Abstract
This article is the second part of a two-part review in which we explore the biomechanics of the sensor–tissue interface as an important aspect of continuous glucose sensor biocompatibility. Part I, featured in this issue of Journal of Diabetes Science and Technology, describes a theoretical framework of how biomechanical factors such as motion and pressure (typically micromotion and micropressure) affect tissue physiology around a sensor and in turn, impact sensor performance. Here in Part II, a literature review is presented that summarizes examples of motion or pressure affecting sensor performance. Data are presented that show how both acute and chronic forces can impact continuous glucose monitor signals. Also presented are potential strategies for countering the ill effects of motion and pressure on glucose sensors. Improved engineering and optimized chemical biocompatibility have advanced sensor design and function, but we believe that mechanical biocompatibility, a rarely considered factor, must also be optimized in order to achieve an accurate, long-term, implantable sensor.
Keywords: biocompatibility, biomechanics, foreign body response, glucose sensor, micromotion, pressure
Introduction
The importance of biomechanics in glucose sensor function is underappreciated. In this review, we look beyond commonly recognized chemical biocompatibility to explore the biomechanics of the sensor–tissue interface as an important aspect of continuous glucose sensor bio-compatibility. Biomechanical factors including motion and pressure give rise to interfacial stresses, which affect tissue physiology around the sensor and in turn, impact sensor performance.
The objective of this article (Part II of a two-part series) is to review the literature that relates biomechanics to sensor performance and to explore application of biomechanical concepts to sensor design. It follows a sister paper1, featured in this issue of Journal of Diabetes Science and Technology, which presents a theoretical framework of biomechanical factors that affect percutaneous and fully implantable glucose sensors. Part I establishes the importance of biomechanics and explores three main contributors to sensor motion and pressure: 1) applied forces, including compressive, tensile, and shear contractile forces; 2) sensordesign, including size, shape, modulus, surface texture, and percutaneous tethering; and 3) subject/patient considerations, including movement in various animal models, tissue compartment, exact sites of implanta-tion within a given compartment, anesthesia or level of sedation, and acute, direct pressure from lying on the sensor. Motion and pressure can affect tissue–sensor contact, interfacial stresses, tissue abrasion, local inflammation and metabolism, cytokines and production of other cellular products, microbleeding, blood flow, lymphatic disruption, interstitial fluid mixing, and development of the foreign body response (FBR), including vessel density and capsule formation.1
In light of the above framework of the biomechanical forces acting on sensors and surrounding tissue, a review of related literature is warranted. Because literature references of continuous sensor data with corresponding biomechanical analysis or even mere recognition that motion or pressure could impact sensor performance are scant (see Table 1), our survey includes other subcutaneously implanted biomaterial studies that relate soft tissue response to motion or pressure. There is a much larger body of literature on biomechanics of hard tissue implants and in particular, the effects of micromotion on bone implants.2–8 However, this review focuses on soft tissue, particularly subcutaneous tissue, as this is the compartment most typical for sensor placement.
Table 1.
In Vivo Studies of Percutaneous (PerQ) and Fully Implanted Subcutaneous (SubQ) Glucose Sensors that Mention the Impact of Motion or Pressure on Sensor Resultsa
| Implant type | Animal model | Number of implants | Duration in vivo | Implant site | Implant sizeb | Measurements/metrics | Results/conclusions that relate to motion and pressure | Reference |
|---|---|---|---|---|---|---|---|---|
| SubQ | Human | 1/person n = 15 | Averaged 144 days | Surgically immobilized in abdomen | Size of AA battery | Sensor performance | Implants surgically immobilized; specifically restricted activity 72 hours and no vigorous activity for 2 weeks in order to limit sensor motion; 13 of 15 sensors functioned | Garg, 200418 |
| SubQ | Human | 1/person n = 5 | 97, 152, 174, 175, 298 days | Abdomen above rectus muscle lateral to midline, inferior to umbilicus | 7 cm L × 3.2 cm W × 1.2 cm H | Sensor performance | Acute artifacts in sensor signal observed during subject movement; lack of tissue–sensor integration and absence of sensor signal attributed to patient motion | Gilligan, 200416 |
| PerQ | Human | 1/person n = 9 2/person n = 6 | 24 hours | Abdomen or upper buttocks | Not reported, estimated 20 mm L × 0.7 mm W × 0.2 mm H | Sensor performance | 60% of continuous glucose monitors reported values <60 mg/dl but serum glucose was not truly low; data support anecdotal reports of inaccurate CGM hypoglycemic values, particularly at night | Mauras, 200411 |
| PerQ | Pig | 4/animal n = 16 | 2, 24, 72 hours and 7 days | Caudoventral direction at 45° to the skin | (L not reported) × 820 μm W × 310 μm H | Capsule Fibrin deposition | Hypothesized that fibrin observed throughout the implantation period was due to sensor damage to vessels during movements | Kvist, 201019 |
| SubQ | Dog | 1/animal n = 27 | 32 ± 9 days | 7 cm interscapular incision; sutured to skin | 8 cm L × 4.5 cm W × 2.5 cm H | Sensor performance (in vivo and in vitro) | Movement and pressure hypothesized to alter sensor sensitivity (disrupt capsule, capillary network, and/or fluid surrounding sensor) | Ward, 199920 |
| SubQ | Dog | 1/animal n = 10 | 20–114 days | Paravertebral thoracic implantation | 7 cm L × 3.2 cm W × 1.2 cm H | Sensor performance (in vivo and in vitro) | Dog falling on table caused rapid dip in sensor signal, followed by rapid return to expected response; firm pressure over sensor also elicited dip in signal | Gilligan, 19949 |
| PerQ and SubQ | Rat | 1/animal n = 12 PerQ n = 3 SubQ | 14, 21 days | 5–7 cm below scapular region on dorsum | 0 mm L × 0.7 mm W × 0.2 mm H | Microvasculature Capsule Sensor performance (PerQ only) | SubQ: 3x less collagen and 3x more vessels in porous vs bare PerQ: constant disregard mechanical stimulation; (bare vs porous microvasculature and capsule not significant); porous sensor signal lower than bare but less variability | Koschwanez, 200821 |
| PerQ and SubQ | Rat | 1/animal n = 11 SubQ n = 17 PerQ | 14 days | 2–3 cm below scapular region (dorsally); sutured to muscle | 20 mm L × 0.7 mm W × 0.2 mm H | Microvasculature Perfusion Sensor performance (PerQ only) | PerQ: constant mechanical stimulation; no difference (vascularity or sensor performance) between bare vs porous; benefits of porous coatings outweighed by motion (PerQ) | Koschwanez, 201022 |
| PerQ | Rat | 1–3/animal n = 22 total | up to 60 days | Various locations on dorsum | 1 mm L × 0.55 mm diameter | Sensor performance | Implantation protocol optimized to minimize long-term macromotion | Long, 200523 |
| PerQ | Rat | 2/animal n = 16 short n = 16 long | 28 days | 1.5 cm laterally to dorsal midline; 3–4 cm caudally | Short: 10 mm Long: 30 mm ~0.55 mm diameter | Sensor performance Capsule | Better longevity and sensitivity was attributed to less micromotion of the shorter electrodes | Ju, 201024 |
No published studies have been designed specifically to study the impact of motion and pressure, but these reports at least acknowledge the role of motion or pressure on sensor performance.
