Abstract
Background:
The Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QOL-DN) is a validated comprehensive questionnaire designed to capture the entire spectrum of DN related to large fiber, small fiber, and autonomic neuropathy not captured in existing instruments. We aimed to determine if the Norfolk QOL-DN could be used to capture changes in QOL that correlate with nerve fiber-specific objective measures in a placebo-controlled trial of two agents that affect different nerve fibers.
Methods:
Sixty patients with DN were allocated to treatment on ruboxistaurin (RBX) (n = 18), topiramate (TPX) (n = 18), or placebo (n = 18). QOL-DN was administered and objective measures of nerve function were performed at entry and end of the study period.
Results:
Total QOL scores improved significantly in the active treatment groups (RBX -9.56 ± 4.13; TPX -12.22 ± 2.76) but not in placebo (-5.56 ± 3.49). There were differences in nerve function improvement between treatments. Neurological symptom scores (NSS) improved with TPX from 5.5 (2.3) to 4.3 (0.65) (p = .007), sensory scores improved with TPX from 15.5 (1.79) to 8.3 (1.19) (p < .001), motor scores did not change, and sensory and motor impairment scores improved with TPX from 18.8 (2.15) to 12.1 (1.71) (p = .003). Total neuropathy scores (TNS) improved with TPX from 24.35 (2.61) to 16.35 (2.02) (p = .001). Neuropathy total symptom score-6 (NTSS-6) changes were significant for both treatments: RBX 4.38 (0.75) to 1.49 (0.38) (p < .001) and TPX 7.57 (1.3) to 4.26 (0.95) (p = .036). Changes in QOL-DN large fiber subscores correlated (Spearman's rank) significantly with changes in NTSS-6 (r = 0.55; p < .0001), NSS (r = 0.31; p < .04), neuropathy impairment score (NIS) (r = 0.35; p < .02), and TNS (r = 0.48; p < .0006). Changes in QOL-DN small fiber subscores correlated significantly with changes in NTSS-6 total scores (r = 0.40; p < .005) and intraepidermal nerve fiber density (IENFD) (r = -0.29; p < .05).
Conclusion:
Ruboxistaurin produced significant improvement in large fiber measures while TPX produced significant changes in small fiber measures. The Norfolk QOL-DN tool differentiated between these changes captured in the fiber-specific domains. Correlations were found between objective measures of neuropathy and total QOL, but those with nerve fiber domain scores were modest and reinforce the need to quantify QOL as an endpoint in neuropathy independent of other measures.
Keywords: diabetic neuropathy, Norfolk QOL-DN, protein kinase C, quality of life, topiramate
Introduction
Diabetic peripheral neuropathy (DPN) is characterized by damage to both large myelinated Aα and Aβ nerve fibers as well as small, thinly myelinated C-fibers. Small fiber damage may occur first in the lower limb and precede large fiber damage, making it one of the earliest indicators of DPN.1–4 Small fiber DPN is associated with increased morbidity and mortality. Symptoms include numbness, pain, and decreased sensation as well as autonomic symptoms such as anhidrotic skin, orthostatic hypotension, resting tachycardia, hypoglycemia unaware-ness, delayed gastric emptying, decreased bladder tone, and impotence.1–5
It is now widely recognized that neuropathy can be associated with diminished physical and emotional functioning and affective symptoms. These can have a direct effect on the perception and interpretation of pain and quality of life (QOL).6,7 There is increasing evidence that diabetic neuropathy (DN) impacts QOL in different domains.8,9 However, the impact of drugs that target different nerve fiber pathways of DN has not been reported.
There are few DN-specific QOL assessment tools.10,11 The Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QOL-DN) is a comprehensive questionnaire that captures the entire spectrum of DN related to large, small, and autonomic nerve fibers. Validated in a large multicenter study the Norfolk-QOL-DN has been shown to correlate well with the total neuropathy score (TNS) as well as nerve fiber-specific functions.8,12 We hypothesized that the Norfolk QOL-DN domain subscores would reflect treatment with drugs that targeted specific nerve fiber populations: topiramate and ruboxistaurin.
