Abstract
Brain-derived neurotrophic factor (BDNF) plays a key role in learning and memory, but its effects on the fiber architecture of the living brain are unknown. We genotyped 455 healthy adult twins and their non-twin siblings (188 males/267 females; age: 23.7±2.1 years, mean±SD) and scanned them with high angular resolution diffusion tensor imaging (DTI), to assess how the BDNF Val66Met polymorphism affects white matter microstructure. By applying genetic association analysis to every 3D point in the brain images, we found that the Val-BDNF genetic variant was associated with lower white matter integrity in the splenium of the corpus callosum, left optic radiation, inferior fronto-occipital fasciculus, and superior corona radiata. Normal BDNF variation influenced the association between subjects’ performance intellectual ability (as measured by Object Assembly subtest) and fiber integrity (as measured by fractional anisotropy; FA) in the callosal splenium, and pons. The BDNF gene may affect intellectual performance by modulating white matter development. This combination of genetic association analysis and large-scale diffusion imaging directly relates a specific gene to the fiber microstructure of the living brain and to human intelligence.
Keywords: BDNF, twins, diffusion imaging, cognition, imaging genomics, white matter
1. Introduction
Growth factors play a crucial role in guiding brain development and connectivity, as they regulate neuronal differentiation and survival, and modulate the plasticity and function of neuronal synapses. Among them, brain-derived neurotrophic factor (BDNF) is critically involved in learning and memory – it modulates hippocampal neurogenesis, synaptic transmission, and activity-induced long-term potentiation and depression (Poo, 2001). In a landmark study, Egan et al. (2003) showed that a common variant in the BDNF gene, a methionine (Met) for valine (Val) substitution at codon 66 in the 5′-proregion of the BDNF protein (Val66Met; dbSNP number rs6265), led to poorer episodic memory and hippocampal activation in a cohort of 641 cognitively intact adults aged 25-45. This amino-acid substitution impairs intracellular trafficking and activity-dependent release of the growth factor, without affecting its synthesis (Chen et al., 2006). The Met allele occurs in around 20% of Caucasians, and 40% of Asians (Shimizu et al., 2004), and is associated with gray matter volume deficits especially in the hippocampus and prefrontal cortex (Pezawas et al., 2004), and with impaired memory even in healthy young people (Hariri et al., 2003). Nevertheless, associations between BDNF Val66Met polymorphism and the fiber integrity of the living brain are still unknown. This is of special interest, as white matter microstructure and intellectual ability were found to be influenced by a partially overlapping set of genes (Chiang et al., 2009).
To study how BDNF Val66Met polymorphism impacts brain microstructure, we acquired high angular resolution diffusion tensor images (DTI) from 455 twins and their non-twin siblings. DTI is a variant of magnetic resonance imaging that is sensitive to the directionally constrained water diffusion in the brain, which occurs preferentially along axons. The fractional anisotropy (FA) of diffusion is a widely accepted index of the microstructural integrity of the white matter (Basser and Pierpaoli, 1996; Beaulieu, 2002). To evaluate the association between BDNF Val66Met genotype data and white matter integrity measured by FA, we applied a general linkage-disequilibrium test for quantitative traits (Abecasis et al., 2000) to each location in the 3D brain images. Given BDNF’s known effects on cortical structure and function, we hypothesized that the BDNF Val66Met polymorphism may contribute to the structural integrity of cerebral white matter.
The influence of the BDNF gene on memory performance has been widely replicated (Hansell et al., 2007), but its association with full-scale IQ has not been detected (Egan et al., 2003). Similarly, another cognition-related gene, the catechol-O-methyltransferase (COMT) gene, was also found to be associated with working memory performance, but not with IQ (Egan et al., 2001). Li et al. (2009) found that the COMT Val158Met polymorphism modulated the association between white matter integrity and IQ – the regression slope for FA against full-scale IQ was higher in Val homozygotes than in Met carriers in the prefrontal regions and in the hippocampal formation, bilaterally. Based on this finding, we expected that gene effects on IQ might be due to indirect interactions between white matter integrity (or other brain structural phenotypes) and IQ. This inability to detect a direct correlation between these specific genes and IQ may be because IQ is a complex phenotype; the endophenotype strategy suggests that it may be easier to identify gene effects if they are mediated by some basic intermediate phenotypes that are readily measured in brain images, e.g., the voxelwise FA in this study. Therefore, our second hypothesis was that the BDNF Val66Met polymorphism may influence intellectual performance via a modulating effect on white matter architecture, similar to the COMT gene. This was an exploratory hypothesis, and we tested it by studying how BDNF genotypes modulated the statistical association between white matter integrity and IQ at every point of the brain, that we previously detected in a subset of the subjects studied here (Chiang et al., 2009).