L, length; W, width; H, height
Table 1 summarizes all glucose sensor studies we identified in the published literature that note some relation between sensor performance and motion or pressure. These sensor references and other examples from related literature that discuss both chronic and acute effects are discussed later. Finally, we describe potential strategies for countering the ill effects of motion and pressure on glucose sensors and describe some of the challenges ahead.
Faulty Sensor Data from Pressure of Lying on Sensors
Evidence of acute compressive effects on continuous glucose monitor (CGM) function was, to our knowledge, first reported in 1994 by Gilligan and colleagues9 in a study using dogs. Castle and Ward10 also observed that direct pressure over the sensor site in canines led to a transient loss of current, which they hypothesized was most likely due to a temporary reduction in local blood flow. In humans, compression (pressure) at the sensor site is believed to be responsible for anomalous hypoglycemic measurements reported at night time, particularly when patients lie directly on the sensor.11
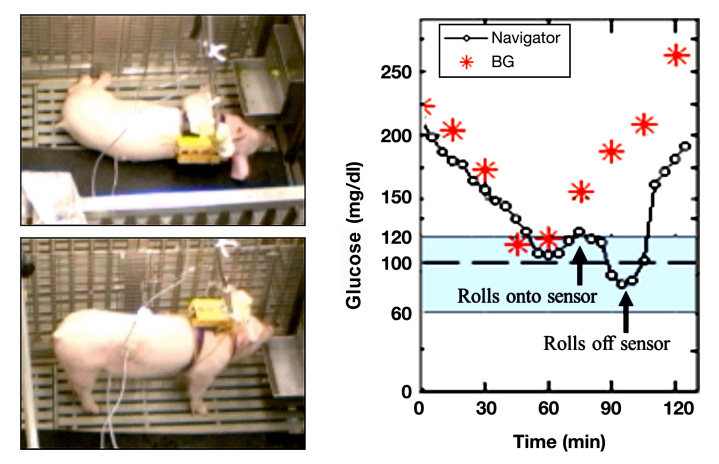
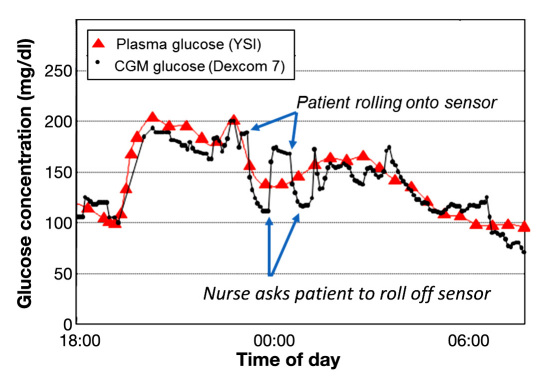
To illustrate acute pressure effects in animals, Figure 1 shows CGM and blood glucose data that was collected as part of a closed-loop experiment.12 Percutaneous (PerQ) sensors (FreeStyle Navigator, Abbott Diabetes Care, Inc.) were inserted into a pig's right and left flanks. As illustrated in Figure 1, the pig lies directly on the sensor during sleep and accordingly, the sensor signal drops. When the pig awakens and rolls off the sensor, the signal recovers. In a second example (Figure 2), CGM data from a female patient wearing a DexCom SEVEN sensor (DexCom Inc., San Diego, CA) on her abdomen is presented (trial details reported elsewhere).13,14 Again, during sleep, the patient rolls onto the sensor and the sensor signal drops as compared to the blood glucose levels. Once the nurse urges the patient to roll off her sensor, compression is relieved and the sensor signal increases. In the authors' own experience, compression arising from restrictive clothing such as a tight beltline directly over a CGM device when in the seated position can also cause a decrease in CGM signal. Petrofsky and colleagues15 examined belt compression on the midsection of human subjects and measured pressures as high as 150 kPa, which were observed to occlude skin circulation. These examples clearly demonstrate the acute effects from direct pressure on the sensor and surrounding tissue.
Figure 1.
Pressure from sleeping on a sensor causes an anomalous dip in signal. PerQ sensors (FreeStyle Navigator) were inserted into the pig's flank using the sensor delivery unit provided with the sensor. When the subject slept on the sensor, the signal dropped markedly (first arrow) and no longer tracked the plasma glucose levels. When the subject rolled off the sensor, the sensor signal recovers (second arrow). Data provided by Dr. Ed Damiano, Boston University. Details are found in a conference abstract.12 BG = blood glucose.
Figure 2.
