounding tissue depends on sensor modules. The strain profiles in the brain tissue resulting from a lateral displacement of 1 μm simulating a tangential tethering force on the electrode that would arise from rotational acceleration of an animal's head are plotted. The models demonstrate significant differences in strain profile in the brain tissues generated by a (A) stiff electrode (elastic modulus, E = 200 GPa) and (B) a hypothetical soft electrode (E = 6 MPa). The maximum principal strain for the stiff electrode is approximately five times greater than the maximum principal strain for the soft electrode (maximum values on color map scale at the top left of each simulation). Importantly, the region of high strain shifts from the stiff electrode tip (A) to the surface of the brain tissue (B). The modulus of brain tissue is approximately 6 kPa (still 1,000 times lower than the hypothetical soft electrode material. These data demonstrate the significant shift in strain magnitude and location for electrodes of different material properties in a soft tissue. Figure adapted from Subbaroyan and colleagues.114
Subject Considerations
Animal Model
The appropriate animal model for sensor and biomaterial implantation studies is a subject of some controversy. There is an expansive body of work performed in the rat (some examples in Part II, Table 1),82 despite the fact that rodent skin has several anatomical and physiological differences from human skin. Even comparisons of the FBR among rodent models have yielded different results.116 Commonly studied loose-skinned small mammals have dense fur, relatively thin epidermal and dermal layers, and a subepithelial layer with bundles of skeletal muscle fibers117 that allow their hair to stand up on end. Profound differences between rats and humans in the FBR and flux of various metabolites in the interstitial fluid at the sensor–tissue interface have been demonstrated over 8 days of SubQ implantation.118,119
To relate the choice of animal models with physiological differences to biomechanical forces acting on the sensor–tissue interface, we consider several factors. First, the SubQ muscle layer that exists in furry animals will be a source of repetitive micromotion. Movement of the loose skin will generate greater shear forces on an implanted sensor compared to a tight skinned animal (e.g., pig, human), which may experience more compressive loading because of the tightness of their skin. Loose-skinned animals may also be more prone to implant migration. Grooming in animals with fur will likely produce more repetitive motion than pigs or humans. For these reasons, the micro- and macromotions will likely be greater in rodents and other haired animals (e.g., dogs). Undoubtedly, a comparative animal study is needed to elucidate FBR differences to implanted sensors over time in the SubQ tissue of various animals and compare these to human data to establish the most appropriate animal model.
The use of rodents is justified in many studies because of their low cost, easy handling, and approximation of human conditions such as diabetes and obesity by genetic modification.119 One caveat to employing genetic modifications is that transgene expression has been demonstrated to change the tissue properties and composition of skin.120 Nevertheless, Klueh and Kreutzer86 have developed a mouse model, which has been validated to produce CGM sensor signals that approximate those observed in humans, though direct FBR comparison was not conducted. This CGM mouse model has enabled exploration into the role of mast cells8 and cytokines121 through the use of knock-out mice, and this research would have been nearly impossible to achieve with more advanced animal models.
A common advanced sensor-testing model is porcine skin, which is structurally similar to human skin, having similar epidermal thickness (30–140 μm in pigs; 50–120 μm in humans) and ratios of dermis to epidermis thickness.122–124 Human dermis thickness ranges from 1 mm on the face to 4 mm on the back125,126 while pig dermis thickness can be up to twice as thick in some regions (6 mm),127 but the dermis of young, 14–15 week-old pigs is thinner (~2 mm).128 Pig skin has been reported to be similar to that of humans in the majority of anatomical and physiological aspects, including paucity of hair, skin thickness, pigmentation, and collagenous tissue frame-work,122,129 and exhibit similar patterns of blood vessels.117 Important to the study of biomechanics in tissue, is the fact that porcine skin contains dermal collagen and elastic content that is more similar to humans than other commonly used mammals.130 Furthermore, experience of some researchers suggest that the collagenous capsule that forms around implants is of similar thickness in pigs and humans (personal correspondence with Dr. Andrew Marshall). Because of their large size, multiple sensors can be tested in a single pig and the implant-to-subject size ratio is comparable to that of human subjects. While porcine skin is compositionally and structurally similar to humans,122 the skin of young pigs is mechanically most similar to humans, making it the preferred model in plastic surgery research.131,132 Beyond biomechanical considerations, which support the porcine model, a direct comparison of CGM performance and FBR in humans and pigs as well as other animals would be helpful toward instilling confidence in the selection of animal models for sensor studies.
Tissue of Implantation
Skin is a complex, viscoelastic tissue composed of three layers: epidermis, dermis, and SubQ tissue, also sometimes referred to as the hypodermis. The mechanical properties of skin are dynamic and vary with age and skin region.133 While skin is often treated as a homogeneous composite, the different layers of skin have distinct mechanical properties.134 Holt and colleagues88 demonstrated that whole skin exhibited rigidly elastic behavior as compared to the more viscous, fluid-like behavior of skin with the epidermis removed. A wide range of elastic modulus has been reported for the various skin layers, including the outer most layer of the epidermis, the stratum corneum (5–1000 MPa with decreasing hydration),135 dermis (56–260 kPa),133,136,137 and SubQ tissue (0.12–23 kPa).136,138 Variation in modulus data arises from differing test methods (e.g., tensile, torsion, suction, indention), tissue sampling (in vivo versus in vitro, sample preparation, and storage), hydration, and the applied testing loads and strains. Also, depending on the tissue of implantation, the composition140 and thickness123,133,140–142 will vary.
As discussed earlier in the Material Modulus section, one strategy for reducing biomechanical forces is to design sensors to more closely match the modulus of the tissues in which they are implanted. This is an extraordinarily difficult materials challenge for PerQ sensors, because they will typically come into contact with all three layers of skin whose moduli can vary by four to five orders of magnitude. Fully implantable sensors are being developed for implantation into specific layers of the skin. For example, Clark and colleagues143 are developing glucose-sensitive nanoparticles for injection into the epidermis, while McShane and Coté144,145 are designing microparticles or smart tattoos for dermal injection. Other groups, such as Gough and colleagues51 and Papadimitrakopoulos and Burgess108 are designing sensors for the SubQ space. The biomechanics of the sensor–tissue interface in each of these compartments will vary greatly. To minimize biomechanical forces due to modulus mismatch, sensors intended for SubQ implantation need to be much softer than those injected into the stiffer epidermis layers.88,146 Even without a perfect modulus match, implantation studies in adipose-rich tissues have exhibited a more benign FBR,58,147 likely due to stress absorptive properties of fat. Of course, size constraints of the desire tissue compartment are important, that is to say, it would be difficult to place a 3-mm-thick sensor in a 1-mm-thick dermal layer. One must also consider the chemical and physiological properties of the tissue compartment.
