Abstract
Biosimilar insulins (BIs) are viewed as commercially attractive products by a number of companies. In order to obtain approval in the European Union or the United States, where there is not a single BI currently on the market, a manufacturer needs to demonstrate that a given BI has a safety and efficacy profile that is similar to that of the “original” insulin formulation that is already on the market. As trivial as this may appear at first glance, it is not trivial at all for a good number of reasons that will be discussed in this commentary. As with protein manufacturing, modifications in the structure of the insulin molecule can take place (which can have serious consequences for the biological effects induced), so a rigid and careful assessment is absolutely necessary. The example of Marvel's failed application with the European Medicines Agency provides insights into the regulatory and clinical challenges surrounding the matter of BI. Although a challenging BI approval process might be regarded as a hurdle to keep companies out of certain markets, it is fair to say that the potential safety and efficacy issues surrounding BI are substantial and relevant and do warrant a careful and evidence-driven approval process.
Keywords: biosimilar insulin, insulin antibodies, insulin formulations, insulin therapy
Introduction
The aim of this commentary is to summarize the current perspective on biosimilar insulins (BIs) and the respective U.S. and E.U. regulations. Based on the fact that not a single BI has yet been approved in the European Union or the United States, the example of the failed application of Marvel's BI in the European Union will be discussed in greater detail. This example highlights the complexity of the approach that regulatory bodies take toward BIs. It is not officially known how many applications for BIs have been filed, but it can be surely assumed that there are at least some.
Confusion of Terms
Different terms are used for BIs, which is a source of confusion:
Follow-on biologics or FoBs. This term is favored in the United States (sometimes, less precisely, named as follow-on proteins or FOPs.
Subsequent entry biologic or SEB. This term is used in Canada.
Similar biotherapeutic products or SBP. This term is used in the WHO guidance, RBP or reference biotherapeutic product.
Similar biological medicinal products or SBMPs. This term is used in Australia.
Biosimilars (the copies versions of biopharmaceuticals that have already been authorized). This term is favored in the European Union.
Biopharmaceuticals (drug products that contain biotechnology-derived proteins as active pharma-ceutical ingredients).
Biopharmaceutical products not subject to regulatory approval or B-NSRA.
Bioidenticals. Same product (“same vessel” in terms of active pharmaceutical ingredient, device might be different) sold under different brand names by different companies.
Interest of Diabetologists in Biosimilar Insulins
For most diabetologists, the efforts required to manufacture a protein-like insulin are not known, and they are not familiar with the complexity of the BI topic. Many regard insulin as a small molecule drug (i.e., generic), despite the considerable differences that exist (Table 1). However, as the example of erythropoietin (EPO) in dialysis patients shows (discussed later), this can be a dangerous misunderstanding. It has to be acknowledged that large international meetings about biosimilars are held each and every year; however, the crosstalk to the diabetes world is currently rather limited. Interestingly, the position/interest toward BIs is very different between countries, possibly reflecting a difference in attitude toward biotechnology or in acknowledging its relevance in general.
Table 1.
Differences between Generics and Biosimilars
| Generics | Biosimilars | |
|---|---|---|
| Product characteristics | Small molecules | Large complex molecules |
| Often very stable | Stability requires special treatment | |
| Typically taken orally | Devices are often the differentiating factor | |
| Production | Produced by chemical synthesis | Produced in living organisms |
| Highly sensitive to manufacturing changes | ||
| Often high production costs | ||
| Development | Very limited clinical trials (only bioequivalence studies) | Significant research and development (i.e. cell lines) |
| Clinical trials to a limited extent | ||
| Regulation | Shorter registration procedures in Europe and the United States | Regulatory pathway defined by the EMA |
| Usually enjoy “substitutability” status | “Comparability” status | |
| In the United States, law approved in March 2010, in force in October 2010 | ||
| Marketing | No or limited detailing to physicians | Detailing to (specialist) physicians required |
| High price reduction | Pharmacists may not substitute | |
| Market substitution in pharmacies | Lower price reduction | |
| Price sensitivity is product specific |
Background
The human insulin (HI) molecule, as a nonglycosylated, disulphide-bonded heterodimer of 51 amino acids, is the result of numerous years of evolution. In order to perform its many biological activities (blood glucose lowering is only one of these), this protein has not only a defined primary structure, but also a well-defined secondary and tertiary structure. Each and every change/modification of this structure may have a serious/relevant impact on the effects of HI.
In past decades, a number of insulin analogs were developed. These are insulin molecules with a primary structure that is different from that of HI. The aim of these developments was to achieve insulin formulations that have improved pharmacokinetic (PK) and pharma-codynamic (PD) properties in comparison to native HI formulations. With these rapid-acting and long-acting insulin analogs, a better coverage of prandial or basal insulin requirements is possible. Ever since insulin analogs were first developed around 1995, there has been a fierce discussion about the potential risks to patients posed by molecular changes introduced in the primary structure of the insulin analogs, (which has consequences for the three-dimensional structure of insulin molecules and the interaction between the molecules as well). The changes introduced are known to impact the intracellular signaling by altering the binding properties of the insulin analogs to the insulin receptor. Also, the higher binding affinity seen with some insulin analogs to the receptor of the insulin-like growth factor-1 was suspected to increase the risk of developing cancer. The developers of insulin analogs were quite careful to introduce changes to the insulin molecule that more or less only affected its self-aggregation properties but not, for example, its binding properties. In other words, it is well-known that changes in the structure of HI can affect the safety and efficacy of this therapeutic protein. Therefore, from a regulatory perspective, obtaining market approval for biosimilar HI (or insulin analogs) is more complex than for generics because they have been manufactured differently from the innovator products that are already on the market.1
Insulin Market
Currently, insulin is manufactured and marketed pre-dominantly by a relatively small number of companies that have been reluctant, for obvious reasons, to provide details on their insulin manufacturing process. However, with the insulin market being driven by an annual sales volume of several billion dollars worldwide, one can assume that more manufacturers will likely make substantial efforts to claim a share of this market. These new insulin manufacturing companies (most of which are located in India and China and with products already on the market in both countries) are interested in gaining a certain market share also in the regulated European and U.S. markets and hence will make efforts to obtain regulatory approval for their (mostly cheaper) products (Table 2). For example, Biocon established its HI plant in India in 2004. From the cost-payer perspective, it would be attractive to have access to insulin at more affordable prices, especially in view of the rapid increase in the number of patients with diabetes who do need insulin treatment. It was forecasted that BIs and insulin analogs will erode $6.1 billion in brand sales in the United States and Europe (France, Germany, Italy, Spain, and United Kingdom) by 2018, saving health care systems $3.8 billion in the process.