Topiramate (TPX)
Topiramate, a structurally novel antiepileptic drug is effective in the treatment of various types of epilepsy and the prevention of migraine headaches. Topiramate has several pharmacologic properties suggesting it may have the potential for overcoming programmed cell death implicated in the pathogenesis of neuropathy.13 It enhances gamma-aminobutyric acid (GABA) activity by interacting with a nonbenzodiazepine site on the GABA receptor, has negative modulatory effects on voltage-activated Na+ channels,14 selectively blocks α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate glutamate receptors, has negative modulatory effects on L-type high voltage-activated Ca++ channels,15 and inhibits the carbonic anhydrase isozymes CA-I and CA-II.16 These multiple mechanisms of action may contribute to its anticonvulsive, antinociceptive, and putative neuroprotective properties. Topiramate has been shown to promote neurite outgrowth and recovery of function after nerve injury, particularly targeting large fibers.17 It relieves pain, and in animal studies, is neurotropic and neuroprotective.18 There may be an added benefit in treating diabetes patients with neuropathy because it exerts both neurotropic and metabolic effects.13,19 We have reported that TPX treatment for 18 weeks induces regeneration of intraepidermal nerve fiber density (IENFD) in patients with diabetic neuropathy.20
Ruboxistaurin (RBX)
Protein kinase C-β (PKC-β) overactivation has been associated with DPN.21–23 Protein kinase C-β is over-activated by hyperglycemia and by disordered fatty acid metabolism, resulting in increased production of vasoconstrictive, angiogenic, and chemotactic cytokines, including transforming growth factor-β, vascular endo-thelial growth factor, endothelin-1, and intercellular adhesion molecules.24
Ruboxistaurin mesylate specifically inhibits PKC-β overactivation, blocking a critical step in the pathogenesis of DPN via its impact on a microvascular mechanism.21 We have demonstrated that treatment with RBX induced improvement in damage to large nerve fiber functions.25
Methods
We entered 60 patients into a placebo-controlled trial of RBX and TPX on DPN carried out for >18 weeks. All subjects had type 2 diabetes, were between 35–75 years of age, and had documented evidence of DPN.20,25 Exclusion criteria included neurological disease or neuro-pathy from a cause other than diabetes, uncontrolled diabetes (more than two episodes of ketoacidosis or hyperosmolar state requiring hospitalization and/or six or more episodes of hypoglycemia requiring assistance within 3 months before study entry), blood pressure >160/95 mm Hg, impaired renal function (creatinine >1.5 mg/dl in males and 1.4 mg/dl in females) or active hepatic disease (transaminases >3x normal), pregnancy or lactation, and suspected carcinoma or 5-year history of carcinoma. Patients were on treatment for hyperglycemia, dyslipidemia, and hypertension, and those with uncontrolled diabetes, hypertension, and dyslipidemia were excluded from the study to ensure that improvements in neuropathy measures could be attributed to the therapeutic agent and not to improvement in diabetes management. Sample size was based on published studies25 using the same methodologies described herein, which demonstrated that 60 patients (20 per arm) needed to be randomized to achieve 80% power for observing statistical significance at the p = .05 level. The Eastern Virginia Medical School Institutional Review Board approved the study and all subjects gave written informed consent prior to any study procedures.
Baseline demographic and clinical data of the 60 patients in each group (n = 20/group) are shown in Table 1. Intraepidermal nerve fiber density, sensory responses, and skin blood flow (SKBF) were assessed before and after treatment in subjects with DPN. After initial evaluation, all patients received active treatment with TPX, titrated to 100 mg/day or the maximum tolerated dose, or treatment with RBX 32 mg/day or placebo. Adverse events were assessed. Of the 60 patients, 54 had complete QOL data and were included in this comparison study. Eighteen were treated with placebo, 18 with RBX, and 18 with TPX. Six patients who failed to complete the QOL questionnaire were not included in the analysis.
Table 1.
Baseline Characteristics
| Characteristic | Placebo (n = 18) | RBX (n = 18) | tpx (n = 18) | p valueb |
|---|---|---|---|---|
| Age (year)a | 57.83 (2.87) | 58.11 (2.02) | 61.06 (1.38) | NS |
| Gender: (female/male) | 3/5 | 3/15 | 5/13 | NS |
| Origin: (cc/aa/other) | 14/4/0 | 14/2/2 | 15/3/0 | NS |
| Height (m)a | 1.77 (0.02) | 1.74 (0.02) | 1.79 (0.02) | NS |
| Weight (kg)a | 103.93 (7.46) | 93.28 (4.37) | 105.19 (5.89) | NS |
| BMI (kg/m2)a | 33.22 (2.21) | 30.71 (1.27) | 32.58 (1.38) | NS |
| Waist circumference (cm)a | 109.62 (5.0) | 108 (3.70) | 113.18 (4.46) | NS |
| HbA1c (%)a | 7.55 (0.25) | 6.93 (0.26) | 7.46 (0.34) | NS |
| SBP (mm Hg)a | 132.17 (3.74) | 133.44 (3.42) | 141.5 (4.50) | NS |
| DBP (mm Hg)a | 73.95 (2.54) | 73.83 (1.78) | 81.83 (2.0) | <.05 |
| Total cholesterol (mg/dl) | 171.0 (9.6) | 174.9 (9.6) | 176.0 (27) | NS |
| LDL (mg/dl) | 99.2 (8.4) | 95.8 (7.1) | 94.0 (7) | NS |
| HDL (mg/dl) | 49.6 (2.9) | 55.1 (4.2) | 46 (3) | NS |
| Triglycerides (mg/dl) | 112.8 (11.7) | 115.7 (18.6) | 176.0 (27) | <.05 |
| NTSS-6 total score (points) | 5.04 (1.12) | 4.82 (0.75) | 7.1 (1.39) | <.05 |
| NSS (points) | 5.8 (0.7) | 5.3 (0.7) | 5.6 (0.65) | NS |
| NIS (points) | 11.3 (1.7) | 7.4 (1.0) | 18.8 (2.15) | <.05 |
| MS (points) | 3.5 (0.4) | 1.7 (0.3) | 3.3 (0.8) | <.05 |
| SS (points) | 7.7 (1.5) | 5.7 (0.8) | 15.5 (1.77) | <.05 |
DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NS not significant; SBP, systolic blood pressure; cc, Caucasian; aa, African American.
