Abstract
Context
Despite the repeated findings of impaired fear conditioning and affective recognition in psychopathic individuals, there has been a paucity of brain imaging research on the amygdala and no evidence suggesting which regions within the amygdala may be structurally compromised in individuals with psychopathy.
Objective
To detect global and regional anatomical abnormalities in the amygdala in individuals with psychopathy.
Design
Cross-sectional design using structural magnetic resonance imaging.
Setting
Participants were recruited from high-risk communities (temporary employment agencies) in the Los Angeles, California, area and underwent imaging at a hospital research facility at the University of Southern California.
Participants
Twenty-seven psychopathic individuals as defined by the Hare Psychopathy Checklist–Revised and 32 normal controls matched on age, sex, and ethnicity.
Main Outcome Measures
Amygdala volumes were examined using traditional volumetric analyses and surface-based mesh modeling methods were used to localize regional surface deformations.
Results
Individuals with psychopathy showed significant bilateral volume reductions in the amygdala compared with controls (left, 17.1%; right, 18.9%). Surface deformations were localized in regions in the approximate vicinity of the basolateral, lateral, cortical, and central nuclei of the amygdala. Significant correlations were found between reduced amygdala volumes and increased total and facet psychopathy scores, with correlations strongest for the affective and interpersonal facets of psychopathy.
Conclusions
Results provide the first evidence, to our knowledge, of focal amygdala abnormalities in psychopathic individuals and corroborate findings from previous lesion studies. Findings support prior hypotheses of amygdala deficits in individuals with psychopathy and indicate that amygdala abnormalities contribute to emotional and behavioral symptoms of psychopathy.
Psychopathy is a clinical condition conceptualized by a combination of core psychopathic personalities (eg, shallow affect, conning and manipulative) and antisocial behavioral outcomes (eg, parasitic lifestyle, poor behavioral control).1–3 Psychopathic individuals are particularly viewed as having a specific emotional and interpersonal style that is characterized by the inability to recognize and experience the emotional significance of social events.4 It was suggested that as a result of their emotional impairments, individuals with psychopathy use a detached, predatory style of antisocial behavior as a strategy to meet their immediate needs without regard for the consequences.4 Consistent with the symptoms of psychopathy, one of the most robust findings in individuals with psychopathy is the abnormal psychophysiological responsivity during the viewing of emotional stimuli and aversive conditioning learning,5–10 suggesting possible deficits in the neurobiological system that governs emotional response, particularly negative emotions, such as fear and anger.11–13
The amygdala has long been known as one of the most important components in the neural circuit underlying emotional processing.14–18 An intact amygdala is found to be necessary for fear conditioning19; thus, impairments in this structure have been hypothesized to contribute to the well-replicated observations of poor fear conditioning in individuals with psychopathy.9,20 In addition, the amygdala is an important component of the neural systems subserving reward learning, social interaction, and moral emotion and reasoning,21–26 where the ability to recognize the emotions signaled by facial expressions is crucial for making advantageous decisions in a complex social environment.19 Therefore, it has also been hypothesized that disturbances in amygdala structure or function may contribute to the social dysfunction and impaired moral decision making in individuals with psychopathy.27
Studies have revealed that the amygdala is not a homogeneous structure and can be differentiated into approximately 13 nuclei. Although the functional specificity of the nuclei in the human amygdala remains unclear, considerable evidence from animal studies suggests that several nuclei of the amygdala are involved in the processing of emotion.28,29 For example, the basolateral and lateral nuclei have been linked to the encoding of sensory-specific features, whereas the central nucleus has been associated with encoding the motivational or affective aspects.30 The importance of the amygdala and its subdivisions in emotional processing and social functioning has led to several predictions that global and regional deficits of the amygdala may contribute to features of psychopathy31–34; however, to our knowledge, no study to date has examined the structural integrity of the amygdala in psychopathic individuals.
Using a community sample, we examined the structure of the amygdala in psychopathic and control groups. It was hypothesized that psychopathic individuals would show a global volume reduction in the amygdala and regional morphological alterations in the approximate locations of basolateral, lateral, and central nuclei. Furthermore, we examined correlations between the amygdala and degree of psychopathy using the 4-facet concept of psychopathy, which includes interpersonal (eg, superficial charm, manipulative), affective (eg, shallow affect, lack of remorse), lifestyle (eg, impulsivity, irresponsibility), and antisocial (eg, poor behavioral control, criminal versatility) facets.35 It was predicted that reduced amygdala volume would correlate more prominently with the core features of psychopathy (ie, interpersonal and affective facets) than the secondary features of antisocial behavior (ie, lifestyle and antisocial facets), which are more common in nonpsychopathic offenders and not as specific to the syndrome of psychopathy.
METHODS
PARTICIPANTS
Eighty-six subjects were recruited from 5 temporary employment agencies in Los Angeles, California.35 Samples from this community have been found to show relatively higher rates of psychopathy/antisocial personality.36 Psychopathy was assessed using the Hare Psychopathy Checklist–Revised (PCL-R)3,35 and supplemented by 5 sources of collateral data that included (1) the Interpersonal Measure of Psychopathy37 ratings; (2) self-reported crime and violence assessed using an adult extension36 of the National Youth Survey self-report delinquency measure38; (3) criminal history transcripts obtained from the Department of Justice; (4) data derived from, and behavioral observations made during, the Structured Clinical Interview for Axis I DSM-IV Disorders and Axis II Personality Disorders39,40; and (5) independent Interpersonal Measure of Psychopathy ratings made by 2 different laboratory assistants during separate phases of testing. A cutoff of 23 (high) and 15 (low) on the total PCL-R score was used to define psychopathy, resulting in a total of 27 individuals with psychopathy (PCL-R score range, 23–40) and 32 controls (PCL-R score range, 5–14). This cutoff was chosen to be consistent with our prior research on this sample36,41–43 and is similar to the optimal cutoff suggested by taxometric analyses of the PCL-R.44 The general term of individuals with psychopathy is used in this article to refer to community individuals with a PCL-R score higher than 23. Demographic, cognitive, and diagnostic characteristic information were collected as follows: (1) age, sex, and ethnicity; (2) socioeconomic status45; (3) full-scale IQ as assessed by the Wechsler Adult Intelligence Scale–Revised46; (4) handedness as assessed by the abbreviated Oldfield Inventory47; (5) lifetime diagnosis of drug/alcohol dependence as assessed by the Structural Clinical Interview for Axis I DSM-IV Disorders39; and (6) the number of alcohol drinks used in the past month as assessed by the alcohol use questionnaire.36 Written informed consent was obtained from all subjects and the study was approved by the University of Southern California institutional review board.