Table 2.
Companies Manufacturing Insulins That Might Become Biosimilar Insulins Once They Have Been Subjected to an Official Comparative Analysis with an Approved Reference Product (Regular HI)
| Name | Country | Insulin marketed (in the home country) |
|---|---|---|
| Wockhardt | India | Regular HI: Wosulin Analog: Glaritus (glargine) |
| Biocon | India | Regular HI: Insugen Analog: Basalog (glargine) |
| Bioton | Poland | Regular HI: Gensulin/Biosulin, SciLin |
| Tonghua DongBao/Gan&Lee | China | Regular HI: Comonlin Analog: Prandilin (insulin lispro), Basalin (glargine) |
| MJ Biopharm (Marvel Life Sciences) | India | Regular HI: Biosulin |
Insulin Manufacturing
For many decades, the major source of (animal) insulin was the pancreases from pigs and cows. After the discovery of the primary structure of the insulin molecule, total chemical synthesis appeared to be the next logical step for insulin production; however, this process is too complex and expensive at this time. Nevertheless, chemical synthesis might be an attractive option for the future, in addition to other novel approaches discussed later. Subsequent to the invention of the biotechnological production of HI in the 1990s, using genetically modified bacteria or yeast as “production machines,” this high-tech approach has been the predominant method for insulin production.
The progress made in production technology of recombinant proteins makes it relatively easy to manufacture BIs nowadays. The main reason for this is that the patents for HI have expired. In addition, many of the insulin analogs will come off patent relatively soon. Also, the methods used to manufacture HI/insulin analogs, which are covered by different patents, will expire. Moreover, manufacturing methods are further developed, and some of these methods actually changed the manufacturing of insulin to a higher yield process, thereby reducing the manufacturing costs.
It is important to understand that the nature of the manufacturing process defines the final product (“process is product”). Each and every manufacturing process differs from each other and to varying degrees. For example, strains of bacteria/yeast used are never identical and incubation technologies/conditions used are not the same. Clearly, this is because the manufacturing details are proprietary knowledge of the innovator. The consequence is that the BI molecules produced cannot be and are not 100% identical to HI; they are similar. The concern is that “minor” alterations in the manufacturing process can have considerable and potentially deleterious effects on the biological effects induced by such proteins (discussed later). In addition to changes of the HI molecule per se, attention must also be given to product-related substances/impurities and process-related impurities; in particular, desamido forms and other forms that may derive from the expression vector or arise from the conversion steps removing the C-peptide and regenerating the three-dimensional structure.
Manufacturing Costs and Market Considerations
The manufacturing costs for HI/BIs are relatively high, at least compared to the costs of a good number of generics. The production technology for BI is complex and typically requires a huge investment. For example, an insulin plant, which can produce the several hundred kilograms per year necessary to supply major markets, can cost more than $150 million. In addition to setting up and managing good manufacturing practice (GMP)-compliant manufacturing facilities, the costs for the clinical development/market approval are considerable too. Even if this can be managed and accomplished, transport of this heat-sensitive product in appropriate cooling chains, storage, distribution, and marketing are cost-intensive elements too. Therefore, the economic advantage that can be achieved with BIs may not be as high as with many generics but will most probably still be significant. It is reasonable to expect that the number of insulin formulations on the market will increase in the future, and the price for insulin will very likely decrease. While the economics might play out to the advantage of the health care sector, the plethora of insulin formulations and choices may actually increase confusion among patients, physicians, and pharmacists.
Looking at the prescription habits of physicians, it appears as if these are not mainly driven by economic considerations. Other factors that need to be considered by BI manufacturers are (1) approval and marketing, (2) the availability of insulin pens, (3) postmarketing infrastructure, and (4) logistics. The guarantee of long-term availability of a given insulin formulation is of relevance too, as physicians typically try to avoid switching patients from one insulin formulation to another without pressing needs.
Regulatory Approval by the European Medicines Agency/Committee for Medicinal Products for Human Use
The key question is whether potential differences between BIs and their already marketed competitors are of clinical relevance or not. Unfortunately, the answer cannot be determined by even the most state-of-the-art in vitro laboratory methods; identifying potential or real difference does require clinical studies with human beings to demonstrate that BIs have an equivalent safety and efficacy profile when compared to the original product.
