Abstract
Background:
Real-time continuous glucose monitoring (RT-CGM) improves hemoglobin A1c (A1C) and hypoglycemia in people with type 1 diabetes mellitus and those with type 2 diabetes mellitus (T2DM) on prandial insulin; however, it has not been tested in people with T2DM not taking prandial insulin. We evaluated the utility of RT-CGM in people with T2DM on a variety of treatment modalities except prandial insulin.
Methods:
We conducted a prospective, 52-week, two-arm, randomized trial comparing RT-CGM (n = 50) versus self-monitoring of blood glucose (SMBG) (n = 50) in people with T2DM not taking prandial insulin. Real-time continuous glucose monitoring was used for four 2-week cycles (2 weeks on/1 week off). All patients were managed by their usual provider. This article reports on changes in A1C 0–12 weeks.
Results:
Mean (±standard deviation) decline in A1C at 12 weeks was 1.0% (±1.1%) in the RT-CGM group and 0.5% (±0.8%) in the SMBG group (p = .006). There were no group differences in the net change in number or dosage of hypoglycemic medications. Those who used the RT-CGM for ≥48 days (per protocol) reduced their A1C by 1.2% (±1.1%) versus 0.6% (±1.1%) in those who used it <48 days (p = .003). Multiple regression analyses statistically adjusting for baseline A1C, an indicator for usage, and known confounders confirmed the observed differences between treatment groups were robust (p = .009). There was no improvement in weight or blood pressure.
Conclusions:
Real-time continuous glucose monitoring significantly improves A1C compared with SMBG in patients with T2DM not taking prandial insulin. This technology might benefit a wider population of people with diabetes than previously thought.
Keywords: type 2 diabetes mellitus, lifestyle, real-time continuous glucose monitoring, self-care of diabetes, self-monitoring of blood glucose
Introduction
Real-time continuous glucose monitoring (RT-CGM) has been used primarily in children, adolescents, and adults with type 1 diabetes mellitus (T1DM). There have now been multiple studies showing improvement in hemoglobin A1c (A1C) using RT-CGM in these populations.1–5 Real-time continuous glucose monitoring may also decrease episodes of severe hypoglycemia that can occur frequently in patients with T1DM and those with type 2 diabetes mellitus (T2DM) on prandial insulin.6,7 The possible benefit of RT-CGM in T2DM patients who are treated with oral agents and/or basal rather than prandial insulin has not been studied, perhaps because these patients are perceived to be less prone to large fluctuations of blood sugars.
Our overarching hypothesis is that RT-CGM conveys a wealth of information about the effects of food, exercise, and other lifestyle events on glucose levels. This wealth of information can provide feedback to the patient and assist him/her in making salutary modifications to their behaviors. In turn, the patient experiences short-term improvements in overall glycemic control, which may be sustained in the long term. This project sought to test a part of this overarching hypothesis, namely, the effects of RT-CGM on short- and long-term glycemic control. We designed a randomized controlled study in patients with T2DM who were not taking prandial insulin to determine if providing RT-CGM monitors for a total of 8 weeks (over a 12-week period) improves glycemic control. This article reports on the short-term results.
Subjects and Methods
Study Design
This is a 52-week, prospective, two-arm, randomized, controlled study investigating the short-term (12-week) and long-term (52-week) relative effectiveness of two methods of blood glucose monitoring on patients with T2DM. The intervention group using RT-CGM was compared to a control group who used only self-monitoring of blood glucose (SMBG). This article reports on the 12-week effectiveness.
Study Population
The study enrolled 100 military health care beneficiaries from the Walter Reed Health Care System, aged 18 years or older, who had T2DM for at least 3 months and an initial A1C ≥ 7% but ≥ 12%. Eligible participants were treated with diet/exercise alone or other glucose-lowering therapies except prandial insulin, were able to independently measure and read finger stick blood glucose levels, and were willing to perform SMBG four times daily. They had all attended an American Diabetes Association (ADA)-recognized diabetes self-management education program. Individuals who were pregnant, lactating, or attempting pregnancy and those on glucocorticoids, amphetamines, anabolic, or weight-reducing medications were excluded. All subjects gave written, informed consent.