MAGNETIC RESONANCE IMAGE ANALYSIS
Structural magnetic resonance images were collected on a 1.5-T Philips (Shelton, Connecticut) S15/ACS scanner (repetition time=34 milliseconds, echo time=12.4 milliseconds, voxel size=0.9×0.9×1.7 mm).36 Before the delineation process, each image volume was first corrected for magnetic field inhomogeneities48 and head tilt and alignment by using 6-parameter rigid-body registrations.44 During the registration procedure, the image volumes were placed in a common coordinate space.49
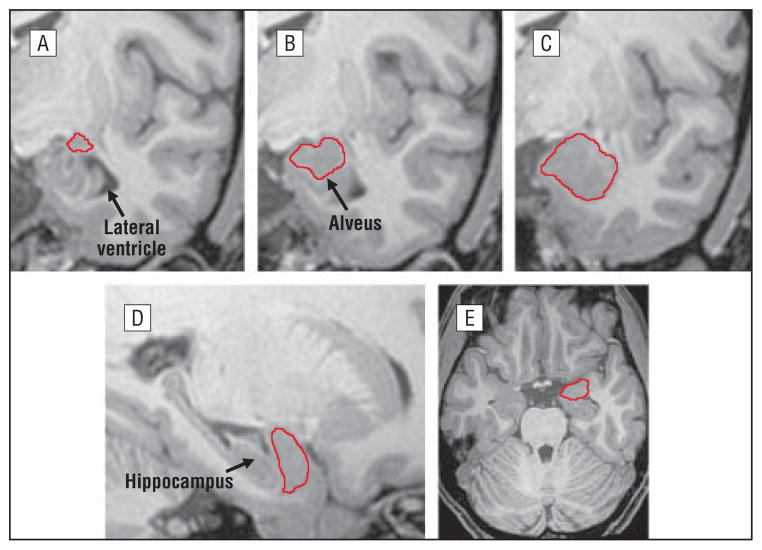
The delineation of the amygdala was conducted by 2 independent technicians (premedical neuroscience trainees) who received extensive training on the delineation protocols under the supervision of one of us (Y.Y.). These 2 technicians, blind to group membership, traced the amygdala following a delineation protocol adopted from previously validated methods50–52 and based on the atlas of the amygdala anatomy by Mai et al.53 The images were first resampled to 1-mm thickness to improve the visibility of anatomical details for identifying the structure. The amygdala was traced in coronal brain slices from posterior to anterior while using the digitized surface contours simultaneously displayed in both sagittal and transverse planes to facilitate a more accurate identification of the boundaries that separate the amygdala from surrounding structures (Figure 1). The amygdala was traced on magnified images (×4) to allow better precision in identifying the anatomical boundaries. Amygdala tracing in each hemisphere began when the structure appeared as a pebble of gray matter superior to the temporal horn of the lateral ventricle and inferior to the white matter of the parietal lobe. As the lateral ventricle moved to a more lateral position as the brain volumes moved anterior, the alveus appeared and was used as the inferior boundary that separates the amygdala from the hippocampus. The inferior boundary shifted to the temporal lobe white matter when the alveus was no longer visible. The tracing of the amygdala was continued anteriorly until the lateral ventricle completely separated the temporal lobe from the rest of the cerebrum. For each subject, right and left amygdala volumes were extracted using these tracings. To assess interrater reliability, 10 different randomly selected cases were traced and the interrater intraclass correlation coefficients for amygdala volumes ranged between 0.91 and 0.95. Whole-brain volumes, obtained as previously described,54 were retained for use as covariates to control for individual differences in brain size in analyses of amygdala morphology.
Figure 1.
The amygdala was traced in high-resolution T1-weighted images using the software MultiTracer (http://air.bmap.ucla.edu/MultiTracer). Tracing was performed in contiguous coronal slices (A–C showing posterior to anterior) while referencing orthogonal sagittal (D) and transverse (E) planes to confirm the correct identification of anatomical boundaries. Even though only the tracings of the right amygdala are illustrated, the left amygdala was traced following the same protocol.
SURFACE-BASED MESH AVERAGING
Surface meshes were constructed for the amygdala using surface-based anatomical modeling, and skeletonizing methods were used to identify regional morphological changes of the amygdala.55 The surface contours representing each individual’s left or right amygdala were made spatially uniform within and across subjects, where each surface was made to represent 15 000 surface points or nodes. Radial distances from the central core (medial axis) of each amygdala to the spatially normalized surface points were then computed within subjects. These distances obtained at each spatially equivalent amygdala surface point were then compared between groups to reveal local surface deformations/expansions and the results were displayed on the group averaged left or right amygdala.
STATISTICAL ANALYSES
Analyses of the amygdala volumes were conducted using SPSS (SPSS Inc, Chicago, Illinois). The general linear model was used to assess the amygdala volume differences between the psychopathy and control groups, while covarying for total brain volume. Socioeconomic status and substance dependence, for which groups differed significantly, were also included in the statistical analyses as covariates. Partial correlations were conducted on the entire sample of 59 to assess the association between amygdala volume and total and facet psychopathy scores, while controlling for total brain volume. All tests of significance were 2-tailed with an α level of .05.
