Abstract
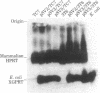
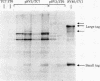
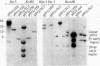
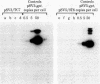
Cultured monkey (TC7) and mouse (3T6) cells synthesize an Excherichia coli enzyme, xanthine-guanine phosphoribosyltransferase (XGPRT; 5-phospho-alpha-D-ribose-1-diphosphate:xanthine phosphoribosyltransferase, EC 2.4.2.22), after transfection with DNA vectors carrying the corresponding bacterial gene, Ecogpt. In contrast to mammalian cells, which do not efficiently use xanthine for purine nucleotide synthesis, cells that produce E. coli XGPRT can synthesize GMP from xanthine via XMP. After transfection with vector-Ecogpt DNAs, surviving cells producing XGPRT can be selectively grown with xanthine as the sole precursor for guanine nucleotide formation in a medium containing inhibitors (aminopterin and mycophenolic acid) that block de novo purine nucleotide synthesis. Cells transformed for Ecogpt arise with a frequency of 10(-4) to 10(-5); they appear to be genetically stable in as much as there is no discernible decrease in XGPRT formation or loss on their ability to grow in selective medium after propagation in nonselective medium. Although several of the vector-gpt DNAs can replicate in monkey and mouse cells, none of the transformants contain autonomously replicating vector-gpt DNA. Rather, the gpt transformants contain one to five copies of the transfecting DNA associated with, and most probably integrated into, cellular DNA sequences. In several transformants, vector-coded gene products for which there was no selection are also synthesized. This suggests that recombinant DNAs containing Ecogpt as a selective marker can be useful for cotransformation of nonselectable genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S., Todaro S. Human diploid cell transformation by DNA extracted from the tumor virus SV40. Science. 1969 Oct 17;166(3903):390–391. doi: 10.1126/science.166.3903.390. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Mantei N., Chambon P. Corrected splicing of a chicken ovalbumin gene transcript in mouse L cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):740–744. doi: 10.1073/pnas.77.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. The inhibition of nucleic acid synthesis by mycophenolic acid. Biochem J. 1969 Jul;113(3):515–524. doi: 10.1042/bj1130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon F., O'Hare K., Perrin F., LePennec J. P., Benoist C., Cochet M., Breathnach R., Royal A., Garapin A., Cami B. Organisation and sequences at the 5' end of a cloned complete ovalbumin gene. Nature. 1979 Mar 29;278(5703):428–434. doi: 10.1038/278428a0. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Berg P. Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells. Cell. 1976 Dec;9(4 Pt 2):695–705. doi: 10.1016/0092-8674(76)90133-1. [DOI] [PubMed] [Google Scholar]
- Graf L. H., Jr, Urlaub G., Chasin L. A. Transformation of the gene for hypoxanthine phosphoribosyltransferase. Somatic Cell Genet. 1979 Nov;5(6):1031–1044. doi: 10.1007/BF01542658. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Davoli D., Thomas C. A., Jr, Fareed G. C. Simian virus 40 carrying an Escherichia coli suppressor gene. J Mol Biol. 1977 May 15;112(2):155–182. doi: 10.1016/s0022-2836(77)80137-x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Papaioannou R., Elion G. B. Human hypoxanthine phosphoribosyltransferase. I. Purification, properties, and specificity. J Biol Chem. 1969 Mar 10;244(5):1263–1270. [PubMed] [Google Scholar]
- Lester S. C., LeVan S. K., Steglich C., DeMars R. Expression of human genes for adenine phosphoribosyltransferase and hypoxanthine-guanine phosphoribosyltransferase after genetic transformation of mouse cells with purified human DNA. Somatic Cell Genet. 1980 Mar;6(2):241–259. doi: 10.1007/BF01538799. [DOI] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Ramsey G. A., Krenitsky T. A., Elion G. B. Guanine phosphoribosyltransferase from Escherichia coli, specificity and properties. Biochemistry. 1972 Dec 5;11(25):4723–4731. doi: 10.1021/bi00775a014. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Howard B. H., Berg P. Synthesis of rabbit beta-globin in cultured monkey kidney cells following infection with a SV40 beta-globin recombinant genome. Nature. 1979 Jan 11;277(5692):108–114. doi: 10.1038/277108a0. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Robins D., Wold B., Sweet R., Jackson J., Lowy I., Roberts J. M., Sim G. K., Silverstein S., Axel R. Altering genotype and phenotype by DNA-mediated gene transfer. Science. 1980 Sep 19;209(4463):1414–1422. doi: 10.1126/science.7414320. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Shander M. H., Manley J. L., Gefter M. L., Maniatis T. Structure and in vitro transcription of human globin genes. Science. 1980 Sep 19;209(4463):1329–1336. doi: 10.1126/science.6158093. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SZYBALSKA E. H., SZYBALSKI W. Genetics of human cess line. IV. DNA-mediated heritable transformation of a biochemical trait. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2026–2034. doi: 10.1073/pnas.48.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Perucho M., Kurtz D., Dana S., Pellicer A., Axel R., Silverstein S. Transformation of mammalian cells with an amplifiable dominant-acting gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3567–3570. doi: 10.1073/pnas.77.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wold B., Wigler M., Lacy E., Maniatis T., Silverstein S., Axel R. Introduction and expression of a rabbit beta-globin gene in mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5684–5688. doi: 10.1073/pnas.76.11.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]