Abstract
Background:
Insulin resistance (IR) and deterioration of beta-cell secretion are main features in the development of type 2 diabetes, which is reflected in increasing serum intact proinsulin levels in later disease stage. Introduction of stable assays that are able to distinguish between intact proinsulin and its specific and unspecific cleavage products has resulted in the finding that serum intact proinsulin values can serve as a direct marker for beta-cell dysfunction, are a highly specific indicator of IR, and can predict cardiovascular risk.
Method:
Determination of fasting intact proinsulin may be used to monitor and optimize antidiabetic therapeutic approaches. Our study group has been involved in a variety of clinical studies investigating drug effects on beta-cell secretory capacity, IR, and intact proinsulin levels. One focus was on the impact of insulin-sensitizing therapy with pioglitazone on the pancreatic beta-cell load.
Results:
Treatment with pioglitazone resulted in significant decreases in elevated proinsulin levels in type 2 diabetes patients. This effect was independent from glycemic control.
Conclusions:
Measurement of fasting intact proinsulin values allows a staging of beta-cell dysfunction and evaluation of IR, thus providing an interesting diagnostic tool for both selection of appropriate therapy and monitoring of treatment success.
Keywords: beta-cell dysfunction, cardiovascular risk, diabetes, insulin resistance, pioglitazone, proinsulin
Introduction
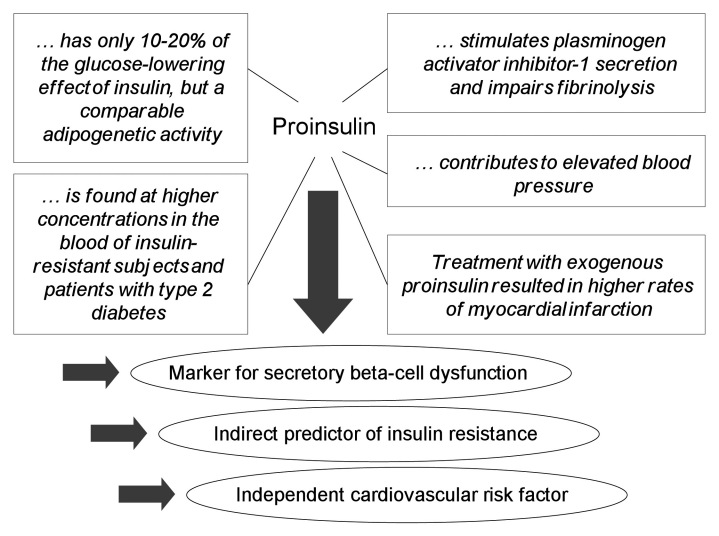
Type 2 diabetes mellitus (T2DM) is characterized initially by a metabolic insulin resistance (IR) and a genetically determined dysfunction of the insulin-secreting pancreatic beta cells. In clinical practice, patients are classified by laboratory markers and symptoms such as hemoglobin A1c (HbA1c), glucose, lipids, blood pressure, and body mass index. However, this specification does not provide insight into the underlying pathophysiological disorders. There has been a rapidly growing interest in new methods for the evaluation of pancreatic beta-cell dysfunction to optimize therapeutic intervention strategies. Conventional means for assessing insulin secretion impairment include homeostasis model assessment (HOMA) score and meal-related functional parameters. Next to this, a current focus is on the defective islet beta-cell processing of the proinsulin molecule that directly reflects the degree of beta-cell dysfunction. Impaired beta-cell secretory capacity induces disproportionately elevated serum proinsulin levels, such as found in subjects with T2DM and impaired glucose tolerance.1–3 Beyond its role as a direct biomarker for beta-cell dysfunction, measurement of intact proinsulin values has also emerged to be an important indirect predictor for IR4,5 and individual cardiovascular risk6 (Figure 1). As a result, determination of fasting intact proinsulin or the proinsulin-to-insulin ratio has become a popular method to describe insulin-resistance-associated beta-cell impairment and the impact of therapeutic interventions on insulin-secreting cells.
Figure 1.
Characteristics of proinsulin: its role as a biomarker.
In our cross-sectional epidemiological SETT2D study (Study for the Evaluation of Treatment Preference in Type 2 Diabetes), we investigated the biochemical and demographic characteristics of 532 individuals with diet or orally treated but insufficiently controlled T2DM. Both IR, as assessed by HOMA-IR score, and disproportionately increased intact proinsulin levels were prevalent in the vast number of patients. These findings underline the close association between beta-cell function and insulin sensitivity.7 Routine assessment of intact proinsulin therefore provides a better understanding of the underlying disease conditions and may allow optimization of antidiabetic therapy beyond simple glucose control.8
Our study group conducted a variety of clinical studies that investigated changes in intact proinsulin levels as a biomarker of beta-cell dysfunction and IR. It is noticeable that patients with T2DM may show a very early increase in fasting intact proinsulin secretion, independent from disease duration or other clinical features.9
This article will report on the role of intact proinsulin measurement for categorizing stages of T2DM progression. Furthermore, we provide an overview of current evidence on how insulin-sensitizing treatment with pioglitazone may interfere with this modern robust biomarker for beta-cell dysfunction, IR, and cardiovascular risk.