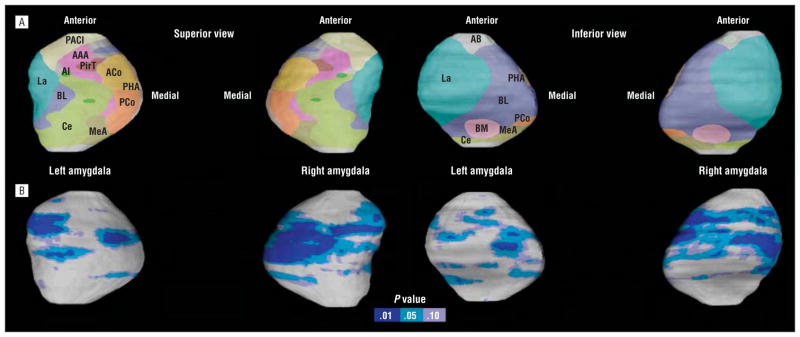
The same statistical models described earlier were implemented using the statistical program R (www.r-project.org) to identify regional changes in amygdala surface anatomy between groups and assess relationships between amygdala surface deformations and psychopathy scores. Uncorrected 2-tailed probability values obtained from these analyses conducted for each amygdala surface point were color coded and displayed on the averaged surface of the entire group to allow initial visualization of localized group differences in amygdala surface structure. Structural variation of the human amygdala and individual differences in the relative position of amygdala nuclei across the surface do not allow the locations of amygdala nuclei to be determined with a high degree of accuracy in imaging data. Thus, the boundaries of the different subnuclei (Figure 2) are approximations as they are based on an extrapolation of the relative nuclei positions in 1 atlas.53 Permutation tests with a threshold of P<.05 were applied to control for multiple spatially correlated comparisons (statistical tests performed at 15 000 amygdala surface locations) to ensure that the overall pattern of effects in the surface-based maps could not have been observed by chance alone.56–58
Figure 2.
The amygdala nuclei and significant regional amygdala surface deformations. A, Schematic representation of the amygdala nuclei is mapped onto the 3-dimensional left and right amygdala surfaces. Structural variation of the human amygdala and individual differences in the relative position of the amygdala nuclei across the surface do not allow the locations of the amygdala nuclei to be determined with a high degree of accuracy in imaging data. Thus, the boundaries of the different subnuclei are approximations based on an extrapolation of the relative nuclei positions derived from the atlas of Mai et al.53 AAA indicates anterior amygdaloid area; AB, accessory basal nucleus; ACo, anterior cortical nucleus; AI, amygdaloid island; BL, basolateral nucleus; BM, basomedial nucleus; Ce, central nucleus; La, lateral nucleus; MeA, medial amygdaloid nucleus; PACl, preamygdalar claustrum; PCo, posterior cortical nucleus; PHA, parahippocampal-amygdaloid transition area; PirT, piriform cortex. B, Statistical maps showing significant regional amygdala surface deformations in individuals with psychopathy (n=27) compared with healthy controls (n=32) as encoded by the color bar (uncorrected P value). No surface expansion in the amygdala in individuals with psychopathy was observed.
RESULTS
PSYCHOPATHIC INDIVIDUALS VS CONTROLS
Subject Characteristics
Groups differed significantly on socioeconomic status and history of substance/alcohol dependence (Table 1). To rule out these potential confounds, they were included as covariates in the general linear models used for the volumetric analyses as well as the surface-based analyses.
Table 1.
Demographic, Cognitive, and Diagnostic Measures for Psychopathy and Control Groups
| Mean (SD) [Range]
|
Statistics | ||
|---|---|---|---|
| Psychopathy (n=27) | Control (n=32) | ||
| Age, y | 32.22 (6.57) | 30.84 (7.14) | F1,57=1.60; P =.21 |
| Socioeconomic status | 30.30 (7.73) | 36.77 (10.81) | F1,57=6.71; P =.01 |
| Ethnicity | ; P =.44 | ||
| White | 12 | 18 | |
| Other | 15 | 14 | |
| Sex | ; P =.27 | ||
| M | 25 | 26 | |
| F | 2 | 6 | |
| Full-scale IQ | 98.11 (14.13) | 103.84 (17.36) | F1,57=1.86; P =.18 |
| Handedness | ; P =.49 | ||
| Right | 23 | 25 | |
| Left | 3 | 7 | |
| Psychopathy | |||
| Total PCL-R3,35 score | 27.96 (4.93) [23–40] | 10.56 (2.83) [5–14] | F1,57=192.6; P < .001 |
| Facet 1 (interpersonal) | 5.41 (1.50) [3–8] | 1.78 (1.43) [0–5] | F1,57=224.5; P < .001 |
| Facet 2 (affective) | 5.26 (1.68) [2–8] | 1.34 (1.15) [0–4] | F1,57=209.1; P < .001 |
| Facet 3 (lifestyle) | 7.19 (2.00) [3–10] | 3.41 (1.88) [0–7] | F1,57=348.0; P < .001 |
| Facet 4 (antisocial) | 7.00 (2.06) [4–10] | 2.13 (1.66) [0–6] | F1,57=287.6; P < .001 |
| Substance/alcohol dependence | ; P < .001 | ||
| Present | 19 | 6 | |
| Absent | 7 | 26 | |
| No. of alcohol drinks/mo | 6.56 (7.32) | 4.29 (6.50) | F1,57=1.56; P =.22 |
Abbreviation: PCL-R, Hare Psychopathy Checklist–Revised.