Protocol
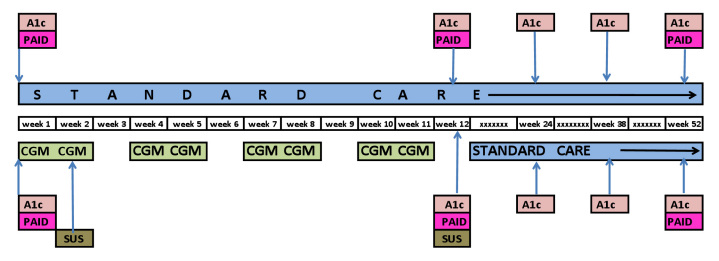
Subjects in the RT-CGM group used the DexComTM SEVEN® (DexCom, Inc., San Diego, CA). The RT-CGM devices were calibrated according to the manufacturer's recommendations. Subjects in the RT-CGM group completed four cycles of 3 weeks each as shown in Figure 1. A cycle consisted of 2 weeks of RT-CGM use and 1 week off. Subjects in this group were also asked to perform SMBG to confirm the RT-CGM value before each meal, at bedtime, for all episodes of hypoglycemia (<70 mg/dl) or hyperglycemia (>180 mg/dl), as well as when not using the RT-CGM.
Figure 1.
Study design.
Patients randomized to SMBG were asked to perform SMBG before each meal and at bedtime. They were provided with and instructed in the use of the AccuChek® Aviva glucometer (Roche Diagnostics Corp., Indianapolis, IN).
Subjects in both groups continued usual care for their diabetes and were instructed to contact their primary care provider for all treatment decisions and consultations, particularly if they developed hypoglycemia (<70 mg/dl). Study staff did not provide any care management.
Additionally, all subjects had baseline and follow-up measurements as shown in Figure 1. Follow-up study visits were performed at 3-week intervals during the first 12 weeks to document adverse effects, to download finger stick data, and to measure weight and blood pressure (BP).
Measures
The primary outcome of the study is A1C. The study was powered to detect a 0.8% [standard deviation (SD) = 1.3%] difference in the reduction of A1C between the two groups. Hemoglobin A1c was measured quarterly using a Roche/Hitachi cobas® c system with a Tina-quant Hemoglobin A1c Gen.2 assay in the Walter Reed Clinical Laboratory.
Secondary outcomes included change in mean and distribution of blood glucose, weight, BP, and diabetes-related stress. Weight was measured on a Scale-Tronix 5005 series scale, and BP was taken using a Welch Allyn Vital Sign 300 series monitor. The Problem Areas in Diabetes (PAID) is a self-administered questionnaire consisting of 20 items that cover a range of emotional problems frequently reported in diabetes. Each item is scored 0 to 4. The system usability scale (SUS) was developed by the Digital Equipment Company, Ltd. It is a 10-item, self-administered questionnaire using a Likert scale that gives subjective assessments of usability. Scores range from 0 to 100, with higher scores indicating greater usability. Weight, BP, and PAID were measured at 0, 12, and 52 weeks. The SUS was administered after patients completed their instruction on RT-CGM but prior to starting to use the device and at 12 weeks.
We calculated the “change-from-baseline” in A1C, weight, BP, and PAID by subtracting the baseline value from the 12-week value.8,9
The study also characterized “usage” of RT-CGM among participants in this group to differentiate between those who followed the protocol and those who did not. The reason for this is our aforementioned overarching hypothesis, which stipulates that access to a continuous stream of information about one's blood glucose can lead to improved knowledge of what affects blood sugar and modifications of behavior. People who followed the protocol had more opportunity to learn from their RT-CGM data. The protocol called for 56 days of usage. Assuming scheduling difficulties or preference to remove the sensor prior to a holiday or weekend, we determined that the minimum number of days to wear the sensor should be 48 to be considered “per protocol.” Thus we created a “usage” variable with the following categories: no usage (SMBG group), <48 days of usage, and ≥48 days of usage (i.e., >85% of time).