Proinsulin: A Pathophysiological Background
Proinsulin is synthesized by the beta cell of the pancreas as a precursor molecule for insulin. Physiologically, virtually all proinsulin molecules are intracellularly cleaved by carboxypeptides into insulin and C-peptide. In healthy subjects, only a minor percentage of uncleaved intact proinsulin is (postprandially) released into the circulation. Progressive IR leads to an increased demand for insulin. Thus the cleavage capacity of the processing enzymes may be exhausted, and the intact precursor or partially processed proinsulin is secreted in addition to insulin and C-peptide.6,10 Intact proinsulin binds to the insulin receptor. However, it has only 10–20% of the glucose-lowering effect of insulin but comparable adipogenetic activity.11
In the past, conventional nonspecific assays showed a high cross reactivity with various fractions of proinsulin-like molecules. This has led to only partial and sometimes incorrect conclusions about the role of proinsulin in the prediction and diagnosis of beta-cell dysfunction and T2DM progression.12 New stable assays have been developed that can distinguish between intact proinsulin and its specific and unspecific cleavage products.6,12–14 Use of these assays in epidemiological and interventional studies has helped to get a better understanding about beta-cell dysfunction and its relation to IR and cardio-vascular risk. Noteworthy is a new specific intact proinsulin enzyme-linked immunosorbent assay (ELISA) that can be easily introduced into routine laboratories and does not require any further specific instrumentation.13,14
Practical Aspects for the Use of Fasting Intact Proinsulin Values in Daily Therapeutic Practice
In later stages of T2DM, proinsulin and proinsulin-like molecules are secreted in increasing amounts with insulin. Based on very specific antibodies, assays are now able to specifically differentiate intact proinsulin from other degradation products, thus allowing a reliable staging of beta-cell dysfunction and IR evaluation. Intact proinsulin is stable in ethylenediaminetetraacetic acid whole blood samples, which can be obtained from the routine sample for HbA1c measurement.
Time point:
Fasting morning state
Specimen:
1.2 ml ethylenediaminetetraacetic acid blood (same specimen as HbA1c)
Result:
Fasting intact proinsulin <11 pmol/liter (ELISA; chemiluminescence assay <10) at manifestation or therapy control = normal value:
No qualitative beta-cell dysfunction
Start or continue therapy to reach glucose target
Repetition after 6 months (sulfonylurea treatment) or 12 months (diet) recommended
Fasting intact proinsulin >11 pmol/liter (ELISA; chemiluminescence assay >10) = elevated value:
Beta-cell dysfunction and IR
Beta-cell protective therapy recommended (exercise, pharmacological insulin-sensitizing strategies, glucagon-like peptide-1 analogs, insulin)
Further diagnosis of cardiovascular risk recommended (e.g. lipids or high-sensitivity C-reactive protein)
Control measurement after 3 months recommended
Elevated Intact Proinsulin Values Reveal Beta-Cell Dysfunction
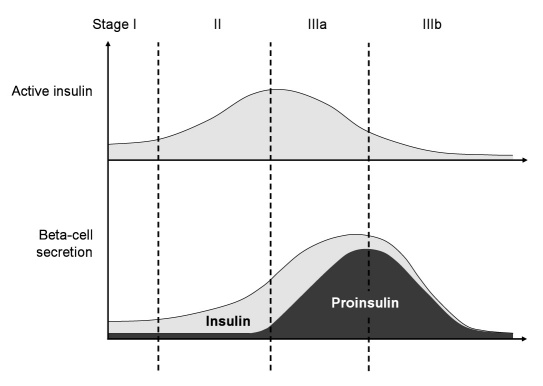
Fasting measures of intact proinsulin values allow for a pathophysiological staging of T2DM based on islet beta-cell processing.6,13,14 Beta-cell dysfunction covers three components: (1) secretion timing disorder, (2) quantitative disorder, and (3) qualitative disorder. A loss of first phase insulin response, an important inhibitory signal for hepatic glucose release, portrays the secretion timing disorder in early stages of T2DM. Disease progression may then lead to a decline in pulsatile insulin release, reflecting an additional secretion timing failure. The quantitative disorder starts when beta cells increase the volume of insulin release based on the progressive external demand. In later stages, exhaustion of the production capacity may result in an almost complete loss of insulin secretion. The quantitative increase in proinsulin secretion will finally induce a deterioration of secretion product composition10 (Figure 2).
Figure 2.
Classification of type 2 diabetes based on the pathophysiology of beta-cell secretion. Insulin-sensitive patients with normal quantitative insulin secretion but lack of the first-phase insulin response would be classified as stage I (timing disorder). When IR is developing, the beta cell may effectively counteract this phenomenon by increased secretion of active insulin (quantitative disorder, stage II). In the further course, the cell may reach the level of saturation of the processing capacity, and intact proinsulin is secreted in an increasing manner (qualitative secretion disorder, stage IIIa). While this contributes to an increased cardiovascular risk, the increasing demand for insulin may finally conclude in a complete exhaustion of beta-cell secretion (stage IIIb). This figure is adapted from Reference 6.
Because of the minor but evident glucose-lowering effect, patients with severe beta-cell dysfunction and high proinsulin output may still have sufficient glucose-lowering capacity to avoid the diagnosis of diabetes mellitus in an oral glucose tolerance test experiment. In fact, beta-cell dysfunction and proinsulin secretion are not consequently correlated with diabetes duration,5,15,16 and proinsulin secretion may precede the onset of clinically overt T2DM.17
Intact Proinsulin Is an Independent Cardiovascular Risk Factor
Current evidence suggests that proinsulin contributes to the excess incidence of cardiovascular disease in T2DM by stimulating plasminogen activator inhibitor-1 secretion and the consecutive inhibition of fibrinolysis6 (Figure 1). Elevated proinsulin concentrations predicted a 200% increased risk for cardiovascular death and morbidity over a 27-year period, independent of other major cardiovascular risk factors in male patients without diabetes.18 In the Hoorn Study, fasting proinsulin levels proved to be significantly associated with all-cause and cardiovascular mortality, independent of glucose tolerance status and IR and largely independent of other cardiovascular risk factors.19 Numerous other clinical trials provide similar results, supporting an independent association between the increase of proinsulin levels and cardiovascular disease.11,15,16,20–23
Intact Proinsulin Predicts Progression of Insulin Resistance
Insulin resistance is a hallmark of T2DM and has been proposed as the common link between glucose metabolism disorder and cardiovascular disease.24 Progression of IR in the course of T2DM leads to increased insulin demands and finally to an impairment of beta-cell function in later stages of the disease. Disproportionately elevated intact proinsulin levels in the peripheral blood serve as an appropriate laboratory marker for this phenomenon by disclosing the exhausted cleavage capacity of intra-cellular processing enzymes.10
Haffner and colleagues4 examined the relation between the fasting proinsulin-to-insulin ratio with a number of metabolic disorders believed to be associated with the IR syndrome. In 423 subjects without diabetes, an increased ratio was significantly associated with hypertension, low high-density lipoprotein cholesterol, high triglyceride levels, and impaired glucose tolerance. These results suggest that even nondiabetic individuals with the IR syndrome not only exhibit hyperinsulinemia as a marker of IR, but also show elevated proinsulin values, which may reflect relative beta-cell failure or malfunction.