Data are presented as mean (± SEM).
Between-group difference was measured by Kruskal-Wallis Test for continuous data and by chi-square test for categorical data.
Measurements
Questionnaires
The Norfolk QOL-DN is a 47-item, self-administered questionnaire designed to measure the relationship between symptomatic DN and QOL from the perspective of the patient. It is composed of two parts: questions related to symptoms experienced by the patient and questions related to the impact of the patient's neuropathy on activities of daily life. A factor analysis performed on the Norfolk QOL-DN separated the questions into five domains: activities of daily living, symptoms, small fiber neuropathy, large fiber neuropathy, and autonomic neuropathy.26,27
The neuropathy total symptom score-6 (NTSS-6) was administered by a trained health professional and used to grade the frequency and intensity of aching, burning, lancinating pain, prickling sensation, numbness, and allodynia in patients' feet and legs.28
Quantitative Neuropathy Testing
All patients had a complete history, physical, and graded neurological examination. Neurological symptom scores (NSS) were generated by completion of a questionnaire. Neurological impairment scores of the lower limb (NIS-LL) were calculated by adding a motor score (MS) and sensory score (SS) obtained from physical and neurological examination. The sum of the NSS and the NIS were calculated to determine the TNS modified from Dyck29 as described by Casellini and colleagues.25 Neuropathy was established by the criteria suggested by the American Diabetes Association (ADA) and American Academy of Neurology.30 Quantitative sensory testing (QST) included measures of temperature and touch perception thresholds. Quantitative autonomic function (QAFT) was assessed by three tests: the heart rate variability during deep breathing at six breaths per minute (E:I ratio), the R-R variation in response to the Valsalva maneuver, and the R-R variation in response to postural change as suggested by the ADA position statement on neuropathy.31
Quantitative sensory testing was performed 2 cm proximal to the skin biopsy site using the Medoc device (TSA2001/VSA3001, Medoc Advanced Medical Systems, Ltd, Ramat Yishai, Israel) following published procedures.31 Touch pressure was measured using graded monofilaments (Semmes-Weinstein).32 The CASE-IV device (WR Medical Electronics, Stillwater, MN) was used to assess vibration perception for the RBX study.
Nerve conduction studies (NCS) were performed on the nondominant peroneal (motor), sural sensory, and ulnar (motor and sensory) nerves using standard techniques.
Laser Doppler Skin Blood Flow Detection of C-Fiber Function
Laser Doppler SkBF assessment of the C-fiber response to varying stimuli was performed as described in other studies33,34 on the dorsum of the nondominant foot and proximal calf at baseline and at the end of treatment. Skin blood flow examinations were also performed at the site of the biopsies and on the dorsum of the dominant foot (TPX) and the distal calf (RBX) with the patient in a reclined position with the legs elevated and completely extended. After a 10-minute baseline period, the temperature was increased to 32 °C for 10 minutes, 40 °C for 10 minutes, and then to 44 °C for 20 minutes.
Quantitative Intraepidermal Nerve Fiber Density
Skin punch biopsies (3 mm) were performed under local anesthesia. Biopsies were collected from each patient's dorsal forearm, lateral thigh (10 cm proximal to patella), lateral proximal leg (10 cm distal to fibular head), and lateral distal leg (10 cm proximal to lateral malleolus). Tissue samples were immediately fixed in 2% buffered paraformaldehyde/lysine/periodate solution for 12–24 hours at 4 °C and then cryoprotected in phosphate buffer with 20% glycerol overnight and frozen for later cryosectioning.
Procedures for identifying neurons in skin biopsies employed immunofluorescence techniques in a modification of the protocol described by McCarthy and colleagues.35,36 The processing and cutting of the sections followed published procedures.36,37
Statistical Methods
The primary endpoints were change in total QOL and the individual QOL domains. Spearman's rank correlation coefficient was used to correlate the total neuropathy score and subscores from different domains of the QOL-DN with symptoms and objective measures of neuropathy. The secondary endpoints were SkBF, QST, and indices of IENFD and length, all of which are continuous (parametric) data. In addition, nonparametric Spearman's rank correlations were performed to examine the relationship among IENFD, SkBF, and measures of neurologic function.
The independent variable was treatment type. Repeated measures of ANOVA (analysis of variance) were used to examine the treatment effects for SkBF, QST, and IENFD. Significance was accepted at p < .05.
Results
Baseline characteristics are reported in Table 1. The only significant differences in the three treatment groups at baseline were diastolic blood pressure and triglycerides. These were higher in the TPX treatment group than in the placebo or RBX groups, which were not significantly different from one another. Of the measures of neuropathy, the NTSS-6, NIS, MS, and SS were significantly higher in the TPX treatment group than in the RBX and placebo groups, and the latter two were not significantly different from each other.
