Abstract
Nosocomial infections are increasingly being recognised as a major patient safety issue. The modern hospital environment and associated health care practices have provided a niche for the rapid evolution of microbial pathogens that are well adapted to surviving and proliferating in this setting, after which they can infect susceptible patients. This is clearly the case for bacterial pathogens such as Methicillin Resistant Staphylococcus aureus (MRSA) and Vancomycin Resistant Enterococcus (VRE) species, both of which have acquired resistance to antimicrobial agents as well as enhanced survival and virulence properties that present serious therapeutic dilemmas for treating physicians. It has recently become apparent that the spore-forming bacterium Clostridium difficile also falls within this category. Since 2000, there has been a striking increase in C. difficile nosocomial infections worldwide, predominantly due to the emergence of epidemic or hypervirulent isolates that appear to possess extended antibiotic resistance and virulence properties. Various hypotheses have been proposed for the emergence of these strains, and for their persistence and increased virulence, but supportive experimental data are lacking. Here we describe a genetic approach using isogenic strains to identify a factor linked to the development of hypervirulence in C. difficile. This study provides evidence that a naturally occurring mutation in a negative regulator of toxin production, the anti-sigma factor TcdC, is an important factor in the development of hypervirulence in epidemic C. difficile isolates, presumably because the mutation leads to significantly increased toxin production, a contentious hypothesis until now. These results have important implications for C. difficile pathogenesis and virulence since they suggest that strains carrying a similar mutation have the inherent potential to develop a hypervirulent phenotype.
Author Summary
Hospital infections are increasingly being recognised as a major patient safety issue with the hospital environment providing a niche for the rapid evolution of microbial pathogens that are well adapted to infecting susceptible patients. The spore-forming Clostridium difficile is one such bacterium, which causes disease in patients undergoing antibiotic therapy. Since 2000, there has been a striking increase in C. difficile infections due to the emergence of hypervirulent isolates that appear to possess extended antibiotic resistance and virulence properties. Here we use a genetic approach to identify a factor linked to the development of hypervirulence in C. difficile. This study shows that a naturally occurring mutation in a negative regulator of toxin production, the anti-sigma factor TcdC, is an important factor contributing to the development of hypervirulence in epidemic isolates, presumably because it leads to significantly increased toxin production. These results have important implications for C. difficile pathogenesis since they suggest that strains carrying a similar mutation have the inherent potential to develop a hypervirulent phenotype. This study has increased our understanding of how these new variant strains cause disease and why they are more harmful, which is critical for the development of improved strategies for preventing and treating these infections.
Introduction
C. difficile is the causative agent of a spectrum of gastrointestinal diseases, collectively known as C. difficile infections, or CDI, that are induced by treatment with antibiotics that disrupt the normal gastrointestinal microbiota. CDI can range from mild diarrhoea, through moderately serious disease, to severe life-threatening pseudomembranous colitis, a chronic, often fatal, gastrointestinal disease [1]. During the past decade, there has been an astonishing increase in the rate and prevalence of C. difficile infections in many parts of the world, including the UK, USA, Canada and Europe, largely due to the emergence of a “hypervirulent” or epidemic group of isolates belonging to the BI/NAP1/027 category [2], [3]. These strains are highly resistant to fluoroquinolones [3] and are associated with more severe disease and higher mortality rates [4]–[7]. C. difficile now also causes disease in those previously not at risk, such as children and pregnant women, with community-associated C. difficile disease being increasingly common [8]–[10].
The reasons for the emergence of these strains, and for their increased virulence, remain largely speculative. The use of fluoroquinolones, and the emergence of fluoroquinolone resistant strains, are undoubtedly driving factors in these new epidemics [11], however, the reasons for the heightened virulence and persistence of these strains are unknown. Genotypic and phenotypic comparison of the hypervirulent BI/NAP1/027 isolates to historical strains has identified numerous differences that may contribute to hypervirulence. Phenotypically, these differences may include the production of a toxin known as binary toxin, or CDT [3], and a higher sporulation rate [12]. Whole genome comparisons have identified numerous genetic differences with BI/NAP1/027 strains having an additional 234 genes compared to the well characterised strain 630 [13], including five unique genetic regions that are absent from both strain 630 and non-epidemic 027 strains [4]. Fundamentally, however, the factors directly resulting in the development of hypervirulence by these strains remain unknown.
The major virulence factors of C. difficile are two members of the large clostridial cytotoxin family, toxin A and toxin B, encoded by the tcdA and tcdB genes, respectively, which are potent monoglucosyltransferases that irreversibly modify members of the Rho family of host regulatory proteins [14]. Two recent studies definitively showed that toxin B plays a major role in the virulence of C. difficile [15], [16]. The role of toxin A in disease was less clear however, with conflicting data concerning toxin A reported [15], [16].
Epidemic strains are reported to produce significantly more toxin A and toxin B than other strains [2]. The tcdA and tcdB genes are located on the chromosome within a region known as the pathogenicity locus or PaLoc [17]. In addition to tcdA and tcdB, the PaLoc encodes three additional genes designated tcdR, tcdE and tcdC, which encode an alternative sigma factor, TcdR [18], a putative holin, TcdE [19], and an anti-sigma factor, TcdC [20], respectively. The expression of toxins A and B is controlled in a complex manner by several factors, including TcdR and TcdC. TcdC is thought to negatively regulate toxin production by interacting with TcdR or with TcdR-containing RNA polymerase holoenzyme or both [20], TcdR is essential for toxin production [18]. BI/NAP1/027 C. difficile strains have a nonsense mutation in tcdC, which results in the production of a truncated protein that no longer negatively regulates TcdR. This mutation is postulated to be responsible for the increased toxin production observed in vitro in these strains [2]. Accordingly, this observation has prompted debate over the importance of the tcdC mutation in the hypervirulent phenotype. However, there is currently a lack of experimental evidence to support this hypothesis, with inconsistent reports in the published literature [20]–[22].
Despite their important impact worldwide on public health little is known about the virulence factors of BI/NAP1/027 strains and many important questions about the pathogenesis of disease caused by these strains remain to be answered, especially the role played by TcdC. BI/NAP1/027 isolates have proven difficult to genetically manipulate, which has hampered our ability to study these strains at the molecular level. To address these questions, here we use a novel Tn916-based plasmid conjugation system to facilitate the efficient transfer of plasmids into BI/NAP1/027 strains of C. difficile. Using this system, we have demonstrated conclusively the role of TcdC as a negative regulator of toxin production in C. difficile. Furthermore, using the hamster model of infection, we provide evidence to show that the tcdC mutation found in BI/NAP1/027 strains is an important factor in the development of hypervirulence by these strains. This study is the first to use isogenic strains to identify a factor involved in the development of a hypervirulent phenotype in C. difficile, and also represents the first in vivo demonstration of the role of TcdC in the pathogenesis of C. difficile disease.
Results
Complementation of the tcdC mutation in a BI/NAP1/027 epidemic isolate in trans
To determine if mutation of the tcdC gene in C. difficile BI/NAP1/027 isolates leads to the development of a hypervirulent phenotype it was necessary to construct isogenic BI/NAP1/027 strains that only differed in their ability to produce a functional TcdC protein. To construct the isogenic strains required for this analysis, genetic manipulation of BI/NAP1/027 isolates was required. The genetic manipulation of these strains has proved difficult and attempts to transfer plasmids into BI/NAP1/027 strains using published methods, which rely on RP4-mediated conjugation from Escherichia coli [23]–[27], were not successful, even though transfer of plasmids into the genetically amenable strains JIR8094, an erythromycin sensitive derivative of strain 630 [24], and CD37 was readily achieved (Table S1). To overcome this barrier and to facilitate DNA transfer into the strains of interest, we developed a novel plasmid transfer system that exploits the conjugation apparatus encoded by the broad-host range transposon Tn916.