Site of Implantation
The exact site of implantation for a given subject is not often carefully controlled and it is common to place multiple implants in a single subject during experimentation. Site-to-site variation with CGM has not been well characterized but has been reported.148 In many experimental cases, biomaterial implants are rotated among the different locations in order to obfuscate site variations.94,111 However, presented here is data that highlight the importance of the exact implant location within the SubQ compartment and demonstrate that varying sites (e.g., SubQ on the back in the scapular region versus SubQ on the back in the middorsal region) can confound experimental outcomes and should be carefully controlled. From the authors' own experience, even the orientation of planar SubQ sensor electrodes (e.g., facing up toward the skin or down toward the muscle) can impact sensor performance (unpublished data). This may be expected based on the FBR data presented in Figure 4 exhibiting a thicker capsule on skin-facing surface of the implant. We believe that some of these site-to-site differences may be due to differential biomechanical forces.
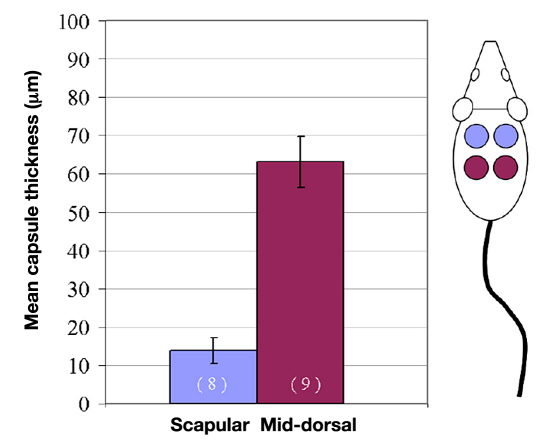
The data in Figure 6 were collected from a study of porous, poly(2-hydroxyethyl methacrylate) (pHEMA) implanted into the SubQ tissue of mice.149 Figure 6 shows mean capsule thickness as a function of implantation site, scapular or middorsal. There was a significant difference in capsule thickness for the two implantation sites.149 The large variation in capsule thickness between implantation sites was unanticipated and is presumed due to the different forces incurred at the scapular and middorsal sites. A thorough discussion of the variance requires a comprehensive evaluation of the site differences, including the measurement of forces (e.g., strain rates during ambulation, compressive forces of skin on the implant, and incidence of scratching at both sites) as well as physiologic variations in the SubQ tissue. The composition of the skin may also vary significantly with position.58 The differences between sites underscore the importance of choosing and controlling a sensor implantation location.
Figure 6.
Implantation site is a primary determinant of capsule thickness surrounding implants placed in the same tissue compartment (SubQ). Four porous pHEMA disks (3.5 mm diameter and 1 mm thick) were implanted subcutaneously into the dorsum through one of two midline incisions. The implants were explanted after 4 weeks and stained with Masson's trichrome, a marker to visualize dense, oriented collagen capsule tissue. The mean capsule thickness is plotted as a function of implant site (scapular and middorsal). Even through all materials were implanted subcutaneously, the exact site of implantation within the subcutis significantly influenced the capsule thickness (error bars represent standard error of the mean). Differences in biomechanical forces at the two different locations are suspected to play a role in the differential capsule thickness. These data highlight the importance of choosing and controlling sensor implantation location in in vivo studies. Figure adapted from Marshall.149
Picha and colleagues58 also observed site-to-site variation in tissue response (Figure 3). Smooth and micropillared silicone disks were rotated among six SubQ sites in a rat model (Figure 3A). Although the tissue response to the micropillared implants was more benign than to smooth implants, the response was found to be highly site-dependent (Figure 3B). The authors hypothesized that in areas of higher fatty content, the compliant, stress-absorbing fat reduced interfacial shear and led to a more benign FBR.58 Site-dependent variation in vascularity around the implants was also observed. Importantly, the size and density of vasculature, for example, one capillary per fat cell in adipose tissue,150 creates a very different glucose supply compared to the few large scattered macrovessels (Figure 3D compared to 3E).
Anesthetics
Anesthetics, including isoflurane and halothane, have been reported to increase blood glucose concentrations, while pentobarbital was found to slightly decrease blood glucose levels.151,152 In addition to chemical effects, there appear to be biomechanical differences between conscious and anesthetized animals due to reduced motion. Ward and colleagues152 noted that fluctuations in sensor signals were markedly reduced in the anesthetized state as compared to the conscious state. While fluctuations in the conscious state could be due to electrochemical inter-ferants, they are most likely caused by local fluctuation of glucose, oxygen, or microvascular blood flow.152 This finding highlights the need for a better understanding of the impact of anesthetics on skin physiology. Sensor developers should be aware that testing in anesthetized animals may not reflect physiological conditions of conscious animals, including motion artifacts.
Acute versus Chronic Subject Motion
Motion artifacts can also arise when acute forces temporarily impact sensor signal. It is hypothesized that applying direct pressure on the sensor and surrounding tissue can cause a temporary reduction of localized blood flow, thus perturbing sensor readings. This acute effect is often short-lived and sensor signal usually resolves once pressure is removed. As we describe in Part II of this review, a common cause of acute motion artifacts is the subject lying or sleeping on the sensor. Chronic motion, however, affects the FBR to the sensor and can alter surrounding tissue. In this case, chronic motion often causes gradual signal loss and may require sensor recalibration.