Errant human CGM data due to compression during sleep. Human CGM and plasma glucose data from a DexCom SEVEN sensor (worn on the abdomen) collected as part of a closed-loop trial performed at the Clinical Research Center at the University of Virginia. Blue arrows denote incidences where patient rolled onto her side and compressed the sensor. When urged by a nurse to roll off the sensor, sensor compression decreased and sensor signal increased. Data provided by Dr. Kovatchev and Dr. Breton. Details of trial are found in conference abstracts.13,14
Patient Activity and Sensor Settling
In a study by Gilligan and colleagues,16 fully implantable sensors were surgically placed into the abdominal subcutis of humans. The authors reported that one of the five sensors never tracked glucose, and upon ex vivo examination, it was discovered that there was no tissue ingrowth into the sensor housing. The authors believe that the subject's high activity levels postimplantation may have generated high shear forces that prevented the implant from anchoring to the tissue and that the lack of vasculature near the sensor interface prevented it from ever functioning properly.17 In a different study with the same type of subcutaneous (SubQ) sensor, Garg and colleagues18 took more cautious measures to limit sensor motion in their human studies of 15 devices. The implants were surgically immobilized and the patients' activity was restricted for 72 hours and vigorous activity prohibited for 2 weeks to allow the tissue around the sensor to heal without mechanical disturbance. Well-designed studies that demonstrate the impact of reduced motion during healing after CGM implantation are lacking.
Sensor Movement within a Mature Capsule
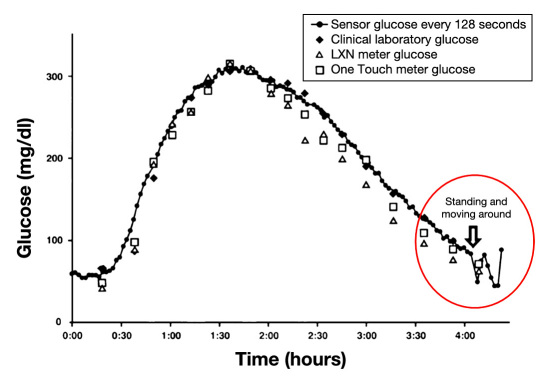
It has also been suggested that if the sensor is not anchored within the foreign body capsule, sensor movement in the capsule occurs and alters sensor performance (personal correspondence with Jim Brauker). Ward and colleagues20 described fluid masses surrounding SubQ implants in dogs and found that fluid and sensor movement affected sensitivity. The authors hypothesized that trauma such as the animal bumping up against the cage may disrupt the foreign body capsule and the local microvasculature, thereby creating functional instability.20 Additionally, the delicate tissue–sensor interface can be disturbed by device migration and other trauma that should be “scrupulously avoided.”20 In a second patient from the Gilligan study, there was a clear change in sensor response when the subject stood up (Figure 3).16 The authors hypothesized that the change in sensor signal was due to postural effects, that is, the compression of the blood supply that produced a blood perfusion and/or oxygen-limited response.16 However, the sensor was most accurate when the patient was sitting, when compression effects seem more likely. Therefore, it is possible that the sensor's movement within the capsule rather than compression of the tissue surrounding the sensor was to blame for sensor signal fluctuations. Sensor movement may cause loss of contact or change in position with the capsule wall. Another reason that could explain these temporal sensor signal fluctuations is mixing of the interstitial fluid (“ISF mixing”),25 particularly if the capsule permitted movement of the sensor within the ISF exudate. Because of the consumptive nature of glucose oxidase sensors, a concentration gradient likely existed around the sensor as was observed by Prichard and colleagues.26 Sliding of the sensor would disturb this gradient, thereby creating a variable signal. Sensor movement could also momentarily aggravate localized cells and cause an upregulation of their glucose consumption, causing the glucose concentration at the sensor interface to change. By reducing the size and mass of the sensor, there will be less device motion, allowing for more rapid integration, reduced settling time, and also resolution of the postural effects.16 Presumably, a smaller, lighter sensor that integrates with the tissue would be less likely to move during postural changes.
Figure 3.
Motion affects sensor performance during a glucose-tracking study in a human subject conducted 103 days postimplantation. The continuous, fully implanted SubQ sensor (-•-) closely tracks blood glucose reference data during a glucose bolus until a significant drop in sensor signal is noted when the patient stands (circled in red). The deviation in sensor signal was attributed to postural effects (i.e., compression of the blood supply in the tissue). However, the change in patient posture could have also caused motion of the sensor relative to the surrounding tissue, triggering changes in tissue–sensor contact and/or mixing of localized glucose concentration in capsule exudate. Figure adapted from Gilligan and colleagues.16
Sensor Motion in Rodent Models
Undoubtedly, large forces are at play in rat studies where sensor migration is evident and even visualized.22 Experimentally, a common failure mode is pulling or removal of sensors,21,23 which can result in abrupt and catastrophic losses of sensor function. Some researchers use specialized gear (e.g., mouse jacket),21 specialized housing (e.g., tether/swivel bowls),27 or specialized catheters,23 and many report using additional sutures or adhesives to help stabilize sensors, especially in small animal models. The intent is primarily to minimize the removal and destruction of sensors by the animal, but despite these efforts, migration due to animal movement and/or loosening of sutures is a common problem in the first 24–48 hours after implantation. Even if sensor removal or destruction is avoided, from our experience, repetitive tugging or scratching leads to greater inflammation around PerQ implants as observed in histological analysis. Anderson28 also describes device motion as being a source of chronic inflammation.
Long and colleagues23 examined a series of PerQ device configurations in rats in an attempt to improve sensor functionality while maintaining animal mobility and welfare. Their sensor design requirements included dura-bility from mechanical forces (e.g., rubbing, scratching, etc.), resisting epidermal migration, and immobilization of the sensor in the tissue without the need for harnesses and tethers connected to swivels.27,29,30 The preferred implantation protocol involved three sensors passing through a single winged catheter hub and was the best of the methods tested for minimizing the size of the PerQ site and providing stable protection and easy access to the miniature sensor wires. Although this PerQ sensor method prevented damage and degradation of the sensors from migration, it is unlikely that there was significant reduction in micromotion, and the authors reported that tissue reaction to the implanted sensors still affected sensor performance.
In another study from Moussy and colleagues24, the impact of sensor motion was evaluated using two different sensors with long or short electrode lengths. After 4 weeks of implantation, 75% of sensors with short electrodes were functioning, while only 50% of long electrodes still tracked glucose (n = 8 each group). There was too much biovariability to draw conclusions about the effect of length and motion on sensor performance. The authors postulated that longer electrodes had a greater range of motion and that motion may have caused tissue damage and affected sensor readings. Since the sensors were tethered at the incision point, it is possible that the stiff, longer electrode rotated about the suture (i.e., a fulcrum). As described in Part I of the review,1 this fulcrum effect was demonstrated in a three-dimensional sensor–tissue model showing that a suture or tethering site may act as a fulcrum about which the sensor pivots and changes the nature and location of stress concentration along the sensor.31 Another important point is that due to the differences in electrode lengths, the actual site of sensing (e.g., distance along animals' dorsum to tip of sensor) varied by at least 20 mm. Precise implantation and the sensing site are also important considerations, which was discussed in Part I. Beyond the work of the Moussy group, there are no known experiments in the literature that were specifically designed to assess the impact of in vivo motion on sensor performance.