Changes in Neurologic Function (Table 2)
Table 2.
| Baseline | Posttreatment | p value | |
|---|---|---|---|
| Neuropathy symptom score (NSS) | |||
| Placebo | 5.8 (0.7) | 5.0 (0.7) | NS |
| RBX-32 | 5.3 (0.7) | 4.2 (0.7) | NS |
| TPX | 5.6 (2.3) | 4.3 (0.65) | .007 |
| Sensory score (SS) | |||
| Placebo | 7.7 (1.5) | 5.9 (1.2) | NS |
| RBX-32 | 5.7 (0.8) | 4.0 (0.8) | NS |
| TPX | 15.5 (1.79) | 8.3 (1.19) | <.001 |
| Motor score (MS) | |||
| Placebo | 3.5 (0.4) | 2.4 (0.4) | .03 |
| RBX-32 | 1.7 (0.3) | 1.6 (0.3) | NS |
| TPX | 3.3 (0.8) | 3.8 (0.9) | NS |
| Neuropathy impairment score (SS + MS) | |||
| Placebo | 11.3 (1.7) | 8.3 (1.3) | NS |
| RBX-32 | 7.4 (1.0) | 5.6 (0.9) | NS |
| TPX | 18.8 (2.15) | 12.1 (1.71) | .003 |
| Total neuropathy score (NIS + NSS) | |||
| Placebo | 17.0 (2.1) | 13.3 (1.8) | NS |
| RBX-32 | 12.7 (1.5) | 9.8 (1.3) | NS |
| TPX | 24.35 (2.61) | 16.35 (2.02) | .001 |
| NTSS-6 | |||
| Placebo | 5.04 (1.12) | 4.38 (1.15) | NS |
| RBX-32 | 4.38 (0.75) | 1.49 (0.38) | <.001 |
| TPX | 7.57 (1.3) | 4.26 (0.95) | .036 |
NS, not significant.
Data are presented as mean (± SEM).
Within-group analysis was performed using Wilcoxon signed-rank test.
Total QOL scores improved significantly in the active treatment groups (RBX -9.56 ± 4.13; TPX -12.22 ± 2.76) but not in placebo (-5.56 ± 3.49). There were differences in nerve function improvement between the two therapeutic agents. The NSS improved with TPX from 5.5 (2.3) to 4.3 (0.65) (p = .007), SS improved with TPX from 15.5 (1.79) to 8.3 (1.19) (p < .001), MS did not change, and sensory and motor impairment scores improved with TPX from 18.8 (2.15) to 12.1 (1.71) (p = .003). The TNS improved with TPX from 24.35 (2.61) to 16.35 (2.02) (p = .001). The changes in NTSS-6 were significant for both agents: RBX 4.38 (0.75) to 1.49 (0.38) (p < .001) and TPX 7.57 (1.3) to 4.26 (0.95) (p = .036). Changes in the QOL-DN large fiber subscore correlated (Spearman's rank) significantly with changes in the NTSS-6 (r = 0.55; p < .0001), NSS (r = 0.31; p < .04), NIS (r = 0.35; p < .02), and TNS (r = 0.48; p < .0006). Changes in the QOL-DN small fiber subscore correlated significantly with changes in the NTSS-6 total score (r = 0.40; p < .005) and IENFD (r = -0.29; p < .05).
Norfolk QOL-DN
Both TPX and RBX groups were compared to the placebo group on factors of the Norfolk QOL-DN. Table 3 shows the differences in each of the QOL domains across each of the three groups. The QOL total score improved significantly at endpoint in the RBX and TPX groups but not in the placebo group. The symptom subscore also improved significantly in both treatment groups but not in the placebo group. The large fiber subscore improved significantly in the TPX group but not in the RBX or placebo group.
Table 3.
QOL-DN Improvement at Endpoint in the Three Groupsa
| QOL domain | Placebo | RBX | TPX | |||
|---|---|---|---|---|---|---|
| Difference | p value | Difference | p value | Difference | p value | |
| Total QOL | -5.56 ± 3.49 | NS | -9.56 ± -4.13 | <.04 | -12.22 ± 2.76 | <.001 |
| Symptoms | -0.28 ± 0.82 | NS | -2.27 ± 0.81 | <.004 | -4.89 ± 0.88 | <.0001 |
| Large fiber | -3.67 ± 2.23 | NS | -4.74 ± 2.69 | NS | -5.61 ± 1.64 | <.05 |
| Small fiber | -1.22 ± 0.69 | NS | -0.5 ± 0.36 | NS | -1.06 ± 0.56 | NS |
| Activities of daily living | -0.39 ± 0.50 | NS | -1.06 ± 0.62 | NS | -0.61 ± 0.54 | NS |
| Autonomic | 0 ± 0.29 | NS | -0.56 ± 0.38 | NS | -0.06 ± 0.26 | NS |
NS, not significant.
Data presented as mean (± SEM).
We have published the results for the neuropathy and metabolic parameters for each treatment group in individual papers.20,38 A summary of these results as they pertain to this segment of the study are provided.