Conclusions
While there are many important factors influencing sensor biocompatibility and affecting performance, we underscore the importance of device–tissue biomechanics. Motion (including micromotion) and pressure, as we have described, can affect the sequelae of the FBR and sensor performance. The contributors to motion and pressure include applied forces (normal, shear, contractile), sensor design (PerQ/SubQ, size, shape, surface topology, modulus), and various subject considerations (species, tissue compartment, exact site, anesthesia). The exact site of sensor implantation can greatly affect the FBR, and we emphasize the importance of controlling site variation in sensor studies. Of course, the biomechanics are just one of many elements that need to be considered in creating a sensor that will function in the biological environment. It will also be critical to perform comparative animal model studies to ensure that the tissue response and sensor performance are representative of human FBR and sensor data. Finite element models could provide a systematic way to evaluate the biomechanics of different sensor designs and aid in minimizing tissue–sensor interfacial stresses to improve FBR and sensor performance. The biomechanics (motion and pressure) of the device–tissue interface influence not only sensor performance but also have implications in the design and development of other long-term implantable devices in soft tissues.
Acknowledgments
The authors wish to acknowledge Dr. Andrew Marshall for discussions on biomaterial biocompatibility and Eric Sussman for histology images.
Abbreviations
- (CGM)
continuous glucose monitoring
- (FBR)
foreign body response
- (PerQ)
percutaneous
- (pHEMA)
poly(2-hydroxyethyl methacrylate)
- (PVC/PAN)
polyvinyl chloride/polyacrylonitride
- (SubQ)
subcutaneous
Funding:
University of Washington Center for Commercialization Fellowship.
Disclosures:
Kristen Helton and Natalie Wisniewski are founders of PROFUSA, Inc. Dr. Wisniewski is affiliated with Becton Dickenson; Alfred Mann Foundation; Arkal Medical; University of Washington; Texas A&M University; Duke University; California State University Long Beach; Stanford University; Karolinska Hospital; American Diabetes Association; and Diabetes Technology Society.
References:
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Peoples T, Perkins BA, Welsh JB, Willi SM, Wood MA. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 3.Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 4.Ju YM, Yu B, West L, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. II. Long-term in vitro/in vivo sensitivity characteristics of sensors with NDGA- or GA-crosslinked collagen scaffolds. J Biomed Mater Res A. 2010;92(2):650–658. doi: 10.1002/jbm.a.32400. [DOI] [PubMed] [Google Scholar]
- 5.Shaw GW, Claremont DJ, Pickup JC. In vitro testing of a simply constructed, highly stable glucose sensor suitable for implantation in diabetic patients. Biosens Bioelectron. 1991;6(5):401–406. doi: 10.1016/0956-5663(91)87004-u. [DOI] [PubMed] [Google Scholar]
- 6.Hoss U, Jeddi I, Schulz M, Budiman E, Bhowgal C, McGarraugh G. Continuous glucose monitoring in subcutaneous tissue using factory-calibrated sensors: a pilot study. Diabetes Technol Ther. 2010;12(8):591–597. doi: 10.1089/dia.2010.0051. [DOI] [PubMed] [Google Scholar]
- 7.Clark LC, Jr, Spokane RB, Homan MM, Sudan R, Miller M. Long-term stability of electroenzymatic glucose sensors implanted in mice. An update. ASAIO Trans. 1988;34(3):259–265. [PubMed] [Google Scholar]
- 8.Klueh U, Kaur M, Qiao Y, Kreutzer DL. Critical role of tissue mast cells in controlling long-term glucose sensor function in vivo. Biomaterials. 2010;31(16):4540–4551. doi: 10.1016/j.biomaterials.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebrin K, Fischer U, Vondorsche HH, von Woetke T, Abel P, Brunstein E. Subcutaneous glucose monitoring by means of electro-chemical sensors: fiction or reality? J Biomed Eng. 1992;14(1):33–40. doi: 10.1016/0141-5425(92)90033-h. [DOI] [PubMed] [Google Scholar]
- 10.Wisniewski N, Moussy F, Reichert WM. Characterization of implantable biosensor membrane biofouling [review] Fresenius J Anal Chem. 2000;366(6-7):611–621. doi: 10.1007/s002160051556. [DOI] [PubMed] [Google Scholar]
- 11.Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J Diabetes Sci Technol. 2008;2(6):1003–1015. doi: 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reichert W, Ratner BD, Anderson J, Coury A, Hoffman AS, Laurencin CT, Tirrell D. 2010 Panel on the biomaterials grand challenges. J Biomed Mater Res A. 2011;96(2):275–287. doi: 10.1002/jbm.a.32969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisniewski N, Reichert M. Methods for reducing biosensor membrane biofouling [review] Colloid Surf B Biointerfaces. 2000;18(3-4):197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 14.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29(12):1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 15.Novak MT, Yuan F, Reichert WM. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem. 2010;398(4):1695–1705. doi: 10.1007/s00216-010-4097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants I. Diffusion properties. J Biomed Mater Res. 1997;37(3):401–412. doi: 10.1002/(sici)1097-4636(19971205)37:3<401::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma-tissue exchange properties. J Biomed Mater Res. 1998;40(4):586–597. doi: 10.1002/(sici)1097-4636(19980615)40:4<586::aid-jbm10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J Biomed Mater Res. 1998;40(4):598–605. doi: 10.1002/(sici)1097-4636(19980615)40:4<598::aid-jbm11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Koschwanez HE, Reichert WM, Klitzman B. Intravital microscopy evaluation of angiogenesis and its effects on glucose sensor performance. J Biomed Mater Res A. 2010;93(4):1348–1357. doi: 10.1002/jbm.a.32630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koschwanez HE, Yap FY, Klitzman B, Reichert WM. In vitro and in vivo characterization of porous poly-L-lactic acid coatings for subcutaneously implanted glucose sensors. J Biomed Mater Res A. 2008;87(3):792–807. doi: 10.1002/jbm.a.31824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridges AW, Garcia AJ. Anti-inflammatory polymeric coatings for implantable biomaterials and devices. J Diabetes Sci Technol. 2008;2(6):984–994. doi: 10.1177/193229680800200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin JH, Marxer SM, Schoenfisch MH. Nitric oxide-releasing sol-gel particle/polyurethane glucose biosensors. Anal Chem. 2004;76(15):4543–4549. doi: 10.1021/ac049776z. [DOI] [PubMed] [Google Scholar]