The oriT region of Tn916 (oriT Tn916) [28] was cloned into the catP-containing C. difficile shuttle plasmid pMTL9361Cm [29], generating pDLL4. This plasmid was introduced into C. perfringens strain JIR4225, which contains five copies of Tn916 [30] and plate matings were performed between this donor strain and several C. difficile strains, including a BI/NAP1/027 strain, M7404, which is a Canadian epidemic isolate [29]. Transconjugants from these matings were isolated on medium supplemented with thiamphenicol and cefoxitin. The efficiency of plasmid transfer into strain M7404 was 1.2×102–4×104 transconjugants/ml of plated culture. Analysis of transconjugants using PCR specific for the catP gene together with restriction analysis confirmed that all putative colonies carried pDLL4 (data not shown), verifying successful plasmid transfer into the BI/NAP1/027 strain M7404. Similar plasmid transfer efficiencies were obtained for numerous other C. difficile strains (Table S1), highlighting the utility of this methodology for the genetic manipulation of clinically relevant strains.
To complement the tcdC mutation in a BI/NAP1/027 strain the intact tcdC gene from strain VPI10463, together with 300 bp of its upstream region, was cloned into the shuttle plasmid pDLL4, generating pDLL17. This plasmid was transferred by Tn916-mediated conjugation from C. perfringens strain JIR4225 to C. difficile strain M7404 as before. PCR was subsequently used to confirm the presence of plasmid pDLL17 in representative transconjugants (data not shown).
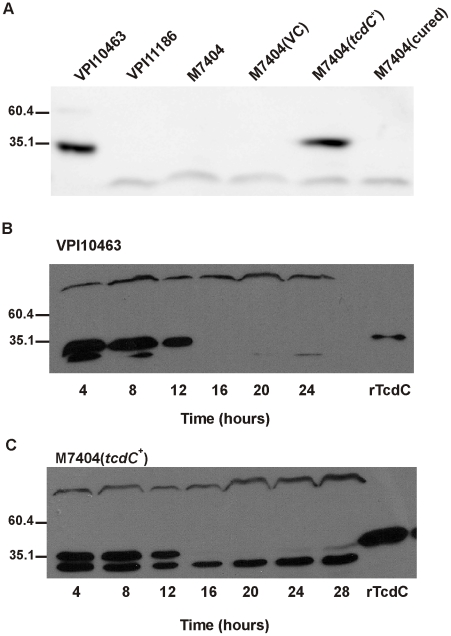
To determine whether the presence of pDLL17 complemented the TcdC deficiency of M7404, Western immunoblots using TcdC-specific antibodies were performed. Lysates were collected from the wild-type M7404, the pDLL4-carrying vector control strain M7404(VC) and the pDLL17-tcdC + strain M7404(tcdC +), as well as strains VPI10463 and the PaLoc-deficient strain VPI11186, which served as positive and negative controls, respectively. An additional control strain, M7404(cured), was generated by serially passaging strain M7404(tcdC +) on non-selective growth medium and curing the plasmid from this strain. Loss of the plasmid was confirmed by sensitivity of the strain to thiamphenicol followed by PCR analysis to verify the absence of several plasmid encoded genes (data not shown). As Figure 1A shows, whilst no TcdC could be detected in the lysates of the negative control strain, the wild-type M7404, M7404(VC) and the plasmid-cured strain M7404(cured), a 34-kDa protein that reacted with TcdC-specific antibodies was detected in lysates from the tcdC +-complemented strain M7404(tcdC +). This band was the same size as the immunoreactive TcdC protein produced by the positive control strain VPI10463, confirming that the tcdC mutation in the BI/NAP1/027 epidemic isolate M7404 was efficiently complemented in trans. Since complementation was performed using a multicopy plasmid, we also quantified TcdC production levels from strain M7404(tcdC +) in comparison to strain VPI10463 using a time-course assay. Previous studies involving transcriptional analysis of PaLoc genes during different growth phases showed that tcdC is expressed in early exponential phase but not in stationary phase, whereas the other PaLoc genes show the opposite expression pattern [31]. VPI10463 (Figure 1B) and M7404(tcdC +) (Figure 1C) exhibited similar TcdC expression patterns, with higher levels of TcdC observed in early exponential phase and negligible amounts detected beyond 16 hours, suggesting that the regulatory regions governing tcdC expression have been retained on the tcdC-carrying fragment used to construct pDLL17. In addition to the kinetics of TcdC expression in strain M7404(tcdC +) mirroring that of VPI10463, a similar amount of protein was also detected at each time point with VPI10463 producing 1.3- to 1.6-fold more protein (Figure 1B) than M7404(tcdC +) (Figure 1C). Therefore, although tcdC complementation was achieved using a multicopy plasmid vector, a physiologically relevant amount of TcdC protein was expressed during the appropriate growth phases in strain M7404(tcdC +).
Figure 1. Western blot analysis of TcdC production by wild-type and complemented C. difficile strains.
(A) Qualitative analysis of TcdC production. VPI10463 is the positive control strain; VPI11186 is the negative control strain; M7404 is a wild-type Canadian BI/NAP1/027 strain; M7404(VC) is the wild-type strain carrying the shuttle plasmid pDLL4; M7404(tcdC +) is the wild-type strain carrying the tcdC expression plasmid pDLL17 and M7404(cured) is the M7404(tcdC +) strain cured of pDLL17. (B) Time course analysis of TcdC production by the positive control strain C. difficile VPI10463. Samples were taken at the indicated times shown in hours. 60 ng of purified recombinant his-tagged TcdC protein (rTcdC) was used as the positive reference sample. (C) Time course analysis of TcdC production by C. difficile strain M7404(tcdC +). Samples were taken at the indicated times shown in hours. 300 ng of purified recombinant his-tagged TcdC protein (rTcdC) was used as the positive reference sample. Western blots were performed with rabbit TcdC-specific antibodies. Size standards are shown (kDa).
TcdC-mediated repression of toxin production in C. difficile
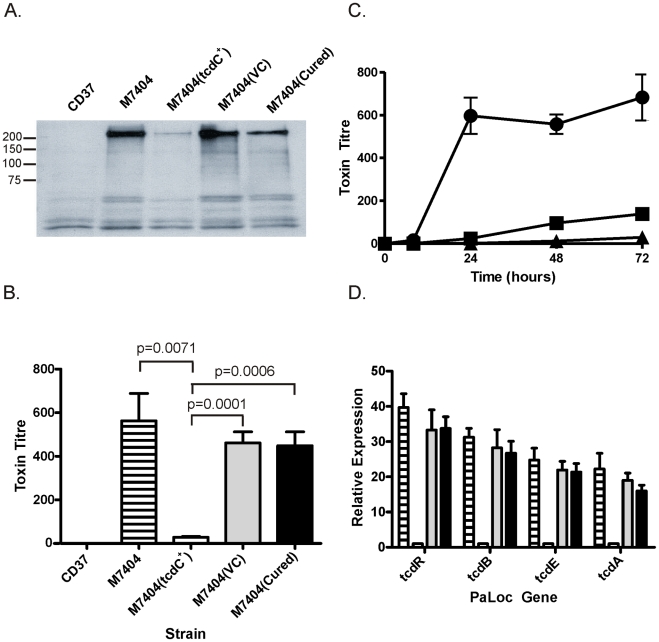
To determine the effect of TcdC on toxin production in strain M7404, a combination of Western immunoblots and cytotoxicity assays were performed using supernatants collected from strain M7404 and isogenic M7404 derivatives carrying the vector pDLL4, pDLL17, the cured strain M7404(cured) and the PaLoc-negative control strain CD37. To assess toxin A production, Western immunoblotting was performed using TcdA-specific antibodies (Figure 2A). The results showed that the presence of the tcdC + plasmid pDLL17 resulted in a dramatic decrease in the amount of toxin A produced by M7404(tcdC+) when compared to the wild-type strain. By contrast, M7404(cured) produced qualitatively similar levels of toxin A to wild-type, as did M7404 carrying the vector plasmid, whereas the PaLoc-negative strain CD37 produced no detectable toxin A, as expected.