Changes in IENFD and SkBF
Treatment with TPX significantly increased mean dendritic length in the forearm and proximal leg and IENFD in proximal leg. This was associated with improvement in TNS, including touch, vibration, and prickling pain thresholds as well as reduction in weight, body mass index (BMI), systolic and diastolic blood pressures, and hemoglobin A1c (HbA1c). Skin blood flow C-fiber detection was measured at baseline and 18 weeks. A significant increase in SkBF was apparent at week 12 but returned to baseline at week 18. Laser Doppler blood flow in the leg increased from 87.7 ± 0.1 to 101.3 ± 0.1 (p < .001) and in the foot from 102.6 ± 0.2 to 143.7 ± 0.6 (p < .001) laser Doppler units at 12 weeks. No correlations were found between the changes in measures of neurologic function, IENFD, and the changes in metabolic parameters.20
As published by Casellini and colleagues,38 RBX had positive within-group treatment effects on endothelium-dependent and C-fiber-mediated SkBF at the distal calf, sensory symptoms (NTSS-6), and measures of the QOL-DN. In addition, RBX had positive between-group treatment effects on change from baseline in NTSS-6 total score and QOL symptom subscore. No significant within- or between-group RBX effects were observed for the remaining efficacy measures—nerve fiber morphometry, QST, QAFT, and NCS.38
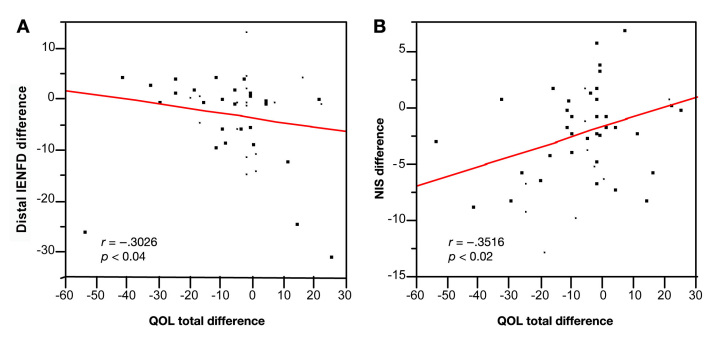
Table 4 shows the correlations between changes in the total QOL score as well as four of its five domains with objective measures of neuropathy in the RBX and TPX groups. The changes in total neuropathy scores correlated significantly with five of the objective measures of neuropathy including NTSS-6, NSS, NIS, TNS, and IENFD as illustrated in Figures 1A and 1B. The fifth domain of the QOL score, autonomic function, is not listed in the table because it did not correlate with any of the objective measures of neuropathy. Skin blood flow measurements are not reported in the table because neither the total QOL score nor its domains correlated significantly with changes in SkBF.
Table 4.
Correlation between Changes in Total QOL and Nerve Fiber Domains with Subjective and Objective Measures of Neuropathy in the RBX and TPX Groupsa
| Total QOL-DN and each domain | |||||
|---|---|---|---|---|---|
| Total | Symptoms | Large fiber | Small fiber | Activities of daily living | |
| NTSS-6 | r = 0.5446 p < .0001 | r = 0.3971 p < .01 | r = 0.5538 p < .0001 | r = 0.4029 p < .005 | r = 0.4121 p < .004 |
| NSS | r = 0.2849 p < .05 | r = 0.1755 p = NS | r = 0.3123 p < .04 | r = 0.1616 p = NS | r = 0.2149 p = NS |
| NIS | r = 0.3081 p < .04 | r = 0.3516 p < .02 | r = 0.3521 p < .02 | r = 0.0540 p = NS | r = 0.2511 p = NS |
| TNS | r = 0.4256 p < .003 | r = 0.4171 p < .004 | r = 0.4843 p < .0006 | r = 0.1250 p = NS | r = 0.3065 p < .04 |
| IENFD | r = -0.3026 p < .04 | r = -0.1157 p = NS | r = -0.2556 p = NS | r = -0.2900 p < .05 | r = -0.2841 p = NS |
NS, not significant.
All correlations were made using Spearman's rank correlation coefficient.
Figure 1.
(A) Spearman's rank test showed that the total QOL-DN difference correlated significantly with the IENFD difference. (B) Spearman's rank test showed that the total QOL-DN difference correlated significantly with the change in NIS.
Discussion
This study showed that the Norfolk QOL-DN successfully detected changes in neuropathy that correlated with the underlying disease state and measures of neuropathy of the patients and was able to distinguish differences in the actions of the two treatments tested in this study. The QOL tool also correlated with objective measures of changes in DN, showing that it may be possible to use this tool as an endpoint in determining the efficacy of new treatments for DPN. The total Norfolk QOL-DN correlated strongly with the TNS, which embraces symptoms of neuropathy and the neurologic examination of motor function, sensory perception, and reflexes. The improvement in patient-reported QOL was associated with an improvement in nerve function assessed using both subjective and objective measures.
Correlations between changes in total QOL and nerve fiber domains with objective measures of neuropathy are modest and reinforce the need to quantify QOL as an endpoint in neuropathy independent of other measures. An improvement in QOL as assessed by the Norfolk QOL-DN can be used as a primary measure of response to therapy in clinical trials as well as clinical practice.