- 23.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81(4):858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117(1):68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002;61(2):180–187. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 26.Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23(7):1649–1656. doi: 10.1016/s0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 27.Galeska I, Kim TK, Patil SD, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess DJ. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005;7(1):E231–E240. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. Int J Pharm. 2010;384(1-2):78–86. doi: 10.1016/j.ijpharm.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 29.Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/pva hydrogel composites for implantable devices. J Diabetes Sci Technol. 2007;1(1):8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward WK, Quinn MJ, Wood MD, Tiekotter KL, Pidikiti S, Gallagher JA. Vascularizing the tissue surrounding a model biosensor: how localized is the effect of a subcutaneous infusion of vascular endothelial growth factor (VEGF)? Biosens Bioelectron. 2003;19(3):155–163. doi: 10.1016/s0956-5663(03)00180-5. [DOI] [PubMed] [Google Scholar]
- 31.Ju YM, Yu BZ, West L, Moussy Y, Moussy F. A dexamethasone-loaded PLGA micirospheres/collagen scaffold composite for implantable glucose sensors. J Biomed Mater Res A. 2010;93(1):200–210. doi: 10.1002/jbm.a.32512. [DOI] [PubMed] [Google Scholar]
- 32.Ward WK, Troupe JE. Assessment of chronically implanted subcutaneous glucose sensors in dogs: the effect of surrounding fluid masses. ASAIO J. 1999;45(6):555–561. doi: 10.1097/00002480-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response [review] AAPS J. 2010;12(2):188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung JY, Barone PW, Kong HJ, Strano MS. Sequential delivery of dexamethasone and VEGF to control local tissue response for carbon nanotube fluorescence based micro-capillary implantable sensors. Biomaterials. 2009;30(4):622–631. doi: 10.1016/j.biomaterials.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 35.Klueh U, Kaur M, Montrose DC, Kreutzer DL. Inflammation and glucose sensors: use of dexamethasone to extend glucose sensor function and life span in vivo. J Diabetes Sci Technol. 2007;1(4):496–504. doi: 10.1177/193229680700100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayant RD, McShane MJ, Srivastava R. In vitro and in vivo evaluation of anti-inflammatory agents using nanoengineered alginate carriers: towards localized implant inflammation suppression. Int J Pharm. 2011;403(1-2):268–275. doi: 10.1016/j.ijpharm.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 37.Ward WK, Hansen JC, Massoud RG, Engle JM, Takeno MM, Hauch KD. Controlled release of dexamethasone from subcutaneously-implanted biosensors in pigs: localized anti-inflammatory benefit without systemic effects. J Biomed Mater Res A. 2010;94(1):280–287. doi: 10.1002/jbm.a.32651. [DOI] [PubMed] [Google Scholar]
- 38.Ward WK, Wood MD, Casey HM, Quinn MJ, Federiuk IF. The effect of local subcutaneous delivery of vascular endothelial growth factor on the function of a chronically implanted amperometric glucose sensor. Diabetes Technol Ther. 2004;6(2):137–145. doi: 10.1089/152091504773731320. [DOI] [PubMed] [Google Scholar]
- 39.Bridges AW. Anti-inflammatory polymeric coatings for implantable biomaterials and devices. J Diabetes Sci Technol. 2008;2(6):984–994. doi: 10.1177/193229680800200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klueh U, Dorsky DI, Kreutzer DL. Enhancement of implantable glucose sensor function in vivo using gene transfer-induced neovascularization. Biomaterials. 2005;26(10):1155–1163. doi: 10.1016/j.biomaterials.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Prichard HL, Schroeder T, Reichert WM, Klitzman B. Bioluminescence imaging of glucose in tissue surrounding polyurethane and glucose sensor implants. J Diabetes Sci Technol. 2010;4(5):1055–1062. doi: 10.1177/193229681000400504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilborn J, Bjursten LM. A new and evolving paradigm for biocompatibitity. J Tissue Eng Regen Med. 2007;1(2):110–119. doi: 10.1002/term.4. [DOI] [PubMed] [Google Scholar]
- 43.Gilligan BC, Shults M, Rhodes RK, Jacobs PG, Brauker JH, Pintar TJ, Updike SJ. Feasibility of continuous long-term glucose monitoring from a subcutaneous glucose sensor in humans. Diabetes Technol Ther. 2004;6(3):378–386. doi: 10.1089/152091504774198089. [DOI] [PubMed] [Google Scholar]
- 44.Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care. 1994;17(8):882–887. doi: 10.2337/diacare.17.8.882. [DOI] [PubMed] [Google Scholar]
- 45.Updike SJ, Shults MC, Gilligan BJ, Rhodes RK. A subcutaneous glucose sensor with improved longevity, dynamic range, and stability of calibration. Diabetes Care. 2000;23(2):208–214. doi: 10.2337/diacare.23.2.208. [DOI] [PubMed] [Google Scholar]
- 46.Updike SJ, Shults MC, Rhodes RK, Gilligan BJ, Luebow JO, von Heimburg D. Enzymatic glucose sensors. Improved long-term performance in vitro and in vivo. ASAIO J. 1994;40(2):157–163. [PubMed] [Google Scholar]
- 47.Garg SK, Schwartz S, Edelman SV. Improved glucose excursions using an implantable real-time continuous glucose sensor in adults with type 1 diabetes. Diabetes Care. 2004;27(3):734–738. doi: 10.2337/diacare.27.3.734. [DOI] [PubMed] [Google Scholar]
- 48. Guiseppi-Elie. Recent developments in bio-smart and responsive materials for implantable biosensors. 2007 [cited 2011 January]. Available from: http://www.tatrc.org/include/conferences/website_irt_nano_07/presentations/1_07_Guiseppi.pdf.