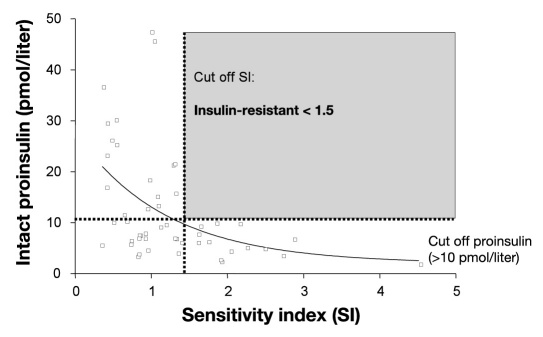
Our group conducted further research including T2DM patients. Data from IRIS-II (study on Insulin Resistance and Insulin Sensitivity – II)] a large epidemiology study with 4270 people with diabetes revealed a calculated specificity of 93.2% (sensitivity 46.9%) for elevated intact proinsulin levels (>10 pmol/liter in the chemiluminescence assay) as an indirect marker of IR. Moreover, patients who presented with elevated proinsulin values demonstrated a higher prevalence of micro- and macro-vascular disease.5 The disproportionate increase in fasting intact proinsulin concentrations even appeared to be a more specific marker for IR and increased cardiovascular risk than suppression of fasting adiponectin.25 Another investigation explored the predictive value of intact proinsulin in 48 T2DM patients (Figure 3). Again, there was a significant correlation between intact proinsulin values and IR measurement (minimal model analysis, p < .05; HOMA, p < .01). Elevation of intact proinsulin values above the reference range (>10 pmol/liter in the chemiluminescence assay) showed a very high specificity (minimal model analysis, 100%; HOMA, 92.9%) and a moderate sensitivity (minimal model analysis, 48.6%; HOMA, 47.1%) as marker for IR.9
Figure 3.
Intact proinsulin is a highly specific marker for IR: correlation between fasting intact proinsulin values and the insulin sensitivity index according to minimal model analysis. Intact proinsulin was determined by means of a specific chemiluminescence test. This figure is adapted from Reference 9.
Effects of Pioglitazone Treatment on Elevated Proinsulin Levels
As demonstrated earlier, high proinsulin values reflect impaired beta-cell secretory capacity, IR, and increased cardiovascular risk. A search for antidiabetic strategies that provide favorable effects on the defective islet beta-cell processing of the proinsulin molecule therefore seems to be useful. We and other study groups have focused on the insulin-sensitizing agent pioglitazone. Here we provide a summary of key clinical results for the influence of pioglitazone treatment on beta-cell function and proinsulin levels (Table 1).
Table 1.
Impact of Pioglitazone Treatment on Proinsulin Levels: Clinical Results in Type 2 Diabetes Patients and Patients without Diabetesa
| Reference number | Treatment arms | Design | Case numbers | Follow-up | Results with pioglitazone |
|---|---|---|---|---|---|
| 26 | PIO versus SU | prospective controlled | N = 55 (T2DM) | 12 weeks | Fasting proinsulin ↓ (from 24.7 ± 12.9 to 14.0 ± 6.2 pmol/liter; p < .01 versus baseline); no significant change with SU |
| 27 | PIO versus PBO | prospective controlled randomized | N = 30 (diet-controlled T2DM) | 12 weeks | Proinsulin/insulin ratio ↓ (-0.057 versus +0.004; p = .03 versus PBO) |
| 28 | PIO versus GLIM | prospective open-label controlled randomized | N = 173 (T2DM) | 24 weeks | Fasting intact proinsulin ↓ (PIO: -24%, p < .001 versus baseline; GLIM: -1.4%, n.s.) |
| 29 | PIO: add-on to MET + SU | prospective open label | N = 54 (T2DM) | 26 weeks | Proinsulin/insulin ratio ↓ (from 0.89 to 0.66, p < .001 versus baseline) |
| 30 | PIO + PBO versus SIM + PBO versus PIO + SIM | prospective double-blind controlled randomized | N = 125 Patients without diabetes and at high cardiovascular risk | 12 weeks | Postprandial intact proinsulin ↓ PIO mono: from 29.5 ± 21.4 to 22.1 ± 17.5 pmol/liter (p < .01 versus baseline); PIO + SIM: from 24.3 ± 27.4 to 21.1 ± 16.5 pmol/liter (p < .05); SIM mono: no significant change |
| 31 | ATOR + PBO versus ATOR + PIO | prospective double-blind controlled randomized | N = 148 Patients without diabetes and at high cardiovascular risk | 24 weeks | Fasting intact proinsulin (↓) ATOR + PBO: from 6.4 ± 0.8 to 5.9 ± 0.5 pmol/liter (n.s.) ATOR+PIO: from 6.3 ± 0.8 to 5.3 ± 0.5 pmol/liter (p = .07 versus baseline) |
| 32 | PIO versus GLIB | prospective double-blind controlled randomized | N = 22 (T2DM) | 20 weeks | Fasting and postprandial proinsulin ↓; no significant change with GLIB |
| 33 | Add-on to MET + SU or glinide: PIO versus PBO | prospective double-blind controlled randomized | N = 299 (T2DM) | 28 weeks | Proinsulin ↓ versus PBO |
| 34 | Patients were switched from insulin to PIO + GLIM | prospective open label | N = 75 (out of 98) completed (T2DM with residual beta-cell function) | 24 weeks | Intravenous glucagon test: C-peptide / intact proinsulin ratio ↑ (5.2% in completers) |
| 35 | PIO + MET after short-term insulin infusion | prospective open label (pilot study) | N = 14 | 16 weeks | Insulin infusion reduced fasting intact proinsulin (-46%); this effect was maintained during PIO + MET |
| 36 | PIO + GLIM versus GLIM up-titration | prospective double-blind controlled randomized | N = 82 (T2DM) | 24 weeks | Fasting intact proinsulin ↓ (from 12.4 ± 10.3 to 7.6 ± 4.8 pmol/liter; p < .05 versus baseline); no significant change with GLIM up-titration |
ATOR, atorvastatin; GLIB, glibenclamide; GLIM, glimepiride; MET, metformin; mono, monotherapy; n.s., not significant; PBO, placebo; PIO, pioglitazone; SIM, simvastatin, SU; sulfonylurea
In 2002, a Japanese study was published that enrolled 55 T2DM patients to clarify the influence of pioglitazone on fasting proinsulin concentrations with a less specific proinsulin assay. The results demonstrated that a 12-week pioglitazone treatment alone or in combination with a sulfonylurea significantly reduced fasting proinsulin levels, whereas sulfonylurea monotherapy did not decrease proinsulin in the third comparator group.26