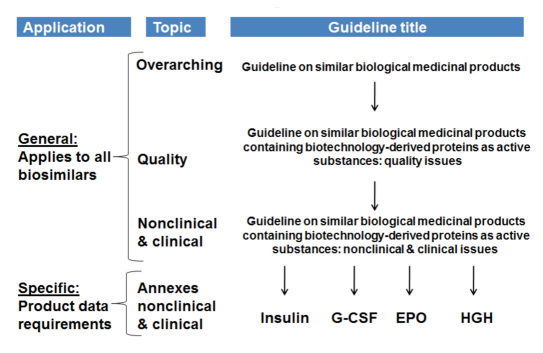
In 2009, in order to address the fact that biosimilars are a completely different class with different compound and manufacturing characteristics when compared to generics, the regulatory bodies in Europe developed a set of guidance documents that are specific to the development of biosimilar medicines. Other regulatory bodies are following Europe's lead or are soon to follow. The Appendix lists all relevant documents issued by the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) for the approval process for biosimilars (Figure 1). A simple comparison of bioavailability that is sufficient for most nonprotein, generic “small molecules” drugs is generally considered not sufficient for biosimilars.
Figure 1.
Overview of the EMA guidelines. Recognition of difference to generics via scientific and clinical class guidelines. G-CSF, granulocyte colony-stimulating factor; HGH, human growth hormone.
One annex to these biosimilar guideline documents is specifically addressing nonclinical and clinical require-ments for soluble insulin-containing products that claim to be similar to another product already on the market. It is of note though that these guidelines deal with only one class of insulin formulations (soluble insulin for prandial insulin therapy) and not with basal insulin formulations and/or any of the human insulin analog formulations.
The EMA requires that biosimilar manufacturers submit data that fully describe the chemical manufacturing characteristics (CMCs) or chemical manufacturing control of their products. Like innovators, manufacturers of biosimilars must completely describe their processes, including detailed and rigorous validation and monitoring of batch-to-batch variability—and especially the effect of any changes they may have introduced to the manufacturing process. The reference product for comparison must be one that is approved for clinical use within the European Union.
Comparison of Pharmacokinetic Properties
In the nonclinical section of the guideline for soluble insulin, the need for comparative in vitro studies is outlined. Topics such as using bioassays and binding assays, as well as toxicological aspects are covered. Clearly, the assays used should have a sensitivity that allows detecting very small differences. A minute difference in the spherical structure of the insulin molecule probably cannot be detected by any analytical method; however, the insulin receptor “sees” this, and the binding properties (i.e., intracellular signaling) might be different. Therefore, in the clinical section, it is required that at least one clinical study provides data on the relative PK properties of the BI and the reference product after subcutaneous (SC) administration. In such a single-dose crossover study, preferably in patients with type 1 diabetes, it should be possible to depict certain data from the time-concentration profile [area under the curve (AUC) as the primary endpoint and Cmax, Tmax, and T1/2 as secondary endpoints]. It is stated that factors contributing to PK variability should be taken into account (insulin dose, site of injection, and thickness of SC fat layer), but the method is not outlined.
Comparison of Pharmacodynamic Properties
To study the PD properties, a euglycemic glucose clamp study should be performed. The PD data collected in such a study are of key importance to demonstrate comparability of a BI. The study should be performed double blind, and PK samples should be collected as well. Interestingly, it is stated that the choice of the study population and the study duration should be justified, but no detailed recommendations or requirements are specified. According to this guideline, the shown clinical comparability in this study is sufficient for market approval for a given BI (i.e., no meaningful differences between this and the reference insulin exist), there is no need for efficacy studies on intermediary or clinical variables, but there is a need to demonstrate a comparable immunogenicity (discussed later).
These recommendations have to be seen in connection with the guideline on the investigation of bioequivalence. In this guideline, details about sample size calculation, endpoints (which differ somewhat for the one for soluble insulin), and statistical methods are outlined.
Regulatory Approval by the Food and Drug Administration
In 2001, the Food and Drug Administration (FDA) announced that it started working on guidelines for pharmaceutical companies to produce copy versions of synthetic insulin and human growth hormone. Despite an intensive discussion (see comments listed in the Appendix), the FDA has not yet issued a guideline document for the approval process of so-called follow-on proteins in the United States. Due to the increasing pressure in the United States to reduce health care costs' among them, the enormous costs for insulin therapy, which are massively increasing due to the enormous increase in the number of patients with diabetes—one can envisage that such a guideline will become available soon. The U.S. Congress is currently considering different bills of how the regulatory process should look like. The assumption was that such a bill will be passed in 2010; however, this was not the case. Most probably, for biosimilars, this will take place in 2011. The question is how long it will take the FDA to make its guidance for specific products. Two competing bills are being discussed in the House of Representatives. The first proposal from 2007, which favors generic producers, was submitted by Rep. Waxmann. An approval of the bill will make it possible for the FDA to approve applications for biosimilars without having to repeat expensive clinical studies. Rep. Eshoo's proposal from March 2008 favors the original producers, as this will require clinical studies to have biosimilars approved and is, in several areas, more restrictive than Rep. Waxmann's proposal.
On March 23, 2010, President Obama signed into law the health care reform legislation entitled, Patient Protection and Affordable Care Act, which had passed the House (as H.R. 3590) on March 21 and which was passed by the Senate (also as H.R. 3590) on December 24, 2009. A memorandum entitled Biologics Price Competition and Innovation summarizes Subtitle A of Title VII, which creates a regulatory approval pathway for biosimilars and a litigation procedure for patent infringement lawsuits brought against Biosimilar applicants.
Any person may submit an application for licensure of a biological product under a new section, 351(k), of the Public Health Service Act. The legislation amends Section 351(a)(1)(A) to provide that a biological product may not be shipped in interstate commerce unless it has a license approved under Subsection (a) or Subsection (k). The definition of “biological product” in Section 351(i) is revised to include proteins, except any chemically synthesized polypeptide. “Biological product” was previously defined as a virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, analogous product, or arsphenamine or derivative of arsphenamine (or any other trivalent organic arsenic compound) applicable to the prevention, treatment, or cure of a disease or condition of human beings. The phrase “reference product” is defined as the single biological product licensed under Subsection (a) against which a biological product is evaluated in an application submitted under Subsection (k).
