Abstract
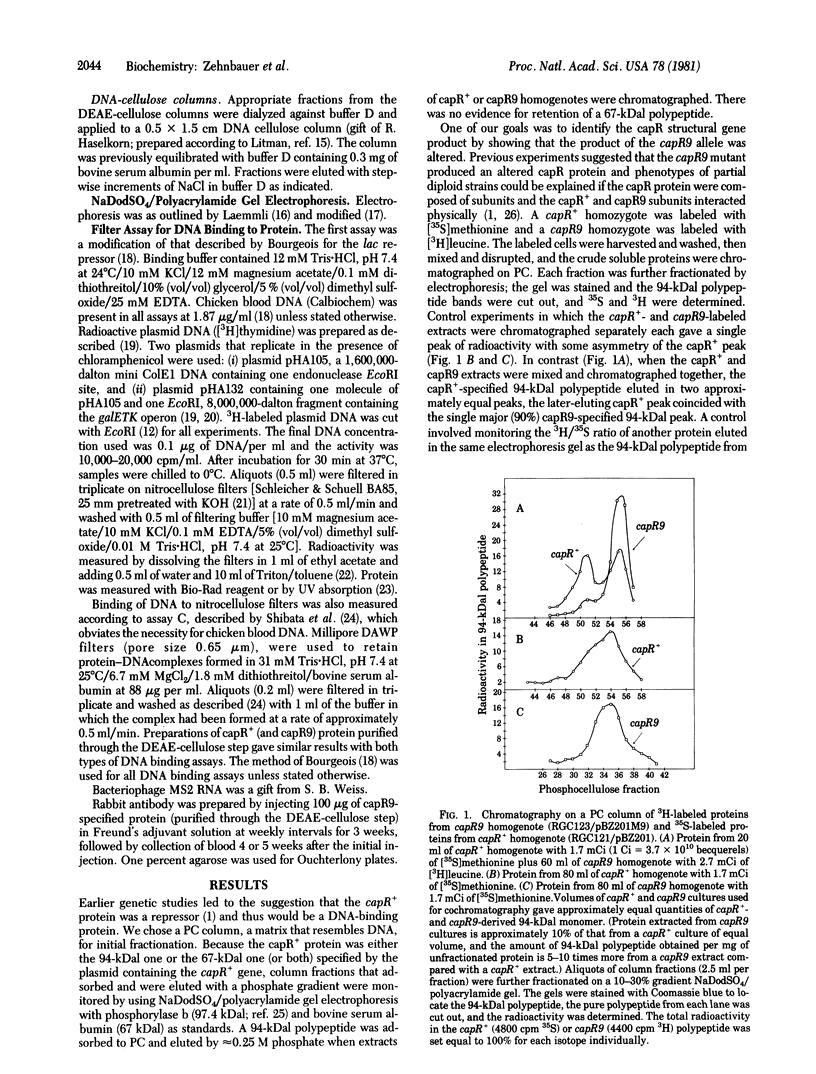
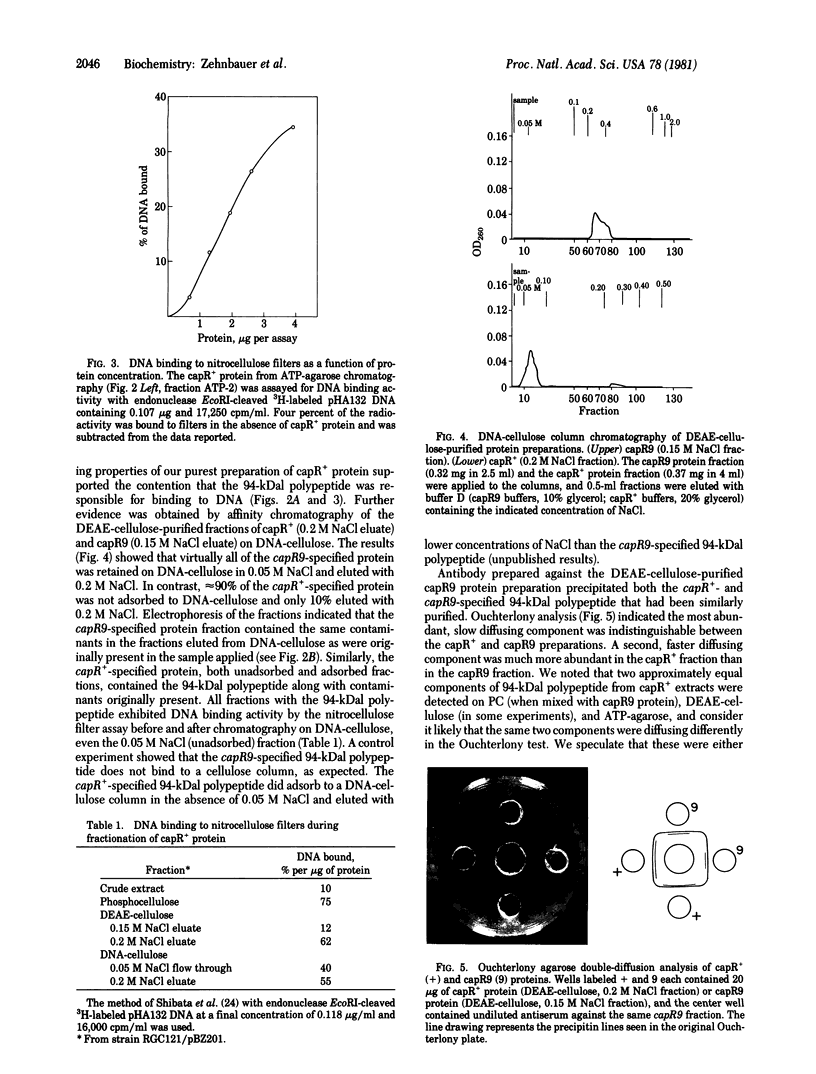
The polypeptide product of the lon (capR) gene was identified and partially purified from bacterial strains homozygous for the capR+ or capR9 (ochre mutation) alleles cloned with pSC101. A 94,000-dalton polypeptide was identified as the lon (capR) gene product. Studies of binding to DNA cellulose columns and nitrocellulose filters indicate that the capR+ and capR9 proteins bind DNA.