Another investigation by Cooper and associates32 examined 22 T2DM patients who were assigned to receive pioglitazone or the sulfonylurea drug glibenclamide for 20 weeks. Sensitization to insulin with pioglitazone treatment resulted in a significant reduction in fasting and postprandial levels of proinsulin, whereas glibenclamide had no effect on the insulin precursor species.
Wallace and coworkers27 randomized 30 subjects with diet-controlled T2DM to 3 months of treatment with pioglitazone or placebo. Pioglitazone increased basal insulin sensitivity by 24.7% (HOMA) versus 2.1% in the placebo group (p = .02). Along with the favorable effect on IR, insulin-sensitizing therapy led to a significant decrease in the proinsulin-to-insulin ratio compared with placebo, suggesting a decline of stress in islet beta cells. Two further studies investigated the effects of pioglitazone as part of oral triple therapy. The insulin-sensitizing agent was administered as add-on to metformin and insulin secretagogues in 54 (uncontrolled study) and 299 (placebo-controlled study) patients with T2DM and inadequate glycemic control. During the follow-up at weeks 26 and 28, respectively, the proinsulin-to-insulin ratio again significantly decreased with pioglitazone therapy, therewith indicating an improvement in beta-cell function.29,33
Our own research supports the encouraging results for an insulin-sensitizing therapeutic strategy, such as pioglitazone treatment. In our studies, we applied the new stable intact proinsulin assays that are able to distinguish between intact proinsulin and its specific and unspecific cleavage products.6,12–14
Pioglitazone in Comparison to Sulfonylurea Administration
In a 6-month parallel randomized single-center open-label study PIONEER (pioneer study for the impact of pioglitazone on atherosclerosis), we evaluated the impact of pioglitazone versus glimepiride on IR and beta-cell dysfunction in 173 T2DM patients. During follow-up, similar reductions in HbA1c were seen in both groups. Treatment with pioglitazone resulted in a significant decrease of HOMA-IR and intact proinsulin levels as markers for IR and beta-cell dysfunction (p < .001 each versus glimepiride), independent from blood glucose control. No such effects were observed in the glimepiride group.28 Another analysis of the PIONEER data substantiated that measurement of intact proinsulin enables an estimation of atherosclerotic risk. We realized a decrease of carotid intima-media thickness by pioglitazone but not glimepiride treatment (p < .0001), despite similar improvements in metabolic control. This effect was correlated with improved insulin sensitivity (r = 0.29; p = .0003) and levels of the intact proinsulin molecule (r = 0.22; p = .006).37
The double-blind, parallel PIOGLIM (pioglitazone and/or glimiperide in diabetes therapy) trial investigated the impact of adding pioglitazone to glimepiride (up-titration to 45 + 4 mg) in comparison to up-titrating the glimepiride dose alone (up-titration to 6 mg). We involved 82 T2DM patients who had been inefficiently treated with a low-dose glimepiride monotherapy. During the 6-month observation period, a significant improvement in IR (HOMA-IR) and intact proinsulin values were documented for the pioglitazone plus glimepiride group (p < .05 each) but not for optimized glimepiride monotherapy. The number of patients with later stage beta-cell dysfunction (as indicated by elevated fasting intact proinsulin levels and/or a HOMA-IR value > 2) was significantly reduced by the combination therapy (from 96% to 75%, p < .05), while it remained unchanged in the glimepiride up-titration group (100% at baseline and endpoint).36
Insulin Treatment Followed by Pioglitazone Therapy
In our PIOSWITCH (pioglitazone allows a switch back from insulin to OAD) study, we reconverted 98 T2DM patients with residual beta-cell function from insulin therapy to oral treatment with pioglitazone and glimepiride. During a 6-month follow-up, 23 patients were prematurely terminated because of an increase in HbA1c from baseline > 0.5% or other reasons. In 75 patients (76%, completers), no deterioration of glucose metabolism occurred. After switching from insulin therapy, the completer group demonstrated improvements in the quality of beta-cell secretion product as measured by the C-peptide-to-proinsulin ratio. Serum intact proinsulin levels had been assessed in the fasting state by means of an intravenous glucagon stimulation test.34
A pilot study with 14 T2DM patients [PIOMET (pioglitazone and/or metformin in diabetes therapy)] examined the efficacy of short-term intravenous insulin intervention followed by oral pioglitazone and metformin therapy to prevent patients from continuous insulin application. Initially, an in-patient 34 h continuous intravenous insulin infusion was performed and metformin was given. We stopped insulin and added pioglitazone at the second in-patient day. Participants were followed for an additional 4 months. Our results demonstrated that a beneficial effect of a short-term intravenous insulin application on glycemic control could be effectively maintained by pioglitazone plus metformin treatment. A 46% decrease in fasting intact proinsulin levels during insulin infusion therapy (p < .05) indicated a significant improvement of beta-cell function. This effect could be maintained with pioglitazone plus metformin treatment.35
