Abstract
Background:
Stress hyperglycemia in the critically ill has been found to be associated with increased morbidity and mortality. Studies have found significant improvements in morbidity and mortality in postsurgical patients whose glucose levels were closely maintained in the euglycemic range. However, subsequent studies, in particular the Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study, found no improvement in subjects with tight glycemic control. In addition to differences in protocol design, patients in the tight glycemic control arm of the NICE-SUGAR study experienced high rates of hypoglycemia compared with other studies. One interpretation of the NICE-SUGAR study results is that it is difficult to achieve normal glycemia in critically ill patients with existing glucose monitoring technology. The purpose of the study reported here was to evaluate the safety and performance of a continuous intravascular glucose sensor that could be used in the future in critically ill patients.
Methods:
A first-generation prototype of an intravascular continuous glucose sensor was evaluated in 29 volunteer subjects with type 1 diabetes mellitus. The sensor operates on the principle of quenched fluorescence. The fluorescent emission from the sensor chemistry is nonlinear, resulting in improved accuracy in the hypoglycemic range. The duration of each study was 8 hours. Sensor output was compared with temporally correlated reference measurements made from venous samples on a laboratory glucose analyzer.
Results:
Data were obtained from 18 of the 29 subjects in the study. Data were analyzed retrospectively using a factory calibration plus a one-point in vivo calibration. The mean absolute relative difference was 7.97%, and 95.1% of all the points were in zone A of the Clarke error grid.
Conclusions:
This pilot study was the first use in human subjects of a prototype of the GluCath Intravascular Continuous Glucose Monitoring System (GluCath System). The GluCath System is based on a novel fluorescent sensor chemistry. The study found the GluCath System had a high level of accuracy as compared with a laboratory reference analyzer.
Keywords: accuracy, fluorescence, hypoglycemia, intravascular continuous glucose monitor, sensor, tight glycemic control
Introduction
Transient hyperglycemia occurs in critically ill patients whether they have a preexisting diagnosis of diabetes or not and is associated with the counterregulatory hormone and cytokine response to trauma and severe illness. Hyperglycemia can be compounded by the high carbohydrate content of total parenteral nutrition, as well as insulin resistance induced by the administration of corticosteroids commonly used in the intensive care unit (ICU). The phenomenon of hyperglycemia in critically ill patients without diabetes was once believed to be an adaptive response to severe illness, guaranteeing an adequate supply of glucose to the brain in the presence of injury and loss of blood.1 Beginning with the work of Furnary and colleagues,2,3 however, it was found that efforts to minimize stress-induced hyperglycemia in postsurgical heart patients reduced complications and mortality in patients with and without diabetes. A landmark paper by Van den Berghe and associates4 compared outcomes of patients who were admitted to a surgical ICU with and without tight glycemic control. In the patients with tight glycemic control, blood sugar was maintained between 80 and 110 mg/dl, resulting in a reduction in mortality to 4.6% compared with 8% in the control group. Studies such as Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) have failed to reproduce the effects seen by Furnary and colleagues and Van den Berghe and associates, and new questions have been raised about the risks and benefits of tight glycemic control in the critically ill.5 A close examination of many of the studies, which did not find a benefit in tight glycemic control, found problems with the protocol design and unacceptable levels of hypoglycemia in the tight glycemic control group.6 It is believed that the advent of continuous glucose monitoring in the critically ill could shed light on these controversies and, more importantly, provide physicians and nurses with the ability to implement tight glycemic control in the hospital without increasing the risk of hypoglycemia.7,8 The study described here examined the accuracy and performance in type 1 diabetes volunteers of a prototype of the GluCath® System, an intravascular continuous glucose monitor intended for use in hospitalized patients. The GluCath System is under development by GluMetrics, Inc. (Irvine, CA). At the time of these studies, the GluCath System was an investigational device limited by United States law to investigational use only.