Most probably, it will become clearer in the near future if the FDA will employ a similar approach to BIs as the EMA.
Insulin Antibodies
Formation of antibodies to HI was not a hot topic for diabetologists for many years, as this occurs frequently but without major consequences for efficacy or safety. The observed increase in antibody titers with adminis-tration of HI via SC injection—which was much more pronounced with animal insulin (from bovine or porcine sources)—or also via the pulmonary route, for example, was not associated with relevant changes in clinical parameters.2 However, as this demonstrates, the immune system is able to detect small differences in the molecule structure of a given protein. Interestingly, the HI applied by this route induced more response of the pulmonary system than insulin analogs (with a different primary structure in at least one amino acid) applied subcutaneously. So it is not easy to predict if immunogenicity of BI may result in insulin antibodies that will have an impact on insulin efficacy and hence glucodynamics. The immunogenicity might also be related to the purity of the given insulin formulation. As the safety concerns about BI relate mainly to the potential of different immunogenicity, clinical trials with a sufficient duration (at least 6–12 months) shall be performed (see the CHMP guideline for HI in the Appendix). The primary outcome measure is the incidence of anti-bodies to the BI and the reference insulin formulation. The study population should have a history of previous insulin exposure. Data should be collected that allow analyzing the correlation of immunogenicity data with clinical data (insulin requirements, metabolic control, and allergic reactions). In case such observations were made in the studies described, additional studies investigating local reactions might be necessary (see the EMA guideline in the Appendix).
Pharmacovigilance Plan and Risk Management Plan
The standards specified by the EMA to prove that a given BI is safe and efficient might be regarded as high. In fact, they may be looked at as measures to protect existing markets for already established insulin manufacturers, as they require a considerable amount of logistics and organization that a company new to the market has issues providing. However, the question really is whether relatively small studies with several hundreds of patients are sufficient to demonstrate safety on a large scale. Clearly, the introduction of a risk management program as suggested by the European guidelines could be aimed to cover this risk. A systematic and prospective postapproval evaluation of a marketed insulin would provide information about safety and efficacy after prolonged periods of usage by larger groups of patients (see the CHMP guideline for HI in the Appendix). However, at least in Europe/Germany, it sometimes seems that pharmacovigilance is not taken very seriously, e.g., by treating physicians, due to all the work involved in reporting a potentially drug-related adverse event. Therefore, the question is, with what certainty could even clinically meaningful differences induced by a BI, e.g., resulting in a difference in metabolic (or other) effects, be identified by a systematic postmarketing (phase 4) study? The differences most probably have to be pronounced (or serious) to be detectable.
Risks Associated with Biosimilar Proteins
One might say that, even if the manufacturing processes for BI production differ to a given extent from those used by the manufacturers of market-approved insulin formulations, the differences in the final molecule are so small that they would not have any clinical relevance. With all the risks involved in analogs, it is worth looking at the experience with other biosimilar proteins; there is at least one prominent example illustrating that there may be considerable risks.
Erythropoietin is used by nephrologists regularly to treat or prevent anemia in dialysis patients. A minor change in the formulation of a given brand of EPO in Europe increased the incidence of pure red cell aplasia, and a number of the dialysis patients treated with the differently processed EPO product died as a consequence from the immune reaction induced by the different product.1,3 The affected patients experienced an antibody-mediated neutralization of endogenous EPO and a complete block in the differentiation of red blood cells. This case has clearly contributed to the awareness that the consequences of introducing process changes have to be monitored quite carefully.
Case Report: Marvel's Insulin
Marvel Lifesciences Private Limited markets active pharma-ceutical ingredients and pharmaceutical finished dose forms for the MJ Group's insulin manufactured in India (http://www.mjbiopharm.com/anti_diabetics.htm). This company (Marvel) has developed a new insulin product synthesized by recombinant deoxyribonucleic acid technology using E. coli cells specially transformed to express the HI gene as the source of the hormone, which is then extracted and purified as the final insulin crystals. Thus Marvel is one of the large generic recombinant HI suppliers in the world and has a long-term tie-up and supply agreement with suppliers of insulin crystals (recombinant human and animal-based crystals) and markets all the types of insulin in a number of countries. Subsequently, in March 2007, Marvel submitted the first European application for a BI for a marketing authorization for recombinant HI in three different formulations: a soluble rapid-acting insulin (Marvel Rapid), a long-acting isophane insulin product (Marvel Long), and a 30:70 mixture (30% soluble, 70% insulin) of these two products (Marvel Mix).
In the end, Marvel officially notified the CHMP in January 2008 that it wished to withdraw its application for their BI formulations (see a list of all documents released by the EMA for this case in the Appendix). This example of a failed application will be discussed in more detail, as a number of lessons can be learned from the three withdrawal reports published (one for each insulin formulation). As the dossiers are not publically available, the subsequently discussed information submitted to EMA is based solely on these reports.4 Unfortunately, many interesting and relevant details are not presented in these reports. It appears as if the dossiers submitted were of suboptimal quality, at least according to the comments by the rapporteurs stated explicitly (“There is a general consensus amongst all assessors that the overall quality of the dossier is very poor”) and more implicitly throughout the three reports.
The CHMP raised numerous concerns about the adequacy of the submission. They also published a list of questions. The major objections raised were about research quality aspects (biosimilarity was not adequately demonstrated, quality failure, and insufficient data), drug substance (process of manufacturing not detailed, e.g., purification), drug product (product not detailed [vials versus cartridges]), and nonclinical aspects (i.e., GMP and CMC was not adequately documented). Probably of similar relevance was that the submission had not followed the guidance documents adequately (e.g., an adequate PK comparison to the reference product had not been carried out).