Figure 2. Analysis of the effect of TcdC complementation on toxin production and PaLoc gene expression by C. difficile.
CD37 is the negative control strain; M7404 is the wild-type BI/NAPI/027 strain; M7404(tcdC +) is the wild-type strain carrying the tcdC expression vector pDLL17; M7404(VC) is the wild-type strain carrying shuttle plasmid pDLL4 and M7404(cured) is the M7404(tcdC +) strain cured of pDLL17. JIR8094 is a derivative of strain 630. (A) Western blot using toxin-A-specific antibodies. Size standards are shown (kDa) (B) Toxin cytotoxicity assays using Vero cells. Strains are as described above. Lined bars represent the wild-type strain M7404; white bars represent M7404(tcdC +); grey bars represent M7404(VC) and black bars represent M7404(cured). Data represent the mean ± s.e.m. (n = 3). (C) Time course of toxin production measured using Vero cell cytotoxicity assays. Strains are as described above and are represented as follows: M7404(tcdC +) (▪), M7404(VC) (•) and JIR8094 (▴). Note that CD37 was included in this analysis but displayed no toxin production; the line representing this strain is therefore not visible. Data represent the mean ± s.e.m. (n = 3). (D) PaLoc gene specific qRT-PCR. Bars correspond to strains as before and PaLoc genes are indicated. Data represent the mean fold-expression ± s.e.m. (n = 3), compared to the M7404(tcdC +) strain.
Vero cell cytotoxicity assays, which predominantly measure toxin B activity [15], were then performed to quantitatively determine the effect of TcdC on toxin production from these strains. As previously observed with toxin A, the amount of toxin produced from strain M7404 was significantly reduced when functional TcdC was restored (Figure 2B). The amount of toxin produced in the TcdC-complemented strain was approximately 16–32-fold less, and therefore significantly lower (p = 0.0001; unpaired t-test, 95% confidence interval), than in the vector control-carrying M7404 derivative. There was, however, no significant difference in toxin activity levels between strains M7404, the vector control strain M7404(VC) or M7404(cured) (Figure 2B). A kinetic analysis of toxin production also clearly showed that the presence of TcdC delayed the onset of toxin production in M7404(tcdC+) in comparison to M7404 carrying the vector plasmid, mirroring the delayed toxin production observed from the tcdC + 630-strain derivative JIR8094 (Figure 2C).
We also determined if TcdC-mediated repression of toxin production was at the transcriptional level and evaluated the effect of tcdC complementation on the expression of the other PaLoc-encoded genes, tcdR and tcdE. Quantitative real-time PCR (qRT-PCR) analysis using RNA extracted from the wild-type strain and its isogenic derivatives was performed to ascertain the relative transcription levels of the tcdA, tcdB, tcdR and tcdE genes. As shown in Figure 2D, an approximate 13- and 23-fold reduction in tcdA- and tcdB-specific mRNA levels, respectively, in strain M7404(tcdC +) was observed compared to M7404. Similar observations were made for tcdR and tcdE expression levels, with 33-fold and 21-fold less tcdR and tcdE mRNA, respectively, in the tcdC-complemented strain compared to the wild-type. No significant differences in the expression levels of these four genes were detected when M7404, the vector-carrying derivative or the cured strain were compared. These data conclusively demonstrate that TcdC negatively regulates toxin production in C. difficile and show that repression occurs at the transcriptional level.
Reduction of the virulence of a BI/NAP1/027 isolate via complementation with TcdC
To define the role of TcdC in the virulence of a BI/NAP1/027 C. difficile isolate, female Golden Syrian hamsters were infected with spores of strain M7404 carrying either the vector control or the tcdC + plasmid (n = 10 and n = 12, respectively). For comparative purposes, a group of hamsters (n = 14) was also infected with strain 630, a strain previously characterised as being less virulent than other clinical isolates [32]. Following infection, all C. difficile strains were found to be equally efficient at colonising the hamsters (data not shown). Infection of colonised hamsters was allowed to proceed and animals were monitored by telemetry. The end point of infection was achieved when the core body temperature of the hamsters dropped to 35°C. This parameter has previously been shown to be a reliable indicator of non-recoverable disease [33]. At this point, the animals were immediately culled for animal ethics reasons. Bacteria were then isolated from the culled hamsters, the bacterial load quantified and isolates subjected to MVLA analysis [33] to confirm that these isolates were the same strain as originally used for infection.
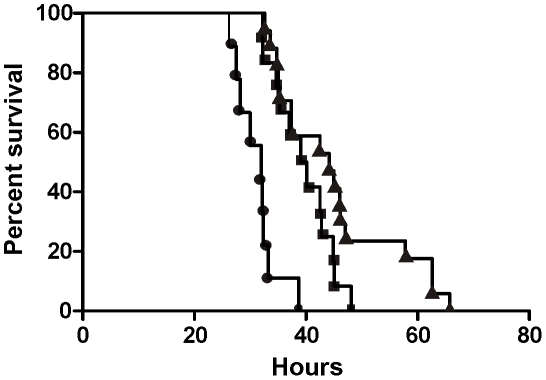
Hamsters infected with the M7404(tcdC +) derivative showed a significant delay (p = 0.0003; Logrank (Mantel-Cox) test; 95% confidence interval) in the mean time taken to reach non-recoverable disease (2370 minutes or 39.5 hours) in comparison to the vector-carrying M7404 group (M7404(VC)), with a mean time of 1869 minutes or 31.15 hours (Figure 3). In one of the hamsters colonised with the M7404(VC) strain, the time taken to reach the end point of infection was substantially longer than the other hamsters in this group (2814 minutes or 46.9 hours). This hamster was shown by statistical analysis (p = 0.0405; Grubbs test; 95% confidence interval) to be an outlier and was therefore excluded from the experimental analysis. Note that statistical significance would be retained upon inclusion of this outlier. Interestingly, whilst the mean time to the end point of infection in the strain 630 group of hamsters (2701 minutes or 45.02 hours) was significantly longer than that of hamsters infected with M7404(VC) (p = 0.0001; Logrank (Mantel-Cox) test; 95% confidence interval), there was no significant difference in the mean time taken to achieve non-recoverable disease in the 630 group compared to the M7404(tcdC +) derivative, indicating that the virulence of the TcdC-complemented strain was equivalent to that of strain 630.
Figure 3. Virulence of C. difficile wild-type and tcdC-complemented strains in hamsters.
Kaplan-Meier survival curve demonstrating time from infection with C. difficile to death. M7404(VC), wild-type M7404 carrying shuttle plasmid pDLL4 (•); M7404(tcdC +), wild-type M7404 carrying tcdC expression plasmid pDLL17 (▪); and strain 630, a C. difficile isolate with known low virulence (▴). Hamsters were infected intragastrically with 10,000 spores from each strain; M7404(VC) (n = 9), M7404(tcdC +) (n = 12) and strain 630 (n = 14).