Traditional Volumetric Analyses
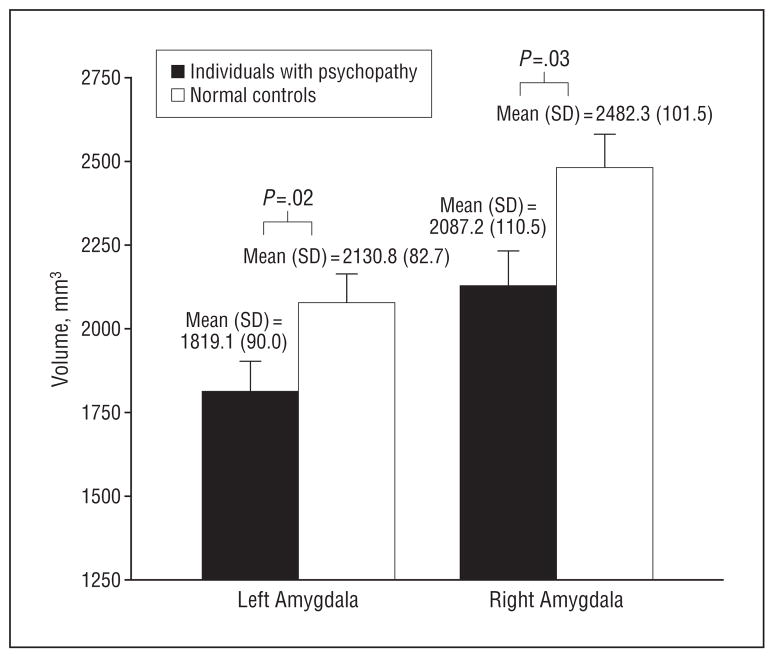
Psychopathic individuals showed a significant volume reduction in the amygdala compared with controls (F2,55=3.85; P =.03), with whole-brain volume as a covariate. As illustrated in Figure 3, individuals with psychopathy showed a 17.14% volume reduction in the left amygdala and an 18.93% volume reduction in the right amygdala compared with controls. While the right amygdala showed a tendency toward greater volumetric reduction than the left, no significant laterality effect was observed (F1,56=0.61; P =.44). Group differences for both left and right amygdala volumes remained significant after correcting for the whole-brain volume, socioeconomic status, and substance/alcohol dependence (P=.02 and P=.02, respectively).
Figure 3.
Amygdala volumes in psychopathic individuals and normal controls, with total brain volume as a covariate. The vertical lines represent the standard error. The group mean (SD) volume is indicated.
Surface-Based Mesh Modeling Methods
Surface-based modeling analysis revealed bilateral shape differences of the amygdala that were consistent with the volumetric findings (left, permutation-corrected P=.047; right, P=.002) (Figure 2). The results remained significant after controlling for whole-brain volume, socioeconomic status, and substance/alcohol dependence (left, permutation-corrected P=.049; right, P=.006). In terms of localization, little evidence could be found for deformations in the vicinity of accessory basal nuclei, basomedial nuclei, medial amygdaloid nuclei, and piriform cortex (Figure 2). Regarding the remaining 9 nuclei, the strongest evidence was found for deformations in the approximate locations of anterior and posterior cortical and central nuclei (superior view) and basolateral and lateral nuclei (superior and inferior view) of the amygdala.
CORRELATION ANALYSES
Traditional Volumetric Analyses
To determine whether the amygdala volume deficit was associated with a particular facet of psychopathy, correlational analyses on the entire sample of 59 subjects were conducted. Findings revealed significant negative correlations between amygdala volumes and the psychopathy total and facet scores, with the correlations strongest for the affective and interpersonal facets (Table 2). All findings remained significant after controlling for whole-brain volume, socioeconomic status, and substance/alcohol dependence (all P< .01).
Table 2.
Correlations Between Amygdala Volumes and the Total and 4 Facet Scores of Psychopathy in the Entire Sample of 59, Controlling for Whole-Brain Volume
| Score | Left Amygdala
|
Right Amygdala
|
||
|---|---|---|---|---|
| Correlation | P Value | Correlation | P Value | |
| Total psychopathy | −0.39 | .002 | −0.40 | .002 |
| Interpersonal facet | −0.45 | <.001 | −0.36 | .005 |
| Affective facet | −0.49 | <.001 | −0.47 | <.001 |
| Lifestyle facet | −0.22 | .10 | −0.34 | .009 |
| Antisocial facet | −0.24 | .07 | −0.27 | .04 |
Surface-Based Mesh Modeling Methods
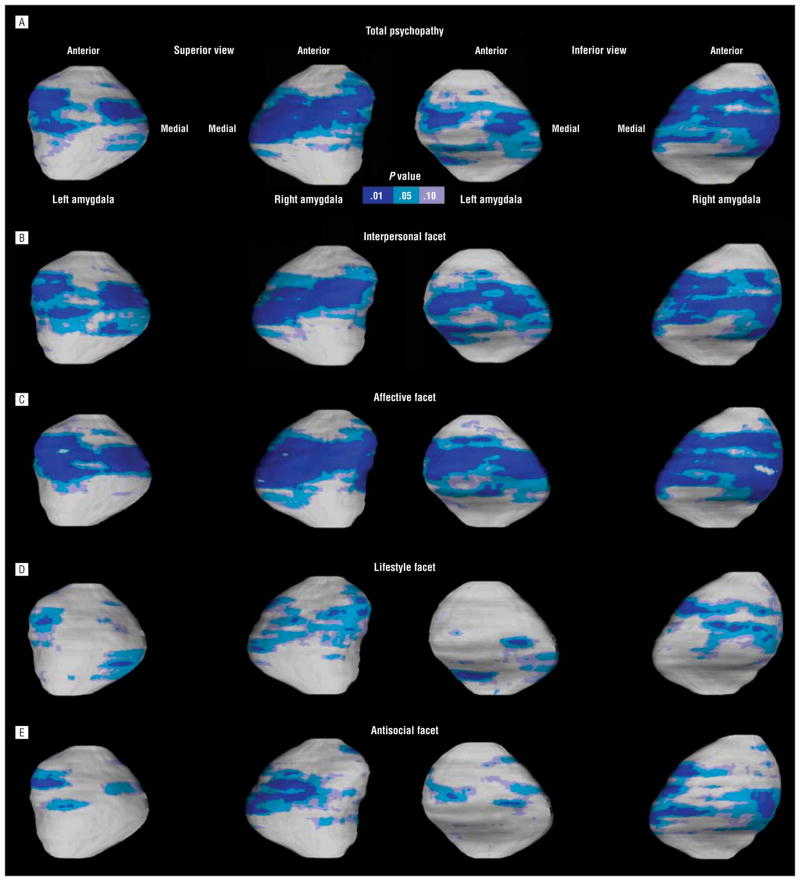
As demonstrated in Figure 4, surface-based analyses revealed similar negative correlations between the amygdala and psychopathy scores, which again were more pronounced for the affective and interpersonal facets. The permutation analyses showed that bilateral amygdala deformations were significantly correlated with total (left, permutation-corrected P=.004; right, P<.001), interpersonal (left, permutation-corrected P<.001; right, P=.001), and affective (left, permutation-corrected P<.001; right, P<.001) scores. The lifestyle and antisocial facets of psychopathy showed significant associations with the left amygdala (permutation-corrected P=.02 and .01, respectively) but not the right amygdala (permutation-corrected P=.12 and .16, respectively). These correlations remained significant after controlling for whole-brain volume, socioeconomic status, and substance/alcohol dependence (all permutation-corrected P < .03). Consistent with group analyses, increased psychopathy scores were associated with increased regional atrophy approximately in the cortical, central, basolateral, and lateral nuclei of the amygdala (Figure 4).