After the review of the data, the CHMP was of the opinion that the Marvel BIs and the reference HIs were not comparable. Moreover, it was unclear whether the comparators used actually valid reference products, e.g., a comparator product with market approval in Europe. The CHMP also noted that the dose-delivery properties of different presentations (vials and cartridges) had not been adequately tested and validated. Furthermore, in the case of Marvel Long, the protamine used to form the isophane crystals was not adequately characterized, and neither the manufacturing process nor the crystallization process was documented in sufficient detail. In the case of Marvel Mix, there were also no details of formulation studies demonstrating a stabilized 30:70 mixture. The CHMP concluded that none of the three products had sufficiently demonstrated its biosimilarity to a properly chosen reference product.
Besides all these more formal aspects (which will not be discussed subsequently in detail), it appears as if the Marvel insulin tends to be more rapidly absorbed than the reference insulin, i.e., there were/are real differences between the Marvel insulin and the reference insulin when it comes to the PD properties. One reason for these results may be that the three-dimensional structure of the two insulins were not identical and that, subsequently, the forces that keep the insulin monomers together as an insulin hexamer (insulin monomers self-assemble themselves to dimers and hexamers) are weaker with Marvel insulin than with other HIs.
In the following, the major focus is on the clinical aspects raised, assuming that these were very relevant to the failure:
The PD study failed to demonstrate equivalent blood glucose lowering effect compared to the reference product.
The efficacy and safety data, which cannot be used to compensate for the failure of PD similarity, showed consistent trends in favor of the reference products.
The immunogenicity of the Marvel insulin products was not properly evaluated.
Three PD studies using a manual euglycemic clamp technique, one for each formulation, were conducted in 24 healthy male volunteers (not in patients with type 1 diabetes as it is recommended for PK studies in the guideline). It is not clear if the same 24 subjects participated in all three studies or if these were different subjects. The studies were performed by a clinical research organization (CRO; FARMOVS-PAREXEL, Bloemfontein, South Africa) with a lot of experience in the euglycemic clamp technique. For each of the formulations, the company carried out a single-dose, randomized, crossover clamp study comparing the Marvel product with a reference originator insulin; however, these studies were not blinded, and endogenous insulin secretion was not suppressed by an intravenous low-dose insulin infusion. Also, no information about the target blood glucose level and the quality of the glucose clamps are available. The PK data were derived from these PD studies, but no independent PK studies were done. In particular, the single-dose crossover comparative study using SC injection recommended by the CHMP was not carried out. The insulin applied (0.2 IU/kg body weight) should be high enough to induce a robust metabolic response, but at least with the isophane insulin, the absolute levels of glucose consumption observed were low. The CHMP specifically mentioned that the applicant did not justify the choice of the classical bioequivalence confidence intervals of 80–125% for the mean AUCs ratio of both PD (primary endpoint) and PK data in the specific context of BIs. In addition, the justification for widening the limits of the interval for the maximum insulin concentration is not acceptable. The primary endpoint [AUC of the glucose infusion rate (GIR) between 0 h (time of insulin injection) and the end of clamp] does not provide any clue about the time-action profile of the insulin applied, i.e., it is not of high relevance for the clinical use of a given insulin.
Marvel Rapid
While total AUC for GIR and PD data for Marvel Rapid was considered equivalent to that of the reference insulin (Humulin R) because it fell within the classical interval of 80–125%, the early AUC within the first 2 h postdose was higher: 45% in the first hour of blood glucose lowering effect (Figure 2). It is of note that the nonequidistant scaling of the y-axis of the PK figure for Marvel Rapid (and Marvel Mix) is that shown in the withdrawal report. Also, that the mean insulin levels were zero before the SC injection is puzzling in healthy subjects; one would have anticipated at least insulin levels in the range of 5–10 μU/ml. That the GIR was zero before 0 min is due to the manual clamp technique used at this site. It requires a certain decline in blood glucose before GIR is started; therefore the onset of the GIR profile is always very rapid.
Figure 2.
Pharmacokinetic (left-hand column) and PD data (right-hand column) generated during euglycemic glucose clamps in 24 healthy subjects. These received single SC injections of three different insulin formulations with Marvels insulin (Marvel Rapid, Marvel Long, and Marvel Mix; black curves) on one study day and injections of the respective HI formulation (used as a reference medicine already authorized in the European Union [Humulin, from Eli Lilly; Humulin R (or S), Humulin N (or I), and Humulin M3, respectively; red curves] on the other study day. Glucose infusion rate was smoothed (adapted from Reference 3).
In this study, there was a good correlation between PK and PD data, e.g., the maximum insulin levels were 16% higher. Also, the elimination half life and residence time were shorter for Marvel Rapid than for the comparator. In other words, Marvel Rapid had a faster absorption, a more potent maximal effect, and a faster elimination than the reference product. The CHMP concluded, “Test insulin was absorbed and eliminated faster than the reference, which resulted in a higher early but shorter effect on blood glucose.” In summary, this is an unacceptable degree of difference with obvious clinical relevance.
Marvel Long
With Marvel Long, the mean insulin profile was super-imposable with those of Humulin N (Figure 2), i.e., the PK parameter shows bioequivalence. However, the CHMP stated that they used a too short sample schedule (which might also explain the different level observed after 24 h) and recommended a multiple dose study. In contrast to the identical PK data, the PD parameter showed that Marvel Long had lower effect on blood glucose than the reference insulin; the total GIR was 27% lower. One has to acknowledge that the metabolic effect induced in general was low (<2 mg/kg/min). From this study, it appears as if Marvel Long has a lower potency than the reference insulin formulation.