Sensor Motion in Porcine Models
In larger animals such as pigs, the shear forces arising from subject ambulation and tissue compression that can arise from skin stretching over the implant are expected to be less significant than in rodents, as the implant is relatively smaller compared to the animal and tissue of implantation. However, even small, chronic forces that generate sensor micromotion may be important throughout the implantation period. Kvist and colleagues19 examined the tissue–sensor (PerQ) interface to characterize the histomorphological changes and quantified gene expression of various immunomodulatory genes in a porcine model of PerQ glucose sensors. Extreme care was taken to minimize the movement of the sensors, including fixing sensors externally to the skin with tape, covering the area with a nonadherent dressing, and fixing sensors with circular bands of adhesive and stretchable tape around the thorax and cranial abdomen.19,32 The results of the gene expression analysis reported by Kvist and colleagues19 coincided with the inflammatory response to implanted medical devices reported by Anderson.28 Interestingly, the presence of fibrin, identified by immunohistochemical staining, was noted at the sensor surface throughout the implantation period (7 days). Fibrin was not expected to be present after the initial wound healing response. The authors hypothesized that the ongoing presence of fibrin was due not only to the increased vascular permeability resulting from inflammation but also from the movement of the sensor in tissue causing physical damage to the vessels and leading to microhemorrhaging in the vicinity of the sensor.19
Reduced Interfacial Stress via Porous Coatings around SubQ Sensors
Koschwanez and colleagues21,22 evaluated the tissue environment surrounding nonfunctional SubQ sensors. The goal of these studies was to reduce fibrosis and promote microvessel formation by coating the sensing element (MiniMed sensor leads) with porous poly-L-lactic acid (PLLA). The porous coating presumably anchored the sensor in the tissue and distributed interfacial stress over a larger surface area. The porous-coated SubQ sensors exhibited significant improvement in vascularity (almost 3x more vessels) and decreased collagen deposition (3x less collagen within 100 μm). In a second study by the same group, a window chamber model was used to allow direct monitoring of the microvasculature surrounding sensors over 14 days.22 Again, PLLA porous-coated and bare sensors (fully implanted, nonfunctional SubQ) were tested and the microvessel number, patency, and orientation were visualized nondestructively using fluorescent dyes. As expected, a significant increase in cumulative microvessel length led to significantly higher laser Doppler flowmetry values adjacent to porous-coated sensors compared to bare sensors. Unfortunately, the fully implanted sensors were nonfunctional (intentional in the design of the experiment) in both experiments, and therefore, it was not possible to evaluate the effect of the porous coatings on sensor performance. Actual sensor data would elucidate the balance between the positive attributes of increased tissue integration and presumably reduced micromotion and the possible negative effects of increased settling times and inflammation, which was likely caused by the degrading porous coatings.
Micromotion of PerQ Sensors Offsets Benefits of Porous Coatings
While the porous coatings on subcutaneously implanted sensors significantly improved FBR compared to noncoated sensors, the same benefit was not observed in the exact same sensors implanted percutaneously. There was no significant difference in the FBR as determined by histological analysis of collagen deposition and vessel density between bare and porous-coated PerQ sensors in either study.21,22 Koschwanez and colleagues21 attributed significant collagen surrounding both PerQ porous and PerQ bare sensors (~1–5x compared to SubQ but highly variable) to the additional mechanical forces on PerQ sensors. The authors also state that suturing the plastic hub connector to the rat dermis may have further contributed to chronic mechanical stress and irritation of the tissue, resulting in increased inflammation.22 They also suggested that increased capillarity (~2–7x compared to SubQ but highly variable) in this case was induced less by surface texturing of the porous coating and more from mechanical irritation resulting from normal rodent movement (i.e., ambulation, grooming) and scratching.22 Therefore, the authors concluded that beneficial effects of the porous coating on tissue response surrounding PerQ sensors were obscured by the effects of constant mechanical stimulation.
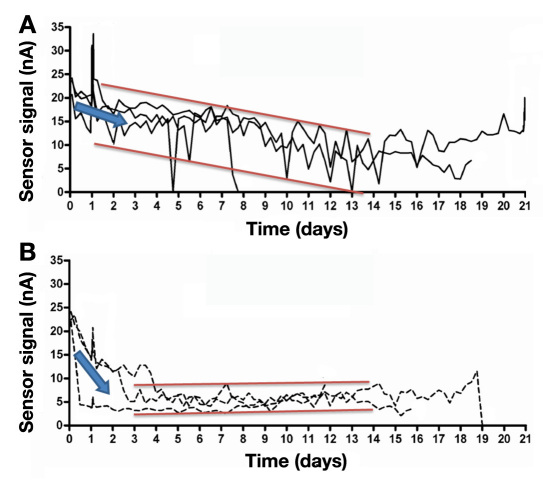
Although there were no statistically significant differences in the highly variable histology, the authors note that the porous-coated sensors experienced a more rapid signal reduction after sensor implantation (Figure 4).21 The signal drop could have been due to the settling period described in other studies.24,33 The consistently lower sensor signals as compared to bare sensors could have been caused by the twofold increase in collagen deposition within 100 μm of the sensor coating as suggested by the authors. Based on our observations, another possible reason for lower signals could be the increased presence of inflammatory cells surrounding the porous, degradable, sensor coating (see Figure 4 in Koschwanez, 2008).21 The authors also observed that porous-coated sensors exhibited less signal fluctuation (noise) during the experiment, which was probably due to better tissue integration preventing sensor displacement and minimizing the shear stress arising from animal motion (Figure 4). Thus, the histological advantages of porous coatings demonstrated in biomaterial studies and for SubQ sensors were not replicated for PerQ sensors. The differences most likely arise from the varying forces affecting PerQ and SubQ sensors and the resultant sensor motion. Therefore, sensor performance was neither extended nor improved through the implementation of degradable porous coatings on PerQ sensors.21,22,24
Figure 4.