- 49. Bioarsis. [cited 2011 January]. Available from: http://www.bio-orasis.com/
- 50.Updike SJ, Shults MC, Gilligan BJ, Rhodes RK. A subcutaneous glucose sensor with improved longevity, dynamic range, and stability of calibration. Diabetes Care. 2000;23(2):208–214. doi: 10.2337/diacare.23.2.208. [DOI] [PubMed] [Google Scholar]
- 51.Gough DA, Kumosa LS, Routh TL, Lin JT, Lucisano JY. Function of an implanted tissue glucose sensor for more than 1 year in animals. Sci Transl Med. 2010;2(42):42ra53. doi: 10.1126/scitranslmed.3001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson JM. Biological responses to materials [review] Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 53.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 54.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials [review] Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morhenn VB, Lemperle G, Gallo RL. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol Surg. 2002;28(6):484–490. doi: 10.1046/j.1524-4725.2002.01273.x. [DOI] [PubMed] [Google Scholar]
- 56.Hori BD, Petrell RJ, Trites AW, Godbey T. Lamination for subdermal implant fixation. J Biomed Mater Res B Appl Biomater. 2009;91(1):17–25. doi: 10.1002/jbm.b.31369. [DOI] [PubMed] [Google Scholar]
- 57.Johansson F, Wallman L, Danielsen N, Schouenborg J, Kanje M. Porous silicon as a potential electrode material in a nerve repair setting: tissue reactions. Acta Biomater. 2009;5(6):2230–2237. doi: 10.1016/j.actbio.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Picha GJ, Drake RF. Pillared-surface microstructure and soft-tissue implants: effect of implant site and fixation. J Biomed Mater Res. 1996;30(3):305–312. doi: 10.1002/(SICI)1097-4636(199603)30:3<305::AID-JBM5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 59.Von Recum A. New aspects of biocompatibility: motion at the interface. In: Lee AJC, Heimke B, Soltesz U, editors. Clinical implant materials: proceedings of the Eighth European Conference on Biomaterials; 1989 September 7-9; Heidelberg, Germany. Amsterdam; New York: Elsevier Science Pub. Co.; 1990. [Google Scholar]
- 60.Ward WK, Slobodzian EP, Tiekotter KL, Wood MD. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials. 2002;23(21):4185–4192. doi: 10.1016/s0142-9612(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 61.Gangjee T, Colaizzo R, von Recum A. Species-related differences in percutaneous wound healing. Ann Biomed Eng. 1985;13(5):451–467. doi: 10.1007/BF02407772. [DOI] [PubMed] [Google Scholar]
- 62.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate [review] Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 63.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli [review] Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 64.Patel AA, Thakar RG, Chown M, Ayala P, Desai TA, Kumar S. Biophysical mechanisms of single-cell interactions with microtopo-graphical cues. Biomed Microdevices. 2010;12(2):287–296. doi: 10.1007/s10544-009-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thakar RG, Chown MG, Patel A, Peng L, Kumar S, Desai TA. Contractility-dependent modulation of cell proliferation and adhesion by microscale topographical cues. Small. 2008;4(9):1416–1424. doi: 10.1002/smll.200701302. [DOI] [PubMed] [Google Scholar]
- 66.Ayala P, Lopez JI, Desai TA. Microtopographical cues in 3D attenuate fibrotic phenotype and extracellular matrix deposition: implications for tissue regeneration. Tissue Eng Part A. 2010;16(8):2519–2527. doi: 10.1089/ten.tea.2009.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang MT, Sniadecki NJ, Chen CS. Geometric considerations of micro- to nanoscale elastomeric post arrays to study cellular traction forces. Adv Mater. 2007;19(20):3119–3123. [Google Scholar]
- 68.Huiskes HWJ, Weinans H, van Rietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop. 1992;274:124–134. [PubMed] [Google Scholar]
- 69.Stopak D, Harris AK. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol. 1982;90(2):383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- 70.Lambert CA, Soudant EP, Nusgens BV, Lapiere CM. Pretranslational regulation of extracellular matrix macromolecules and collagenase expression in fibroblasts by mechanical forces. Lab Invest. 1992;66(4):444–451. [PubMed] [Google Scholar]
- 71.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolodney MS, Wysolmerski RB. Tissue reaction to soft-tissue anchored percutaneous implants in rabbits. J Cell Biol. 1992;117(1):73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Gimbrone MA. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A. 1986;83(7):2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ookawa K, Sato M, Ohshima N. Changes in the microstructure of cultured porcine aortic endothelial cells in the early stage after applying a fluid-imposed shear stress. J Biomech. 1992;25(11):1321–1328. doi: 10.1016/0021-9290(92)90287-b. [DOI] [PubMed] [Google Scholar]
- 75.Ando J, Nomura H, Kamiya A. The effect of fluid shear stress on the migration and proliferation of cultured endothelial cells. Microvasc Res. 1987;33(1):62–70. doi: 10.1016/0026-2862(87)90007-0. [DOI] [PubMed] [Google Scholar]
- 76.Worthen GS, Smedly LA, Tonnesen MG, Ellis D, Voelkel NF, Reeves JT, Henson PM. Effects of shear stress on adhesive interaction between neutrophils and cultured endothelial cells. J Appl Physiol. 1987;63(5):2031–2041. doi: 10.1152/jappl.1987.63.5.2031. [DOI] [PubMed] [Google Scholar]