Statistical Analyses
The study groups were tested for their equality with respect to baseline characteristics using t-tests and chi-square tests. We then examined the distributions of the change-from-baseline variables and compared the equality of their means using t-tests for the comparison of the two treatment groups and analysis of variance (ANOVA) for the comparison of the three RT-CGM usage groups. We also tested the distributions of the outcomes using comparable nonparametric tests, Wilcoxon–Mann–Whitney tests, and Kruskal–Wallis tests. Lastly, we performed a series of separate multiple regression analyses for each outcome. The first series regressed change-from-baseline values on participants' baseline characteristics known to affect the outcomes, the baseline value of the outcome, and the main independent variable, treatment group (intention-to-treat) or usage group. The regression analyses included the baseline measures of the outcomes, because often the biggest predictor of follow-up health status is prior health status, and rates of change might differ according to baseline status because it is generally more difficult to change a health outcome that is close to normal than it is to change a health outcome that is far from normal. The second series added an interaction effect—group × baseline value for the health outcome—to the multiple regression analyses to address the question of whether baseline status affects how group affects change-from-baseline. That is, did baseline status and group membership interact to produce an additional effect?
Results
Table 1 presents the baseline characteristics of the study sample by group. The groups were similar with respect to baseline weight, body mass index, BP, A1C, and diabetes therapy. The participants in the RT-CGM group were slightly younger on average, and there were more men in the RT-CGM group than in the SMBG group.
Table 1.
Characteristics of the Study Participants by Treatment Group
| Variable | Treatment Group | P Value | |
|---|---|---|---|
| SMBG | RT-CGM | ||
| (n = 50) | (n = 50) | ||
| Age [mean (SD)]a | 60.0 (11.9) | 55.5 (9.6) | 0.04 |
| Male (n)b | 22/50 | 33/50 | 0.03 |
| Therapyb | 0.76 | ||
| Diet and exercise only (n) | 4/50 | 3/50 | |
| Oral medications only (n) | 27/50 | 24/50 | |
| Oral medications/Byetta (n) | 5/50 | 4/50 | |
| Basal insulin, alone or in combination (n) | 14/50 | 19/50 | |
| Body mass index—baseline [mean (SD)] | 32.7 (7.7) | 31.9 (5.8) | 0.54 |
| Weight at baseline [mean (SD)]a | 197.3 (46.4) | 206.5 (35.7) | 0.27 |
| Weight at 12 weeks [mean (SD)]a | 196.5 (43.1) | 202.6 (32.3) | 0.42 |
| Systolic BP at baseline [mean (SD)]a | 132.5 (19.3) | 130.8 (16.2) | 0.46 |
| Systolic BP at 12 weeks [mean (SD)]a | 129.5 (18.0) | 129.3 (16.7) | 0.95 |
| Diastolic BP at baseline [mean (SD)]a | 77.6 (9.8) | 79.0 (8.9) | 0.46 |
| Diastolic BP at 12 weeks [mean (SD)]a | 76.2 (8.3) | 77.7 (11.3) | 0.46 |
| A1C at baseline [mean (SD)]a | 8.2 (1.1) | 8.4 (1.3) | 0.24 |
| A1C at 12 weeks [mean (SD)]a | 7.7 (1.2) | 7.4 (1.0) | 0.23 |
P value is from a t-test.
P value is from a chi-square test.
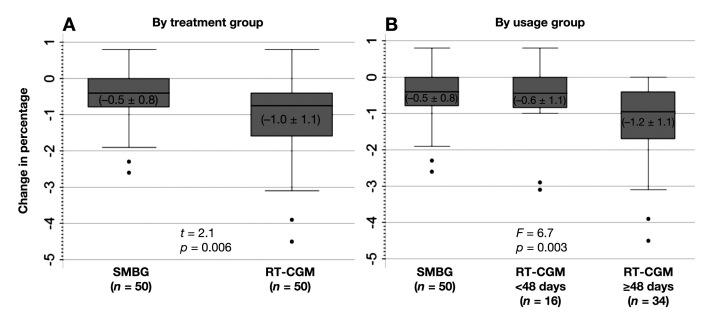
Changes-from-baseline in A1C are shown in Figure 2A. Mean (± SD) A1C decreased by 1.0% (±1.1%) for the RT-CGM group and 0.5% (±0.8%) for the SMBG group (p = .006). The median change-from-baseline in the RT-CGM and SMBG groups were -0.75% and -0.40%, respectively (z = 2.51 and p = .01). Note that three participants in the RT-CGM group opted not to use the technology following randomization but still are included in the RT-CGM group in these analyses. In multiple regression analyses, in which change-from-baseline for each outcome was regressed on age, gender, therapy, baseline value for the outcome, and treatment group, the RT-CGM group was a significant predictor of change in A1C. Specifically, the RT-CGM group had a statistically adjusted decline in A1C of 0.60% greater than the SMBG group (p = .002). Baseline A1C status was also a significant predictor (p < .0001).