Effects of sensor design on continuous monitoring of interstitial glucose. PerQ (A) bare and (B) porous-coated sensors (Medtronic MiniMed) were implanted into the dorsum of rats and data continuously monitored for up to 21 days. Porous-coated sensors initially experienced a more apparent and rapid signal reduction compared with bare sensors (slope of blue arrows). However, unlike bare sensors, sensors with porous coatings exhibited less signal fluctuation (noise between red bars) over time. Figure adapted from Koschwanez and colleagues.21
Micromotion from Breathing and Pulsatile Blood Flow
Although glucose sensor literature is limited in its discussion of micromotion, it has been recognized as a primary concern for implants in neurosurgery and neuronal electrophysiology.17,34 The effects have been quantitated under various physiological conditions17 and computationally modeled.31 Micromotion around implanted brain electrodes has been shown to affect sensor performance, and Gilletti and colleagues17 measured micromotion normal to the brain surface and its dependence on surgical and physiological conditions (e.g., craniotomy size, integrity of dura mater, electrode position). Micromotion of 10–30 μm due to respiration and 2–4 μm due to pulsatile blood flow were measured.17 The authors also noted that anesthesia had an effect on tissue displacement and measured a difference of approxi-mately 60 μm between anesthetized and conscious states. Unfortunately, the neural electrode performance was not characterized in this study, so the impact of brain micromotion on electrode signals is not clear. However, the authors concluded that these displacement data provide insights into the biomechanical stressors affecting long-term neural implant functionality. Although these findings were generated with electrodes in brain tissue, they highlighted the importance of recognizing and systematically evaluating micromotion and underscored the importance of extending this work to subcutaneously implanted glucose sensors.
Application of Biomechanical Concepts to Sensor Design
In addition to the host of other strategies to enhance biocompatibility and minimize the FBR, improvements to sensor design should include strategies for reducing mechanical interfacial stresses. We purport that any development effort considering only chemical strategies to enhance sensor–tissue interactions will be limited by a partial view of sensor biocompatibility. To reduce external forces and interaction with the device, a fully implanted sensor design is preferred. Sensor design specifications need to be considered that will improve the in vivo soft tissue response including stabilization in the tissue, modulus matching, surface topography, and sensor size. After understanding the factors contributing to motion, some sensor prototypes designed for the research animal model (e.g., methods for securing to skin, tunneling leads under skin, etc.) may require form factor redesign specifically for use in a given animal model and subsequent optimization for clinical trialing in humans. Pressure sensors, skin blood indicators (e.g., laser Doppler), and oxygen sensors may be valuable tools to enhance our understanding of the short-term forces that affect blood and oxygen perfusion around the sensor. Micropressure and/or microshear sensors (e.g., strain gauges) and motion analysis techniques35 would enable rigorous study of micromotion and pressure on sensor performance. Placement of redundant sensors on different parts of the body is another strategy to circumvent acute pressure perturbations in CGM signal.
Ideally, to minimize biomechanical forces, a sensor would be made of materials that closely match the modulus of the implantation tissue. In fact, von Recum and colleagues36 suggested choosing first a material based on its mechanical properties and then adjusting its surface chemistry to improve biocompatibility. One approach to improving biocompatibility has been the use of thermo-responsive hydrogel coatings as self-cleaning membranes to minimize biofouling.37 Yet to be determined, however, is the impact on biocompatibility of biomechanical forces from the swelling and deswelling of the hydrogel on the surrounding tissue. Flexible sensor designs have been described for long-term subcutaneous implantation,38 yet many materials used in the medical device industry have a modulus in the range of 1–200 GPa and above.39 Even polymers such as polyurethanes (~60–600 MPa) are much stiffer40 than the viable layers of skin (0.1 to ~260 kPa, excluding the stratum corneum).39,41–51 Furthermore, skin is a complex tissue comprising several different layers, each with different mechanical properties.47 Skin properties also vary with location on the body45 and healed skin can differ significantly from native tissue39,52 and will change over time. Therefore, matching the properties of skin under dynamic conditions poses a formidable engineering challenge.
Surface topography (e.g., porous or pillared) has been shown to improve the vascularity near implants and reduce fibrosis,53–56 although these effects have been less obvious for PerQ devices.21,22,24 In cases where porous-coated sensors were fully implanted, long-term data are reported,9,16,18,57,58 albeit after several weeks after surgical implantation and under constrained physical activity. Still, increasing the surface area of the sensor through microtexturing will distribute the mechanical stress over a larger surface area and reduce the magnitude of forces transmitted to any one point in the surrounding tissue. Future SubQ sensor design efforts should include reduction in size59,60 and thickness.58 Smaller sensors can be injected or implanted through microincisions that are less disruptive to the local tissue and require less time to heal. Thin sensors may avoid mechanical discontinuity between tissue layers61 that causes stress concentrations and microtrauma.62 Finally, corners and edges have been recognized as locales of high stress concentration and increased collagen density.63 While collagen density at corners is high, the capsule appears thinner (see Figure 4B in Part I of this article). The trade-off is not clear between capsule thickness and density, but we note that thickness and density vary with location. Transport to a sensing element at a corner or tip having a thin, dense capsule will likely differ from transport to a sensing element along a smooth, planar surface where a thicker, less dense capsule is expected. Based on the modeling of Novak and colleagues,64 vessel density, which to our knowledge has not been characterized at corners compared to smooth planar surfaces, will be more critical to driving transport than the differential capsule thickness or density.
Conclusions
Although limited, there is discussion in the literature of tissue biomechanics affecting continuous glucose monitors. A broader exploration of the biomaterial literature demonstrates the impact of not only large-scale forces but also the detrimental effects of small-magnitude forces arising from subject activity and mismatch of material–tissue properties. However, the mechanobiology of the tissue response is still poorly understood. Future studies that quantify the impact of applied forces would be of great interest, and this type of mechanistic insight would benefit future engineering efforts. Sensor design criteria should include the reduction of interfacial stresses and biomechanical factors should be considered in experimental models (e.g., sensor site, animal mobility, etc.). To enhance biomechanical biocompatibility, a sensor should be soft in order to closely match tissue modulus, small to reduce tissue displacement, and microtextured (porous or pillared surfaces) to reduce interfacial stress concentrations. To achieve these design input require-ments, there remain significant engineering challenges, not least of which are development of novel materials and further miniaturization of componentry. With Part I1 of this article providing a theoretical framework, and this article (Part II) providing specific examples from literature, we hope to raise awareness of critical biomechanical considerations in implantable sensor design and testing.