Figure 4.
Statistical maps showing significant negative correlations between the amygdala surface structure and total psychopathy (A) and interpersonal (B), affective (C), lifestyle (D), and antisocial (E) facet scores across the entire sample of 59 subjects. The uncorrected P values corresponding to significant r values are encoded by the color bar. The regional deformations were confirmed by permutations indicating an overall pattern of significant correlations between deformations in the amygdala and the increase in the total and facet scores.
COMMENT
To our knowledge, this study is the first to show localized structural abnormalities of the amygdala in individuals with psychopathy. In this study, psychopathic individuals were found to show bilateral amygdala volume reductions compared with controls. Significant regional deformations were found to be most prominent in the vicinity of the basolateral, lateral, cortical, and central nuclei of the amygdala in individuals with psychopathy. In addition, negative correlations were found between amygdala volumes and all 4 psychopathy facet scores, with correlations being most pronounced for the affective and interpersonal facets of psychopathy. Results could not be attributed to demographic group differences in socioeconomic status or substance/alcohol dependence. Findings provide initial evidence indicating amygdala structural abnormalities as 1 key element in the neurobiological bases of psychopathy.
Findings of amygdala volume reductions in individuals with psychopathy are consistent with lesion studies showing that damage to the amygdala results in emotional impairments similar to those of psychopathy. Animals with selective lesions to the amygdala have been found to show substantial impairments in fear conditioning and abnormal responsivity to threatening objects. For example, Hitch-cock and Davis59 demonstrated that lesions to the amygdala impaired fear-potentiated startle response in rats. In a recent study, Machado and Bachevalier60 found that rhesus monkeys with selective lesions to the amygdala showed profound behavioral changes that precluded positive social interactions (eg, decreased affiliation and popularity within the group) and decreased reactivity to threatening gestures. Similar findings have been reported showing that monkeys with bilateral amygdala lesions displayed blunted emotional response (ie, lack of fear) and decreased defensive behavior to threats (ie, a fake snake).61,62 These behavioral changes found in animals with amygdala lesions may suggest, albeit indirectly, that amygdala structural abnormalities may contribute to the classic findings of poor fear conditioning and impaired emotion recognition observed in individuals with psychopathy.7,9,63
In humans, selective lesions to the amygdala are rare, making it difficult to draw clear conclusions regarding the role such lesions play in modulating the emotion and behavior of patients. However, evidence suggests that there are similarities between the affective and social impairments resulting from amygdala lesions and the behavioral dysfunctions characterizing individuals with psychopathy. For example, Adolphs et al19 demonstrated in a case study that extensive bilateral amygdala damage (as a result of Urbach-Wiethe disease) led to difficulty in recognizing emotions in facial expressions, particularly when the patient was responding to a highly arousing facial expression such as fear, similar to the impairments observed in individuals with psychopathy.64 Several subsequent studies reported similar findings and show a consistent pattern of intact facial identification and impaired recognition of the facial expression of fear in patients with amygdala lesions such as those caused by disease (eg, encephalitis) and/or resection (eg, lobectomy or amygdalotomy).15,65–71
Observations from studies of patients with temporal lobe epilepsy (TLE) may also provide evidence in support of our findings linking altered amygdala morphology with psychopathy. Although amygdala damage in patients with TLE often occurs in conjunction with damage to other regions of the brain, pathological findings are regionally isolated in some cases.72–74 Volumetric reductions indicating pathology of the amygdala are frequently documented in patients with TLE (with reports ranging between 10%–57% amygdala volume reductions),75 where the lateral and basal nuclei appear most susceptible to seizure-induced damage.72,75–77 Patients who have TLE and exhibit concomitant amygdala damage have been found to show impairments in emotion recognition. For example, Houghton et al78 reported that impairments in the ability to recognize negative emotional expressions were associated with reduced amygdala volume in patients with TLE. In addition, several studies have shown that TLE-induced amygdala damage also results in impaired social judgment (ie, rate aversive facial appearances as more positive) because of the difficulty in recognizing negative facial emotions,79,80 again consistent with behavioral dysfunction reported in individuals with psychopathy. These human lesion studies demonstrate that amygdala lesions lead to outcomes of affective impairments that parallel to some extent the profile of individuals with psychopathy7,12,81 and support the findings of this study in suggesting that structural impairments in the amygdala may predispose to the emotional and social dysfunction in individuals with psychopathy.
The surface-based mesh modeling analyses indicate regional atrophy in amygdala structure in the vicinity of the basolateral, lateral, central, and cortical nuclei in individuals with psychopathy, echoing the complexity of the clinical descriptions of this disorder. The most robust evidence suggests that the lateral, basolateral, and central nuclei of the amygdala are independently and interactively involved in emotional processing, including fear conditioning and autonomic reactivity to affective stimuli,28–30 while other studies have further linked these nuclei to reward-related learning, behavioral inhibition, and decision making.28 With regard to the cortical nuclei, the very few findings are suggestive that this region may be involved in parenting, social interaction, and the regulation of stress and anxiety.28 Therefore, the regional morphological abnormalities of the amygdala observed in this study may reflect a variety of emotional and behavioral features in individuals with psychopathy. Future research using higher-resolution imaging will be needed to identify the structural alterations within each nucleus of the amygdala, but the even greater challenge will be to delineate the human functional significance of such deformations based on the behavioral neuroscience animal literature.