Although symptoms of neuropathy are not suitable to serve as independent diagnostic criteria for DPN, it has been suggested that the development of innovative tools for specific symptom evaluation may be important in the assessment of treatment outcomes for investigational drugs and as a marker of disease progression.28 The Norfolk QOL-DN was designed to help fill this need. Some clinical trials have used short healthcare-administered symptom-only questionnaires such as the NTSS-6, which does not cover the total complex of DPN-related QOL.39 The Neuro-QOL questionnaire has been used to measure the impact of foot ulcers on QOL and depression9 and has shown that depression predicts the first foot ulcer but not recurrent foot ulceration. The tool does not, however, address the remaining components of neuropathy on QOL.40 Other instruments, developed for a comprehensive evaluation of symptom domains have been used in clinical trials only in conjunction with complete neurological examinations as the primary endpoint. These tools have failed to demonstrate a correlation between the underlying changes in the symptom score and the underlying disease state.28 The Norfolk QOL-DN proved to be responsive to changes in the patients' neurologic status over a 6-month time period and these changes correlated with the patients' underlying disease state. This is the first trial in which a comprehensive neuropathy QOL tool has shown the capability to detect changes in neuropathy symptoms and nerve fiber-specific domains in response to pharmacological intervention independent of other objective measures.
Topiramate was shown here to improve IENFD as well as TNS, including touch, vibration, and prickling pain thresholds, indicating that TPX has effects on small, unmyelinated epidermal nerve fibers in the skin but also exercises an effect on large fiber neuropathy. The large fiber domain of the Norfolk QOL-DN was able to detect this difference and correlate with the improvement in the underlying disease state. This is also the first QOL tool that has been able to correlate QOL changes with changes in the underlying disease state while at the same time distinguishing the effects of two mechanistically different neuropathy treatments. These findings suggest that comprehensive QOL tools such as the Norfolk QOL-DN could be used as a primary outcome measure in head-to-head neuropathy trials. Such tools also elucidate symptom domains not captured by traditional measures of neuropathy, a cardinal issue for measuring the impact of an intervention on patients' well-being.
The ability of the Norfolk QOL-DN to show clinically significant changes in this short 6-month trial may prove to be of great importance in large efficacy trials such as the MBBQ study on RBX. Data from this cohort of DPN patients suggest that treatment of patients with neuropathy with the isoform-specific PKC inhibitor may reduce sensory symptoms and improve endothelial-dependent SkBF. However, the study failed to meet its primary endpoints. This failure may have been caused by the observance of placebo effects that were consistent with other studies of short duration on DPN, most likely due to the variability in the responses measured and the longer duration required to show changes in large fiber measures such as nerve conduction studies.
Conclusions
Our study shows that although longer duration studies of nerve function are warranted and necessary to detect consistent changes in neuropathy, the Norfolk QOL-DN could be used as a primary endpoint to detect significant differences in QOL that correlate with improvement in underlying diseases states, even in studies of short duration. Based on our other studies on QOL, we show that, in terms of percent reduction of QOL to TNS, the TNS score of an average patient would drop approximately one point, representing a 1% improvement in the QOL-DN score.
Our study has limitations. Because the patient population was well-defined at baseline, it is unclear whether the same RBX and TPX effects would be observed after short-term treatment in the general population of DPN patients, including those with more severe disease. Also, because the study was only 6 months in duration, it is unclear what the effect of RBX and TPX would be on SkBF and other measures of DPN at later time points. Studies of longer duration are necessary to confirm and extend our observations to affirm that the observed changes are sustained over the long term and that the Norfolk QOL-DN continues to detect domain-specific changes that correlate with changes in the underlying disease state. It is possible that the changes in symptoms could precede and predict changes in the underlying nerve function and that the Norfolk QOL-DN would subserve to function as a surrogate for measures such as nerve conduction, notorious for their relative insensitivity to short-term intervention. Nerve conduction measures require studies of long duration to determine efficacy and are costly, time-consuming, and difficult to carry out. Tools such as the Norfolk QOL-DN are capable of providing acceptable endpoints for short-term studies, which are surrogates for changes in the structure and function of the neuropathic process.
Abbreviations
- (ADA)
American Diabetes Association
- (BMI)
body mass index
- (DN)
diabetic neuropathy
- (DPN)
diabetic peripheral neuropathy
- (GABA)
gamma-aminobutyric acid
- (HbA1c)
hemoglobin A1c
- (IENFD)
intraepidermal nerve fiber density
- (MS)
motor score
- (NCS)
nerve conduction studies
- (NIS)
neuropathy impairment score
- (NIS-LL)
neurological impairment scores of the lower limb
- (Norfolk QOL-DN)
Norfolk Quality of Life Diabetic Neuropathy
- (NS)
not significant
- (NSS)
neurological symptom score
- (NTSS-6)
neuropathy total symptom score-6
- (PKC-β)
protein kinase C-β
- (QAFT)
quantitative autonomic function
- (QOL)
quality of life
- (QST)
quantitative sensory testing
- (RBX)
ruboxistaurin
- (SEM)
standard error of the mean
- (SkBF)
skin blood flow
- (SS)
sensory score
- (TNS)
total neuropathy score
- (TPX)
topiramate
Disclosures:
Publication of study results was not contingent on the sponsor's approval or censorship of the article. The Norfolk QOL-DN is owned and Copyrighted by Eastern Virginia Medical School. A portion of the funding from this study came from Eli Lilly and Company.