2. Methods
2.1. Subject description and Genotyping
The BDNF Val66Met polymorphism was identified as rs6265 in the dbSNP public database (http://www.ncbi.nlm.nih.gov/projects/SNP/). Genotype data were available for 785 healthy adult twins and their non-twin siblings (385 males / 400 females) from 439 families with the following ancestry: British Isles 76.2%, Northern Europe 12.6%, Mediterranean 5.4%, unknown 5.8%, and were determined using primer extension in the Sequenom Mass-Array system. Genotyping error rate was < 0.1%. The frequency of the BDNF Val allele was 81%, and was similar for all ancestral groups (British Isles: 82%, Northern Europe: 77%, Mediterranean: 76%, unknown ancestry: 79%). The probability of correct zygosity assignment was > 99.99% (Hansell et al., 2007). To find the association between image measures and BDNF genotype data, we acquired DTI scans from 455 subjects (188 males/267 females; age: 23.7±2.1 years, mean±SD). BDNF genotypes were Val/Val for 316 subjects (69% of the total), Val/Met for 118 subjects (26%), and Met/Met for 21 subjects (5%). To test the robustness and reproducibility of our findings, we split the 455 subjects into two independent sub-groups, Groups 1 and 2, with a similar sample size: Group 1 had 234 subjects (99 males/135 females; age: 23.7±1.9 years) from 110 different nuclear families. BDNF Val/Met genotypes were Val/Val for 161 subjects (68% of the total), for 60 subjects (26%), and Met/Met for 13 subjects (6%); Group 2 had 221 subjects (89 males/132 females; age: 23.7±2.2 years) from 128 families unrelated to those in Group 1. BDNF genotypes were Val/Val for 155 subjects (70% of the total), Val/Met for 58 subjects (26%), and Met/Met for 8 subjects (4%). The twin/sibling composition of the families in both sub-groups is described in Table 1. The BDNF genotype distribution in all 455 subjects and in both sub-groups followed the Hardy-Weinberg equilibrium (P > 0.01). All subjects were screened to exclude cases of pathology known to affect brain structure, a history of significant head injury, a neurological or psychiatric illness, substance abuse or dependence, or a psychiatric disorder in any first-degree relative.
Table 1.
Twin/sibling composition for the participating families
| Number of families |
||
|---|---|---|
| Group 1* | Group 2# | |
| MZ pairs | 33 | 44 |
| MZ pair plus one non-twin sibling | 4 | 5 |
| DZ pairs | 54 | 23 |
| DZ trizygotic triplets | 2 | 1 |
| DZ pair plus one non-twin sibling | 9 | 1 |
| DZ pair plus two non-twin siblings | 1 | 0 |
| Single participants (not paired) | 3 | 43 |
| Two non-twin siblings | 4 | 10 |
| Three non-twin siblings | 0 | 1 |
MZ: monozygotic, DZ: dizygotic.
BDNF genotypes were available for both parents in 91 families, for only one parent in 16 families, and for neither parent in 3 families.
BDNF genotypes were available for both parents in 101 families, for only one parent in 12 families, and for neither parent in 15 families; knowing the parental genotypes enhances statistical power for within-family association tests, even when phenotypes (DTI scans here) are not available for them.
