Abstract
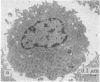
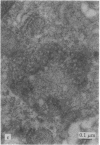
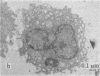
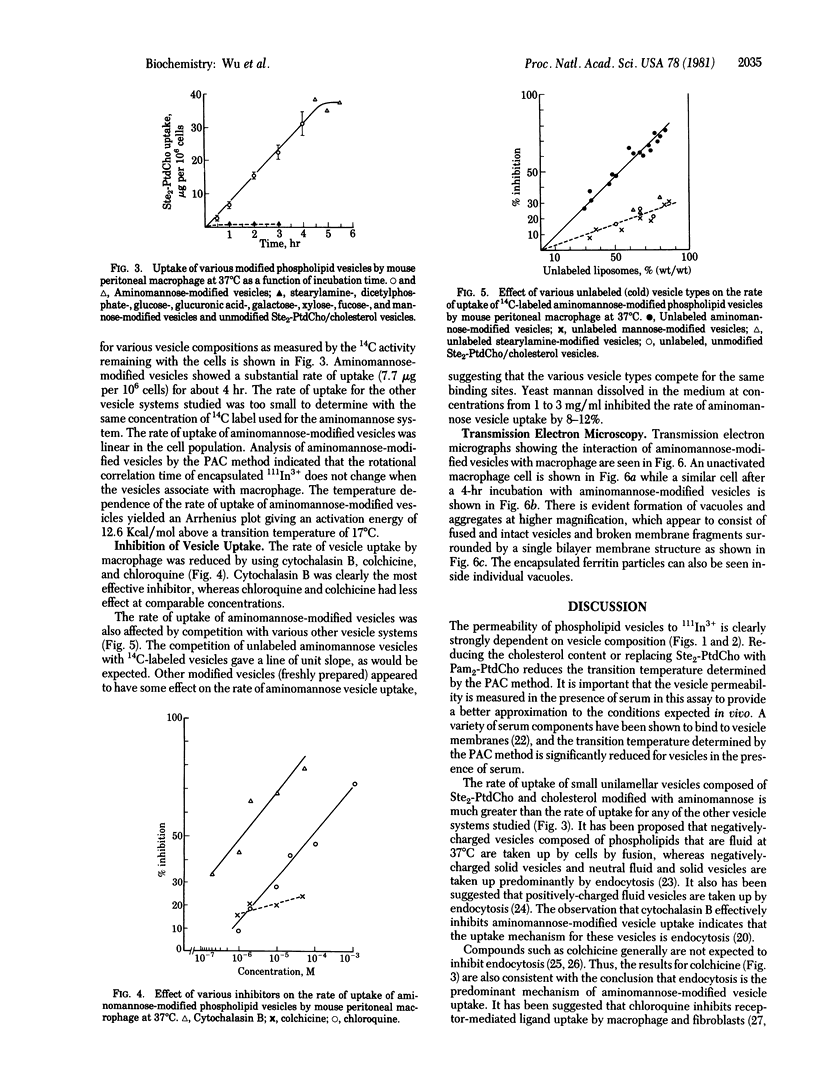
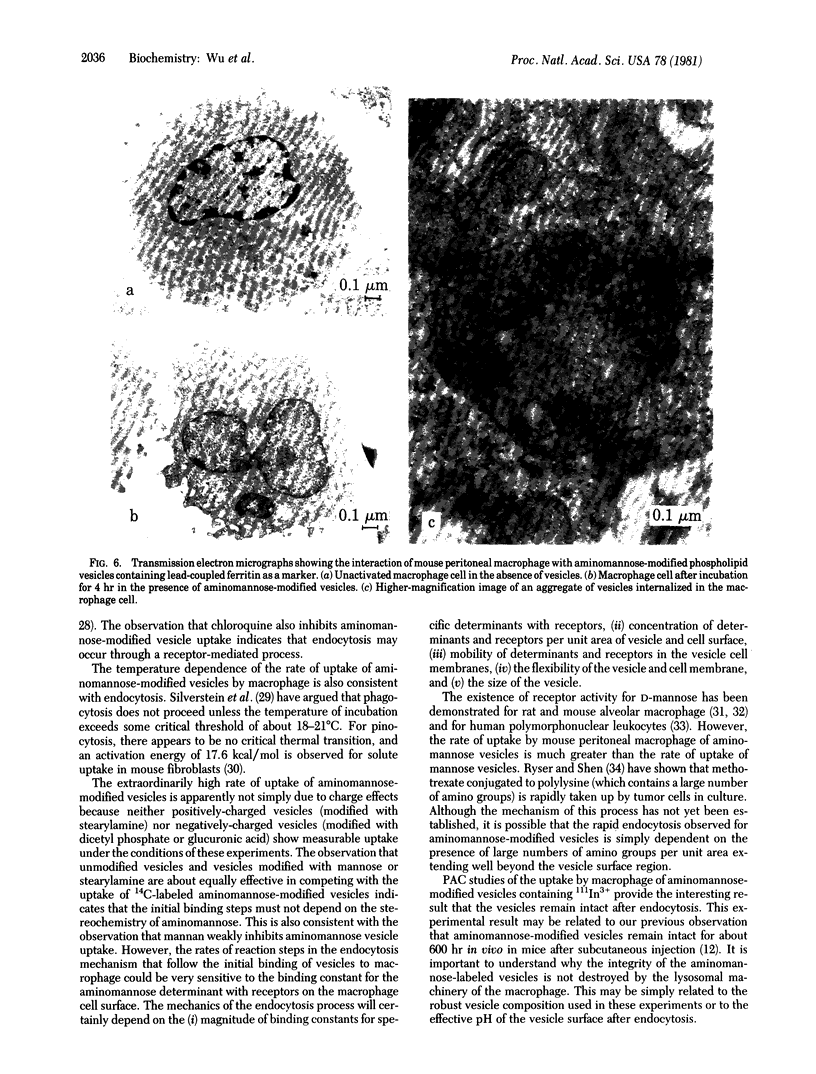
Modification of the surface of distearoyl phosphatidylcholine vesicles with synthetic glycolipids dramatically affects the rate of uptake of these vesicles by mouse peritoneal macrophage. The high rate of uptake of 6-aminomannose-modified vesicles is effectively inhibited by cytochalasin B and chloroquine but not by colchicine, indicating that the mechanisms of vesicle uptake is phagocytosis. Other modified vesicles appear to have some effect on the rate of uptake of 6-aminomannose-modified vesicles suggesting that the various vesicle types compete for the same initial binding sites. Analysis of 6-aminomannose-modified vesicles by gamma-ray perturbed angular correlation spectroscopy shows that the rotational correlation time of the encapsulated 111In3+ does not change when the vesicles associate with macrophage. This result is consistent with transmission electron microscopy, which indicates that the aminomannose-modified vesicles remain intact after phagocytosis as aggregates of fused and intact vesicles surrounded by a single bilayer membrane structure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Sly W. S. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem Biophys Res Commun. 1977 Jul 11;77(1):409–415. doi: 10.1016/s0006-291x(77)80213-1. [DOI] [PubMed] [Google Scholar]
- Alving C. R., Schneider I., Swartz G. M., Jr, Steck E. A. Sporozoite-induced malaria: therapeutic effects of glycolipids in liposomes. Science. 1979 Sep 14;205(4411):1142–1144. doi: 10.1126/science.382358. [DOI] [PubMed] [Google Scholar]
- Alving C. R., Steck E. A., Chapman W. L., Jr, Waits V. B., Hendricks L. D., Swartz G. M., Jr, Hanson W. L. Liposomes in leishmaniasis: therapeutic effects of antimonial drugs, 8-aminoquinolines, and tetracycline. Life Sci. 1980 Jun 30;26(26):2231–2238. doi: 10.1016/0024-3205(80)90207-6. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhisey A. N., Freed J. J. Altered movement of endosomes in colchicine-treated cultured macrophages. Exp Cell Res. 1971 Feb;64(2):430–438. doi: 10.1016/0014-4827(71)90097-8. [DOI] [PubMed] [Google Scholar]
- Bussian R. W., Wriston J. C., Jr Influence of incorporated cerebrosides on the interaction of liposomes with HeLa cells. Biochim Biophys Acta. 1977 Dec 1;471(2):336–336. doi: 10.1016/0005-2736(77)90261-9. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Therapy of spontaneous metastases by intravenous injection of liposomes containing lymphokines. Science. 1980 Jun 27;208(4451):1469–1471. doi: 10.1126/science.7384789. [DOI] [PubMed] [Google Scholar]