It is apparent from these virulence experiments that the expression of TcdC in a BI/NAP1/027 isolate has an important effect on virulence, resulting in a significant delay in the time needed to reach non-recoverable disease. These data therefore provide compelling evidence that the naturally occurring mutation of tcdC in BI/NAP1/027 isolates is an important factor in the development of a hypervirulent phenotype by these strains.
TcdC status of clinical isolates does not predict toxin production level
The results presented here show that a BI/NAP1/027 strain complemented with tcdC is not as virulent as its isogenic vector-carrying control, suggesting that any C. difficile strain that acquires a null TcdC phenotype has the potential to develop a hypervirulent phenotype. Furthermore, phylogenetic studies have shown C. difficile to be a genetically diverse species, with disease-causing isolates seemingly arising from multiple lineages, suggesting that virulence in these strains may have evolved independently [4], [34]. The tcdC status of a diverse group of clinical isolates was therefore determined in parallel to the genetic studies described above. One hundred Australian clinical isolates were initially analysed by toxinotyping, a typing method which categorises strains according to variation in the PaLoc region; BI/NAP1/027 strains belong to toxinotype III [35]. Approximately 5% of these strains were found to belong to a toxinotype associated with a tcdC mutation. PCR and sequence analysis was then used to confirm the presence of tcdC mutations in each of these isolates. A single BI/NAP1/027 was identified in this survey, designated strain KI [36]. One other strain, DLL3053, was particularly interesting since it belonged to toxinotype group III but was not a BI/NAP1/027 isolate. This strain harboured the well documented single base pair deletion at nucleotide position 117 of tcdC, and the 18 base pair in-frame deletion from nucleotide 330 to 347 [37]. Two strains, DLL3054 and DLL3055, were toxinotype V strains with a nonsense mutation at nucleotide position 184 (C184T) and a 39 base pair deletion from nucleotides 341 to 379 [37] whereas strain DLL3056 was toxinotype XIV and possessed a nonsense mutation at nucleotide position 191 (C191A) and an in-frame 36 base pair deletion from nucleotide 300 to 336.
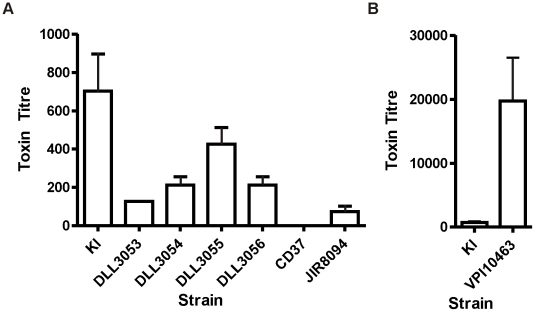
To examine the impact of tcdC mutations on toxin production in these clinical isolates, Vero cell cytotoxicity assays were performed. These assays, which predominantly detect toxin B, determine the relative amounts of toxin produced by each strain since the expression of toxins A and B is coordinately regulated [23], [31]. Strains JIR8094, a derivative of strain 630 [24], and VPI10463 [38], which both possess intact tcdC genes, were used as positive reference controls, and the PaLoc-negative strain CD37 was used as a negative control. Strains were grown in glucose-free medium since glucose has previously been shown to repress toxin production [23]. As shown in Figure 4A, the relative amount of toxin produced by the tcdC clinical isolates varied over approximately a 10-fold range. In agreement with the work of others [12], [22], these results show that the presence of mutations within tcdC was not directly correlated with high level toxin production. Strain DLL3053 for example, did not produce levels of toxin significantly different from that of strain JIR8094, which is considered to be a low toxin producer [39]. Of all the isolates with tcdC mutations, strain KI [36] produced the most toxin, and at significantly (p = 0.0406; unpaired t-test, 95% confidence interval) higher levels that were approximately 10-fold more than strain DLL3053 even though both strains have identical tcdC alleles.
Figure 4. Comparative analysis of toxin production by naturally occurring tcdC clinical isolates.
Vero cell cytotoxicity assays were used to determine toxin production levels. (A) Strain JIR8094 is a tcdC + control strain, CD37 is a PaLoc-negative control strain and KI is an Australian BI/NAPI/027 isolate [36]. All other strains (DLL3053-DLL3056) are clinical isolates carrying naturally occurring tcdC mutations, collected from Australian hospitals. (B) Toxin production by the tcdC + control strain VPI10463 is shown on a separate bar chart due to the much higher levels of toxin produced. For comparative purposes strain KI is represented on both bar charts. Data represent the mean±s.e.m. (n = 3).
In comparison to the TcdC-positive reference strains, all of the tcdC-deficient clinical isolates produced more toxin than strain JIR8094 apart from strain DLL3053 (Figure 4A). Conversely, however, all strains produced significantly less toxin than VPI10463 (p = 0.0099–0.0202; unpaired t-test, 95% confidence interval), including the BI/NAP1/027 strain KI. VPI10463 produced over 100-fold more toxin than strains JIR8094 and DLL3053 and over 30-fold more than KI (Figure 4B). This observation is in agreement with recently published findings, which showed that VPI10463 produced significantly more toxin than other strains [12]; however, in that study, strains were grown using glucose-rich BHI medium so differential effects of glucose on toxin production by each strain could not be ruled out. In agreement with other studies [12], [22], [37], our data therefore suggest that the tcdC-status alone of C. difficile isolates is not an accurate predictor of high-level toxin production.
Discussion
The hypothesis that the naturally-occurring tcdC mutation in epidemic BI/NAP1/027 isolates contributes to hypervirulence is widely accepted, despite a lack of supportive experimental evidence. Indeed, the exact role of TcdC in the pathogenesis of C. difficile disease has remained controversial with conflicting findings reported in the literature [20]–[22]. As a result, several published studies have suggested that there is a need to assess isogenic tcdC strains in order to conclusively determine the role of this gene in the virulence of C. difficile [12], [22], [40]. We have now constructed such isogenic strains and compared them in an animal model. The results conclusively show that TcdC negatively regulates toxin production in C. difficile. Most importantly, complementation of the tcdC mutation in the BI/NAP1/027 epidemic isolate M7404 clearly showed that this mutation is an important factor in the development of hypervirulence by this strain since the genetic complementation of tcdC reduced virulence in comparison to the wild-type strain.
To elucidate the role of TcdC in hypervirulence, it was necessary to construct an isogenic panel of BI/NAP1/027 strains that were identical except for the presence or absence of the wild-type tcdC gene. Despite the publication of studies describing the successful transfer of plasmids into the BI/NAP1/027 isolate R20291 [25], [26], [41], this group of strains has remained difficult to work with at the molecular genetic level. As such, a new system that utilised the conjugation apparatus of Tn916 was developed in this study and used successfully to genetically manipulate a number of clinically relevant isolates, including a BI/NAP1/027 strain of C. difficile. Tn916 is a broad host-range conjugative transposon that was recently used to transfer plasmids into genetically intractable strains of Enterococcus faecium [28] and has been shown to transfer into C. difficile [42], [43]. C. perfringens was chosen for use as a donor strain in anticipation that it may be more proficient for the transfer of plasmids into C. difficile in comparison to the more distantly related E. coli. The addition of oriT Tn916 onto the shuttle vector pMTL9361Cm facilitated the efficient transfer of this plasmid into strain M7404 from a Tn916-carrying C. perfringens strain. Furthermore, this system has been successfully used to transfer shuttle plasmids into every C. difficile isolate tested so far (Table S1). Most importantly, this new technology facilitated the complementation of the tcdC mutation in strain M7404 enabling the role of TcdC in the virulence of BI/NAP1/027 strains of C. difficile to be investigated.