Acknowledgments
The authors wish to thank Dr. Edward Damiano for CGM pressure data in pigs, Dr. Boris Kovatchev and Dr. Marc Breton for CGM pressure data in humans, and Dr. James Brauker for discussions on human glucose sensor studies.
Abbreviations
- (CGM)
continuous glucose monitor
- (FBR)
foreign body response
- (ISF)
interstitial fluid
- (PerQ)
percutaneous
- (PLLA)
poly-L-lactic acid
- (SubQ)
subcutaneous
Funding:
Dr. Helton received funding from the University of Washington Center for Commercialization Fellowship.
Disclosures:
Kristen Helton and Natalie Wisniewski are founders of PROFUSA, Inc. Dr. Wisniewski is affiliated with Becton Dickenson; Alfred Mann Foundation; Arkal Medical; University of Washington; Texas A&M University; Duke University; California State University Long Beach; Stanford University; Karolinska Hospital; American Diabetes Association; and Diabetes Technology Society.
References:
- 1.Helton KL, Ratner BD, Wisniewski N. Biomechanics of the sensor–tissue interface—effects of motion, pressure and design on sensor performance and the foreign body response—part I: theoretical framework. J Diabetes Sci Technol. 2011;5(3):632–646. doi: 10.1177/193229681100500317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mjöberg B. The theory of early loosening of hip prostheses [review] Orthopedics. 1997;20(12):1169–1175. doi: 10.3928/0147-7447-19971201-12. [DOI] [PubMed] [Google Scholar]
- 3.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 4.Szmukler-Moncler S, Salama H, Reingewirtz Y, Dubruille JH. Timing of loading and effect of micromotion on bone-dental implant interface: review of experimental literature. J Biomed Mater Res. 1998;43(2):192–203. doi: 10.1002/(sici)1097-4636(199822)43:2<192::aid-jbm14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Huiskes R, Weinans H, Vanrietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Rel Res. 1992;274:124–134. [PubMed] [Google Scholar]
- 6.Søballe K, Hansen ES, Rasmussen HB, Jørgensen PH, Bünger C. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res. 1992;10(2):285–299. doi: 10.1002/jor.1100100216. [DOI] [PubMed] [Google Scholar]
- 7.Aspenberg P, Goodman S, Toksviglarsen S, Ryd L, Albrektsson T. Intermittent micromotion inhibits bone ingrowth. Titanium implants in rabbits. Acta Orthop Scand. 1992;63(2):141–145. doi: 10.3109/17453679209154809. [DOI] [PubMed] [Google Scholar]
- 8.Prendergast PJ, Huiskes R, Søballe K. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech. 1997;30(6):539–548. doi: 10.1016/s0021-9290(96)00140-6. [DOI] [PubMed] [Google Scholar]
- 9.Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care. 1994;17(8):882–887. doi: 10.2337/diacare.17.8.882. [DOI] [PubMed] [Google Scholar]
- 10.Castle JR, Ward WK. Amperometric glucose sensors: sources of error and potential benefit of redundancy. J Diabetes Sci Technol. 2010;4(1):221–225. doi: 10.1177/193229681000400127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauras N, Beck RW, Ruedy KJ, Kollman C, Tamborlane WV, Chase P, Buckingham BA, Tsalikian E, Weinzimer SA, Booth AD, Xing DY. Lack of accuracy of continuous glucose sensors in healthy, nondiabetic children: results of the Diabetes Research in Children Network (DirecNet) accuracy study. J Pediatr. 2004;144(6):770–775. doi: 10.1016/j.jpeds.2004.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Khatib FH, Jiang J, Damiano ER. Automated blood-glucose regulation in diabetic swine using bi-hormonal and insulin-only closed-loop control systems. 69th Scientific Sessions of the American Diabetes Association; 2009 Jun 5-9; New Orleans (LA). 2009. Abstract Number: 426-P. [Google Scholar]
- 13.Breton MD, Patek SD, Farret A, Place J, Demartini S, Brown S, Anderson S, Kovatchev BP, Renard E. Safety supervision system: first clinical trials. Proceedings of the Fourth Advanced Technologies and Treatments for Diabetes Annual Meeting; 2011 Feb 16-19; London, UK. Diabetes Technol Ther. 2011;13(2):176. [Google Scholar]
- 14.Renard EM, Farret A, Place J, Cobelli C, Kovatchev BP, Breton MD. Closed-loop insulin delivery using subcutaneous infusion and glucose sensing, and equipped with a dedicated safety supervision algorithm, improves safety of glucose control in type 1 diabetes. Proceedings of the 46th Annual Meeting of the European Association for the Study of Diabetes; 2010 Sep 20-24; Stockholm, Sweden. [Google Scholar]
- 15.Petrofsky JS, McLellan K, Prowse M, Bains G, Berk L, Lee S. The effect of body fat, aging, and diabetes on vertical and shear pressure in and under a waist belt and its effect on skin blood flow. Diabetes Technol Ther. 2010;12(2):153–160. doi: 10.1089/dia.2009.0123. [DOI] [PubMed] [Google Scholar]
- 16.Gilligan BC, Shults M, Rhodes RK, Jacobs PG, Brauker JH, Pintar TJ, Updike SJ. Feasibility of continuous long-term glucose monitoring from a subcutaneous glucose sensor in humans. Diabetes Technol Ther. 2004;6(3):378–386. doi: 10.1089/152091504774198089. [DOI] [PubMed] [Google Scholar]
- 17.Gilletti A, Muthuswamy J. Brain micromotion around implants in the rodent somatosensory cortex. J Neural Eng. 2006;3(3):189–195. doi: 10.1088/1741-2560/3/3/001. [DOI] [PubMed] [Google Scholar]
- 18.Garg SK, Schwartz S, Edelman SV. Improved glucose excursions using an implantable real-time continuous glucose sensor in adults with type 1 diabetes. Diabetes Care. 2004;27(3):734–738. doi: 10.2337/diacare.27.3.734. [DOI] [PubMed] [Google Scholar]