Figure 2.
Changes in A1C from baseline to 12 weeks, (A) by treatment group and (B) by RT-CGM usage group. The figure shows boxplots. The boxes themselves contain the 25th, 50th, and 75th percentiles. The whiskers of the boxes show the minimum and maximum values, with the dots beneath the whiskers indicating possible outlying values. Means ± SDs are shown in parentheses within each box. P values are from t-tests or ANOVA comparing the group's mean changes. In multiple regression analyses in which change scores for each outcome were regressed on age, gender, therapy, baseline value for the outcome, and treatment group, group was a significant predictor of change in A1C, -0.48 meaning the RT-CGM group had an adjusted decline in A1C of 0.48% greater than the SMBG group (p = .006). For the RT-CGM group ≥ 48 days, adjusted decline in A1C was -0.60 (p = .002) relative to the SMBG group. Group was not significant for the other outcomes, weight, and BP.
The analyses of usage found that 16/50 used the RT-CGM <48 days and 34/50 used it ≥48 days. Mean (± SD) A1C decreased by 0.6% (±1.1%) for the participants in the RT-CGM <48 days group and 1.2% (±1.1%) for participants who used the technology per protocol. Figure 2B shows that the median A1C change-from-baseline in those using RT-CGM <48 days versus ≥48 days was -0.45% and -0.95%, respectively (chi-square 11.33 with two degrees of freedom and p = .005). The difference among those in the SMBG group, the RT-CGM <48 days group, and the RT-CGM ≥48 days group was significant (p = .002) in the ANOVA and persisted in the multiple regression models, which found that the RT-CGM group had an average, statistically adjusted decline in A1C of -0.48% greater than the SMBG group (p = .006) net of baseline A1C status, which was also significant (p < .0001).
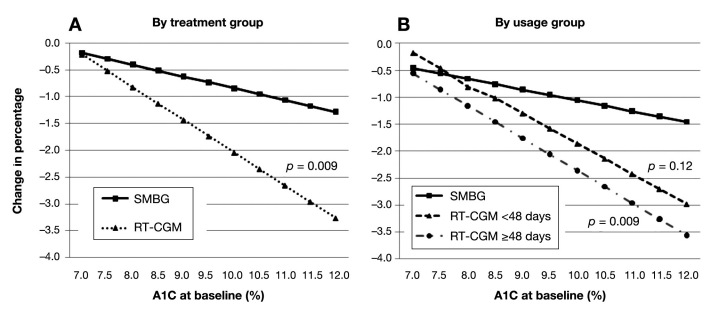
The extent of the effect of treatment group and usage group on A1C differed by baseline A1C (Figure 3) so that a higher A1C predicted a better response. Further, the difference in A1C decline between the SMBG group and the group that used RT-CGM ≥48 days was narrowest among participants with a low baseline A1C and widest among participants with a high baseline A1C (Figure 2B).
Figure 3.
Interaction of baseline A1C with group in the prediction of 12-week change in A1C. Figures were derived from multiple regression analyses. P values for interaction effects are shown in parentheses in the graphs and refer to the difference from the SMBG group. The coefficient (standard error) for the A interaction term, group × baseline A1C, was -0.39 (0.14). The coefficient (standard error) for the B interaction terms, (RT-CGM < 48 days) × (A1C at baseline) and (RT-CGM ≥ 48 days) × (A1C at baseline), were -0.36 (0.23) and -0.40 (0.15), respectively. Equations for the plotting of the lines assumed mean age, male gender, and oral medications only.
Table 2 shows the mean glucose in the RT-CGM group taken both from their RT-CGM readings and their accompanying SMBG as well as from the SMBG group. The RT-CGM group tested 2.9 times per day while the SMBG group tested 2.4 times per day. Although data from the RT-CGM and SMBG readings are not directly comparable, we present this information because it is typically reported in RT-CGM studies and provides a context for interpreting the A1C results.
Table 2.