Previous in vitro studies have shown that TcdC is able to sequester the TcdR sigma factor, preventing its association with core RNA polymerase and blocking toxin gene expression [20]. These experiments suggested that TcdC was important in the regulation of toxin production by C. difficile, but the in vivo role of this protein was not determined. Conversely, several studies on C. difficile clinical isolates [22], [37] showed that the absence of a functional tcdC gene was not an accurate predictor of high level toxin production or increased disease severity, indicating that TcdC may not play an important role in virulence in these strains [22], [37]. The analysis of Australian clinical isolates in the present study is in accordance with these latter studies in that isolates with naturally occurring tcdC mutations were found to produce toxin at a range of different levels that were not necessarily high. However, since these strains, and those in the other studies [22], [37], are not isogenic it is not possible to draw conclusions about the importance of tcdC in the context of toxin yield or virulence. By contrast, the isogenic tcdC strains studied here clearly show that TcdC is a negative regulator of toxin production since the tcdC complemented BI/NAP1/027 C. difficile strain produced significantly less toxin A and B than the non-complemented control strains. The finding that TcdC-status is not correlated with toxin production in clinical isolates highlights the limitation of accurately assigning gene function by studying non-isogenic strains, particularly in a highly heterogeneous species such as C. difficile. In this context, it might be of interest to study the function of tcdC in isogenic strains generated in a different genetic background such as a ribotype 078 isolate.
Analysis of PaLoc gene expression by qRT-PCR demonstrated that TcdC exerts regulatory control of toxin production at the transcriptional level, and this is in keeping with its proposed role as an anti-sigma factor [20]. The observation that the expression of tcdR and tcdE is reduced in the tcdC-complemented strain, together with tcdA and tcdB, is probably because of autoregulation of tcdR since TcdR upregulates its own expression and that of the other PaLoc genes [23].
The virulence of strain M7404 was reduced upon complementation of tcdC, clearly demonstrating that the tcdC mutation in BI/NAP1/027 strains has a significant impact on virulence and is likely to be an important factor in the development of hypervirulence by these strains. Surprisingly, the virulence of M7404(tcdC +) was found to be equivalent to that of strain 630, which has been shown in other studies to be reduced in virulence in comparison to other isolates, including three other BI-type strains [32]. These findings have important implications for C. difficile virulence since they suggest that strains carrying tcdC mutations have the inherent potential to develop hypervirulence. The recent emergence of a new class of hypervirulent strains, ribotype 078 [44], may be one such example. These isolates encode a non-functional TcdC protein [37], produce significantly more toxin than non-epidemic strains, are associated with more severe disease as well as higher rates of mortality and are increasingly being identified as the causative agent of CDI [44], [45].
Although these experiments show that TcdC-status alone can modulate virulence it is probable that multiple factors working synergistically are necessary for the development of hypervirulence in the BI/NAP1/027 strains. It is likely that the accumulation of multiple genetic changes in addition to the tcdC mutation has enabled BI/NAP1/027 strains to become the predominant disease-causing isolates in numerous countries. Of particular importance might be variations in the functional activity of the encoded toxins since these isolates were recently shown to produce a toxin B that shows variation across the C-terminal receptor binding domain of the protein [46], resulting in more potent activity across a wider range of cell lines in comparison to toxin B from the historical, non-epidemic strain 630 [4]. Furthermore, using the zebrafish embryo model of intoxication, the BI/NAP1/027 toxin B was recently shown to have pronounced in vivo cytotoxic activity in comparison to toxin B from VPI10463, another historical non-epidemic isolate, with greater tissue tropism and more extensive tissue destruction observed [47]. Since toxin B is thought to be one of the major virulence factors of C. difficile [15], [16] these observations suggest that TcdB variations might play an important role in the hypervirulent phenotype.
There are other factors that may influence C. difficile hypervirulence. The BI/NAP1/027 strains encode an additional toxin known as binary toxin or CDT [2]. The role of this toxin in CDI remains to be elucidated but a recent study showed that CDT induces the formation of microtubule-based protrusions on the host cell surface thereby increasing C. difficile adherence to epithelial cells. Moreover, intestinal colonisation of gnotobiotic mice with a BI/NAP1/027 C. difficile strain was significantly reduced in mice treated with CDT-neutralising antibodies in comparison to control mice [48]. These findings suggest that CDT may be an important colonisation factor, enhancing the ability of BI/NAP1/027 strains to initiate infection as well as causing adjunctive tissue damage during later stages of infection, potentially leading to more severe disease. Many BI/NAP1/027 strains are also more proficient at sporulation than non-epidemic C. difficile strains [12], [49]. C. difficile spores are highly infectious [50] and play a critical role in the transmission of CDI and perhaps in disease relapse, which is a serious problem in patients with CDI [51]. In this context, enhanced sporulation is ostensibly an important adaptation by BI/NAP1/027 isolates, which would result in larger numbers of spores being shed from infected patients and an increased environmental spore load, ultimately leading to higher transmission rates. Finally, the development of fluoroquinolone resistance, in particular to moxifloxacin and gatifloxacin, is unquestionably a major factor in epidemics caused by BI/NAP1/027 strains [3], [11]. In this regard, the hypothetical co-evolution of enhanced virulence traits and antibiotic resistance in C. difficile mirrors trends seen with other significant nosocomial pathogens such as Methicillin Resistant Staphylococcus aureus (MRSA) [52] and Vancomycin Resistant Enterococcus (VRE) species [53]. In summary, it is clear that our findings represent an important breakthrough in our understanding of the development of hypervirulence in prevailing C. difficile isolates and will provide a significant reference point for future studies on epidemic strains and their control.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the United Kingdoms Home Office Animals (Scientific Procedures) Act of 1986 which outlines the regulation of the use of laboratory animals for the use of animals in scientific procedures. The experiments described were subject to approval by the University of Glasgow Ethics Committee and by a designated Home Office Inspector (Project Number 60/4218). All experiments were subject to the 3 R consideration (refine, reduce and replace) and all efforts were made to minimize suffering.
Bacterial strains and growth conditions
The characteristics and origins of all recombinant strains and plasmids are shown in Table 1 and Table S2, respectively. All bacteriological culture media were obtained from Oxoid. C. difficile strains were cultured in BHIS [54] or TY medium [15], unless otherwise stated, in an atmosphere of 10% H2, 10% CO2, and 80% N2 at 37°C in a Coy anaerobic chamber. Escherichia coli was cultured in 2×YT medium aerobically at 37°C, with shaking for broth cultures. All antibiotics were purchased from Sigma-Aldrich and were used at the following concentrations: cycloserine (Cs, 250 µg/ml), cefoxitin (Cf, 8 µg/ml), thiamphenicol (Tm, 10 µg/ml) or tetracycline (Tc, 10 µg/ml), chloramphenicol (Cm, 25 µg/ml).