- 19.Kvist PH, Iburg T, Dawson HD, Jensen HE. Effect of subcutaneous glucose sensor implantation on skin mRNA expression in pigs. Diabetes Technol Ther. 2010;12(10):791–799. doi: 10.1089/dia.2010.0041. [DOI] [PubMed] [Google Scholar]
- 20.Ward WK, Troupe JE. Assessment of chronically implanted subcutaneous glucose sensors in dogs: the effect of surrounding fluid masses. ASAIO J. 1999;45(6):555–561. doi: 10.1097/00002480-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Koschwanez HE, Yap FY, Klitzman B, Reichert WM. In vitro and in vivo characterization of porous poly-L-lactic acid coatings for subcutaneously implanted glucose sensors. J Biomed Mater Res A. 2008;87(3):792–807. doi: 10.1002/jbm.a.31824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koschwanez HE, Reichert WM, Klitzman B. Intravital microscopy evaluation of angiogenesis and its effects on glucose sensor performance. J Biomed Mater Res A. 2010;93(4):1348–1357. doi: 10.1002/jbm.a.32630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long N, Yu B, Moussy Y, Moussy F. Strategies for testing long-term transcutaneous amperometric glucose sensors. Diabetes Technol Ther. 2005;7(6):927–936. doi: 10.1089/dia.2005.7.927. [DOI] [PubMed] [Google Scholar]
- 24.Ju YM, Yu BZ, West L, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. II. Long-term in vitro/in vivo sensitivity character-istics of sensors with NDGA- or GA-crosslinked collagen scaffolds. J Biomed Mater Res A. 2010;92(2):650–658. doi: 10.1002/jbm.a.32400. [DOI] [PubMed] [Google Scholar]
- 25.Henning T. Commercially available continuous glucose monitoring systems. In: Cunningham DD, Stenken JA, editors. in vivo glucose sensing, volume 174. Hoboken (NJ): John Wiley & Sons, Inc; 2009. [Google Scholar]
- 26.Prichard HL, Schroeder T, Reichert WM, Klitzman B. Biolumi-nescence imaging of glucose in tissue surrounding polyurethane and glucose sensor implants. J Diabetes Sci Technol. 2010;4(5):1055–1062. doi: 10.1177/193229681000400504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klueh U, Liu ZH, Cho B, Ouyang TM, Feldman B, Henning TP, Kaur M, Kreutzer D. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther. 2006;8(3):402–412. doi: 10.1089/dia.2006.8.402. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JM. Biological responses to materials [review] Ann Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 29.Thomé-Duret V, Aussedat B, Reach G, Gangnerau MN, Lemonnier F, Klein JC, Zhang Y, Hu Y, Wilson GS. Continuous glucose monitoring in the free-moving rat. Metabolism. 1998;47(7):799–803. doi: 10.1016/s0026-0495(98)90115-9. [DOI] [PubMed] [Google Scholar]
- 30.Ward WK, Jansen LB, Anderson E, Reach G, Klein JC, Wilson GS. A new amperometric glucose microsensor: in vitro and short-term in vivo evaluation. Biosens Bioelectron. 2002;17(3):181–189. doi: 10.1016/s0956-5663(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 31.Subbaroyan J, Martin DC, Kipke DR. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J Neural Eng. 2005;2(4):103–113. doi: 10.1088/1741-2560/2/4/006. [DOI] [PubMed] [Google Scholar]
- 32.Kvist PH, Iburg T, Bielecki M, Gerstenberg M, Buch-Rasmussen T, Hasselager E, Jensen HE. Biocompatibility of electrochemical glucose sensors implanted in the subcutis of pigs. Diabetes Technol Ther. 2006;8(4):463–475. doi: 10.1089/dia.2006.8.463. [DOI] [PubMed] [Google Scholar]
- 33.Updike SJ, Shults MC, Gilligan BJ, Rhodes RK. A subcutaneous glucose sensor with improved longevity, dynamic range, and stability of calibration. Diabetes Care. 2000;23(2):208–214. doi: 10.2337/diacare.23.2.208. [DOI] [PubMed] [Google Scholar]
- 34.Maynard EM, Fernandez E, Normann RA. A technique to prevent dural adhesions to chronically implanted microelectrode arrays. J Neurosci Methods. 2000;97(2):93–101. doi: 10.1016/s0165-0270(00)00159-x. [DOI] [PubMed] [Google Scholar]
- 35.Mahmud J, Holt CA, Evans SL. An innovative application of a small-scale motion analysis technique to quantify human skin deformation in vivo. J Biomech. 2010;43(5):1002–1006. doi: 10.1016/j.jbiomech.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Von Recum A. New aspects of biocompatibility: motion at the interface. In: Lee AJC, Heimke B, Soltesz U, editors. Clinical implant materials: proceedings of the Eighth European Conference on Biomaterials, 1989 Sep 7-9. Heidelberg, Germany. Amsterdam; New York: Elsevier Science Pub. Co; 1990. [Google Scholar]
- 37.Gant RM, Abraham AA, Hou Y, Cummins BM, Grunlan MA, Coté GL. Design of a self-cleaning thermoresponsive nanocomposite hydrogel membrane for implantable biosensors. Acta Biomater. 2010;6(8):2903–2910. doi: 10.1016/j.actbio.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 38.Yu BZ, Long N, Moussy Y, Moussy F. A long-term flexible minimally-invasive implantable glucose biosensor based on an epoxy-enhanced polyurethane membrane. Biosens Bioelectron. 2006;21(12):2275–2282. doi: 10.1016/j.bios.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Hilborn J, Bjursten LM. A new and evolving paradigm for biocompatibitity. J Tissue Eng Regen Med. 2007;1(2):110–119. doi: 10.1002/term.4. [DOI] [PubMed] [Google Scholar]
- 40.Sanders JE, Bale SD, Neumann T. Tissue response to microfibers of different polymers: Polyester, polyethylene, polylactic acid, and polyurethane. J Biomed Mater Res. 2002;62(2):222–227. doi: 10.1002/jbm.10285. [DOI] [PubMed] [Google Scholar]