- 77.Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts [review] Biochim Biophys Acta-Mol Cell Res. 2009;1793(5):911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 78.Bott K, Upton Z, Schrobback K, Ehrbar M, Hubbell JA, Lutolf MP, Rizzi SC. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials. 2010;31(32):8454–8464. doi: 10.1016/j.biomaterials.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 79.Buxboim A, Rajagopal K, Brown AEX, Discher DE. How deeply cells feel: methods for thin gels. J Phys Condes Matter. 2010;22(19):194116. doi: 10.1088/0953-8984/22/19/194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ingber DE. Tensegrity: The architectural basis of cellular mechanotransduction [review] Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 81.Ingber DE, Dike L, Hansen L, Karp S, Liley H, Maniotis A, McNamee H, Mooney D, Plopper G, Sims J, Wang N. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int Rev Cytol. 1994;150:173–224. doi: 10.1016/s0074-7696(08)61542-9. [DOI] [PubMed] [Google Scholar]
- 82.Helton KL, Ratner BD, Wisniewski N. Biomechanics of the sensor-tissue interface'effects of motion, pressure and design on sensor performance and the foreign body response'part II: examples and application. J Diabetes Sci Technol. 2011;5(3):647–656. doi: 10.1177/193229681100500318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanders JE, Goldstein BS, Leotta DF. Skin response to mechanical stress: adaptation rather than breakdown–a review of the literature [review] J Rehabil Res Dev. 1995;32(3):214–226. [PubMed] [Google Scholar]
- 84.Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61(1):29–35. doi: 10.1016/s0306-9877(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 85.Gilletti A, Muthuswamy J. Brain micromotion around implants in the rodent somatosensory cortex. J Neural Eng. 2006;3(3):189–195. doi: 10.1088/1741-2560/3/3/001. [DOI] [PubMed] [Google Scholar]
- 86.Klueh U, Liu ZH, Cho B, Ouyang TM, Feldman B, Henning TP, Kaur M, Kreutzer D. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther. 2006;8(3):402–412. doi: 10.1089/dia.2006.8.402. [DOI] [PubMed] [Google Scholar]
- 87.Geerligs M, Peters GW, Ackermans PA, Oomens CW, Baaijens FP. Does subcutaneous adipose tissue behave as an (anti-)thixotropic material? J Biomech. 2010;43(6):1153–1159. doi: 10.1016/j.jbiomech.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 88.Holt B, Tripathi A, Morgan J. Viscoelastic response of human skin to low magnitude physiologically relevant shear. J Biomech. 2008;41(12):2689–2695. doi: 10.1016/j.jbiomech.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Von Recum AF. Applications and failure modes of percutaneous devices: a review. J Biomed Mater Res. 1984;18(4):323–336. doi: 10.1002/jbm.820180403. [DOI] [PubMed] [Google Scholar]
- 90.Rosengren A, Danielsen N, Bjursten LM. Reactive capsule formation around soft-tissue implants is related to cell necrosis. J Biomed Mater Res. 1999;46(4):458–464. doi: 10.1002/(sici)1097-4636(19990915)46:4<458::aid-jbm3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 91.Kvist PH, Bielecki M, Gerstenberg M, Rossmeisl C, Jensen HE, Rolin B, Hasselager E. Evaluation of subcutaneously-implanted glucose sensors for continuous glucose measurements in hyper-glycemic pigs. In vivo. 2006;20(2):195–203. [PubMed] [Google Scholar]
- 92.Klueh U, Liu Z, Ouyang T, Cho B, Feldman B, Henning TP, Kreutzer D. Blood-induced interference of glucose sensor function in vitro: implications for in vivo sensor function. J Diabetes Sci Technol. 2007;1(6):842–849. doi: 10.1177/193229680700100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Delvoye P, Wiliquet P, Leveque JL, Nusgens BV, Lapiere CM. Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J Invest Dermatol. 1991;97(5):898–902. doi: 10.1111/1523-1747.ep12491651. [DOI] [PubMed] [Google Scholar]
- 94.Walboomers XF, Croes HJE, Ginsel LA, Jansen JA. Microgrooved subcutaneous implants in the goat. J Biomed Mater Res. 1998;42(4):634–641. doi: 10.1002/(sici)1097-4636(19981215)42:4<634::aid-jbm21>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 95.Adams WP., Jr Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg. 2009;36(1):119–126. doi: 10.1016/j.cps.2008.08.007. vii. [DOI] [PubMed] [Google Scholar]
- 96.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 97.Kvist PH, Iburg T, Dawson HD, Jensen HE. Effect of subcutaneous glucose sensor implantation on skin mRNA expression in pigs. Diabetes Technol Ther. 2010;12(10):791–799. doi: 10.1089/dia.2010.0041. [DOI] [PubMed] [Google Scholar]
- 98.Jansen JA, Paquay YG, van der Waerden JP. Tissue reaction to soft-tissue anchored percutaneous implants in rabbits. J Biomed Mater Res. 1994;28(9):1047–1054. doi: 10.1002/jbm.820280909. [DOI] [PubMed] [Google Scholar]
- 99.Paquay Y, de Ruijter JE, van der Waerden J, Jansen JA. Titanium fiber mesh anchorage for percutaneous devices applicable for peritoneal dialysis. J Biomed Mater Res. 1994;28(11):1321–1328. doi: 10.1002/jbm.820281110. [DOI] [PubMed] [Google Scholar]
- 100.Von Recum AF, Park JB. Permanent percutaneous devices. Crit Rev Bioeng. 1981;5(1):37–77. [PubMed] [Google Scholar]
- 101.Gerritsen M. Problems associated with subcutaneously implanted glucose sensors [editorial] Diabetes Care. 2000;23(2):143–145. doi: 10.2337/diacare.23.2.143. [DOI] [PubMed] [Google Scholar]
- 102.Marshall AJ, Irvin CA, Barker T, Sage EH, Hauch KD, Ratner BD. Biomaterials with tightly controlled pore size that promote vascular in-growth. Polym Prepr. 2004;45(2):100–101. [Google Scholar]
- 103.Campbell CE, von Recum AF. Microtopography and soft tissue response. J Invest Surg. 1989;2(1):51–74. doi: 10.3109/08941938909016503. [DOI] [PubMed] [Google Scholar]
- 104.Dungel P, Long N, Yu B, Moussy Y, Moussy F. Study of the effects of tissue reactions on the function of implanted glucose sensors. J Biomed Mater Res A. 2008;85(3):699–706. doi: 10.1002/jbm.a.31593. [DOI] [PubMed] [Google Scholar]
