Abstract
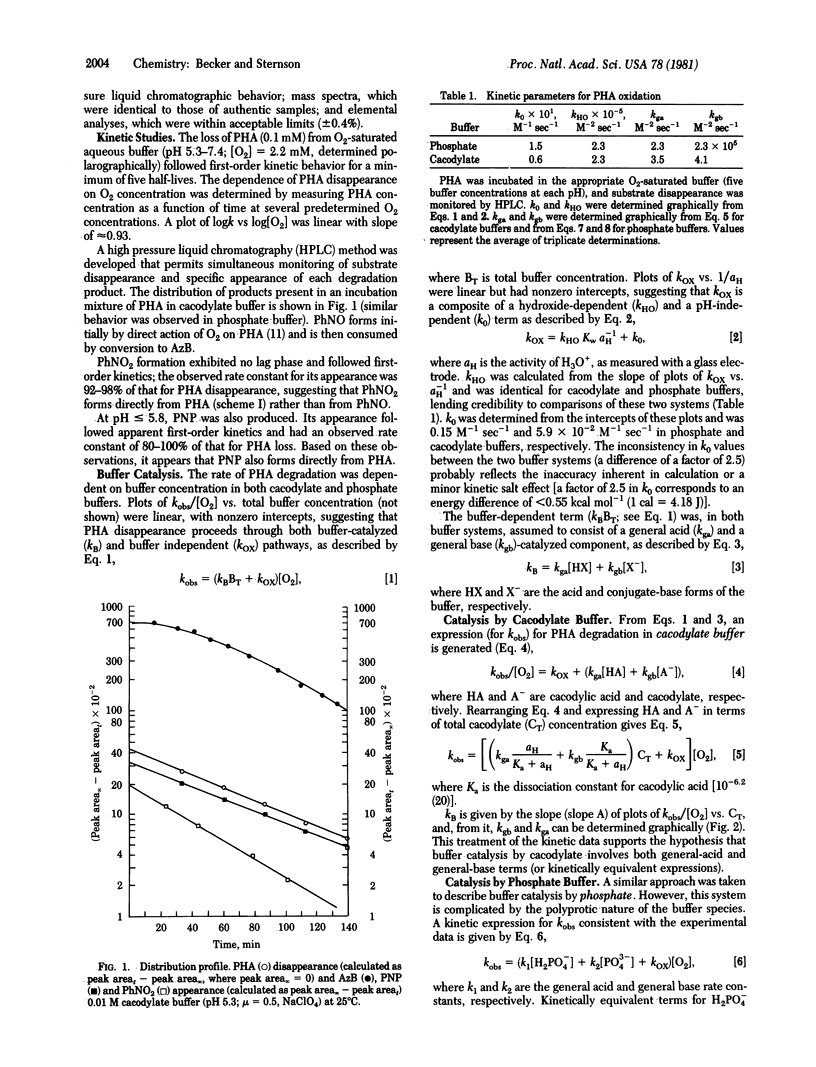
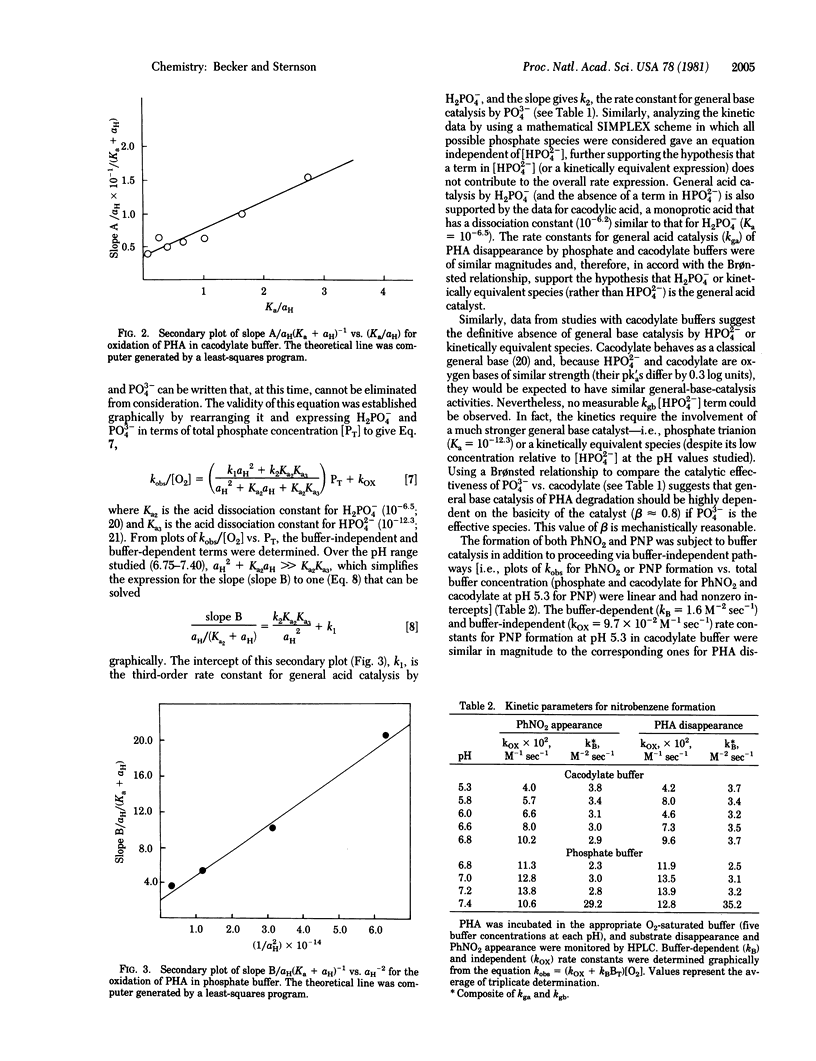
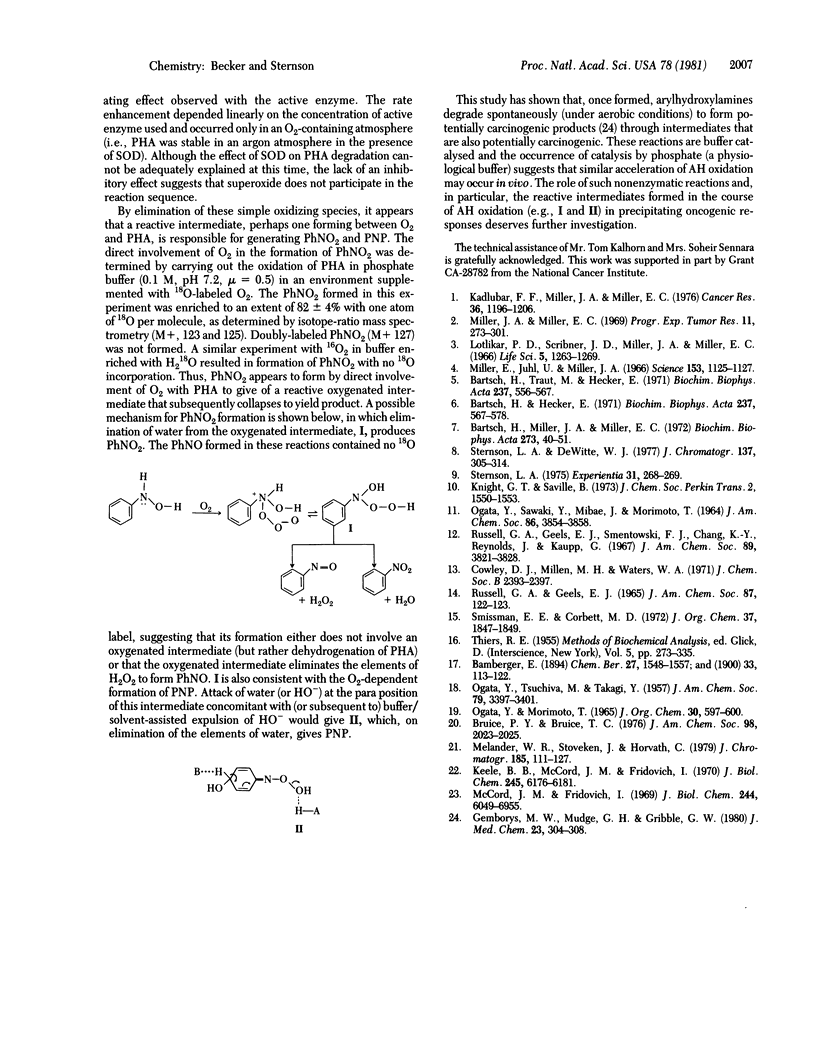
Phenylhydroxylamine is degraded in aqueous phosphate buffers at physiological pH values (6.8-7.4) to give nitrosobenzene, nitrobenzene, and azoxybenzene. The reaction is O2 dependent and subject to general acid and general base catalysis. At pH less than or equal to 5.8 in cacodylate buffer, it is converted to p-nitrosophenol in addition to nitrosobenzene, nitrobenzene, and azoxybenzene. Nitrobenzene and p-nitrosophenol appear to form directly from phenylhydroxylamine. A common intermediate generated from phenylhydroxylamine and O2 is suggested to account for the formation of nitrobenzene, nitrosobenzene, and p-nitrosophenol and is consistent with kinetic studies and 18O-labeling experiments. The results suggest that neither hydrogen peroxide nor superoxide (O-2) are involved in the oxidation sequence.