References:
- 1.Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuro-pathies. Diabetologia. 2000;43(8):957–963. doi: 10.1007/s001250051477. [DOI] [PubMed] [Google Scholar]
- 2.Vinik A, Erbas T, Stansberry KB, Pittenger G. Small fiber neuro-pathy and neurovascular disturbances in diabetes mellitus. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S451–S473. doi: 10.1055/s-2001-18602. [DOI] [PubMed] [Google Scholar]
- 3.Al-Shekhlee A, Chelimsky TC, Preston DC. Review: small-fiber neuropathy. Neurologist. 2002;8(4):237–253. doi: 10.1097/00127893-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Vinik A, Ullal J, Parson HK, Casellini CM. Diabetic neuropathies: clinical manifestations and current treatment options. Nat Clin Pract Endocrinol Metab. 2006;2(5):269–281. doi: 10.1038/ncpendmet0142. [DOI] [PubMed] [Google Scholar]
- 5.Lacomis D. Small-fiber neuropathy [abstract] Muscle Nerve. 2002;26(2):173–188. doi: 10.1002/mus.10181. [DOI] [PubMed] [Google Scholar]
- 6.Benbow SJ, Wallymahmed ME, MacFarlane IA. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91(11):733–737. doi: 10.1093/qjmed/91.11.733. [DOI] [PubMed] [Google Scholar]
- 7.Grootenhuis PA, Snoek FJ, Heine RJ, Bouter LM. Development of a type 2 diabetes symptom checklist: a measure of symptom severity. Diabet Med. 1994;11(3):253–261. doi: 10.1111/j.1464-5491.1994.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 8.Vinik EJ, Stansberry KB, Zarrabi L, Witherspoon CAG, McNitt PM, Vinik AI. Development of a sensitive, specific quality of life inventory for peripheral neuropathy [abstract] Diabetes. 2000;49:A819. [Google Scholar]
- 9.Vileikyte L, Peyrot M, Bundy C, Rubin RR, Leventhal H, Mora P, Shaw JE, Baker P, Boulton AJ. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care. 2003;26(9):2549–2555. doi: 10.2337/diacare.26.9.2549. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264–AS279. [PubMed] [Google Scholar]
- 11.Guyatt GH, Bombardier C, Tugwell PX. Measuring disease-specific quality of life in clinical trials. CMAJ. 1986;134(8):889–895. [PMC free article] [PubMed] [Google Scholar]
- 12.Vinik E, Stansberry K, Vinik A. Use of neuropathy quality of life tool (Norfolk QOL-DN) in a large clinical trial: comparison with symptom scores (NSS), nerve impairment (NISS), quantitative sensory (QST), autonomic (QAFT) tests and electrophysiology [abstract]. Abstracts of the 63rd Scientific Sessions of the American Diabetes Association. June 13–17, 2003, New Orleans, Louisiana, USA. Diabetes. 2003;52(Suppl 1):A198. [Google Scholar]
- 13.Raskin P, Donofrio PD, Rosenthal NR, Hewitt DJ, Jordan DM, Xiang J, Vinik AI. Topiramate vs placebo in painful diabetic neuro-pathy: analgesic and metabolic effects. Neurology. 2004;63(5):865–873. doi: 10.1212/01.wnl.0000137341.89781.14. [DOI] [PubMed] [Google Scholar]
- 14.Chong MS, Libretto SE. The rationale and use of topiramate for treating neuropathic pain. Clin J Pain. 2003;19(1):59–68. doi: 10.1097/00002508-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 15.White HS. Molecular pharmacology of topiramate: managing seizures and preventing migraine. Headache. 2005;45(Suppl 1):S48–S56. doi: 10.1111/j.1526-4610.2005.4501006.x. [DOI] [PubMed] [Google Scholar]
- 16.Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41(Suppl 1):S35–S39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 17.Donofrio PD, Raskin P, Rosenthal NR, Hewitt DJ, Jordan DM, Xiang J, Vinik AI. Safety and effectiveness of topiramate for the management of painful diabetic peripheral neuropathy in an open-label extension study. Clin Ther. 2005;27(9):1420–1431. doi: 10.1016/j.clinthera.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Shadiack AM, Molino LJ, Yagel SK, Shank R, Rogers KP, Codd EE, Raffa RB, Wild KD. The novel anticonvulsant topiramate is anti-allodynic in a rat model of neuropathic pain. Analgesia. 1999;4:173–179. [Google Scholar]
- 19.Ben-Menachem E, Axelsen M, Johanson EH, Stagge A, Smith U. Predictors of weight loss in adults with topiramate-treated epilepsy. Obes Res. 2003;11(4):556–562. doi: 10.1038/oby.2003.78. [DOI] [PubMed] [Google Scholar]
- 20.Boyd AL, Barlow PM, Pittenger GL, Simmons KF, Vinik AI. Topiramate improves neurovascular function, epidermal nerve fiber morphology, and metabolism in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2010;3:431–437. doi: 10.2147/DMSOTT.S13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Way KJ, Katai N, King GL. Protein kinase C and the development of diabetic vascular complications. Diabet Med. 2001;18(12):945–959. doi: 10.1046/j.0742-3071.2001.00638.x. [DOI] [PubMed] [Google Scholar]