Marvel Mix
With this insulin mixture, the insulin profiles were super- imposable except in first 4 h; they were slightly higher than with the reference insulin. This fits the observation made with Marvel Rapid (30% of this insulin mixture is soluble insulin). The main PK parameter showed bioequivalence. However, in this study, again no good correlation between PK and PD parameters were observed, i.e., the PD parameters differed again (like with Marvel Long). It is of note that, in this study (in contrast to Marvel Long), Marvel Mix (which consists of 70% of Marvel Long) had higher effect on blood glucose; the total GIR was 23% higher. Marvel attributed this apparent inconsistency to batch-to-batch variability.
Marvel also presented the results of a single efficacy and safety multicenter clinical study in 526 patients with type 1 or type 2 diabetes who received either the Marvel insulins or the reference HIs. This consisted of a 6-month, double-blind, comparative phase testing all three Marvel insulin formulations against their respective reference products, followed by an open-label, 6-month extension whose results were not part of the dossier.
Marvel tried to compensate for the disappointing PD studies (i.e., no biosimilarity) by this clinical efficacy and safety study in patients with diabetes, an approach not at all accepted by the CHMP. One wonders why Marvel has undertaken this approach. It appears as if Marvel started the development of this application before the specific annex document for soluble insulin was issued. They also asked for the scientific advice of the national authorities of three European Union member states: Finland, Sweden, and Netherlands. Thus the applicant considered the analysis of efficacy of this clinical trial as pivotal to demonstrate BI.
In contrast, the CHMP stated that the sensitivity to detect differences between insulin products is higher for euglycemic clamp PK/PD studies than for clinical efficacy trials. Clinical efficacy data are considered to be only supportive to PK/PD studies, and safety data are considered as pivotal.
In this trial, patients received either soluble and isophane insulin in flexible doses (“free”) or the fixed-dose combination (“fixed”). They were stratified into four strata according to the type of combination (fixed or free) and the type of diabetes (1 or 2; see Table 3). The primary efficacy endpoint of the study was the hemoglobin A1c level at 24 weeks. Safety endpoints included treatment-emergent adverse events and the development of IgG anti-insulin antibodies.
Table 3.
Distribution of the Treatment Combinationsa
| Free combination | Fixed | Total | |||
|---|---|---|---|---|---|
| Test | Ref | Test | Ref | ||
| Type 1 | 106 | 103 | 17 | 17 | 243 |
| Type 2 | 38 | 39 | 104 | 102 | 283 |
| Total | 144 | 142 | 121 | 119 | 526 |
Full analysis set; test, Marvel insulin; ref, approved insulin formulation.
The study was coordinated by a CRO based in Germany (CCDRD AG). Twenty-seven clinical centers participated; 3 were based in Germany, 10 in Poland, 7 in Bulgaria, and another 7 in Serbia. The number of randomized patients by country was as follows: 234 in Poland, 153 in Bulgaria, 101 in Serbia, and 38 in Germany. When its comes to recruitment per center, there were considerable differences: 2 to 78 patients. The four most important centers (recruited >30 patients, 40% of the study population) were located in Poland (1), Bulgaria (2), and Serbia (1).
It appears as if the quality of the study was not optimal; in the study report, there was no statement about study monitoring/site audits. The number of patients who dropped out and/or had major protocol deviations was high: 18% of the randomized population. Interestingly, this figure varied between centers from 0% to 47%. There were significantly more withdrawals from the study in the Marvel groups than in the comparator groups (12% versus 7%). There was also frequent noncompliance with the sequential randomization. In addition, the dosing data were not analyzed in enough detail to conclude that patients in the test and comparator groups had actually received comparable doses of insulin. There was also an unusually low reporting rate of adverse events and a discrepancy detected in the safety listings. In the end, this resulted in this suggestion of the CHMP: “GCP inspection of the CRO and/or the most important centre(s) is deemed appropriate.”
The results of this trial showed that the Marvel insulins appear progressively to lose efficacy between 12 and 24 weeks, in clear contrast to the reference product (Table 4). However, hemoglobin A1c levels at 24 weeks did not differ statistically between the two groups. The CHMP was not in favor of the high equivalence margin of 0.6% used; however, the trends favoring the reference insulin formulation except the free combination in patients with type 2 diabetes.
Table 4.
Primary Endpoint Hemoglobin A1c after 24 Weeks of Treatment
| Per protocol set | Adjusted meana | |||
|---|---|---|---|---|
| Test | Humulin | Difference | 95% confidence interval | |
| Type 1 fixed | 8.43 | 8.16 | 0.28 | (-0.54, 1.09) |
| Type 1 free | 8.53 | 8.30 | 0.22 | (-0.15, 0.60) |
| Total type 1 | 8.51 | 8.29 | 0.22 | (-0.12, 0.56) |
| Type 2 fixed | 7.73 | 7.52 | 0.21 | (-0.04, 0.47) |
| Type 2 free | 7.33 | 7.68 | -0.35 | (-0.85, 0.15) |
| Total type 2 | 7.65 | 7.56 | 0.09 | (-0.15, 0.32) |
From ANCOVA adjusting for screening value.
Regarding safety outcomes in this trial, the Marvel products and the reference comparators (from Eli Lilly) were associated with similar rates of adverse events in patients with type 2 diabetes (25% versus 31%, respectively) and with similar rates of new antibody formation in the first 24 weeks (10.7% versus 12.5%, respectively), but those with type 1 diabetes had substantially higher rates of adverse events (24% versus 12%) and of new antibody formation (21.9% versus 14.0%) with the Marvel products. The CHMP concluded that immunogenicity was not fully evaluated; for example, the assay and its validation were not described, treatment-naïve patients were excluded, only new antibodies were considered, and the impact of antibodies on safety and efficacy was not analyzed. Finally, the pharmacovigilance plan and the risk management program submitted in this dossier were not considered to fulfill the requirements of EMA guidance documents.