- 105.Matlaga BF, Yasenchak LP, Salthouse TN. Tissue response to implanted polymers: the significance of sample shape. J Biomed Mater Res. 1976;10(3):391–397. doi: 10.1002/jbm.820100308. [DOI] [PubMed] [Google Scholar]
- 106.Li DJ, Ohsaki K, Ii K, Cui PC, Ye Q, Baba K, Wang QC, Tenshin S, Takano-Yamamoto T. Thickness of fibrous capsule after implantation of hydroxyapatite in subcutaneous tissue in rats. J Biomed Mater Res. 1999;45(4):322–326. doi: 10.1002/(sici)1097-4636(19990615)45:4<322::aid-jbm6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 107.Kvist PH, Iburg T, Aalbaek B, Gerstenberg M, Schoier C, Kaastrup P, Buch-Rasmussen T, Hassel ager E, Jensen HE. Biocompatibility of an enzyme-based, electrochemical glucose sensor for short-term implantation in the subcutis. Diabetes Technol Ther. 2006;8(5):546–559. doi: 10.1089/dia.2006.8.546. [DOI] [PubMed] [Google Scholar]
- 108.Vaddiraju S, Tomazos I, Burgess DJ, Jain FC, Papadimitrakopoulos F. Emerging synergy between nanotechnology and implantable biosensors: a review. Biosens Bioelectron. 2010;25(7):1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanders JE, Stiles CE, Hayes CL. Tissue response to single-polymer fibers of varying diameters: evaluation of fibrous encapsulation and macrophage density. J Biomed Mater Res. 2000;52(1):231–237. doi: 10.1002/1097-4636(200010)52:1<231::aid-jbm29>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 110.Sanders JE, Rochefort JR. Fibrous encapsulation of single polymer microfibers depends on their vertical dimension in subcutaneous tissue. J Biomed Mater Res A. 2003;67(4):1181–1187. doi: 10.1002/jbm.a.20027. [DOI] [PubMed] [Google Scholar]
- 111.Den Braber ET, de Ruijter JE, Jansen JA. The effect of a subcutaneous silicone rubber implant with shallow surface microgrooves on the surrounding tissues in rabbits. J Biomed Mater Res. 1997;37(4):539–547. doi: 10.1002/(sici)1097-4636(19971215)37:4<539::aid-jbm13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 112.Liao KC, Chang SC, Chiu CY, Chou YH. Acute response in vivo of a fiber-optic sensor for continuous glucose monitoring from canine studies on point accuracy. Sensors. 2010;10(8):7789–7802. doi: 10.3390/s100807789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanders JE, Bale SD, Neumann T. Tissue response to microfibers of different polymers: polyester, polyethylene, polylactic acid, and polyurethane. J Biomed Mater Res. 2002;62(2):222–227. doi: 10.1002/jbm.10285. [DOI] [PubMed] [Google Scholar]
- 114.Subbaroyan J, Martin DC, Kipke DR. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J Neural Eng. 2005;2(4):103–113. doi: 10.1088/1741-2560/2/4/006. [DOI] [PubMed] [Google Scholar]
- 115.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon micro-electrode arrays. Exp Neurol. 2005;195(1):115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 116.Kidd KR, Dal Ponte DB, Kellar RS, Williams SK. A comparative evaluation of the tissue responses associated with polymeric implants in the rat and mouse. J Biomed Mater Res. 2002;59(4):682–689. doi: 10.1002/jbm.10032. [DOI] [PubMed] [Google Scholar]
- 117.Perez R, Davis SC. Relevance of animal models for wound healing [review] Wounds. 2008;20(1):3–8. [PubMed] [Google Scholar]
- 118.Wisniewski N, Rajamand N, Adamsson U, Lins PE, Reichert WM, Klitzman B, Ungerstedt U. Analyte flux through chronically implanted subcutaneous polyamide membranes differs in humans and rats. Am J Physiol Endocrinol Metab. 2002;282(6):E1316–E1323. doi: 10.1152/ajpendo.00259.2001. [DOI] [PubMed] [Google Scholar]
- 119.Ekberg NR, Brismar K, Malmstedt J, Hedblad MA, Adamson U, Ungerstedt U, Wisniewski N. Analyte flux at a biomaterial-tissue interface over time: implications for sensors for type 1 and 2 diabetes mellitus. J Diabetes Sci Technol. 2010;4(5):1063–1072. doi: 10.1177/193229681000400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Serrat MA, Vinyard CI, King D. Alterations in the mechanical properties and composition of skin in human growth hormone transgenic mice. Connect Tissue Res. 2007;48(1):19–26. doi: 10.1080/03008200601021373. [DOI] [PubMed] [Google Scholar]
- 121.Klueh U, Liu Z, Feldman B, Kreutzer D. Importance of interleukin-1 and interleukin-1 receptor antagonist in short-term glucose sensor function in vivo. J Diabetes Sci Technol. 2010;4(5):1073–1086. doi: 10.1177/193229681000400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meyer W, Schwarz R, Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol. 1978;7:39–52. doi: 10.1159/000401274. [DOI] [PubMed] [Google Scholar]
- 123.Sandby-Moller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol. 2003;83(6):410–413. doi: 10.1080/00015550310015419. [DOI] [PubMed] [Google Scholar]
- 124.Morris GM, Hopewell JW. Epidermal cell kinetics of the pig: a review. Cell Tissue Kinet. 1990;23(4):271–282. doi: 10.1111/j.1365-2184.1990.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 125.Reihsner R, Balogh B, Menzel EJ. Two-dimensional elastic properties of human skin in terms of an incremental model at the in vivo configuration. Med Eng Phys. 1995;17(4):304–313. doi: 10.1016/1350-4533(95)90856-7. [DOI] [PubMed] [Google Scholar]
- 126.Tan CY, Statham B, Marks R, Payne PA. Skin thickness measure-ment by pulsed ultrasound: its reproducibility, validation and variability. Br J Dermatol. 1982;106(6):657–667. doi: 10.1111/j.1365-2133.1982.tb14702.x. [DOI] [PubMed] [Google Scholar]
- 127.Shergold OA, Fleck NA, Radford D. The uniaxial stress versus strain response of pig skin and silicone rubber at low and high strain rates. Int J Impact Eng. 2006;32(9):1384–1402. [Google Scholar]
- 128.Winter GD. Maibach HI, Rovee DT. Epidermal wound healing. Chicago: Year Book Medical Publishers; 1972. Epidermal regeneration studied in the domestic pig. [Google Scholar]