In addition to the significant amygdala volume reductions in individuals with psychopathy, reduced volumes were found to correlate with increased total psychopathy scores across the entire sample. The correlations were found to be most pronounced for the affective and interpersonal facets of psychopathy. These findings are consistent with 1 abstract reporting a correlation between reduced volumes in the right amygdala and increased affective-interpersonal scores of psychopathy in a violent offender sample.82 Results are further supported by evidence from functional imaging studies showing a higher degree of abnormal activation in the amygdala to be associated with an increase in the psychopathy score during affect recognition, prisoner’s dilemma tasks, and moral decision making.83–85 The associations between reduced amygdala volume and increased psychopathy total and facet scores are in line with evidence indicating that, in addition to emotional processing, the amygdala mediates a variety of brain functions, including attention, memory, social judgment, and moral decision making, through its neural connections with the prefrontal cortex and other subcortical structures.79,86–89 Therefore, our findings support the hypothesis that a higher degree of structural abnormalities in the amygdala may contribute to a greater manifestation of the affective-interpersonal impairments and, to a lesser degree, an increase in antisocial behavior and lifestyle in individuals with psychopathy.
There are several potential limitations of the present study that need to be taken into account when interpreting the results. First, the sample size is modest, thus raising the risk of potential type II error. However, the sample size is comparable with those used in other magnetic resonance imaging studies, and the use of correlation analyses on the entire sample of 59 subjects increases the power for detecting any association between amygdala volume and psychopathy. Second, there was the lack of absolute objectivity due to the nature of manual tracing. However, the method was used to test the specific hypotheses of regional morphological deformations in the amygdala in individuals with psychopathy, which would not have been possible if an automated imaging analysis method, such as voxel-based morphometry, was used. Third, although we controlled for socioeconomic status and substance dependence, a possibility remains that other confounds may contribute to the amygdala structural alterations observed in individuals with psychopathy, such as pharmacological treatments received prior to the scanning. Another limitation of this study is that even though we were able to conduct the group comparisons on a surface point-by-point level to elucidate the approximate locations of the amygdala deformations, we were unable to test the significance values for each distinct nucleus. Therefore, while surface maps indicate a more pronounced amygdala deformation in the cortical, central, basolateral, and lateral nuclei, the possibility remains that the findings also reflect deformations of nuclei relatively less represented on the surface (eg, medial amygdaloid and basomedial nuclei). Last, the standard resolution of structural imaging data does not yet allow reliable localization of structural alterations within specific amygdala nuclei. The precise identification of the amygdala nuclei in humans requires a combination of histological/immunohistochemical methods and neurochemical and cytoarchitectonic criteria,90 which was not possible in this study using in vivo imaging with a 1.5-T scanner. As a result, it needs to be made clear that the labeling of the schematic representation of the amygdala nuclei shown in this study was based on information obtained from a single human brain atlas53 and has not been validated in any probabilistic manner. Nonetheless, this approach was chosen taking into account the methodological and technological difficulties to best illustrate the approximate locations of regional amygdala deformations in individuals with psychopathy and illuminate the need for future studies to examine the amygdala as a heterogeneous structure and identify subtle and localized structural changes within the amygdala. Although the precise localization of structural changes within specific nuclei awaits further research, this study is the first, to our knowledge, to provide an examination of localized morphological alterations in the amygdala in individuals with psychopathy. The advance in the understanding of the neuropathology in psychopathy could greatly benefit from future studies examining the mediating risk factors (eg, autonomic fear conditioning response) together with structural imaging data to translate amygdala structural impairments directly into functional impairments in individuals with psychopathy.
CONCLUSIONS
It is increasingly argued that deficits in the amygdala may be a crucial component in the neurobiological mechanisms underlying psychopathy.31–34 The findings in this study provide the first evidence, to our knowledge, showing significant volume reductions and regional morphological alterations in bilateral amygdala in psychopathic individuals and indicate that structural impairments are most prominent in the vicinity of basolateral, lateral, central, and cortical nuclei. The amygdala reductions may particularly predispose to core psychopathic features of emotional and social dysfunctions. Although future prospective longitudinal research is required to determine the neural mechanisms in psychopathy, this study highlights the importance of the amygdala as a potentially critical neural substrate for both emotional and behavioral features of psychopathy.
Acknowledgments
Funding/Support: This study was supported by National Research Service Award 1F31MH079592 (Dr Yang), grants K02 MH01114-06 and MH50940 (Dr Raine), and Career Development Award K01 MH073990 from the National Institute of Mental Health, research grant P41 RR13642 from the National Center for Research Resources, and grant U54 RR021813 from the National Institutes of Health through the NIH Roadmap for Medical Research.
Footnotes
Financial Disclosure: None reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Additional Contributions: Shannon Bell, BA, Sheri Leung, BA, and Owen Phillips, BS, assisted with data scoring and analysis.