Table 1. Bacterial strains.
| Strain | Characteristics | Source/Reference |
| E. coli | ||
| DH5α | F-φ80Δ lacZdM15Δ(lacZYA-argF)U169 endA1recA1 hsdr17(τκ −mκ +)deoR thi-1 supE44 gyrA96 relA1 | Life Technologies |
| TOP10 | F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galU galK rpsL(StrR) endA1 λ− | |
| Invitrogen | ||
| HB101 | thi-1 hsdS20(rB − mB −) supE44 recAB ara-14 leuB5 proA2 lacY1 galK rpsL20 (Smr) xyl-5 mtl-1 | [60] |
| C. perfringens | ||
| JIR4225 | C. perfringens strain JIR325 with 5 chromosomal copies of Tn916 | [30] |
| C. difficile | ||
| M7404 | Canadian BI/NAP1/027 isolate | [29] |
| 630 | Wild-type C. difficile strain; first C. difficile genome available | [61] |
| JIR8094 | Erythromycin sensitive derivative of strain 630 | [24] |
| VPI10463 | PaLoc-positive C. difficile isolate | [38] |
| VPI11186 | PaLoc-negative C. difficile isolate | [38] |
| CD37 | PaLoc-negative C. difficile isolate | [62] |
| KI | Australian BI/NAP1/027 isolate | [36] |
| DLL3053 | Australian toxinotype III clinical isolate | This study |
| DLL3054 | Australian toxinotype V clinical isolate | This study |
| DLL3055 | Australian toxinotype V clinical isolate | This study |
| DLL3056 | Australian toxinotype XIV clinical isolate | This study |
| M7404(VC) | DLL3001 (M7404 carrying shuttle plasmid pDLL4) | This study |
| M7404(tcdC +) | DLL3002 (M7404 carrying tcdC expression plasmid pDLL17) | This study |
| M7404(cured) | DLL3003 (M7404(tcdC +) cured of plasmid pDLL17) | This study |
Molecular biology and PCR techniques
Plasmid DNA was isolated using a QIAprep spin miniprep kit (Qiagen). Genomic DNA was prepared using a DNeasy tissue kit (Qiagen). Standard methods for the digestion, modification, ligation, and analysis of plasmid and genomic DNA were used [55]. Nucleotide sequence analysis was carried out using a PRISM BigDye Terminator cycle sequencing kit (Applied Biosystems) and detection was performed by Micromon at Monash University. Oligonucleotide primer sequences are listed below. Unless otherwise stated, all PCR experiments were carried out with Phusion DNA polymerase (New England Biolabs) and the 2× Failsafe PCR buffer E (Epicentre) according to the manufacturer's instructions.
Construction of recombinant plasmids
For construction of the Tn916 transferrable clostridial shuttle vector, PCR was performed using primers DLP33 (5′-GAATTCGCCCTTTTTTATACTCCCCTTG-3′) and DLP34 (5′-GAATTCGCCCTCAAAGGACGAATATGTCGC-3′) and chromosomal DNA extracted from Clostridium perfringens strain JIR4225 [30]. The resulting 700 bp DNA fragment, which contained the oriT region of Tn916, was TOPO-cloned into pCR-Blunt II-TOPO according to the manufacturer's instructions (Invitrogen). The fragment was then excised from pCR-Blunt II-TOPO using EcoRI and cloned into the equivalent sites of plasmid pMTL9361Cm [29], resulting in plasmid pDLL4.
For construction of the tcdC-carrying plasmid, PCR was performed using primers DLP35 (5′-CTGCAGCCACCTCTAAATCACTGAGTCACTTAATTAC-3′) and DLP36 (5′-CTGCAGAGCCTTGTAACTGTTTATTTGC-3′) and C. difficile strain VPI10463 genomic DNA in order to amplify a 1085 bp fragment encompassing the tcdC gene and upstream region. This fragment was then TOPO-cloned into pCR-Blunt II-TOPO, before being excised with PstI and subcloned into the equivalent site of plasmid pDLL4, resulting in the final construct pDLL17.
Transfer of plasmid DNA into C. difficile by conjugation
The conjugation procedure utilising E. coli HB101(pVS520) as the conjugative donor was carried out as previously described [29]. Recombinant plasmids were introduced into C. perfringens strain JIR4225 as before [56]. Conjugations utilising C. perfringens JIR4225 were then performed as follows: separate 90 ml BHIS broth cultures were inoculated with 1 ml aliquots from an overnight C. difficile recipient strain or C. perfringens donor strain starter culture and grown to mid-exponential phase. Approximately 1 ml was removed from each culture, mixed and centrifuged. The cell pellet was then resuspended in phosphate-buffered saline (PBS), spread onto a BHIS agar plate and incubated overnight at 37°C. Bacterial growth was harvested in sterile PBS before being spread onto BHIS agar supplemented with thiamphenicol and incubated overnight as before. Bacterial growth was again harvested with PBS and dilutions spread onto BHIS agar supplemented with cefoxitin and thiamphenicol or tetracycline, and the plates incubated under anaerobic conditions for 24 to 72 h.
Toxin A-specific Western blots
The toxins were partially purified by ammonium sulphate precipitation from culture supernatants harvested after growth for 72 hours and toxin A was then detected by Western blotting as described previously [15].
TcdC-specific Western blots
For non-quantitative TcdC-specific Western Blots, crude extracts of C. difficile were prepared by sonication of samples taken from cultures that had been grown for 12 hours under anaerobic conditions. The crude extracts from each strain were then subjected to electrophoresis in a 15% SDS-PAGE gel and transferred to a nitrocellulose membrane using standard methods [55]. Membranes were treated with anti-TcdC antibody [57] and detected following treatment with goat anti-mouse IgG-alkaline phosphatase conjugated secondary antibody using standard procedures. For quantitative TcdC-specific Western blots, cultures of C. difficile VPI10463 and C. difficile M7404(tcdC +) were grown under anaerobic conditions and samples were removed every 4 hours for 24 or 28 hours respectively. Each sample was normalized to an optical density (600 nm) of 0.9 prior to lysis, to ensure that the same number of cells was present. Lysates were then prepared by sonication prior to SDS-PAGE gel electrophoresis, transfer and detection, as described above. Following detection, the amount of TcdC in each lysate was quantified by densitometric analysis, using purified recombinant TcdC protein (rTcdC) as the standard and the ImageJ software package, according to published methods [58].
Vero cell cytotoxicity assays
Toxin B was detected in C. difficile culture supernatants harvested after growth for 72 hours by Vero cell cytotoxicity assays as described previously [15], except that each well was seeded with 1×105 cells.
RNA extraction and reverse transcription
Total RNA was extracted from C. difficile cultures grown for 12 h in TY media. Reverse transcription was performed using AMV Reverse Transcriptase (Promega) using random hexamer oligonucleotides primers and 2 µg template RNA. The cDNA samples were then purified using Qiaquick Columns (Qiagen).
qRT-PCR assay design and real time PCR
PaLoc gene specific primers were designed using Primer 3 software (Geneious Software). qRT-PCR was performed using an AB7300 real-time PCR instrument (Applied Biosystems). Reactions were carried out using the FastStart Universal SYBR Green Master Mix (Roche) with 40 ng of cDNA as template. Standard curves were generated for each primer pair using C. difficile genomic DNA, and melt curve analysis was performed following each qRT-PCR reaction to verify amplification specificity. Samples were normalised using the C. difficile rrnA gene.
Preparation of spores for animal infection
Spores were prepared from C. difficile cultures grown in 500 ml of BHI broth. Cultures were pelleted by centrifugation for 10 mins and re-suspended in 50% ethanol. The material was then vortexed every 10 min for 1 h before centrifugation for 10 mins. The pellet was then treated with 1% Sarkosyl in PBS for 1 h at room temperature and again pelleted by centrifugation, followed by incubation overnight at 37°C with lysozyme (10 mg/ml) in 125 mM Tris-HCl buffer (pH 8.0). The sample was treated in a sonicating water bath (3 pulses of 3 min each; 1510 Branson) before centrifugation through a 50% sucrose gradient for 20 mins. The pellet was incubated in 2 ml of PBS containing 200 mM EDTA, 300 ng/ml proteinase K and 1% Sarkosyl for 30 mins at 37°C before centrifugation through a 50% sucrose gradient for 20 mins. The final pellet was then washed twice in sterile distilled water before finally being resuspended in 1 ml of sterile water. Spore preparations were stored at −80°C prior to use.