- Furbish F. S., Blair H. E., Shiloach J., Pentchev P. G., Brady R. O. Enzyme replacement therapy in Gaucher's disease: large-scale purification of glucocerebrosidase suitable for human administration. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3560–3563. doi: 10.1073/pnas.74.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. T. Cell adhesion mediated by glycolipids. Nature. 1978 Dec 7;276(5688):624–626. doi: 10.1038/276624a0. [DOI] [PubMed] [Google Scholar]
- Hwang K. J., Mauk M. R. Fate of lipid vesicles in vivo: a gamma-ray perturbed angular correlation study. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4991–4995. doi: 10.1073/pnas.74.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansons V. K., Weis P., Chen T., Redwood W. R. In vitro interaction of L1210 cells with phospholipid vesicles. Cancer Res. 1978 Mar;38(3):530–535. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINETTI G. V., ERBLAND J., STOTZ E. The quantitative analysis of plasmalogens by paper chromatography. Biochim Biophys Acta. 1959 Jan;31(1):251–252. doi: 10.1016/0006-3002(59)90464-0. [DOI] [PubMed] [Google Scholar]
- Magee W. E. Potentiation of interferon production and stimulation of lymphocytes by polyribonucleotides entrapped in liposomes. Ann N Y Acad Sci. 1978;308:308–324. doi: 10.1111/j.1749-6632.1978.tb22032.x. [DOI] [PubMed] [Google Scholar]
- Magee W. E., Talcott M. L., Straub S. X., Vriend C. Y. A comparison of negatively and positively charged liposomes containing entrapped polyinosinic-polycytidylic acid for interferon induction in mice. Biochim Biophys Acta. 1976 Dec 21;451(2):610–618. doi: 10.1016/0304-4165(76)90156-2. [DOI] [PubMed] [Google Scholar]
- Mauk M. R., Gamble R. C., Baldeschwieler J. D. Targeting of lipid vesicles: specificity of carbohydrate receptor analogues for leukocytes in mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4430–4434. doi: 10.1073/pnas.77.8.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk M. R., Gamble R. C., Baldeschwieler J. D. Vesicle targeting: timed release and specificity for leukocytes in mice by subcutaneous injection. Science. 1980 Jan 18;207(4428):309–311. doi: 10.1126/science.7350660. [DOI] [PubMed] [Google Scholar]
- Mauk M. R., Gamble R. C. Preparation of lipid vesicles containing high levels of entrapped radioactive cations. Anal Biochem. 1979 Apr 15;94(2):302–307. doi: 10.1016/0003-2697(79)90364-6. [DOI] [PubMed] [Google Scholar]