- 129.Kvist PH, Iburg T, Bielecki M, Gerstenberg M, Buch-Rasmussen T, Hasselager E, Jensen HE. Biocompatibility of electrochemical glucose sensors implanted in the subcutis of pigs. Diabetes Technol Ther. 2006;8(4):463–475. doi: 10.1089/dia.2006.8.463. [DOI] [PubMed] [Google Scholar]
- 130.Heinrich W, Lange PM, Stirtz T, Iancu C, Heidemann E. Isolation and characterization of the large cyanogen bromide peptides from the alpha1- and alpha2-chains of pig skin collagen. FEBS Lett. 1971;16(1):63–67. doi: 10.1016/0014-5793(71)80687-7. [DOI] [PubMed] [Google Scholar]
- 131.Belkoff SM, Naylor EC, Walshaw R, Lanigan E, Colony L, Haut RC. Effects of subcutaneous expansion on the mechanical properties of porcine skin. J Surg Res. 1995;58(2):117–123. doi: 10.1006/jsre.1995.1019. [DOI] [PubMed] [Google Scholar]
- 132.Sasaki GH, Pang CY. Pathophysiology of skin flaps raised on expanded pig skin. Plast Reconstr Surg. 1984;74(1):59–67. doi: 10.1097/00006534-198407000-00008. [DOI] [PubMed] [Google Scholar]
- 133.Diridollou S, Black D, Lagarde JM, Gall Y, Berson M, Vabre V, Patat F, Vaillant L. Sex- and site-dependent variations in the thickness and mechanical properties of human skin in vivo. Int J Cosmet Sci. 2000;22(6):421–435. [PubMed] [Google Scholar]
- 134.Flynn C, McCormack BA. Finite element modelling of forearm skin wrinkling. Skin Res Technol. 2008;14(3):261–269. doi: 10.1111/j.1600-0846.2008.00289.x. [DOI] [PubMed] [Google Scholar]
- 135.Wu KS, van Osdol WW, Dauskardt RH. Mechanical properties of human stratum corneum: effects of temperature, hydration, and chemical treatment. Biomaterials. 2006;27(5):785–795. doi: 10.1016/j.biomaterials.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 136.Hendriks FM, Brokken D, van Eemeren JT, Oomens CW, Baaijens FP, Horsten JB. A numerical-experimental method to characterize the non-linear mechanical behaviour of human skin. Skin Res Technol. 2003;9(3):274–283. doi: 10.1034/j.1600-0846.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 137.Barel A, Courage W, Clarys P. Suction method for measurement of skin mechanical properties: the cutometer. In: Serup J, Jemec G, editors. Handbook of non-invasive methods and the skin. Boca Raton (FL): CRC Press; 1995. pp. 335–340. [Google Scholar]
- 138.Van Houten EEW, Doyley MM, Kennedy FE, Weaver JB, Paulsen KD. Initial in vivo experience with steady-state subzone-based MR elastography of the human breast. J Magn Reson Imaging. 2003;17(1):72–85. doi: 10.1002/jmri.10232. [DOI] [PubMed] [Google Scholar]
- 139.Groenendaal W, von Basum G, Schmidt KA, Hilbers PA, van Riel NA. Quantifying the composition of human skin for glucose sensor development. J Diabetes Sci Technol. 2010;4(5):1032–1040. doi: 10.1177/193229681000400502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kanehisa H, Miyatani M, Azuma K, Kuno S, Fukunaga T. Influences of age and sex on abdominal muscle and subcutaneous fat thickness. Eur J Appl Physiol. 2004;91(5-6):534–537. doi: 10.1007/s00421-003-1034-9. [DOI] [PubMed] [Google Scholar]
- 141.Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6):1519–1530. doi: 10.1185/03007995.2010.481203. [DOI] [PubMed] [Google Scholar]
- 142.Rajadhyaksha M, Gonzalez S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113(3):293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 143.Balaconis MK, Billingsley K, Dubach MJ, Cash KJ, Clark HA. The design and development of fluorescent nano-optodes for in vivo glucose monitoring. J Diabetes Sci Technol. 2011;5(1):68–75. doi: 10.1177/193229681100500110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McShane M, Ritter D. Microcapsules as optical biosensors. J Mater Chem. 2010;20(38):8189–8193. [Google Scholar]
- 145.Russell RJ, Pishko MV, Gefrides CC, McShane MJ, Cote GL. A fluorescence-based glucose biosensor using concanavalin A and dextran encapsulated in a poly(ethylene glycol) hydrogel. Anal Chem. 1999;71(15):3126–3132. doi: 10.1021/ac990060r. [DOI] [PubMed] [Google Scholar]
- 146.Patel PN, Smith CK, Patrick CW. Rheological and recovery properties of poly(ethylene glycol) diacrylate hydrogels and human adipose tissue. J Biomed Mater Res A. 2005;73(3):313–319. doi: 10.1002/jbm.a.30291. [DOI] [PubMed] [Google Scholar]
- 147.Williams SK, Berman SS, Kleinert LB. Differential healing and neovascularization of ePTFE implants in subcutaneous versus adipose tissue. J Biomed Mater Res. 1997;35(4):473–481. doi: 10.1002/(sici)1097-4636(19970615)35:4<473::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 148.Castle JR, Ward WK. Amperometric glucose sensors: sources of error and potential benefit of redundancy. J Diabetes Sci Technol. 2010;4(1):221–225. doi: 10.1177/193229681000400127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marshall AJ. Seattle: University of Washington; 2004. Porous hydrogels with well-defined pore structure for biomaterials applications [doctoral dissertation] [Google Scholar]
- 150.Földi M, Földi E, Strössenreuther RHK, Kubik S. München, Germany: Elsevier, Urban & Fischer Verlag; 2006. Földi's textbook of lymphology for physicians and lymphedema therapists. [Google Scholar]
- 151.Koschwanez HE, Reichert WM. In vitro, in vivo and post explantation testing of glucose-detecting biosensors: current methods and recommendations. Biomaterials. 2007;28(25):3687–3703. doi: 10.1016/j.biomaterials.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ward WK, Wood MD, Troupe JE. Understanding spontaneous output fluctuations of an amperometric glucose sensor: effect of inhalation anesthesia and use of a nonenzyme containing electrode. ASAIO J. 2000;46(5):540–546. doi: 10.1097/00002480-200009000-00006. [DOI] [PubMed] [Google Scholar]