Methods
The GluCath System consists of a sensor, a monitor, a connecting cable between the monitor and the sensor, and a sterile calibration solution. The sensor consists of a 250 μm thick optical fiber containing the glucose-sensing chemistry. The sensor chemistry, which is based on the principle of quenched fluorescence, is immobilized in a hydrogel at the distal tip of the fiber. Light emitting diodes located in the monitor excite the chemistry, allowing for it to fluoresce in the presence of glucose. The sensor contains a temperature probe adjacent to the optical fiber to measure the temperature in the vicinity of the sensor and correct for the effect of temperature on the glucose response. Differences in the fluorescent sensor response at two distinct excitation wavelengths are used to measure the pH and to correct for the effect of pH on the glucose response.
The sensor is surrounded by a biocompatible microporous membrane that excludes red blood cells, thus providing a direct measurement of plasma glucose. The sensor is coated with a heparin-based antithrombogenic coating to inhibit fibrin and thrombus formation on the sensor. The total outer diameter of the sensor is 430 μm.
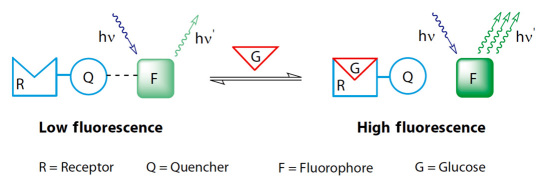
The sensor chemistry consists of three molecular components as shown in Figure 1: a fluorophore, a quencher, and a boronic-acid-based glucose receptor.
Figure 1.
Three molecular components of the GluCath sensor chemistry shown in the absence of glucose (low fluorescence) and in the presence of glucose (high fluorescence).
The fluorophore is excited by blue light at 420 and 470 nm and emits green light at 530 nm. The quencher is bound to the glucose receptor in a single molecule, the receptor quencher. In the absence of glucose, the receptor quencher binds to the fluorophore and reduces the fluorescence emission. In the presence of glucose, the interaction between receptor quencher and fluorophore weakens, resulting in an increase in fluorescence intensity. The intensity of the fluorescence therefore depends on the concentration of glucose.9
The primary challenge for continuous glucose monitoring in the ICU is sensor accuracy at low glucose concentration. An accurate, continuous glucose sensor could enable clinicians to maintain their patients' glucose levels in tight control without increasing the risk of hypoglycemia. The nonlinearity of the GluCath sensor fluorescence intensity as a function of glucose is directly responsible for the high accuracy in the hypoglycemic range. The greatest fractional increase in fluorescence as a function of glucose is observed in the hypoglycemic and euglycemic range. When the glucose value changes by 150 mg/dl from 50 to 200 mg/dl, the increase in fluorescence is 40%, resulting in a 2 mg/dl resolution in that range. When the glucose changes by 150 mg/dl from 250 to 400 mg/dl, there is an increase in fluorescence of 6%, resulting in a 10 mg/dl resolution in that range.
An 8 hour outpatient feasibility study was designed to evaluate the sensor. The study was approved by the Western Institutional Review Board (Olympia, WA). All subjects gave informed witnessed consent to participate in the study. The subjects were otherwise healthy volunteers with type 1 diabetes mellitus. Subjects were asked to arrive at the clinic with normal glycemia, but some arrived in hypoglycemia and some arrived in hyperglycemia. During the study, the subjects were in a semirecumbent position in an adjustable hospital bed except for occasional walks to and from a bathroom. The sensors remained indwelling during such times, and the monitors traveled with the patients either in a fanny pack or on a movable IV pole. Per the study protocol, the subjects administered insulin on the advice of a physician to achieve mild hypoglycemia (<70 mg/dl). Otherwise, the subjects adhered to their previous diabetes management regimens. All the subjects ate lunch approximately 3 to 5 hours after sensor insertion and gave insulin by pump or injection per their preexisting diabetes regimen.