References
- 1.Cleckley H. The Mask of Sanity. St Louis, MO: Mosby; 1941. [Google Scholar]
- 2.Cooke DJ, Michie C, Hart SD. Facets of clinical psychopathy: toward clearer measurement. In: Patrick CJ, editor. Handbook of Psychopathy. New York, NY: The Guilford Press; 2005. [Google Scholar]
- 3.Hare RD. Manual for the Hare Psychopathy Checklist–Revised. Toronto, ON, Canada: Multi-Health Systems; 1991. [Google Scholar]
- 4.Patrick CJ. Emotional processes in psychopathy. In: Raine A, Sanmartín J, editors. Violence and Psychopathy. New York, NY: Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- 5.Arnett PA. Autonomic responsivity in psychopaths: a critical review and theoretical proposal. Clin Psychol Rev. 1997;17(8):903–936. doi: 10.1016/s0272-7358(97)00045-7. [DOI] [PubMed] [Google Scholar]
- 6.Kiehl KA, Hare RD, McDonald JJ, Brink J. Semantic and affective processing in psychopaths: an event-related potential (ERP) study. Psychophysiology. 1999;36(6):765–774. [PubMed] [Google Scholar]
- 7.Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: startle reflex modulation. J Abnorm Psychol. 1993;102(1):82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- 8.Raine A. Evoked potentials and psychopathy. Int J Psychophysiol. 1989;8(1):1–16. doi: 10.1016/0167-8760(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 9.Raine A. Crime, conditioning, and arousal. In: Nyborg H, editor. The Scientific Study of Human Nature: Tribute to Hanes J. Eysenck. Oxford, England: Elsevier; 1997. [Google Scholar]
- 10.Stanford MS, Houston RJ, Barratt ES. Psychophysiological correlates of psychopathic disorders. In: Felthous AR, Saß H, editors. International Handbook on Psychopathic Disorders and the Law. Hoboken, NJ: John Wiley & Sons Inc; 2007. [Google Scholar]
- 11.Patrick CJ. Emotion and psychopathy: startling new insights. Psychophysiology. 1994;31(4):319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 12.Patrick CJ, Cuthbert BN, Lang PJ. Emotion in the criminal psychopath: fear image processing. J Abnorm Psychol. 1994;103(3):523–534. doi: 10.1037//0021-843x.103.3.523. [DOI] [PubMed] [Google Scholar]
- 13.Williamson S, Harpur TJ, Hare RD. Abnormal processing of affective words by psychopaths. Psychophysiology. 1991;28(3):260–273. doi: 10.1111/j.1469-8986.1991.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 14.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, Anderson A, Lee GP, Damasio AR. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37(10):1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 16.Brothers L, Ring B, Kling A. Response of neurons in the macaque amygdala to complex social stimuli. Behav Brain Res. 1990;41(3):199–213. doi: 10.1016/0166-4328(90)90108-q. [DOI] [PubMed] [Google Scholar]
- 17.Kling A, Green PC. Effects of neonatal amygdalectomy in the maternally reared and maternally deprived monkey. Nature. 1967;213(5077):742–743. doi: 10.1038/213742b0. [DOI] [PubMed] [Google Scholar]
- 18.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 19.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 20.Blair RJ. The roles of orbitofrontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55(1):198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 21.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 22.Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Davis M. The role of the amygdala in conditioned fear. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York, NY: Wiley-Liss; 1992. [Google Scholar]
- 24.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 25.Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14(2):148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 26.LeDoux JE. The Emotional Brain. New York, NY: Simon & Schuster; 1996. [Google Scholar]
- 27.Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Soc Cogn Affect Neurosci. 2006;1(3):203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87(4):1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- 29.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 30.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29(5):272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Blair RJ, Jones L, Clark F, Smith M. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology. 1997;34(2):192–198. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 32.Damasio A. Descartes’ Error: Emotion, Reason, and the Human Brain. New York, NY: GP Putnam’s Sons; 1994. [Google Scholar]
- 33.Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142(2–3):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Raine A. Functional and structural brain imaging research on psychopathy. In: Felthous AH, Sass H, editors. International Handbook on Psychopathic Disorders and the Law. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 35.Hare RD. Manual for the Revised Psychopathy Checklist. 2. Toronto, ON, Canada: Multi-Health Systems; 2003. [Google Scholar]
- 36.Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57(2):119–127. doi: 10.1001/archpsyc.57.2.119. discussion 128-129. [DOI] [PubMed] [Google Scholar]
- 37.Kosson DS, Steuerwald BL, Forth AE, Kirkhart KJ. A new method for assessing the interpersonal behavior of psychopathic individuals: preliminary validation studies. Psychol Assess. 1997;9(2):89–101. [Google Scholar]
- 38.Elliott DS, Ageton SS, Huizinga D, Knowles BA, Canter RJ. The Prevalence and Incidence of Delinquent Behavior: 1976–1980—National Estimates of Delinquent Behavior by Sex, Race, Social Class and Other Selected Variables. Boulder, CO: Behavior Research Institute; 1983. National Youth Survey Report No. 26. [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID, Version 2.0) New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- 40.First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II, Version 2.0) New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- 41.Yang Y, Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biol Psychiatry. 2005;57(10):1103–1108. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, LaCasse L, Colletti P. Hippocampal structural asymmetry in unsuccessful psychopaths. Biol Psychiatry. 2004;55(2):185–191. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- 43.Raine A, Lencz T, Taylor K, Hellige JB, Bihrle S, Lacasse L, Lee M, Ishikawa S, Colletti P. Corpus callosum abnormalities in psychopathic antisocial individuals. Arch Gen Psychiatry. 2003;60(11):1134–1142. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- 44.Harris GT, Rice ME, Quinsey VL. Psychopathy as a taxon: evidence that psychopaths are a discrete class. J Consult Clin Psychol. 1994;62(2):387–397. doi: 10.1037//0022-006x.62.2.387. [DOI] [PubMed] [Google Scholar]
- 45.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]
- 46.Wechsler D. Wechsler Adult Intelligence Scale–Revised. San Antonio, TX: Psychological Corp; 1981. [Google Scholar]
- 47.Bryden MP. Measuring handedness with questionnaires. Neuropsychologia. 1977;15(4–5):617–624. doi: 10.1016/0028-3932(77)90067-7. [DOI] [PubMed] [Google Scholar]
- 48.Sled JG, Pike GB. Standing-wave and RF penetration artifacts caused by elliptic geometry: an electrodynamic analysis of MRI. IEEE Trans Med Imaging. 1998;17(4):653–662. doi: 10.1109/42.730409. [DOI] [PubMed] [Google Scholar]