2.2. Image processing and registration
All MR images were collected using a 4 Tesla Bruker Medspec MRI scanner (Bruker Medical, Ettingen, Germany), with a transverse electromagnetic (TEM) headcoil, at the Center for Magnetic Resonance (University of Queensland, Australia). High angular resolution diffusion-weighted scans were acquired using single-shot echo planar imaging with a twice-refocused spin echo sequence, to reduce eddy-current induced distortions. Imaging parameters were: 21 axial slices (5 mm thick), FOV = 23 cm, TR/TE 6090/91.7 ms, 0.5 mm gap, with a 128×100 acquisition matrix. 30 images were acquired: 3 with no diffusion sensitization (i.e., T2-weighted images) and 27 diffusion-weighted images (b = 1145.7 s/mm2) in which the gradient directions were evenly distributed on an imaginary hemisphere. The reconstruction matrix was 128×128, yielding a 1.8×1.8 mm2 in-plane resolution. Total scan time was 3.05 minutes. We used the FMRIB software library (FSL, http://www.fmrib.ox.ac.uk/fsl/) for pre-processing and affine alignment of the diffusion images. For each participant, motion artifacts were corrected by linearly registering all the T2-weighted and diffusion-weighted images to one of the T2-weighted images (the “eddy_correct” command). Then the three T2-weighted images were averaged and stripped of non-brain tissues to yield a binary brain extraction mask (cerebellum included), using the Brain Extraction Tool (BET) (Smith, 2002), followed by expert manual editing, if necessary. The masked T2-weighted image was then registered to a standardized high-resolution brain MRI template defined in the International Consortium for Brain Mapping space (ICBM) (Holmes et al., 1998) with a 9-parameter linear transformation using the software FLIRT (Jenkinson and Smith, 2001). The resulting transformation parameters were used to rotationally reorient the diffusion tensors (computed from diffusion-weighted images using the “DTIFIT” command) at each voxel (Alexander et al., 2001). The tensor-valued images were linearly realigned based on trilinear interpolation of the log-transformed tensors (Arsigny et al., 2005), and resampled to isotropic voxel resolution (with dimensions: 128×128×93 voxels, resolution: 1.7×1.7×1.7 mm3). The FA image derived from the affine-registered DT image (Basser and Pierpaoli, 1996) was then fluidly registered to a randomly selected participant’s FA image (Chiang et al., 2007).
We averaged the fluidly-registered FA images across all the subjects from Groups 1 and 2 (n = 455) and restricted subsequent data analysis to regions with average FA > 0.2, as in Smith et al (2006), to focus our regions of interest on major white matter fiber structures. Each participant’s FA map was smoothed using an isotropic Gaussian filter with full width at half maximum (FWHM) = 6 mm (Smith et al., 2006).
2.3. Association between diffusion anisotropy and BDNF polymorphisms
We followed the method of Abecasis et al. (2000) to analyze associations between the BDNF polymorphism and FA at each voxel. To eliminate spurious associations due to population stratification, the additive genetic values that coded different variants of the BDNF polymorphism (g; Val/Val = 1, Val/Met = 0, and Met/Met = −1) were decomposed into orthogonal between-family (b) and within-family (w) components. For sibling j in family i, FA value at each voxel (yij), after adjusted for age and sex effects using the general linear model, was modeled as yij = μ + βbbi + βwwij, where μ is the overall phenotypic mean value. bi is the between-family component, or the expected family average, of the additive genetic values of all the siblings in family i. The within-family component for each participant is given by wij = gij – bi. The covariance matrix of FA for family i, denoted by Φi, was based on known genetic similarity between relatives, written in terms of the different sources of variance:
Here σa2 is the variance due to the additive effects of the BDNF polymorphism and σA2 is the variance due to the residual additive of all other genes on the genome. σC2 and σE2 are respectively the variance that comes from environmental factors that are common to all family members, and that are unique for each individual (experimental errors are included in σE2). πijk is the expected proportion of alleles that arise from the same ancestor allele, i.e., identical by descent (IBD) for participants j and k. Estimation of πijk is detailed in (Haseman and Elston, 1972).
The above association model was fitted using the maximum-likelihood method (Abecasis et al., 2000). βw ≠ 0 indicates that there is genuine association of the additive effect of the BDNF polymorphism on FA, i.e., the genetic marker (the BDNF Val66Met polymorphism) is either the trait locus for FA, or is considered to be in linkage disequilibrium with the trait locus. The significance of the genuine association coefficient βw was determined by minus two times this difference between the full model and the restricted (βw was excluded) model, which is asymptotically distributed approximately as a chi-squared distribution with one degree of freedom.
Here the association between the BDNF gene and FA was estimated with each voxel regarded as an independent unit; no tracking of white matter fibers was performed. Linking diffusion measures defined using tractography (Behrens et al., 2007) with genotype data may elucidate genetic influences on cortical connectivity. Nevertheless, tract labeling has not yet been fully automated and is labor intensive, so here we performed association analysis using voxel-based methods. Moreover, we only considered BDNF gene effects on brain fiber architecture; contributions from other genes, or epistatic effects between BDNF and other genes were not included. Future genome-wide association studies (GWAS) (Benyamin et al., 2009) are warranted to study joint effects of multiple genes on diffusion imaging measures.