- 22.Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA. 2002;288(20):2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 23.Setter SM, Campbell RK, Cahoon CJ. Biochemical pathways for microvascular complications of diabetes mellitus. Ann Pharmacother. 2003;37(12):1858–1866. doi: 10.1345/aph.1D002. [DOI] [PubMed] [Google Scholar]
- 24.Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr EJ 3rd; MBBQ Study Group. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C β-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clinical Therapeutics. 2005;27(8):1164–1180. doi: 10.1016/j.clinthera.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Casellini C, Barlow P, Rice A, Casey M, Simmons K, Pittenger G, Bastyr E, Vinik A. Effect of ruboxistaurin (RBX) on quantitative measures of diabetic peripheral neuropathy (DPN) Diabetes. 2006:791-P. doi: 10.2337/dc06-1699. [DOI] [PubMed] [Google Scholar]
- 26.Vinik EJ, Hayes RP, Oglesby A, Bastyr E, Barlow P, Ford-Molvik SL, Vinik AI. The development and validation of the Norfolk QOL-DN, a new measure of patients' perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther. 2005;7(3):497–508. doi: 10.1089/dia.2005.7.497. [DOI] [PubMed] [Google Scholar]
- 27.Vinik EJ, Paulson JF, Ford-Molvik SL, Vinik AI. German-Translated Norfolk Quality of Life (QOL-DN) Identifies the Same Factors as the English Version of the Tool and Discriminates Different Levels of Neuropathy Severity. J Diabetes Sci Technol. 2008 doi: 10.1177/193229680800200616. Vinik EJ, Paulson JF, Ford-Molvik SL, Vinik AI. German-Translated Norfolk Quality of Life (QOL-DN) Identifies the Same Factors as the English Version of the Tool and Discriminates Different Levels of Neuropathy Severity. J Diabetes Sci Technol. 2008 November; 2(6): 1075–1086 2(6):1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastyr EJ 3rd, Price KL, Bril V; MBBG Study Group. Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther. 2005;27(8):1278–1294. doi: 10.1016/j.clinthera.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Dyck PJ. Small-fiber neuropathy determination. Muscle Nerve. 1988;11(9):998–1000. doi: 10.1002/mus.880110911. [DOI] [PubMed] [Google Scholar]
- 30.Consensus statement: report, recommendations of the San Antonio conference on diabetic neuropathy. American Diabetes Association American Academy of Neurology. Diabetes Care. 1988;11(7):592–597. doi: 10.2337/diacare.11.7.592. [DOI] [PubMed] [Google Scholar]
- 31.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 32.Vinik AI, Suwanwalaikorn S, Stansberry KB, Holland MT, McNitt PM, Colen LE. Quantitative measurement of cutaneous perception in diabetic neuropathy. Muscle Nerve. 1995;18(6):574–584. doi: 10.1002/mus.880180603. [DOI] [PubMed] [Google Scholar]
- 33.Stansberry KB, Hill M, McNitt PM, Bhatt BA, Vinik AI. Skin blood flow reactivity and neuropathy [abstract] Diabetes. 1994;43(Suppl 1):107A. [Google Scholar]
- 34.Stansberry KB, Hill MA, Shapiro SA, McNitt PM, Bhatt BA, Vinik AI. Impairment of peripheral blood flow responses in diabetes resembles an enhanced aging effect. Diabetes Care. 1997;20(11):1711–1716. doi: 10.2337/diacare.20.11.1711. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45(10):1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 36.Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intra-epidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care. 2004;27(8):1974–1979. doi: 10.2337/diacare.27.8.1974. [DOI] [PubMed] [Google Scholar]
- 37.Pittenger G, Mehrabyan A, Simmons K, Dublin C, Barlow P, Vinik AI. Small fiber neuropathy is associated with the metabolic syndrome. Metab Syndr Relat Disord. 2005;3(2):113–121. doi: 10.1089/met.2005.3.113. [DOI] [PubMed] [Google Scholar]
- 38.Casellini CM, Barlow PM, Rice AL, Casey M, Simmons K, Pittenger G, Bastyr EJ 3rd, Wolka AM, Vinik AI. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007;30(4):896–902. doi: 10.2337/dc06-1699. [DOI] [PubMed] [Google Scholar]
- 39.Sinnreich M, Taylor BV, Dyck PJ. Diabetic neuropathies. Classification, clinical features, and pathophysiological basis. Neurologist. 2005;11(2):63–79. doi: 10.1097/01.nrl.0000156314.24508.ed. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez JS, Vileikyte L, Ulbrecht JS, Rubin RR, Garrow AP, Delgado C, Cavanagh PR, Boulton AJ, Peyrot M. Depression predicts first but not recurrent diabetic foot ulcers. Diabetologia. 2010;53(10):2241–2248. doi: 10.1007/s00125-010-1821-x. [DOI] [PubMed] [Google Scholar]