- Mauk M. R., Gamble R. C. Stability of lipid vesicles in tissues of the mouse: a gamma-ray perturbed angular correlation study. Proc Natl Acad Sci U S A. 1979 Feb;76(2):765–769. doi: 10.1073/pnas.76.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L., Axline S. G. Phagolysosome formation in normal and colchicine-treated macrophages. J Exp Med. 1975 Oct 1;142(4):903–913. doi: 10.1084/jem.142.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G., Papahadjopoulos D. Lipid vesicles as carriers for introducing materials into cultured cells: influence of vesicle lipid composition on mechanism(s) of vesicle incorporation into cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1603–1607. doi: 10.1073/pnas.73.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznansky M., Lange Y. Transbilayer movement of cholesterol in dipalmitoyllecithin-cholesterol vesicles. Nature. 1976 Feb 5;259(5542):420–421. doi: 10.1038/259420a0. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Slama J., Bangerter F. W. Functional incorporation of synthetic glycolipids into cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2510–2513. doi: 10.1073/pnas.77.5.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottini G., Cian F., Soranzo M. R., Albrigo R., Patriarca P. Evidence for the involvement of human polymorphonuclear leucocyte mannose-like receptors in the phagocytosis of Escherichia coli. FEBS Lett. 1979 Sep 15;105(2):307–312. doi: 10.1016/0014-5793(79)80636-5. [DOI] [PubMed] [Google Scholar]
- Rottini G., Cian F., Soranzo M. R., Albrigo R., Patriarca P. Evidence for the involvement of human polymorphonuclear leucocyte mannose-like receptors in the phagocytosis of Escherichia coli. FEBS Lett. 1979 Sep 15;105(2):307–312. doi: 10.1016/0014-5793(79)80636-5. [DOI] [PubMed] [Google Scholar]
- Ryser H. J., Shen W. C. Conjugation of methotrexate to poly(L-lysine) increases drug transport and overcomes drug resistance in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3867–3870. doi: 10.1073/pnas.75.8.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Titus-Dillon P., Hall C. W., Neufeld E. F. Inhibition of receptor-mediated uptake of a lysosomal enzyme into fibroblasts by chloroquine, procaine and ammonia. Exp Cell Res. 1979 Mar 15;119(2):359–364. doi: 10.1016/0014-4827(79)90364-1. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Chloroquine and ammonium ion inhibit receptor-mediated endocytosis of mannose-glycoconjugates by macrophages: apparent inhibition of receptor recycling. Biochem Biophys Res Commun. 1980 Mar 13;93(1):1–8. doi: 10.1016/s0006-291x(80)80237-3. [DOI] [PubMed] [Google Scholar]
- Warr G. A. A macrophage receptor for (mannose/glucosamine)-glycoproteins of potential importance in phagocytic activity. Biochem Biophys Res Commun. 1980 Apr 14;93(3):737–745. doi: 10.1016/0006-291x(80)91139-0. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Magin R. L., Yatvin M. B., Zaharko D. S. Liposomes and local hyperthermia: selective delivery of methotrexate to heated tumors. Science. 1979 Apr 13;204(4389):188–191. doi: 10.1126/science.432641. [DOI] [PubMed] [Google Scholar]