Quality Assurance
If the process is the product, the point to be seen is that, in daily practice, it is quite cumbersome (and expensive) to maintain the same level of production quality for each and every batch of BI manufactured. Even if all laboratory methods used to check the quality of the final product according to GMP are documented and in place, one is tempted to ask if this is sufficient to guarantee identical effects of the protein product in the human body. Clearly, this does not only hold true for new insulin manufacturers but for established ones as well. Therefore, the “robustness” of the manufacturing process (production of one “good” batch of BI is not sufficient) is extremely important in order to achieve a consistently reliable product. Measures to assess the “precision”/”reproducibility” of the manufacturing process have yet to be defined.
It needs to be considered that complex manufacturing processes are not static; the procedures continuously progress to optimize the yield of the process. But is the outcome of the modified process always completely identical? How large are the batch-to-batch variations? Also, materials necessary for the manufacturing process (like certain peptidases, e.g., trypsin) may differ from batch to batch in their properties. Therefore, the manufacturing processes are not a stereotypic simple and straightforward story but have a more astonishing, dynamic complexity, and one wonders about what can go wrong at every step, resulting in a whole batch of useless or nonidentical proteins.
The practical relevance of the aspects elaborated on here have been illustrated in a publication about the manufacturing of insulin glargine by Biocon, where Kannan and colleagues5 reported that this long-acting insulin analog that normally is not glycosylated had three sugar molecules (mannose) attached to it, following a different manufacturing process.
Substitution
Automatic substitution allows for the dispensing by pharmacists of generic drugs in place of prescribed innovator products without the knowledge or consent of the treating physician. Where this approach can be appropriate for generics, this may not to be appropriate for BIs. Switching from one product to the other might be associated with certain risks, e.g., immune responses with efficacy implications. An interesting question in this context is who would be liable in the case of adverse events that were triggered by a substituted product. Is it the prescribing physician, the pharmacist, or the manufacturer? In a comment by the EMA about biosimilars, it is stated that, “since biosimilar and biological reference medicines are similar but not identical, the decision to treat a patient with a reference or a biosimilar medicine should be taken following the opinion of a qualified healthcare professional.”
Novel Approaches for Insulin Manufacturing
In the past, peptides such as insulin were manufactured by introducing certain vectors into bacteria or yeast cells. The additional genetic information forces these “production machines” to produce single-chain peptides (in most cases). After harvesting these peptides and cleaning them, the three-dimensional formation of the peptide must be introduced. Such typical biotechno-logical procedures are taking place in large tanks under highly controlled conditions.
Attempts were started to avoid many of these complex procedures and instead transfer certain production steps from yeast or bacteria into plants. For example, the Canadian company SemBioSys announced that they are making good progress with their innovative plant-based production technology for BI (SBS-1000). In March 2009, SemBioSys announced that, in a phase I/II trial performed in the United Kingdom in 2008, SBS-1000 was deemed bioequivalent to Eli Lilly's Humulin R.6
It remains to be seen if, from a regulatory perspective, the proteins provided by this substantially different approach are regarded as BIs (because they are not manufactured in typical processes used for biologicals/biopharmaceutical proteins) or if a full-fledged clinical development process is required. One can argue that the end product is HI and nothing else. Most probably, the EMA will focus on an appropriate GMP. The FDA appears not to regard this insulin as a new chemical entity (“Discussions with the US Food and Drug Administration (FDA) have confirmed that safflower-produced insulin is eligible to follow a shortened drug approval process”7).
Conclusions
In summary, a careful analysis of the story of BI results in some sense of ambiguity. One tends to believe that BIs are as safe as other insulins, probably because it is not readily apparent how much thought and effort needs to go into a rather detailed assessment of safety and efficacy of BIs. Based on the complexities of the product, its manufacturing process, and the drug safety and efficacy considerations that need to be applied, it is not at all surprising that the requirements developed by the regulatory bodies are high and demanding. In the only example of an application for market approval of a BI in Europe, it is obvious that the application was of mediocre quality in many aspects, but at the same time, the insulin demonstrated real differences in PD properties as shown in glucose clamp studies (and also in a clinical trial). A more detailed look at BIs uncovers aspects that substantiate the careful and thoughtful approach regulators are taking with BI. Considering certain potential risks (e.g., immunogenicity providing antibodies impacting PD), more extensive clinical trials would be of help and could be part of a risk management program as suggested, e.g., by the EMA. Nevertheless, it is well-known that adverse events such as immune responses might not be detected even by such studies. It is of interest to note that the majority of marketing applications for biosimilars (others than BI) were, in fact, successful (they got market approval). These approvals were for more complex proteins than insulin.
In any case, from a clinical and regulatory standpoint, it is obvious why, without sufficient safety and efficacy data, BI can not be approved or used in clinical practice. However, one can be relatively sure that BIs will become available in regulated markets such as the United States and the European Union in the future.
Abbreviations
- (AUC)
area under the curve
- (BI)
biosimilar insulin
- (CHMP)
Committee for Medicinal Products for Human Use
- (CMC)
chemical manufacturing characteristic
- (CRO)
clinical research organization
- (EMA)
European Medicines Agency
- (EPO)
erythropoietin
- (GIR)
glucose infusion rate
- (FDA)
Food and Drug Administration
- (GMP)
good manufacturing practice
- (HI)
human insulin
- (PD)
pharmacodynamic
- (PK)
pharmacokinetic
- (SC)
subcutaneous
Appendix
European Medicines Agency Guidelines
Committee for Medicinal Products for Human Use. Guideline on similar biological medicinal products. London: European Medicines Agency; 2005. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf. Accessed September 5, 2010.