Research in Individuals without Diabetes and at High Cardiovascular Risk
In nondiabetic patients at cardiovascular risk, treatment with pioglitazone did not significantly change fasting intact proinsulin levels. Interestingly, this was opposed by a pronounced reduction of the postprandial increase in intact proinsulin as measured during a standardized oral glucose load.30 In the double-blinded PIOSTAT (pioglitazone and statin therapy for reduction of inflam-matory disease) study, we randomized 125 individuals without diabetes to pioglitazone, pioglitazone and simvastatin, or simvastatin alone. Glucose, insulin, and intact proinsulin levels were measured, subsequent to an oral glucose load. In contrast to the only moderately elevated intact proinsulin levels at baseline (upper normal limit), a remarkable increase in this marker could be observed after the oral glucose challenge. Although fasting intact proinsulin concentrations remained unchanged, the increase in postprandial intact proinsulin levels turned out to be significantly reduced during pioglitazone and pioglitazone plus simvastatin treatment. No such effect was seen with simvastatin monotherapy.30
Another trial [PIOVASC (pioglitazone and vascular function)] investigated the impact of atorvastatin mono-therapy and combined treatment with atorvastatin and pioglitazone. We randomized 148 patients without diabetes and monitored fasting plasma levels of intact proinsulin over 6 months. At baseline, proinsulin values were within the upper normal range. Addition of pioglitazone to atorvastatin resulted in a tendency toward further reduction in fasting intact proinsulin levels (p = .07). The biomarker remained unchanged with atorvastatin treatment alone.31
Results with Other Insulin-Sensitizing Strategies and Insulin Therapy
Current evidence for rosiglitazone, the other thiazo-lidinedione drug, supports the pronounced benefit of insulin-sensitizing therapy on beta-cell function and beta-cell processing of the proinsulin molecule.38–43
Diet,44 exercise,45 and metformin therapy46,47 have been shown to decrease IR and proinsulin levels in T2DM patients as well, though to a lesser extent than with thiazolidinedione treatment. In contrast to insulin-sensitizing strategies, sulfonylureas have been reported not to change or to increase plasma proinsulin values.26,28,31,36,38,41,48–50
Preliminary studies revealed that the fasting proinsulin serum concentration was significantly higher in T2DM patients treated with sulfonylureas than in a well-matched group receiving insulin only.49 One of our studies explored the immediate effect of supplementary insulin treatment on beta-cell function in 20 T2DM patients with glimepiride monotherapy. The patients were randomized either to continue with their oral treatment or to switch to a fixed-dose supplementary insulin application. Oral glucose tolerance tests after drug uptake were performed at days 7 and 14, with measurement of intact and total proinsulin. Significant reductions from baseline were seen in the supplementary insulin therapy group for the fasting and postprandial values of intact proinsulin, while no changes occurred at all in the sulfonylurea group.50
Conclusions and Summary
Available study data suggest that fasting intact proinsulin is a reliable and robust biomarker for beta-cell dysfunction, IR, and cardiovascular risk in T2DM patients. Measurement of serum intact proinsulin levels with the new specific assays allows for a staging of T2DM according to impaired beta-cell secretory capacity. This classification may help to select and optimize daily therapy of the individual patient. Elevated intact proinsulin values indicate an advanced stage of beta-cell exhaustion. Given the close relation between beta-cell function and insulin sensitivity, measurement of intact proinsulin has also been identified as a very specific indirect marker for clinically relevant IR in individuals with and without diabetes.
While drugs enhancing insulin secretion can be used in earlier stages of T2DM (stages I and II), their application in beta-cell dysfunction stage IIIa or IIIb should not be recommended, because further proinsulin secretion may result in an increased cardiovascular risk. Insulin-sensitizing strategies and insulin, by contrast, reduce the pancreatic beta-cell load. For example, treatment with the oral insulin-sensitizing agent pioglitazone resulted in a pronounced decrease of elevated proinsulin values and improved cardiovascular risk profile, while the levels remained high during sulfonylurea therapy. It is important to understand that this pathophysiological phenomenon is independent from glucose control, which is to date the commonly accepted therapeutic target. Currently, lateral-flow-based point-of-care rapid tests are under development (IR2Dx Inc., Moraga, CA), which allow for determination of the critical cut-off value (11 pmol/liter in Caucasians). Their availability may be useful for a broader use of this robust biomarker in treatment selection and monitoring of T2DM.
In conclusion, fasting intact proinsulin, as measured by means of the new stable chemiluminescence or ELISAs, serves as an interesting diagnostic tool for both selection of an appropriate therapy and monitoring of treatment success in patients with T2DM. A decrease in proinsulin concentrations by beta-cell protective therapeutic approaches may be considered a worthwhile target of modern diabetes treatment.