Keywords: plasmids, radiation sensitivity, cell division, proteolysis, capsular polysaccharide
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni H., Berg P. E., Markovitz A. New mini-ColE1 as a molecular cloning vehicle. J Bacteriol. 1977 Jan;129(1):358–366. doi: 10.1128/jb.129.1.358-366.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni H., Markovitz A. Characterization of a mini ColE1 cloning vector. Plasmid. 1979 Apr;2(2):225–236. doi: 10.1016/0147-619x(79)90041-6. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E., Hua S. S., Avni H., Markovitz A. Transcriptional control of the calactose operon by the capR (lon) and capT genes. J Bacteriol. 1973 May;114(2):891–893. doi: 10.1128/jb.114.2.891-893.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Ultraviolet sensitivity gene of Escherichia coli B. J Bacteriol. 1968 May;95(5):1555–1559. doi: 10.1128/jb.95.5.1555-1559.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda R. C., Markovitz A. Cloned DNA fragment specifying major outer membrane protein a in Escherichia coli K-12. J Bacteriol. 1978 Oct;136(1):369–380. doi: 10.1128/jb.136.1.369-380.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S. S., Markovitz A. Multiple regulator gene control of the galactose operon in Escherichia coli K-12. J Bacteriol. 1972 Jun;110(3):1089–1099. doi: 10.1128/jb.110.3.1089-1099.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanka E., Edelbluth C., Schlicht M., Schuster H. Escherichia coli dnaB protein. Affinity chromatography on immobilized nucleotides. J Biol Chem. 1978 Aug 25;253(16):5847–5851. [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to non-operator DNA: detailed studies and a comparison of eequilibrium and rate competition methods. J Mol Biol. 1972 Dec 30;72(3):671–690. doi: 10.1016/0022-2836(72)90184-2. [DOI] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- Mackie G., Wilson D. B. Regulation of the gal operon of Escherichia coli by the capR gene. J Biol Chem. 1972 May 25;247(10):2973–2978. [PubMed] [Google Scholar]
- Markovitz A., Rosenbaum N. A regulator gene that is dominant on an episome and recessive on a chromosome. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1084–1091. doi: 10.1073/pnas.54.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. C., Ficht T. A., Holladay L. A., Moyer R. W. The purification and properties of a double-stranded DNA-binding protein encoded by the gene D5 of bacteriophage T5. J Biol Chem. 1979 Aug 25;254(16):8042–8051. [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Cunningham R. P., DasGupta C., Radding C. M. Homologous pairing in genetic recombination: complexes of recA protein and DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5100–5104. doi: 10.1073/pnas.76.10.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shineberg B., Zipser D. The ion gene and degradation of beta-galactosidase nonsense fragments. J Bacteriol. 1973 Dec;116(3):1469–1471. doi: 10.1128/jb.116.3.1469-1471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Koide A., Hermann J., Ericsson L. H., Kumar S., Wade R. D., Walsh K. A., Neurath H., Fischer E. H. Complete amino acid sequence of rabbit muscle glycogen phosphorylase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4762–4766. doi: 10.1073/pnas.74.11.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Conditional mutations involving septum formation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):107–114. doi: 10.1128/jb.93.1.107-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Genetics of Resistance to Radiation in ESCHERICHIA COLI. Genetics. 1947 May;32(3):221–248. doi: 10.1093/genetics/32.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnbauer B. A., Markovitz A. Cloning of gene lon (capR) of Escherichia coli K-12 and identification of polypeptides specified by the cloned deoxyribonucleic acid fragment. J Bacteriol. 1980 Aug;143(2):852–863. doi: 10.1128/jb.143.2.852-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]