The sensor was inserted into a peripheral vein (e.g., cephalic or basilic vein) of the forearm using a standard 22G over-the-needle splittable introducer. After the sensor was inserted into the vessel through the introducer, the introducer was retracted from the vessel, split apart, and discarded, leaving only the sensor in the vessel. The sensor was held in place with surgical adhesive tape. In some subjects, a second sensor was placed in a different peripheral vessel in the arm using the same procedure. In all subjects, an intravenous catheter was placed in the contralateral arm for the purpose of obtaining venous blood samples for measurement on a laboratory reference analyzer. Venous samples were taken once every 15 min (±5 min) for measurement on an ABL 805 Flex Blood Gas Analyzer (Radiometer, Copenhagen, Denmark). A total of 32 venous samples were collected over the 8 h duration of the study. In this study, the blood gas analyzer was operated by research personnel and was not certified for clinical laboratory use. Accordingly, the samples were also measured with a SureStep Flexx hospital blood glucose meter (LifeScan, Milpitas, CA). These measurements were made by appropriately trained clinic personnel. All clinical decisions regarding the management of the subject's glycemic levels were made using the SureStep Flexx meter. Data from the GluCath System was compared to the blood gas analyzer and not the SureStep blood glucose meter.
The prototype monitors used in the study stored the detected fluorescence emission but did not display the calibrated data in real time. The sensors were calibrated retrospectively using a simulated prospective calibration based on a factory calibration plus a one-point in vivo calibration obtained 30 min after sensor insertion. The in vivo calibration utilized a venous blood sample measured with the ABL Radiometer 805 blood gas analyzer. Data from the GluCath System was compared with the ABL Radiometer values and analyzed using accepted methods for continuous glucose monitoring systems such as the mean absolute relative difference (MARD), the Clarke error grid, as well as conformity with the International Organization of Standardization (ISO) 15197 standard for the clinical accuracy of glucose meters.
Results
Performance of the first-generation prototype GluCath System was evaluated in a total of 29 subjects. There were 12 female subjects and 17 male subjects. The subjects ranged in age from 19 to 68 years with a mean of 48 years. The duration of diabetes in the subjects ranged from 5 to 44 years with a mean of 26 years.
A total of 45 sensors were inserted into these subjects. The prototype device was found to be sensitive to a number of motion-related artifacts, particularly as related to the optical connectors. Data from 25 sensors were excluded from the analysis presented here because of motion artifacts (n = 16) or sensor damage (n = 9). The studies described here were also used to optimize the method of sensor insertion into the vessel. The data reported here consist of the results from 20 sensors inserted into 18 different subjects. These data were used for assessment of sensor accuracy and performance. There were only two subjects in which both sensors functioned properly for the entire duration of the study. This is not a sufficient number to do a credible analysis of the intrasensor reliability or performance. In one subject, the MARD was 6.15% on one sensor and 7.04% on the other sensor. In the other subject, the MARD was 7.7% on one sensor and 9.2% on the other sensor.
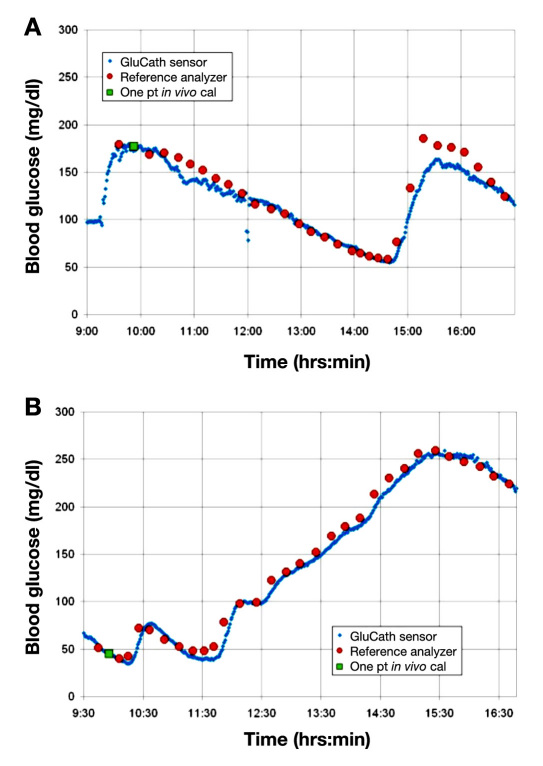
In Figure 2, the temporal traces of the calibrated GluCath System are shown for two different subjects and compared with the laboratory reference measurements. Analysis of the data found a high percentage of points in zone A of the Clarke error grid for these subjects (96.8% and 93.3%, respectively). The MARD was also found to be low for both of these subjects (6.28% and 7.0%, respectively).
Figure 2.