Abbreviations
- (ELISA)
enzyme-linked immunosorbent assay
- (HbA1c)
hemoglobin A1c
- (HOMA)
homeostasis model assessment
- (IR)
insulin resistance
- (T2DM)
type 2 diabetes mellitus
References
- 1.Reaven GM, Chen YD, Hollenbeck CB, Sheu WH, Ostrega D, Polonsky KS. Plasma insulin, C-peptide, and proinsulin concentrations in obese and nonobese individuals with varying degrees of glucose tolerance. J Clin Endocrinol Metab. 1993;76(1):44–48. doi: 10.1210/jcem.76.1.8421101. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Bowsher RR, Mykk¨nen L, Hazuda HP, Mitchell BD, Valdez RA, Gingerich R, Monterossa A, Stern MP. Proinsulin and specific insulin concentration in high- and low-risk populations for NIDDM. Diabetes. 1994;43(12):1490–1493. doi: 10.2337/diab.43.12.1490. [DOI] [PubMed] [Google Scholar]
- 3.Røder ME, Porte D, Jr, Schwartz RS, Kahn SE. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(2):604–608. doi: 10.1210/jcem.83.2.4544. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Mykk¨nen L, Valdez RA, Stern MP, Holloway DL, Monterrosa A, Bowsher RR. Disproportionately increased proinsulin levels are associated with the insulin resistance syndrome. J Clin Endocrinol Metab. 1994;79(6):1806–1810. doi: 10.1210/jcem.79.6.7989488. [DOI] [PubMed] [Google Scholar]
- 5.Pfützner A, Standl E, Hohberg C, Konrad T, Strotmann HJ, Lübben G, Langenfeld MR, Schulze J, Forst T. IRIS II study: intact proinsulin is confirmed as a highly specific indicator for insulin resistance in a large cross-sectional study design. Diabetes Technol Ther. 2005;7(3):478–486. doi: 10.1089/dia.2005.7.478. [DOI] [PubMed] [Google Scholar]
- 6.Pfützner A, Pfützner AH, Larbig M, Forst T. Role of intact proinsulin in diagnosis and treatment of type 2 diabetes mellitus. Diabetes Technol Ther. 2004;6(3):405–412. doi: 10.1089/152091504774198124. [DOI] [PubMed] [Google Scholar]
- 7.Pfützner A, Pfützner AH, Stridde E, Huppertz E, Reimer T, Derwahl M, Forst T, Petrak F. Insulin resistance and b-cell-dysfunction in insufficiently controlled type 2 diabetes: the SETT2D Trial. Diabetes Stoffwechsel Herz. 2007;2:91–97. [Google Scholar]
- 8.Pfützner A, Weber MM, Forst T. A biomarker concept for assessment of insulin resistance, beta-cell function and chronic systemic inflammation in type 2 diabetes mellitus. Clin Lab. 2008;54(11-12):485–490. [PubMed] [Google Scholar]
- 9.Pfützner A, Kunt T, Hohberg C, Mondok A, Pahler S, Konrad T, Lübben G, Forst T. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care. 2004;27(3):682–687. doi: 10.2337/diacare.27.3.682. [DOI] [PubMed] [Google Scholar]
- 10.Pfützner A, Kann PH, Pfützner AH, Kunt T, Larbig M, Weber MM, Forst T. Intact and total proinsulnew aspects for diagnosis and treatment of type 2 diabetes mellitus and insulin resistance. Clin Lab. 2004;50(9-10):567–573. [PubMed] [Google Scholar]
- 11.Galloway JA, Hooper SA, Spradlin CT, Howey DC, Frank BH, Bowsher RR, Anderson JH. Biosynthetic human proinsulin. Review of chemistry, in vitro and in vivo receptor binding, animal and human pharmacology studies, and clinical trial experience. Diabetes Care. 1992;15(5):666–692. doi: 10.2337/diacare.15.5.666. [DOI] [PubMed] [Google Scholar]
- 12.Pfützner A, Kunt T, Langenfeld M, Löbig M, Knesovic M, Forst T. Clinical and laboratory evaluation of specific chemiluminescence assays for intact and total proinsulin. Clin Chem Lab Med. 2003;41(9):1234–1238. doi: 10.1515/CCLM.2003.189. [DOI] [PubMed] [Google Scholar]
- 13.Pfützner A, Pfützner AH, Kann PH, Stute R, Löbig M, Yang JW, Mistry J, Forst T. Clinical and laboratory evaluation of a new specific ELISA for intact proinsulin. Clin Lab. 2005;51(5-6):243–249. [PubMed] [Google Scholar]
- 14.Siebenhaar R, Weise A, Safinowski M, Reisinger K, Musholt PB, Reimer T, Pfützner A, Forst T. Clinical and laboratory evaluation of a new specific ELISA for intact proinsulin. Diabetes Stoffwechsel Herz. 2008;2:275–281. [Google Scholar]
- 15.Lindahl B, Dinesen B, Eliasson M, Røder M, Jansson JH, Huhtasaari F, Hallmans G. High proinsulin concentration precedes acute myocardial infarction in a nondiabetic population. Metabolism. 1999;48(9):1197–1202. doi: 10.1016/s0026-0495(99)90138-5. [DOI] [PubMed] [Google Scholar]
- 16.Lindahl B, Dinesen B, Eliasson M, Røder M, Hallmans G, Stegmayr B. High proinsulin levels precede first-ever stroke in a nondiabetic population. Stroke. 2000;31(12):2936–2941. doi: 10.1161/01.str.31.12.2936. [DOI] [PubMed] [Google Scholar]
- 17.Nijpels G, Popp-Snijders C, Kostense PJ, Bouter LM, Heine RJ. Fasting proinsulin and 2-h post-load glucose levels predict the conversion to NIDDM in subjects with impaired glucose tolerance: the Hoorn Study. Diabetologia. 1996;39(1):113–118. doi: 10.1007/BF00400421. [DOI] [PubMed] [Google Scholar]
- 18.Zethelius B, Byberg L, Hales CN, Lithell H, Berne C. Proinsulin is an independent predictor of coronary heart disease: report from a 27-year follow-up study. Circulation. 2002;105(18):2153–2158. doi: 10.1161/01.cir.0000015855.04844.e7. [DOI] [PubMed] [Google Scholar]