Full text
PDF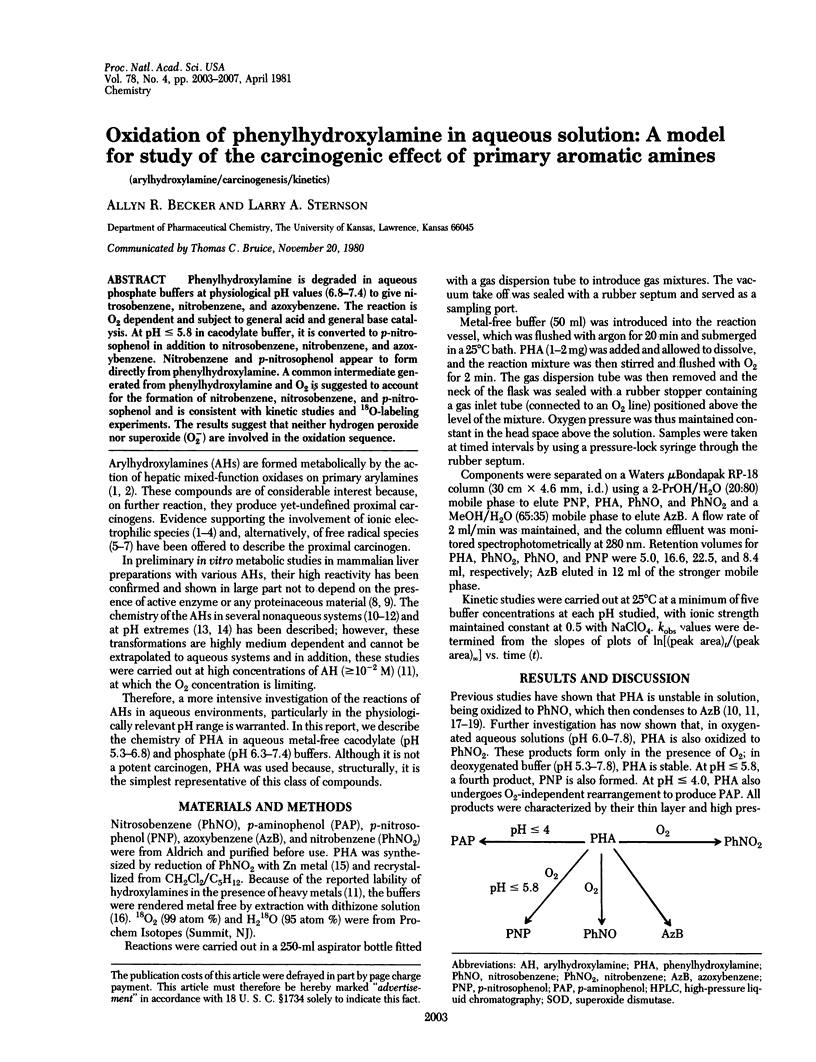

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch H., Hecker E. On the metabolic activation of the carcinogen N-hydroxy-N-2-acetylaminofluorene. 3. Oxidation with horseradish peroxidase to yield 2-nitrosofluorene and N-acetoxy-N-2-acetylaminofluorene. Biochim Biophys Acta. 1971 Jun 22;237(3):567–578. doi: 10.1016/0304-4165(71)90277-7. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Miller J. A., Miller E. C. N-acetoxy-N-acetylaminoarenes and nitrosoarenes. One-electron non-enzymatic and enzymatic oxidation products of various carcinogenic aromatic acethydroxamic acids. Biochim Biophys Acta. 1972 Jun 26;273(1):40–51. doi: 10.1016/0304-4165(72)90189-4. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Traut M., Hecker E. On the metabolic activation of N-hydroxy-N-2-acetylamino-fluorene. II. Simultaneous formation of 2-nitrosofluorene and N-acetoxy-N-2-acetylaminofluorene from N-hydroxy-N-2-acetylaminofluorene via a free radical intermediate. Biochim Biophys Acta. 1971 Jun 22;237(3):556–566. doi: 10.1016/0304-4165(71)90276-5. [DOI] [PubMed] [Google Scholar]
- Gemborys M. W., Mudge G. H., Gribble G. W. Mechanism of decomposition of N-hydroxyacetaminophen, a postulated toxic metabolite of acetaminophen. J Med Chem. 1980 Mar;23(3):304–308. doi: 10.1021/jm00177a019. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F., Miller J. A., Miller E. C. Microsomal N-oxidation of the hepatocarcinogen N-methyl-4-aminoazobenzene and the reactivity of N-hydroxy-N-methyl-4-aminoazobenzene. Cancer Res. 1976 Mar;36(3):1196–1206. [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Miller E. C., Juhl U., Miller J. A. Nucleic acid guanine: reaction with the carcinogen N-acetoxy-2-acetylaminofluorene. Science. 1966 Sep 2;153(3740):1125–1127. doi: 10.1126/science.153.3740.1125. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Miller E. C. The metabolic activation of carcinogenic aromatic amines and amides. Prog Exp Tumor Res. 1969;11:273–301. doi: 10.1159/000391399. [DOI] [PubMed] [Google Scholar]
- Sternson L. A., DeWitte W. J. High-pressure liquid chromatographic analysis of aniline and its metabolites. J Chromatogr. 1977 Jul 21;137(2):305–314. doi: 10.1016/s0021-9673(00)81353-9. [DOI] [PubMed] [Google Scholar]
- Sternson L. A. Detection of arylhydroxylamines as intermediates in the metabolic reduction of nitro compounds. Experientia. 1975 Mar 15;31(3):268–270. doi: 10.1007/BF01922532. [DOI] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]