Summary Statistics of Glucose Readings, Baseline to 12 Weeks
| RT-CGM | SMBG (n = 47)c | ||
|---|---|---|---|
| From RT-CGM (n = 47)a | From SMBG (n = 44)b | ||
| Average number of readings/day | every 5 min | 2.9 | 2.4 |
| Mean glucose mg/dl | 147.3 | 150.4 | 161.9 |
| % <50 mg/dl | 0.2 | 1.9 | 2.1 |
| % <70 mg/dl | 2.1 | 3.6 | 2.7 |
| % >180 mg/dl | 22.6 | 24.3 | 28.7 |
| % >240 mg/dl | 6.1 | 7.4 | 12.1 |
| % within target range | 75.3 | 72.1 | 68.6 |
Three people in the RT-CGM group opted not to use the technology after randomization, thus n = 47.
Six people in the RT-CGM group opted not to do and/or provide SMBG data, hence n = 44; 3/6 includes the 3 people who did not use the RT-CGM.
Three people in the SMBG group did not do finger stick checks and/or provide the data, hence n = 47.
We calculated the net change in hypoglycemic medica-tion prescriptions by subtracting the number of discontinu-ations from the number of initiations. We similarly calculated the number of dose changes of existing medications by subtracting the number of decreases from increases. The medications used during the study by the RT-CGM and SMBG groups, respectively, were metformin (37 and 43), glipizide (26 and 29), glyburide (2 and 4), glimepiride (2 and 3), pioglitazone (15 and 11), exenatide (10 and 1), liraglutide (0 and 1), sitagliptin (13 and 9), acarbose (1 and 0), and basal insulin (either glargine or detemir) (21 and 25). The RT-CGM group had about the same net change in new diabetes medications (11 versus 12) and similar increases in doses of existing medications (20 versus 23). However, only 6% (3/50) of patients in the RT-CGM group, compared with 16% (8 of 50) in the SMBG group, were begun on basal insulin.
The mean SUS score at baseline was 72.4 ± 13.5, suggesting moderately good usability. It was unchanged at 12 weeks (74.8 ± 14.6). There was a weak correlation of -0.27 (p = .07) between the baseline SUS score and change in A1C.
Changes-from-baseline of weight, BP, and PAID scores did not differ by treatment group or usage.
Discussion
This study tested the hypothesis that the information provided by RT-CGM would benefit patients with T2DM who were not using prandial insulin. This is the first study to investigate RT-CGM use in a T2DM patient population whose medication use is typical of the United States population with T2DM.10 The data show significant improvement in A1C at 12 weeks in those afforded RT-CGM as compared to SMBG in patients who were managed by their regular diabetes care provider and had no management input or recommendations from the study staff. This finding suggests that real-time feedback about the glycemic effects of meals and exercise may teach lifestyle skills that result in better glycemic control for patients with T2DM in the short term. Further support of this notion is that the improvement in A1C occurred without any net change in hypoglycemic therapy. Given that a A1C improvement of ≥0.5% would be considered significant given measurement error in clinical laboratories,11 the data suggest that most patients with A1C ≥ 8.0% would benefit from RT-CGM if used as prescribed. Furthermore, current treatment regimen and age were not significant predictors of A1C change at 12 weeks, suggesting that it is possible that those using a variety of therapies and all age groups may benefit from this type of an intervention. However, this study was not designed nor powered to determine that. Unlike most of the previous RT-CGM studies, which looked at continuous use of RT-CGM over a 3–6 month period, our study used four cycles of RT-CGM over a 12-week period. As expected, those who used RT-CGM per protocol during their cycle on RT-CGM had the largest improvement in A1C. The Juvenile Diabetes Research Foundation (JDRF)1 and the O'Connell and associates5 studies also found that the greatest predictor of A1C change was frequency of sensor use.
We were unable to determine the shortest RT-CGM use needed to achieve a significant decrease in A1C. In addition, we cannot predict if this intervention will have long-term “legacy” effects and, if so, what factors might be predictive of that benefit.