- 41.Geerligs M, Peters GW, Ackermans PA, Oomens CW, Baaijens FP. Does subcutaneous adipose tissue behave as an (anti-)thixotropic material? J Biomech. 2010;43(6):1153–1159. doi: 10.1016/j.jbiomech.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 42.Agache PG, Monneur C, Leveque JL, Derigal J. Mechanical properties and Young's modulus of human skin in vivo. Arch Dermatol Res. 1980;269(3):221–232. doi: 10.1007/BF00406415. [DOI] [PubMed] [Google Scholar]
- 43.Barel A, Courage W, Clarys P. Suction method for measurment of skin mechanical: the cutometer. In: Serup J, Jemec G, editors. Handbook of non-invasive methods and the skin. Boca Raton (FL): CRC Press; 1995. pp. 335–340. [Google Scholar]
- 44.Diridollou S, Berson M, Vabre V, Black D, Karlsson B, Auriol F, Gregoire JM, Yvon C, Vaillant L, Gall Y, Patat F. An in vivo method for measuring the mechanical properties of the skin using ultrasound. Ultrasound Med Biol. 1998;24(2):215–224. doi: 10.1016/s0301-5629(97)00237-8. [DOI] [PubMed] [Google Scholar]
- 45.Diridollou S, Black D, Lagarde JM, Gall Y, Berson M, Vabre V, Patat F, Vaillant L. Sex- and site-dependent variations in the thickness and mechanical properties of human skin in vivo. Int J Cosmet Sci. 2000;22(6):421–435. [PubMed] [Google Scholar]
- 46.Gennisson JL, Baldeweck T, Tanter M, Catheline S, Fink M, Sandrin L, Cornillon C, Querleux B. Assessment of elastic parameters of human skin using dynamic elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(8):980–989. doi: 10.1109/tuffc.2004.1324402. [DOI] [PubMed] [Google Scholar]
- 47.Hendriks FM, Brokken D, van Eemeren J, Oomens CWJ, Baaijens FPT, Horsten J. A numerical-experimental method to characterize the non-linear mechanical behaviour of human skin. Skin Res Technol. 2003;9(3):274–283. doi: 10.1034/j.1600-0846.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 48.Patel PN, Smith CK, Patrick CW. Rheological and recovery properties of poly(ethylene glycol) diacrylate hydrogels and human adipose tissue. J Biomed Mater Res A. 2005;73(3):313–319. doi: 10.1002/jbm.a.30291. [DOI] [PubMed] [Google Scholar]
- 49.Van Houten EEW, Doyley MM, Kennedy FE, Weaver JB, Paulsen KD. Initial in vivo experience with steady-state subzone-based MR elastography of the human breast. J Magn Reson Imaging. 2003;17(1):72–85. doi: 10.1002/jmri.10232. [DOI] [PubMed] [Google Scholar]
- 50.Wu JZ, Cutlip RG, Welcome D, Dong RG. Estimation of the viscous properties of skin and subcutaneous tissue in uniaxial stress relaxation tests. Biomedical Mater Eng. 2006;16(1):53–66. [PubMed] [Google Scholar]
- 51.Wu KS, van Osdol WW, Dauskardt RH. Mechanical properties of human stratum corneum: Effects of temperature, hydration, and chemical treatment. Biomaterials. 2006;27(5):785–795. doi: 10.1016/j.biomaterials.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Levenson SM, Geever EF, Crowley LV, Oates JF 3rd, Berard CW, Rosen H. The healing of rat skin wounds. Ann Surg. 1965;161:293–308. doi: 10.1097/00000658-196502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29(12):1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 54.Marshall AJ, Irvin CA, Barker T, Sage EH, Hauch KD, Ratner BD. Biomaterials with tightly controlled pore size that promote vascular in-growth. Polym Prepr. 2004;45(2):100–101. [Google Scholar]
- 55.Picha GJ, Drake RF. Pillared-surface microstructure and soft-tissue implants: effect of implant site and fixation. J Biomed Mater Res. 1996;30(3):305–312. doi: 10.1002/(SICI)1097-4636(199603)30:3<305::AID-JBM5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 56.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma-tissue exchange properties. J Biomed Mater Res. 1998;40(4):586–597. doi: 10.1002/(sici)1097-4636(19980615)40:4<586::aid-jbm10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 57.Updike SJ, Shults MC, Gilligan BJ, Rhodes RK. A subcutaneous glucose sensor with improved longevity, dynamic range, and stability of calibration. Diabetes Care. 2000;23(2):208–214. doi: 10.2337/diacare.23.2.208. [DOI] [PubMed] [Google Scholar]
- 58.Ward WK, Slobodzian EP, Tiekotter KL, Wood MD. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials. 2002;23(21):4185–4192. doi: 10.1016/s0142-9612(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 59.Sanders JE, Stiles CE, Hayes CL. Tissue response to single-polymer fibers of varying diameters: evaluation of fibrous encapsulation and macrophage density. J Biomed Mater Res. 2000;52(1):231–237. doi: 10.1002/1097-4636(200010)52:1<231::aid-jbm29>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 60.Vaddiraju S, Tomazos I, Burgess DJ, Jain FC, Papadimitrakopoulos F. Emerging synergy between nanotechnology and implantable biosensors: a review. Biosens Bioelectron. 2010;25(7):1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders JE, Rochefort JR. Fibrous encapsulation of single polymer microfibers depends on their vertical dimension in subcutaneous tissue. J Biomed Mater Res A. 2003;67(4):1181–1187. doi: 10.1002/jbm.a.20027. [DOI] [PubMed] [Google Scholar]
- 62.Holt B, Tripathi A, Morgan J. Viscoelastic response of human skin to low magnitude physiologically relevant shear. J Biomech. 2008;41(12):2689–2695. doi: 10.1016/j.jbiomech.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matlaga BF, Yasenchak LP, Salthouse TN. Tissue response to implanted polymers: the significance of sample shape. J Biomed Mater Res. 1976;10(3):391–397. doi: 10.1002/jbm.820100308. [DOI] [PubMed] [Google Scholar]
- 64.Novak MT, Yuan F, Reichert WM. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem. 2010;398(4):1695–1705. doi: 10.1007/s00216-010-4097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]