2.4. How does BDNF polymorphism modify the association between white matter anisotropy and intellectual performance?
To study how the BDNF polymorphism influences intellectual performance, we assessed the subjects’ general intellectual ability at age 16 using the Multidimensional Aptitude Battery (MAB) (Jackson, 1984), a measure highly correlated with the Wechsler Adult Intelligence Scale. Duration of education (in months) was recorded at the same time. We examined three verbal (information, arithmetic, and vocabulary) and two performance (spatial and object assembly) IQ sub-scales. Each subtest gave a raw score; verbal (VIQ), performance (PIQ), and full-scale (FIQ) intelligence quotient standardized scores were derived from these sub-scales. The IQ data were available in 440 subjects. There was no difference in FIQ (Val/Val: 114.1 ± 12.4, n = 304; Val/Met: 114.6±12.8, n = 116; Met/Met: 109.2±15.8, n = 20; mean±SD) or in other IQ sub-scales among the three genotype groups.
The BDNF polymorphism may affect IQ via its modulatory effect on the association between brain white matter structure and IQ, i.e., the regression slope (for FA versus IQ), may differ in people with different BDNF genotypes. This was modeled by adding an interaction term w IQ into FA-IQ regression, using the following moderated regression equation:
Subjects’ IQ and voxel FA values were adjusted for age and sex, and the IQ score was further adjusted for the duration of school education. Positive interaction effect, i.e. βint > 0, means that subjects with wij > 0 have a greater slope than subjects with wij < 0 in FA regressed against the IQ score. This may be clearer if we consider the partial derivative of FA with respect to IQ in the above equation, given by ∂FA/∂IQ = βIQ + βint · w; the slope of the regression for FA on IQ is then shown to depend on w. The covariance matrix is identical to that in the previous section.
To eliminate collinearity between the regressors, we used principal component regression, where the design matrix of a linear regression is transformed into a matrix whose columns are the eigenvectors weighted by the eigenvalues of the original design matrix, and thus are linearly independent of each other (Slinker and Glantz, 1985). The moderated regression equation above may be written in matrix form. For family i with ni members, yi = Xiβ; here yi=[FAi,1 FAi,2 … FAi,ni]T, β = [μ βIQ βb βw βint]T, and Xi consists of five columns that are ni-element vectors of values of 1, and values of IQ, bi, wij, and (IQij·wij) of all ni members (1 ≤ j ≤ ni), in order. The superscript T indicates matrix transpose. Let the design matrix for the total of n families be , and matrix V consists of columns that are normalized eigenvectors of X. Given VVT = I (the identity matrix), we may rewrite the regression equation so that (XV) is the new design matrix whose columns are linearly independent, and b = VTβ is the new vector of the regression coefficients to obtain. We removed the columns of V that corresponded to eigenvalues of X that were smaller than a pre-defined threshold (10−13 times the maximum eigenvalue of X), if any, to avoid singularity that may arise due to collinearity between columns of X.
The significance of the modulatory effect of BDNF polymorphism on FA and IQ, or βint, was determined by the Wald-type statistic (Vonesh and Carter, 1992). Given βint = l(b) = V(5)b, where V(5) is the fifth row of V, the null hypothesis βint = 0 may be tested using the Wald test statistic: T2 = lT[LΩLT]−1l, where L(b) = [∂l(b)/∂b], and Ω is the covariance matrix of b. We refer readers to (Vonesh and Carter, 1992) for the derivation of Ω. T2 follows the F distribution F (1, n – q), where q = 5 is the dimension of β.
We applied the above method to evaluate how the BDNF polymorphism modulated the association between FA and subjects’ VIQ, PIQ, and FIQ scores, as we hypothesized that these three IQ scores respectively reflected subjects’ verbal, performance, and overall intellectual performance. Moreover, we also estimated BDNF interactions with FA and the object assembly (OBJ) sub-scale, as in our previous study (Chiang et al., 2009) we found that the cross-trait correlation between FA and FIQ, PIQ, and OBJ IQ scores was strongly mediated by overlapping genetic factors.