Many studies have shown that intensive diabetes treatment reduces the chronic complications of both T1DM and T2DM.12–16 Accordingly, tight glucose management has become today's standard of care, and monitoring glucose has become a critically important tool in achieving this goal. The ADA recommends SMBG as an essential aspect of diabetes management in insulin-treated patients and a desirable aspect in non-insulin-treated patients with diabetes.17 However, there is controversy over the benefit of SMBG in those patients with T2DM who are not taking insulin. Several studies have shown a small or no effect of SMBG.18–20 Systematic review and meta-analyses of SMBG in non-insulin-treated patients with T2DM concluded that use of SMBG was associated with small reductions in A1C of 0.24% to 0.4%.21,22 The reason that studies of SMBG have not been shown to have a greater effect in this patient population has been attributed to the fact that most of the studies have been “passive,” i.e., blood glucose goals were not set and the data was not actionable, i.e., no therapeutic changes were made as a result of the information.23 However, Polonsky and colleagues24 have shown significant improvement of A1C using a structured approach to SMBG, presumably because it led to more treatment changes.
The use of RT-CGM has been demonstrated to provide significant benefits to patients with T1DM. The JDRF trial, a large 26-week randomized trial of 322 patients with T1DM showed that continuous glucose monitoring reduced A1C by 0.5% as compared with usual SMBG in their age 25 years or older population using RT-CGM.1 Two smaller studies showed a similar change in A1C in those patients with T1DM using RT-CGM, typically -0.4% to -0.6% change greater than the standard care group.3,5 A study that was done in both T1DM and T2DM participants showed a more modest -0.3% change in A1C.7
The JDRF trial also found that the times per day “in range” significantly improved. However, we did not find that either mean glucose or percentage of values “in range” differed between the RT-CGM and SMBG groups. We did not ask our SMBG patients to obtain postprandial glucose measurements. We believe that the discrepancy in mean glucose versus A1C results may be explained by not having the postprandial data, as postprandial glucose levels are a major determinant of A1C.
The strengths of our study are its prospective, randomized design and the inclusion of a typical cohort of patients with T2DM. One of the limitations of this study is the absence of “masked” baseline continuous glucose monitoring data to compare with the real-time information. However, it has been shown that unmasking patients with T1DM resulted in marked improvement in glycemic control.25 Another limitation is the absence of post-prandial blood glucose data in the SMBG group, which would be a fairer comparison and has been shown to improve A1C more than preprandial testing as well as cause a greater reduction in carotid intima-media thickness.26 But the study reflects “real-world” conditions in which subjects did not even comply with testing four times per day as required by the study design. Nevertheless, the number of times per day that those in the SMBG group actually tested is greater than is typical for most patients with T2DM who are not on prandial insulin. A further limitation of our study is the absence of self-care data. Future studies should evaluate whether or not postprandial SMBG, when used as a “learning” tool, is comparable to RT-CGM in its ability to improve glycemic control.
In summary, this is the first randomized controlled trial to demonstrate a clinically meaningful reduction in A1C using RT-CGM in T2DM patients not on prandial insulin. Our data suggest that most patients with T2DM with A1C ≥ 7.0 may benefit from short-term RT-CGM use and that the result is achievable without an increase in the number or dose of hypoglycemic medications. If our results are confirmed, this technique might be employed routinely in a typical diabetes clinic population as a safe and effective nonpharmacologic intervention. Further studies are currently underway to determine if the benefit of short-term RT-CGM use causes long-term improvement in glycemic control.
Acknowledgments
We are indebted to the dedicated research staff and subjects who participated in this clinic research project.
Abbreviations
- (A1C)
hemoglobin A1c
- (ADA)
American Diabetes Association
- (ANOVA)
analysis of variance
- (BP)
blood pressure
- (JDRF)
Juvenile Diabetes Research Foundation
- (PAID)
Problem Areas in Diabetes
- (RT-CGM)
real-time continuous glucose monitoring
- (SD)
standard deviation
- (SMBG)
self-monitoring of blood glucose
- (SUS)
system usability scale
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
Funding:
DexCom, Inc. provided financial and in-kind support for this investigator-initiated study.
Disclaimer:
The opinions expressed in this paper reflect the personal views of the authors and not the official views of the United States Army or the Department of Defense.