Hamster experiments
Female Golden Syrian hamsters purchased from Harlan Olac UK were used for all animal experiments. Telemetry chips (Vitalview Emitter) were inserted by laparotomy into the body cavity of the animals at least 3 weeks before infection with C. difficile. Animal experiments were then carried out as described previously [33], except that animals received 1×104 spores of C. difficile. Animals were culled when core body temperature dropped below 35°C. This study was carried out in strict accordance with the recommendations in the United Kingdoms Home Office Animals (Scientific Procedures) Act of 1986 which outlines the regulation of the use of laboratory animals for the use of animals in scientific procedures. The experiments described were subject to approval by the University of Glasgow Ethics Committee and by a designated Home Office Inspector (Project Number 60/4218). All experiments were subject to the 3 R consideration (refine, reduce and replace) and all efforts were made to minimize suffering.
Quantification of bacterial load
To estimate colonisation, hamsters were sacrificed and the gut region from the caecum to the anus removed. The tissues were homogenised in PBS using a Stomacher and viable counts were performed on the homogenate as described previously [33].
Confirmation of infecting strains
To confirm that the bacteria isolated from the hamster were the same strain as originally used for infection, genomic DNA was isolated and subjected to MVLA as described previously [59]. Plasmid rescue was performed as previously described [29] followed by restriction digest analysis to confirm plasmid integrity.
Supporting Information
Efficiency of RP4 or Tn 916 -mediated plasmid transfer to C. difficile strains. The efficiency of RP4 or Tn916-mediated plasmid transfer from E. coli or C. perfringens donors, respectively, to C. difficile recipient strains is shown, calculated as described in Materials and Methods and expressed as transconjugants per ml.
(DOC)
Bacterial plasmids. Bacterial plasmids used in this study, together with the genetic features relevant to this work, are shown.
(DOC)
Acknowledgments
We thank Christoph von-Eichel Streiber for kindly providing toxin A-specific antibodies and Rachael Poon and Tongted Phumoonna-Das for assistance with Vero cell cytotoxicity assays.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by Project Grants from the Australian National Health and Medical Research Council and the Australian Research Council (Monash University), Grant AI057637 from the United States National Institute of Allergy and Infectious Diseases (Monash University and Institut Pasteur) and by Project and Programme Grants from Institut Pasteur, The Wellcome Trust and a personal fellowship for GRD from the Royal Society of Edinburgh (Glasgow University). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Borriello SP. Pathogenesis of Clostridium difficile infection. J Antimicrob Chemother. 1998;41:13–19. doi: 10.1093/jac/41.suppl_3.13. [DOI] [PubMed] [Google Scholar]
- 2.Warny M, Pepin J, Fang A, Killgore G, Thompson A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 3.McDonald LC, Killgore GE, Thompson A, Owens RC, Kazakova SV, et al. An epidemic, toxin gene–variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 4.Stabler RA, He M, Dawson L, Martin M, Valiente E, et al. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 2009;10:R102. doi: 10.1186/gb-2009-10-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 6.Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–280. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 7.Kuijper EJ, Coignard B, Tull P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(Suppl 6):2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 8.Rouphael NG, O'Donnell JA, Bhatnagar J, Lewis F, Polgreen PM, et al. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198:635 e631–636. doi: 10.1016/j.ajog.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 9.Zilberberg MD, Tillotson GS, McDonald C. Clostridium difficile infections among hospitalized children, United States, 1997–2006. Emerg Infect Dis. 2010;16:604–609. doi: 10.3201/eid1604.090680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker SS, Faden H, Sayej W, Patel R, Baker RD. Increasing Incidence of Community-Associated Atypical Clostridium difficile Disease in Children. Clin Pediatr (Phila) 2010;49:644–647. doi: 10.1177/0009922809360927. [DOI] [PubMed] [Google Scholar]
- 11.Riley TV. Is Clostridium difficile a threat to Australia's biosecurity? Med J Aust. 2009;190:661–662. doi: 10.5694/j.1326-5377.2009.tb02630.x. [DOI] [PubMed] [Google Scholar]
- 12.Merrigan M, Venugopal A, Mallozzi M, Roxas B, Viswanathan VK, et al. Human Hypervirulent Clostridium difficile Strains Exhibit Increased Sporulation as Well as Robust Toxin Production. J Bacteriol. 2010;192:4904–4911. doi: 10.1128/JB.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N, et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet. 2006;38:779–786. doi: 10.1038/ng1830. [DOI] [PubMed] [Google Scholar]
- 14.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 15.Lyras D, O'Connor JR, Howarth PK, Sambol SP, Carter GP, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–1179. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, et al. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 17.Braun V, Hundsberger T, Leukel P, Sauerborn M, Eichel-Streiber Cv. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene. 1996;181:29–38. doi: 10.1016/s0378-1119(96)00398-8. [DOI] [PubMed] [Google Scholar]
- 18.Mani N, Dupuy B. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc Natl Acad Sci U S A. 2001;98:5844–5849. doi: 10.1073/pnas.101126598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan KS, Wee BY, Song KP. Evidence for holin function of tcdE gene in the pathogenicity of Clostridium difficile. J Med Microbiol. 2001;50:613–619. doi: 10.1099/0022-1317-50-7-613. [DOI] [PubMed] [Google Scholar]
- 20.Matamouros S, England P, Dupuy B. Clostridium difficile toxin expression is inhibited by the novel regulator TcdC. Mol Microbiol. 2007;64:1274–1288. doi: 10.1111/j.1365-2958.2007.05739.x. [DOI] [PubMed] [Google Scholar]
- 21.Dupuy B, Govind R, Antunes A, Matamouros S. Clostridium difficile toxin synthesis is negatively regulated by TcdC. J Med Microbiol. 2008;57:685–689. doi: 10.1099/jmm.0.47775-0. [DOI] [PubMed] [Google Scholar]
- 22.Murray R, Boyd D, Levett PN, Mulvey MR, Alfa MJ. Truncation in the tcdC region of the Clostridium difficile PathLoc of clinical isolates does not predict increased biological activity of Toxin B or Toxin A. BMC Infect Dis. 2009;9:103. doi: 10.1186/1471-2334-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mani N, Lyras D, Barroso L, Howarth P, Wilkins T, et al. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. J Bacteriol. 2002;184:5971–5978. doi: 10.1128/JB.184.21.5971-5978.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor JR, Lyras D, Farrow KA, Adams V, Powell DR, et al. Construction and analysis of chromosomal Clostridium difficile mutants. Mol Microbiol. 2006;61:1335–1351. doi: 10.1111/j.1365-2958.2006.05315.x. [DOI] [PubMed] [Google Scholar]
- 25.Burns DA, Heap JT, Minton NP. SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. J Bacteriol. 2010;192:657–664. doi: 10.1128/JB.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartman ST, Minton NP. A mariner-based transposon system for in vivo random mutagenesis of Clostridium difficile. Appl Environ Microbiol. 2010;76:1103–1109. doi: 10.1128/AEM.02525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, et al. The ClosTron: Mutagenesis in Clostridium refined and streamlined. J Microbiol Methods. 2010;80:49–55. doi: 10.1016/j.mimet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Nallapareddy SR, Singh KV, Murray BE. Construction of improved temperature-sensitive and mobilizable vectors and their use for constructing mutations in the adhesin-encoding acm gene of poorly transformable clinical Enterococcus faecium strains. Appl Environ Microbiol. 2006;72:334–345. doi: 10.1128/AEM.72.1.334-345.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter GP, Lyras D, Allen DL, Mackin KE, Howarth PM, et al. Binary toxin production in Clostridium difficile is regulated by CdtR, a LytTR family response regulator. J Bacteriol. 2007;189:7290–7301. doi: 10.1128/JB.00731-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awad MM, Rood JI. Isolation of a-toxin, q-toxin and k-toxin mutants of Clostridium perfringens by Tn916 mutagenesis. Microb Pathogen. 1997;22:275–284. doi: 10.1006/mpat.1996.0115. [DOI] [PubMed] [Google Scholar]