Temporal traces of the calibrated GluCath intravascular continuous glucose sensor for two subjects with type 1 diabetes compared with ABL Radiometer 805 Blood Gas Analyzer.
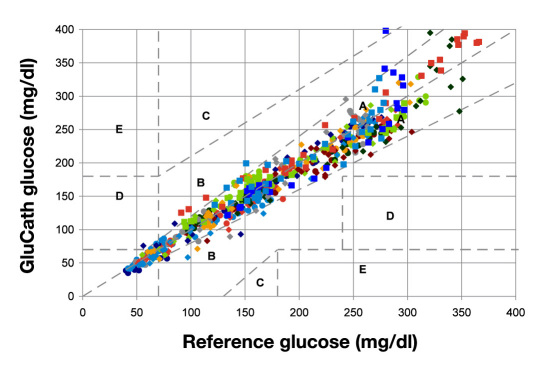
Figure 3 shows the composite Clarke error grid for 18 subjects and 20 sensors. As noted earlier, this is a subset of the total data excluding subjects and sensors that were designated for purposes of evaluating changes in sensor hardware. Data from sensors for which there were gross malfunctions in the optical fiber or instrument were also excluded. In Figure 3, 95% of all the points were in zone A of the Clarke error grid, and the MARD was 7.9%. In the lower left quadrant of the grid (less than 120 mg/dl), 95.6% of all points were in zone A of the Clarke error grid, and the MARD was approximately 7.7%.
Figure 3.
Composite Clarke error grid for 18 subjects and 20 sensors. Ninety-five percent of all the points were in zone A of the Clarke error grid, and the MARD was 7.9%.
Accuracy of continuous glucose sensors for tight glycemic control, as noted earlier, is most important in the hypo-glycemic range because of the need to provide timely and reliable warnings for actual or impending hypoglycemia. In the data shown in Figure 3, there were 11 paired points with a MARD of 7.4% for reference analyzer measurements less than 50 mg/dl. There were 66 paired points with a MARD of 8.7% between 51 and 80 mg/dl. Finally, there were 83 paired points between 81 and 120 mg/dl with a MARD equal to 7.8%. The different colors and symbols in the figure denote data from separate sensors.
These data are summarized further in Tables 1 and 2. Table 1 gives the accuracy of the sensor compared with ISO 15197 for values below 75 mg/dl and above 75 mg/dl. While ISO 15197 was not designed for continuous glucose sensors, it is the relevant accuracy standard that point-of-care glucose monitoring systems must currently meet and thus provides a starting point for assessing individual (temporally discrete) readings of a continuous glucose sensor.
Table 1.
GluCath Sensor Data Stratified per ISO 15197
| <75 mg/dl | ≤75 mg/dl | ||
|---|---|---|---|
| Relative difference | Number of samples | Relative difference | Number of samples |
| ≤ ±5 mg/dl | 40/66 (60.6%) | ≤ ±5% | 203/508 (40.0%) |
| ≤ ±10 mg/dl | 57/66 (86.4%) | ≤ ±10% | 361/508 (71.1%) |
| ≤ ±15 mg/dl | 65/66 (98.5%) | ≤ ±15% | 445/508 (87.6%) |
| — | ≤ ±20% | 483/508 (95.1%) | |
Table 2.
GluCath Sensor Accuracy and Performancea
| Glucose (mg/dl) | Number of samples | Clarke A Zone | MARD |
|---|---|---|---|
| ≤50 | 11 | 11/11 (100%) | 7.40% |
| 51–80 | 66 | 62/66 (93.9%) | 8.74% |
| 81–120 | 83 | 75/83 (90.4%) | 7.80% |
| 121–240 | 252 | 243/252 (96.4%) | 7.85% |
| ≤241 | 162 | 155/162 (95.7%) | 7.96% |
| All results | 574 | 546/574 (95.1%) | 7.97% |
a Zone A of the Clarke error grid and MARD stratified by glucose level.
According to the standard, a glucose measurement device is clinically accurate if 95% or more of all points less than 75 mg/dl fall within ±15 mg/dl and if 95% of all points equal to or greater than 75 mg/dl fall within ±20% of the reference sample measurements. Data from the GluCath System shown earlier conforms to the ISO 15197 standard for clinical accuracy of blood glucose measurements.