- 19.Alssema M, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ; Hoorn Study. Proinsulin concentration is an independent predictor of all-cause and cardiovascular mortality: an 11-year follow-up of the Hoorn Study. Diabetes Care. 2005;28(4):860–865. doi: 10.2337/diacare.28.4.860. [DOI] [PubMed] [Google Scholar]
- 20.B¨venholm P, Proudler A, Tornvall P, Godsland I, Landou C, de Faire U, Hamsten A. Insulin, intact and split proinsulin, and coronary artery disease in young men. Circulation. 1995;92(6):1422–1429. doi: 10.1161/01.cir.92.6.1422. [DOI] [PubMed] [Google Scholar]
- 21.Wareham NJ, Byrne CD, Hales CN. Role of insulin and proinsulin in diabetic vascular disease. Metabolism. 1995;44(10 Suppl 4):76–82. doi: 10.1016/0026-0495(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 22.Yudkin JS, May M, Elwood P, Yarnell JW, Greenwood R, Davey Smith G; Caerphilly Study. Concentrations of proinsulin like molecules predict coronary heart disease risk independently of insulprospective data from the Caerphilly Study. Diabetologia. 2002;45(3):327–336. doi: 10.1007/s00125-001-0756-7. [DOI] [PubMed] [Google Scholar]
- 23.Katz RJ, Ratner RE, Cohen RM, Eisenhower E, Verme D. Are insulin and proinsulin independent risk markers for premature coronary artery disease? Diabetes. 1996;45(6):736–741. doi: 10.2337/diab.45.6.736. [DOI] [PubMed] [Google Scholar]
- 24.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007;49(21):2112–2119. doi: 10.1016/j.jacc.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 25.Langenfeld MR, Forst T, Standl E, Strotmann HJ, Lübben G, Pahler S, Kann P, Pfützner A; IRIS II study. IRIS II Study: sensitivity and specificity of intact proinsulin, adiponectin, and the proinsulin/adiponectin ratio as markers for insulin resistance. Diabetes Technol Ther. 2004;6(6):836–843. doi: 10.1089/dia.2004.6.836. [DOI] [PubMed] [Google Scholar]
- 26.Kubo K. Effect of pioglitazone on blood proinsulin levels in patients with type 2 diabetes mellitus. Endocr J. 2002;49(3):323–328. doi: 10.1507/endocrj.49.323. [DOI] [PubMed] [Google Scholar]
- 27.Wallace TM, Levy JC, Matthews DR. An increase in insulin sensitivity and basal beta-cell function in diabetic subjects treated with pioglitazone in a placebo-controlled randomized study. Diabet Med. 2004;21(6):568–576. doi: 10.1111/j.1464-5491.2004.01218.x. [DOI] [PubMed] [Google Scholar]
- 28.Pfützner A, Hohberg C, Lübben G, Pahler S, Pfützner AH, Kann P, Forst T. Pioneer study: PPARgamma activation results in overall improvement of clinical and metabolic markers associated with insulin resistance independent of long-term glucose control. Horm Metab Res. 2005;37(8):510–515. doi: 10.1055/s-2005-870320. [DOI] [PubMed] [Google Scholar]
- 29.Dorkhan M, Magnusson M, Frid A, Grubb A, Groop L, Jovinge S. Glycaemic and nonglycaemic effects of pioglitazone in triple oral therapy of patients with type 2 diabetes. J Intern Med. 2006;260(2):125–133. doi: 10.1111/j.1365-2796.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 30.Forst T, Pfützner A, Lübben G, Weber M, Marx N, Karagiannis E, Koehler C, Baurecht W, Hohberg C, Hanefeld M. Effect of simvastatin and/or pioglitazone on insulin resistance, insulin secretion, adiponectin, and proinsulin levels in nondiabetic patients at cardiovascular risk–the PIOSTAT Study. Metabolism. 2007;56(4):491–496. doi: 10.1016/j.metabol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Forst T, Wilhelm B, Pfützner A, Fuchs W, Lehmann U, Schaper F, Weber M, Müller J, Konrad T, Hanefeld M. Investigation of the vascular and pleiotropic effects of atorvastatin and pioglitazone in a population at high cardiovascular risk. Diab Vasc Dis Res. 2008;5(4):298–303. doi: 10.3132/dvdr.2008.043. [DOI] [PubMed] [Google Scholar]
- 32.Cooper MB, Al Majali K, Bailey CJ, Betteridge DJ. Reduced postprandial proinsulinaemia and 32-33 split proinsulinaemia after a mixed meal in type 2 diabetic patients following sensitization to insulin with pioglitazone. Clin Endocrinol (Oxf) 2008;68(5):738–746. doi: 10.1111/j.1365-2265.2007.03113.x. [DOI] [PubMed] [Google Scholar]
- 33.Charpentier G, Halimi S, F-PIO-100 Study Investigators Earlier triple therapy with pioglitazone in patients with type 2 diabetes. Diabetes Obes Metab. 2009;11(9):844–854. doi: 10.1111/j.1463-1326.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 34.Hohberg C, Pfützner A, Forst T, Lübben G, Karagiannis E, Borchert M, Schöndorf T. Successful switch from insulin therapy to treatment with pioglitazone in type 2 diabetes patients with residual beta-cell function: results from the PioSwitch study. Diabetes Obes Metab. 2009;11(5):464–471. doi: 10.1111/j.1463-1326.2008.00975.x. [DOI] [PubMed] [Google Scholar]