- 49.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J The International Consortium for Brain Mapping (ICBM) A probabilistic atlas of the human brain: theory and rationale for its development. Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 50.Narr KL, Thompson PM, Sharma T, Moussai J, Blanton R, Anvar B, Edris A, Krupp R, Rayman J, Khaledy M, Toga AW. Three-dimensional mapping of temporolimbic regions and the lateral ventricles in schizophrenia: gender effects. Biol Psychiatry. 2001;50(2):84–97. doi: 10.1016/s0006-3223(00)01120-3. [DOI] [PubMed] [Google Scholar]
- 51.Bartzokis G, Mintz J, Marx P, Osborn D, Gutkind D, Chiang F, Phelan CK, Marder SR. Reliability of in vivo volume measures of hippocampus and other brain structures using MRI. Magn Reson Imaging. 1993;11(7):993–1006. doi: 10.1016/0730-725x(93)90218-3. [DOI] [PubMed] [Google Scholar]
- 52.Levitt JG, Blanton RE, Caplan R, Asarnow R, Guthrie D, Toga AW, Capetillo-Cunliffe L, McCracken JT. Medial temporal lobe in childhood-onset schizophrenia. Psychiatry Res. 2001;108(1):17–27. doi: 10.1016/s0925-4927(01)00108-1. [DOI] [PubMed] [Google Scholar]
- 53.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diego, CA: Academic Press; 1997. [Google Scholar]
- 54.Yang Y, Raine A, Narr KL, Lencz T, LaCasse L, Colletti P, Toga AW. Localisation of increased prefrontal white matter in pathological liars. Br J Psychiatry. 2007;190:174–175. doi: 10.1192/bjp.bp.I06.025056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, Kumar A. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165(2):229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21(4):1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Thompson PM, Hayashi KM, De Zubicaray GL, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer’s disease. Neuroimage. 2004;22(4):1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 58.Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18(1):32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- 59.Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 1986;100(1):11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- 60.Machado CJ, Bachevalier J. The impact of selective amygdala, orbitofrontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120(4):761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- 61.Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral physiological components of anxious temperament. J Neurosci. 2001;21(6):2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25(37):8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hare HD. Electrodermal and cardiovascular correlates of psychopathy. In: Hare RD, Schalling D, editors. Psychopathic Behavioral: Approaches to Research. New York, NY: Wiley; 1978. [Google Scholar]
- 64.Marsh AA, Blair RJ. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci Biobehav Rev. 2008;32(3):454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14(8):1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 66.Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14(4):526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- 67.Anderson AK, Phelps EA. Intact recognition of vocal expressions of fear following bilateral lesions of the human amygdala. Neuroreport. 1998;9(16):3607–3613. [PubMed] [Google Scholar]
- 68.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 69.Broks P, Young AW, Maratos EJ, Coffey PJ, Calder AJ, Isaac CL, Mayes AR, Hodges JR, Montaldi D, Cezayirli E, Roberts N, Hadley D. Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia. 1998;36(1):59–70. doi: 10.1016/s0028-3932(97)00105-x. [DOI] [PubMed] [Google Scholar]
- 70.Young AW, Hellawell DJ, Van De Wal C, Johnson M. Facial expression processing after amygdalotomy. Neuropsychologia. 1996;34(1):31–39. doi: 10.1016/0028-3932(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 71.Schmolck H, Squire LR. Impaired perception of facial emotions following bilateral damage to the anterior temporal lobe. Neuropsychology. 2001;15(1):30–38. [PubMed] [Google Scholar]
- 72.Hudson LP, Munoz DG, Miller L, McLachlan RS, Girvin JP, Blume WT. Amygdaloid sclerosis in temporal lobe epilepsy. Ann Neurol. 1993;33(6):622–631. doi: 10.1002/ana.410330611. [DOI] [PubMed] [Google Scholar]
- 73.Miller LA, McLachlan RS, Bouwer MS, Hudson LP, Munoz DG. Amygdalar sclerosis: preoperative indicators and outcome after temporal lobectomy. J Neurol Neurosurg Psychiatry. 1994;57(9):1099–1105. doi: 10.1136/jnnp.57.9.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guerreiro C, Cendes F, Li LM, Jones-Gotman M, Andermann F, Dubeau F, Piazzini A, Feindel W. Clinical patterns of patients with temporal lobe epilepsy and pure amygdalar atrophy. Epilepsia. 1999;40(4):453–461. doi: 10.1111/j.1528-1157.1999.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 75.Pitkänen A, Tuunanen J, Kälviäinen R, Partanen K, Salmenperä T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32(1–2):233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 76.Meyer A, Beck E, Shepherd M. Unusually severe lesions in the brain following status epilepticus. J Neurol Neurosurg Psychiatry. 1955;18(1):24–33. doi: 10.1136/jnnp.18.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Margerison JH, Corsellis JA. Epilepsy and the temporal lobes: a clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89(3):499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- 78.Houghton JM, Broks P, Wing A, Eldridge P, Walsh R, Davies P, Jukes R, Kuczynski A, Reynders H. Does temporal lobe epilepsy impair the ability to recognize cues to the emotional state of others? Epilepsia. 2000;41(suppl 7):249. [Google Scholar]
- 79.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 80.Reynders HJ, Broks P, Dickson JM, Lee CE, Turpin G. Investigation of social and emotion information processing in temporal lobe epilepsy with ictal fear. Epilepsy Behav. 2005;7(3):419–429. doi: 10.1016/j.yebeh.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 81.Plutchik R. Emotions and sociopathy. Behav Brain Sci. 1995;18(3):570–571. [Google Scholar]
- 82.Tiihonen J, Hodgins S, Vaurio O. Amygdaloid volume loss in psychopathy. Abstr Soc Neurosci. 2000:2017. [Google Scholar]
- 83.Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biol Psychiatry. 2004;56(7):516–521. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 84.Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, Lilienfeld SO. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biol Psychiatry. 2007;61(11):1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 85.Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Mol Psychiatry. 2009;14(1):5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- 86.Anderson AK, Phelps EA. Expression without recognition: contributions of the human amygdala to emotional communication. Psychol Sci. 2000;11(2):106–111. doi: 10.1111/1467-9280.00224. [DOI] [PubMed] [Google Scholar]
- 87.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 88.Sarter M, Markowitsch HJ. Involvement of the amygdala in learning and memory: a critical review, with emphasis on anatomical relations. Behav Neurosci. 1985;99(2):342–380. doi: 10.1037//0735-7044.99.2.342. [DOI] [PubMed] [Google Scholar]
- 89.Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11(12):499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 90.Savander V, Go C, Ledoux JE, Pitkänen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol. 1995;361(2):345–368. doi: 10.1002/cne.903610211. [DOI] [PubMed] [Google Scholar]