Table 2 shows the accuracy and performance of the GluCath System compared with the reference measure-ments for different ranges of glucose.
In the data set shown in the composite Clarke error grid (Figure 3) and Table 2, there were a total of 574 paired points. The percentage of points in zone A of the Clarke error grid was 95.1%, and the overall MARD was 7.97%.
Discussion
Table 2 shows a relatively constant MARD across all glucose ranges. The lowest MARD, 7.4%, was found in the lowest glucose range less than or equal to 50 mg/dl. This observation is consistent with the nonlinear response of the sensor that results in the largest fractional change in fluorescence per unit of glucose in the hypoglycemic range. This finding is in contrast with typical published results from electrochemical enzymatic glucose sensors in which the highest MARD is usually found in the lowest glucose range. The highest MARD, 8.74%, was found in the range of 51 to 80 mg/dl. The small but discernible increase in MARD in this range was associated with a slight reduction in accuracy at the high rates of change (>3 mg/dl/min) induced by treatment of hypoglycemia in this range and below.
While the results reported here are encouraging, there are a number of limitations in this study. This study was done with an early prototype of the GluCath monitor, which was susceptible to motion artifacts and other instrument errors. Sensor reliability was relatively poor. Highly accurate data for the entire 8 hour duration of the study was obtained in only 18 of 29 subjects. A second-generation version of the GluCath monitor has been found to be relatively insusceptible to motion artifacts. In addition, the prototype monitor used in the study stored the raw fluorescent return signal proportional to glucose and did not perform the mathematical transformation of the fluorescent signal to a calibrated glucose signal in real time. Accordingly, the data in this study were retrospectively analyzed using a simulated prospective calibration. Subsequent studies with a second-generation instrument containing a full prospective calibration found no difference between a retrospective simulated prospective calibration and a true prospective calibration.
Another important limitation of the present study is its relatively short 8-hour duration. The ultimate use of this technology is envisioned for 24 to 48 hours, consistent with the clinical need for patients in the ICU. The ability of the sensor chemistry to measure glucose correctly depends in part on maintaining the thromboresistance of the antithrombogenic coating. The antithrombogenic coating used in these studies consisted of ionically bound heparin applied to the outer membrane of the sensor. There was no evidence of fibrin or thrombus on the sensors after 8 hours of use in this study. Animal studies in an ovine model have suggested good thromboresistance up to 48 hours. Human clinical studies are currently underway to assess sensor performance at 24 hours duration.
Finally, this study was done in healthy volunteers with type 1 diabetes mellitus using a relatively small set of concomitant medications. No effect was seen on sensor performance with any of the medications used by this small set of subjects (e.g., cholesterol-lowering drugs, blood pressure medication, birth control pills, prophylactic aspirin, and hormone replacement therapy). Additional studies will be needed in the ICU to evaluate the effect, if any, of commonly used medications in the critically ill on sensor performance and accuracy. In addition, subjects in this study were at normal physiological temperatures and pH. The sensor is sensitive in principle to variations in blood temperature. The temperature sensor adjacent to the optical fiber found variations in the peripheral blood vessel temperatures, but over a relatively narrow range compared with what is likely to be encountered in the ICU (e.g., 30 to 37 °C). In the ICU, iatrogenically induced hypothermia is likely to result in significantly lower peripheral blood temperatures. Sensor accuracy and performance will need to be validated over the full range of temperatures encountered in that setting. Similarly, the sensor is sensitive to variations in pH. In a study in healthy volunteer subjects, only minimal pH excursions were observed. Sensor accuracy and performance will also need to be verified over the full range of pH values seen in critically ill patients (e.g., 6.8 to 7.8).
Conclusions
This was the first pilot study in humans of the GluCath System, a novel intravascular continuous glucose monitoring system intended for hospitalized patients. This study demonstrated that the GluCath System is capable of achieving a high level of accuracy as shown in the temporal traces, the composite Clarke error grid, and the ISO 15197 tables. The use of quenched fluorescence for glucose sensing is an innovative and fundamentally different method of measurement than typical electro-chemical enzymatic glucose sensors. The GluCath System is highly selective for glucose, and it gives a continuous and direct measurement of plasma glucose in flowing blood. The nonlinear response provides an enhanced accuracy in hypoglycemia.