Committee for Medicinal Products for Human Use. Guidelines on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues. London: European Medicines Agency; 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003953.pdf. Accessed September 5, 2010.
Committee for Medicinal Products for Human Use. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. London: European Medicines Agency; 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003920.pdf. Accessed September 5, 2010.
Committee for Medicinal Products for Human Use. Annex guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. Guidance on similar medicinal products containing recombinant human soluble insulin. London: European Medicines Agency; 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003957.pdf. Accessed September 5, 2010.
European Medicines Agency. International Conference on Harmonization Topic Q7: good manufacturing practice for active pharmaceutical ingredients. Note for guidance on good manufacturing practice for active pharmaceutical ingredients (CPMP/ICH/4106/00). London: European Medicines Agency; 2000. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002825.pdf. Accessed September 5, 2010.
European Medicines Agency. International Conference on Harmonization Topic Q5A (R1): quality of biotechnological products: viral safety evaluation of biotechnology products derived from cell lines of human or animal origin. Note for guidance on quality of biotechnological products: viral safety evaluation of biotechnology products derived from cell lines of human or animal origin (CPMP/ICH/295/95). London: European Medicines Agency; 1997. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002801.pdf. Accessed September 5, 2010.
European Medicines Agency. International Conference on Harmonization Topic Q6B: specifications: test procedures and acceptance criteria for biotechnological/biological products. Note for guidance on specifications: test procedures and acceptance criteria for biotechnological/biological products (CPMP/ICH/365/96). London: European Medicines Agency; 1999. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002824.pdf. Accessed September 5, 2010.
European Medicines Agency. Questions and answers on biosimilar medicines (similar biological medicinal products). London: European Medicines Agency; 2008 http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/12/WC500020062.pdf. Accessed September 5, 2010.
Committee for Medicinal Products for Human Use. Guideline on the investigation of bioequivalence. London: European Medicines Agency; 2010 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf. Accessed September 5, 2010.
Marvel Case
European Medicines Agency. Pre-authorisation evaluation of medicines for human use. Withdrawal assessment report for Insulin Human Rapid Marvel. London: European Medicines Agency; 2008. http://www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2010/01/WC500067086.pdf. Accessed September 5, 2010.
European Medicines Agency. Pre-authorisation evaluation of medicines for human use. Withdrawal assessment report for Insulin Human Long Marvel. London: European Medicines Agency; 2008. http://www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2010/01/WC500067170.pdf. Accessed September 5, 2010.
European Medicines Agency. Pre-authorisation evaluation of medicines for human use. Withdrawal assessment report for Insulin Human 30/70 Mixed Marvel. London: European Medicines Agency; 2008. http://www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2010/01/WC500067169.pdf. Accessed September 5, 2010.
European Medicines Agency. Press release: Marvel LifeSciences Ltd withdraws its marketing authorisation applications for Insulin Human Rapid Marvel, Insulin Human Long Marvel and Insulin Human 30/70 Mix Marvel. London: European Medicines Agency; 2008. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500015335.pdf. Accessed September 5, 2010.
European Medicines Agency. Questions and answers on the withdrawal of the marketing authorisation application for Insulin Human Rapid Marvel, Insulin Human Long Marvel, Insulin Human 30/70 Mix Marvel. London: European Medicines Agency; 2008. http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/11/WC500015341.pdf. Accessed September 5, 2010.
Disclosures:
Lutz Heinemann is partner of Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany, and Profil Institute for Clinical Research, Inc., San Diego, CA. He is also an employee of the latter institute. Marcus Hompesch is CEO of Profil Institute for Clinical Research, Inc., and partner. These institutes perform clinical trials in cooperation with many pharmaceutical companies. Both authors are members of advisory boards and speakers bureaus and have received honoraria from such companies. Lutz Heinemann and Marcus Hompesch are not stockholders in any of the companies in which the institutes performs clinical trials.
References:
- 1.Kuhlmann M, Covic A. The protein science of biosimilars. Nephrol Dial Transplant. 2006;21(Suppl 5):v4–v8. doi: 10.1093/ndt/gfl474. [DOI] [PubMed] [Google Scholar]
- 2.Heise T, Bott S, Tusek C, Stephan JA, Kawabata T, Finco-Kent D, Liu C, Krasner A. The effect of insulin antibodies on the metabolic action of inhaled and subcutaneous insula prospective randomized pharmacodynamic study. Diabetes Care. 2005;28(9):2161–2169. doi: 10.2337/diacare.28.9.2161. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, Michaud P, Papo T, Ugo V, Teyssandier I, Varet B, Mayeux P. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346(7):469–475. doi: 10.1056/NEJMoa011931. [DOI] [PubMed] [Google Scholar]
- 4.Kuhlmann M, Marre M. Lessons learned from biosimilar epoetins and insulins. Br J Diabetes Vasc Dis. 2010;10(2):90–97. [Google Scholar]
- 5.Kannan V, Narayanaswamy P, Gadamsetty D, Hazra P, Khedkar A, Iyer H. A tandem mass spectrometric approach to the identification of O-glycosylated glargine glycoforms in active pharmaceutical ingredient expressed in Pichia pastoris. Rapid Commun Mass Spectrom. 2009;23(7):1035–1042. doi: 10.1002/rcm.3965. [DOI] [PubMed] [Google Scholar]
- 6. SemBioSys. Spring 2010 fact sheet. http://www.sembiosys.com/pdf/fact_sheet_spring_2010.pdf. Accessed March 16, 2011.
- 7. SemBioSys. Our solution for diabetes. http://www.sembiosys.com/Products/Diabetes.aspx. Accessed March 16, 2011.