References:
- 1.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 2.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, Buckingham B, Chase P, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Huang ES, Kollman C, Kowalski AJ, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer SA, Wilson DM, Wolpert H, Wysocki T, Xing D. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32(8):1378–1383. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 4.Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9(3):203–210. doi: 10.1089/dia.2007.0205. [DOI] [PubMed] [Google Scholar]
- 5.O'Connell MA, Donath S, O'Neal DN, Colman PG, Ambler GR, Jones TW, Davis EA, Cameron FJ. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250–1257. doi: 10.1007/s00125-009-1365-0. [DOI] [PubMed] [Google Scholar]
- 6.Zick R, Petersen B, Richter M, Haug C, SAFIR Study Group. Comparison of continuous blood glucose measurement with conventional documentation of hypoglycemia in patients with type 2 diabetes on multiple daily insulin injection therapy. Diabetes Technol Ther. 2007;9(6):483–492. doi: 10.1089/dia.2007.0230. [DOI] [PubMed] [Google Scholar]
- 7.Chico A, Vidal-Ríos P, Subirà M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with Type 1 and Type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care. 2003;26(4):1153–1157. doi: 10.2337/diacare.26.4.1153. [DOI] [PubMed] [Google Scholar]
- 8.Fitzmaurice GM, Laird NM, Ware JH. Hoboken: Wiley; 2004. Applied longitudinal analysis. [Google Scholar]
- 9.Rogosa D. Myths about longitudinal research. In: Schaie KW, Campbell RT, Meredith W, Rawlings SC, editors. Methodological issues in aging research. New York: Springer; 1988. pp. 171–209. [Google Scholar]
- 10.Centers for disease Control and Prevention. Diabetes data & trends. Treating eiabetes (insulin and oral medication use): percentage using diabetes medication by type of medication. http://www.cdc.gov/diabetes/statistics/treating_national.htm. [Google Scholar]
- 11.Little RR, Rohlfing CL, Sacks DB, National Glycohemoglobin Standardization Program (NGSP) Steering Committee Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205–214. doi: 10.1373/clinchem.2010.148841. [DOI] [PubMed] [Google Scholar]
- 12.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 13.Reichard P, Pihl M. Mortality and treatment side-effects during long-term intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study. Diabetes. 1994;43(2):313–317. doi: 10.2337/diab.43.2.313. [DOI] [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 15.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, Holman R, Kinmonth AL, Neil A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335(7611):132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Kane MJ, Bunting B, Copeland M, Coates VE; ESMON Study Group. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336(7654):1174–1177. doi: 10.1136/bmj.39534.571644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insula blinded, randomized trial. Am J Med. 2005;118(4):422–425. doi: 10.1016/j.amjmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Poolsup N, Suksomboon N, Rattanasookchit S. Meta-Analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: an update. Diabetes Technol Ther. 2009;11(12):775–784. doi: 10.1089/dia.2009.0091. [DOI] [PubMed] [Google Scholar]
- 21.Towfigh A, Romanova M, Weinreb JE, Munjas B, Suttorp MJ, Zhou A, Shekelle PG. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insula meta-analysis. Am J Manag Care. 2008;14(7):468–475. [PubMed] [Google Scholar]
- 22.Welschen LM, Bloemendal E, Nijpels G, Dekker JM, Heine RJ, Stalman WA, Bouter LM. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insula systematic review. Diabetes Care. 2005;28(6):1510–1517. doi: 10.2337/diacare.28.6.1510. [DOI] [PubMed] [Google Scholar]
- 23.Klonoff DC, Bergenstal R, Blonde L, Boren SA, Church TS, Gaffaney J, Jovanovic L, Kendall DM, Kollman C, Kovatchev BP, Leippert C, Owens DR, Polonsky WH, Reach G, Renard E, Riddell MC, Rubin RR, Schnell O, Siminiero LM, Vigersky RA, Wilson DM, Wollitzer AO. for the Coalition for Clinical Research. Consensus report of the coalition for clinical research-self-monitoring of blood glucose. J Diabetes Sci Technol. 2008;2(6):1030–1053. doi: 10.1177/193229680800200612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Petersen B, Schweitzer M, Wagner RS. Structured self-monitoring of blood glucose significantly reduces A1c levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262–267. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a trans-cutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 26.Mohan V, Ravikumar R, Poongothai S, Amutha A, Sowmya S, Karkhuzali K, Parkin CG. A single-center, open, comparative study of the effect of using self-monitoring of blood glucose to guide therapy on preclinical atherosclerotic markers in type 2 diabetic subjects. J Diabetes Sci Technol. 2010;4(4):942–948. doi: 10.1177/193229681000400425. [DOI] [PMC free article] [PubMed] [Google Scholar]