There is a growing interest in the use of continuous glucose sensors in the intensive care setting. Published results suggest that current commercially available electro-chemical sensors based in the interstitial fluid may have comparable accuracy in critically ill hospitalized patients as in otherwise healthy ambulatory patients. In a study by Bridges and coworkers10 that used the Medtronic Guardian sensor and measured interstitial fluid in critically ill children, the percentage of points in zone A of the Clarke error grid was 74.4%, and the MARD was 15.3%. The results reported in this article from the GluCath System achieved a higher level of accuracy than reported with continuous glucose sensors inserted in subcutaneous adipose tissue. This improved accuracy is likely due primarily to the fact that the GluCath System is intravascular and makes a direct measurement of blood glucose as opposed to interstitial fluid glucose. However, the improved accuracy in hypoglycemia of the GluCath System compared with the current generation of electrochemical enzymatic sensors may be another reason for the difference in accuracy and performance overall.
It remains to be seen what the actual requirements are for a clinically and commercially viable continuous glucose sensor in the critical care environment. Clearly, the system must be easy to use, must be reliable, and must conform to standard nursing practice in the ICU. The exact accuracy requirements for continuous glucose sensors in the ICU are not known from either a clinical or regulatory perspective. The field of tight glycemic control has been the subject of great controversy, and glycemic target levels have been raised out of concern for the deleterious effect of hypoglycemia. Despite the controversy, the advent of accurate and reliable continuous intravascular glucose sensors are likely to play a major role in resolving the question of optimal glycemic targets in the critically ill and providing improved care for patients in the ICU.
Acknowledgments
The sponsor, GluMetrics, wishes to acknowledge support by the Industry University Cooperative Research Program of the University of California.
Abbreviations
- (ICU)
intensive care unit
- (ISO)
International Organization for Standardization
- (MARD)
mean absolute relative difference
- (NICE-SUGAR)
Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation
Funding:
This work was funded by GluMetrics, Inc.
Disclosures:
Howard Zisser, Lois Jovanovič, Uzma Khan, and Wendy Bevier were paid by GluMetrics to conduct the clinical study. They do not own stock in GluMetrics, and they did not receive any other compensation. At the time of the study, Thomas Peyser, Matt Romey, Jeff Suri, Paul Strasma, Stephanie Tiaden, and Soya Gamsey were full-time employees of GluMetrics, Inc.
References:
- 1.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyper-glycemia. Crit Care Clin. 2001;17(1):107–124. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 2.Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63(2):356–361. doi: 10.1016/s0003-4975(96)01044-2. [DOI] [PubMed] [Google Scholar]
- 3.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 5.NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 6.Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, Mesotten D. Clinical review: intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. 2009;94(9):3163–3170. doi: 10.1210/jc.2009-0663. [DOI] [PubMed] [Google Scholar]
- 7.Krinsley JS. Glycemic variability in critical illness and the end of Chapter 1. Crit Care Med. 2010;38(4):1206–1208. doi: 10.1097/CCM.0b013e3181d3aba5. [DOI] [PubMed] [Google Scholar]
- 8.Mebis L, Gunst J, Langouche L, Vanhorebeek I, Van den Berghe G. Indication and practical use of intensive insulin therapy in the critically ill. Curr Opin Crit Care. 2007;13(4):392–398. doi: 10.1097/MCC.0b013e3281c1c9c8. [DOI] [PubMed] [Google Scholar]
- 9.Gamsey S, Suri JT, Wessling RA, Singaram B. Continuous glucose detection using boronic acid-substituted viologens in fluorescent hydrogels: linker effects and extension to fiber optics. Langmuir. 2006;22(21):9067–9074. doi: 10.1021/la0617053. [DOI] [PubMed] [Google Scholar]
- 10.Bridges BC, Preissig CM, Maher KO, Rigby MR. Continuous glucose monitors prove highly accurate in critically ill children. Crit Care. 2010;14(5):R176. doi: 10.1186/cc9280. [DOI] [PMC free article] [PubMed] [Google Scholar]