- 35.Musholt PB, Schöndorf T, Pfützner A, Hohberg C, Kleine I, Fuchs W, Hehenwarter S, Dikta G, Kerschgens B, Forst T. Combined pioglitazone and metformin treatment maintains the beneficial effect of short-term insulin infusion in patients with type 2 diabetes: results from a pilot study. J Diabetes Sci Technol. 2009;3(6):1442–1450. doi: 10.1177/193229680900300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfützner A, Derwahl M, Jacob S, Hohberg C, Blümner E, Lehmann U, Fuchs W, Forst T. Limitations of the HOMA-B score for assessment of beta-cell functionality in interventional trials–results from the PIOglim study. Diabetes Technol Ther. 2010;12(8):599–604. doi: 10.1089/dia.2010.0019. [DOI] [PubMed] [Google Scholar]
- 37.Forst T, Hohberg C, Fuellert SD, Lübben G, Konrad T, Löbig M, Weber MM, Sachara C, Gottschall V, Pfützner A. Pharmacological PPARgamma stimulation in contrast to beta cell stimulation results in an improvement in adiponectin and proinsulin intact levels and reduces intima media thickness in patients with type 2 diabetes. Horm Metab Res. 2005;37(8):521–527. doi: 10.1055/s-2005-870322. [DOI] [PubMed] [Google Scholar]
- 38.Smith SA, Porter LE, Biswas N, Freed MI. Rosiglitazone, but not glyburide, reduces circulating proinsulin and the proinsulinsulin ratio in type 2 diabetes. J Clin Endocrinol Metab. 2004;89(12):6048–6053. doi: 10.1210/jc.2004-0705. [DOI] [PubMed] [Google Scholar]
- 39.Ovalle F, Bell DS. Effect of rosiglitazone versus insulin on the pancreatic beta-cell function of subjects with type 2 diabetes. Diabetes Care. 2004;27(11):2585–2589. doi: 10.2337/diacare.27.11.2585. [DOI] [PubMed] [Google Scholar]
- 40.Pfützner A, Schöndorf T, Seidel D, Winkler K, Matthaei S, Hamann A, Forst T. Impact of rosiglitazone on beta-cell function, insulin resistance, and adiponectin concentrations: results from a double-blind oral combination study with glimepiride. Metabolism. 2006;55(1):20–25. doi: 10.1016/j.metabol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Hanefeld M, Patwardhan R, Jones NP; Rosiglitazone Clinical Trials Study Group. A one-year study comparing the efficacy and safety of rosiglitazone and glibenclamide in the treatment of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2007;17(1):13–23. doi: 10.1016/j.numecd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Hamann A, Garcia-Puig J, Paul G, Donaldson J, Stewart M. Comparison of fixed-dose rosiglitazone/metformin combination therapy with sulphonylurea plus metformin in overweight individuals with type 2 diabetes inadequately controlled on metformin alone. Exp Clin Endocrinol Diabetes. 2008;116(1):6–13. doi: 10.1055/s-2007-984441. [DOI] [PubMed] [Google Scholar]
- 43.Hanley AJ, Zinman B, Sheridan P, Yusuf S, Gerstein HC; Diabetes Reduction Assessment With Ramipril, Rosiglitazone Medication (DREAM) Investigators. Effect of rosiglitazone and ramipril on {beta}-cell function in people with impaired glucose tolerance or impaired fasting glucose: the DREAM Trial. Diabetes Care. 2010;33(3):608–613. doi: 10.2337/dc09-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies MJ, Metcalfe J, Day JL, Grenfell A, Hales CN, Gray IP. Improved beta cell function, with reduction in secretion of intact and 32/33 split proinsulin, after dietary intervention in subjects with type 2 diabetes mellitus. Diabet Med. 1994;11(1):71–78. doi: 10.1111/j.1464-5491.1994.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 45.Byberg L, Zethelius B, McKeigue PM, Lithell HO. Changes in physical activity are associated with changes in metabolic cardio-vascular risk factors. Diabetologia. 2001;44(12):2134–2139. doi: 10.1007/s001250100022. [DOI] [PubMed] [Google Scholar]
- 46.Nagi DK, Ali VM, Yudkin JS. Effect of metformin on intact proinsulin and des 31,32 proinsulin concentrations in subjects with non-insulin-dependent (type 2) diabetes mellitus. Diabet Med. 1996;13(8):753–757. doi: 10.1002/(SICI)1096-9136(199608)13:8<753::AID-DIA163>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 47.Hermann LS, Ranstam J, Vaaler S, Melander A. Effects of antihyperglycaemic therapies on proinsulin and relation between proinsulin and cardiovascular risk factors in type 2 diabetes. Diabetes Obes Metab. 1999;1(4):227–232. doi: 10.1046/j.1463-1326.1999.00034.x. [DOI] [PubMed] [Google Scholar]
- 48.Davies MJ, Metcalfe J, Day JL, Grenfell A, Hales CN, Gray IP. Effect of sulphonylurea therapy on plasma insulin, intact and 32/33 split proinsulin in subjects with type 2 diabetes mellitus. Diabet Med. 1994;11(3):293–298. doi: 10.1111/j.1464-5491.1994.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 49.Dworacka M, Abramczyk M, Winiarska H, Kuczynski S, Borowska M, Szczawinska K. Disproportionately elevated proinsulin levels in type 2 diabetic patients treated with sulfonylurea. Int J Clin Pharmacol Ther. 2006;44(1):14–21. doi: 10.5414/cpp44014. [DOI] [PubMed] [Google Scholar]
- 50.Pfützner A, Lorra B, Abdollahnia MR, Kann PH, Mathieu D, Pehnert C, Oligschleger C, Kaiser M, Forst T. The switch from sulfonylurea to preprandial short-acting insulin analog substitution has an immediate and comprehensive beta-cell protective effect in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2006;8(3):375–384. doi: 10.1089/dia.2006.8.375. [DOI] [PubMed] [Google Scholar]