- 31.Hundsberger T, Braun V, Weidmann M, Leukel P, Sauerborn M, et al. Transcription analysis of the genes tcdA-E of the pathogenicity locus of Clostridium difficile. European J Bioch. 1997;244:735–742. doi: 10.1111/j.1432-1033.1997.t01-1-00735.x. [DOI] [PubMed] [Google Scholar]
- 32.Razaq N, Sambol S, Nagaro K, Zukowski W, Cheknis A, et al. Infection of hamsters with historical and epidemic BI types of Clostridium difficile. J Infect Dis. 2007;196:1813–1819. doi: 10.1086/523106. [DOI] [PubMed] [Google Scholar]
- 33.Goulding D, Thompson H, Emerson J, Fairweather NF, Dougan G, et al. Distinctive profiles of infection and pathology in hamsters infected with Clostridium difficile strains 630 and B1. Infect Immun. 2009;77:5478–5485. doi: 10.1128/IAI.00551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He M, Sebaihia M, Lawley TD, Stabler RA, Dawson LF, et al. Evolutionary dynamics of Clostridium difficile over short and long time scales. Proc Natl Acad Sci U S A. 2010;107:7527–7532. doi: 10.1073/pnas.0914322107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rupnik M, Avesani V, Janc M, von Eichel-Streiber C, Delmee M. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J Clin Microbiol. 1998;36:2240–2247. doi: 10.1128/jcm.36.8.2240-2247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards M, Knox J, Elliott B, Mackin K, Lyras D, et al. Severe infection with Clostridium difficile PCR ribotype 027 acquired in Melbourne, Australia. Med J Australia. 2011;194:369–371. doi: 10.5694/j.1326-5377.2011.tb03012.x. [DOI] [PubMed] [Google Scholar]
- 37.Curry SR, Marsh JW, Muto CA, O'Leary MM, Pasculle AW, et al. tcdC genotypes associated with severe TcdC truncation in an epidemic clone and other strains of Clostridium difficile. J Clin Microbiol. 2007;45:215–221. doi: 10.1128/JCM.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyerly DM, Barroso LA, Wilkins TD, Depitre C, Corthier G. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect Immun. 1992;60:4633–4639. doi: 10.1128/iai.60.11.4633-4639.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter GP, Rood JI, Lyras D. The role of toxin A and toxin B in Clostridium difficile-associated disease: Past and present perspectives. Gut Microbes. 2010;1:58–64. doi: 10.4161/gmic.1.1.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913–1924. doi: 10.1053/j.gastro.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 41.Heap JT, Pennington OJ, Cartman ST, Carter GP, NP. M. The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Meth. 2007;70:452–464. doi: 10.1016/j.mimet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Mullany P, Wilks M, Tabaqchali S. Transfer of Tn916 and Tn916DE into Clostridium difficile: demonstration of a hot-spot for these elemnets in the C. difficile genome. FEMS Microbiol Lett. 1991;79:191–194. doi: 10.1016/0378-1097(91)90084-n. [DOI] [PubMed] [Google Scholar]
- 43.Minton N, Carter G, Herbert M, O'Keeffe T, Purdy D, et al. The development of Clostridium difficile genetic systems. Anaerobe. 2004;10:75–84. doi: 10.1016/j.anaerobe.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Goorhuis A, Debast SB, van Leengoed LA, Harmanus C, Notermans DW, et al. Clostridium difficile PCR ribotype 078: an emerging strain in humans and in pigs? J Clin Microbiol. 2008;46:1157; author reply 1158. doi: 10.1128/JCM.01536-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson LF, Valiente E, Wren BW. Clostridium difficile–a continually evolving and problematic pathogen. Infect Genet Evol. 2009;9:1410–1417. doi: 10.1016/j.meegid.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Stabler RA, Dawson LF, Phua LT, Wren BW. Comparative analysis of BI/NAP1/027 hypervirulent strains reveals novel toxin B-encoding gene (tcdB) sequences. J Med Microbiol. 2008;57:771–775. doi: 10.1099/jmm.0.47743-0. [DOI] [PubMed] [Google Scholar]
- 47.Lanis JM, Barua S, Ballard JD. Variations in TcdB activity and the hypervirulence of emerging strains of Clostridium difficile. PLoS Pathog. 2010;6:e1001061. doi: 10.1371/journal.ppat.1001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwan C, Stecher B, Tzivelekidis T, van Ham M, Rohde M, et al. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5:e1000626. doi: 10.1371/journal.ppat.1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akerlund T, Persson I, Unemo M, Noren T, Svenungsson B, et al. Increased sporulation rate of epidemic Clostridium difficile Type 027/NAP1. J Clin Microbiol. 2008;46:1530–1533. doi: 10.1128/JCM.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawley TD, Clare S, Walker AW, Goulding D, Stabler RA, et al. Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect Immun. 2009;77:3661–3669. doi: 10.1128/IAI.00558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:403–410. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Lindsay JA. Genomic variation and evolution of Staphylococcus aureus. Int J Med Microbiol. 2010;300:98–103. doi: 10.1016/j.ijmm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Bonten MJ, Willems R, Weinstein RA. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect Dis. 2001;1:314–325. doi: 10.1016/S1473-3099(01)00145-1. [DOI] [PubMed] [Google Scholar]
- 54.Smith CJ, Markowitz SM, Macrina FL. Transferable tetracycline resistance in Clostridium difficile. Antimicrob Agents and Chemother. 1981;19:997–1003. doi: 10.1128/aac.19.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 56.Awad MM, Bryant AE, Stevens DL, Rood JI. Virulence studies on chromosomal a-toxin and q-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of a-toxin in Clostridium perfringens-mediated gas gangrene. Mol Microbiol. 1995;15:191–202. doi: 10.1111/j.1365-2958.1995.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 57.Govind R, Vediyappan G, Rolfe RD, Fralick JA. Evidence that Clostridium difficile TcdC is a membrane-associated protein. J Bacteriol. 2006;188:3716–3720. doi: 10.1128/JB.188.10.3716-3720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics Internatl. 2004;11:36–42. [Google Scholar]
- 59.Marsh JW, O'Leary MM, Shutt KA, Pasculle AW, Johnson S, et al. Multilocus variable-number tandem-repeat analysis for investigation of Clostridium difficile transmission in Hospitals. J Clin Microbiol. 2006;44:2558–2566. doi: 10.1128/JCM.02364-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 61.Wust J, Sullivan NM, Hardegger U, Wilkins TD. Investigation of an outbreak of antibiotic-associated colitis by various typing methods. J Clin Microbiol. 1982;16:1096–1101. doi: 10.1128/jcm.16.6.1096-1101.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mullany P, Wilks M, Lamb I, Clayton C, Wren B, et al. Genetic analysis of a tetracycline resistance element from Clostridium difficile and its conjugal transfer to and from Bacillus subtilis. J Gen Microbiol. 1990;136:1343–1349. doi: 10.1099/00221287-136-7-1343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Efficiency of RP4 or Tn 916 -mediated plasmid transfer to C. difficile strains. The efficiency of RP4 or Tn916-mediated plasmid transfer from E. coli or C. perfringens donors, respectively, to C. difficile recipient strains is shown, calculated as described in Materials and Methods and expressed as transconjugants per ml.
(DOC)
Bacterial plasmids. Bacterial plasmids used in this study, together with the genetic features relevant to this work, are shown.
(DOC)