2.5. Correction for multiple comparisons
Given the very large number of tests performed (~80,000 voxels), we controlled for type I error due to multiple comparisons across voxels using the topological false discovery rate (FDR) method (Chumbley et al., 2010; Chumbley and Friston, 2009). Chumbley et al. (2010) recently showed that voxelwise FDR, which is still quite widely used in statistical brain mapping, does not control adequately for regional effects and recommended topological FDR, to provide better assurance that the false positive rates are strictly controlled. The topological FDR method controls the expected proportion of false positive findings over all clusters, and it is not based on a test of the overall volume of the set of supra-threshold voxels, at different statistical thresholds (as standard FDR is). A cluster was defined as a set of contiguous voxels where the size of the tested effect at every voxel surpassed a certain threshold. Here we set the threshold to Z > 3, where Z was the standard Normal variate corresponding to the voxelwise P-value (e.g., the P-maps derived from BDNF-FA associations; note this also defines a threshold in the P-maps). We then used the FMRISTAT toolbox developed by Keith Worsley (http://www.math.mcgill.ca/keith/fmristat/) to compute the significance P-value for each cluster, derived from the Gaussian random field theory (Friston et al., 1994). This P-value indicates how improbable it is to detect any other clusters with a greater volume (i.e., containing more voxels) than that cluster, based on the null hypothesis that there is no tested effect but only Gaussian noise in the whole image field. P-values for all clusters were then inputted into the traditional FDR procedure (Benjamini and Hochberg, 1995). Here we controlled the FDR of clusters within 5%, which means that only 5% of the clusters that are declared to be significant tend, on average, to be false positive findings. All computational processes were executed using a 306-node, dual-processor SUN Microsystems V20z cluster. Each compute node has a dual 64-bit 2.4 GHz AMD Opteron CPU.
3. Results
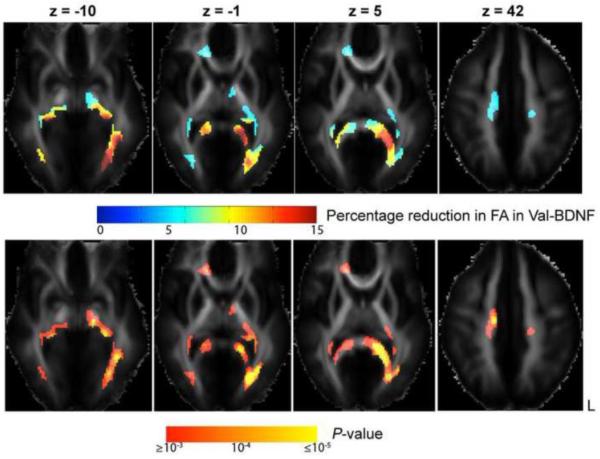
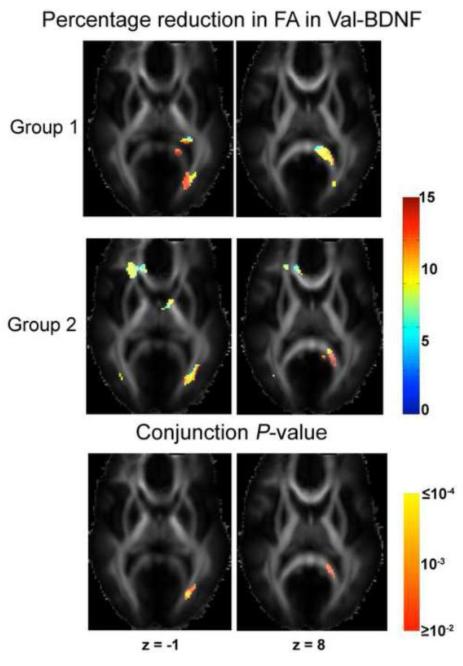
By associating BDNF genotype data with white matter anisotropy in all 455 subjects, we found remarkable and widespread BDNF gene effects across major white matter fibers. Figure 1 shows that the Val genetic variant of BDNF is associated with lower FA in several key white matter tracts (βw < 0), such as the splenium of the corpus callosum and left posterior thalamic radiation/optic radiation (z = −1 and z = 5; in Montreal Neurological Institute (MNI) coordinates, expressed in mm), the junction of the genu of the corpus callosum and the anterior corona radiata on the right (z = 5), left inferior fronto-occipital fasciculus (IFO)/inferior longitudinal fasciculus (ILF), midbrain (which contains corticobulbar and corticospinal tracts) and fornix bilaterally (z = −10), and superior corona radiata on the right (z = 42). FA in subjects whose Val allele exceeded their expected family average (w > 0; n = 90) had up to 15% lower FA than whose Met allele exceeded their expected family average (w < 0; n = 80). In other words, the BDNF allele that is normally considered favorable (Val, not Met) conferred a detectable and systematic deficit in white matter anisotropy of up to 15% regionally. We also tested BDNF-FA associations in the two sub-groups respectively, to detect brain regions where the BDNF effects were robust enough to reproduce in smaller samples. Figure 2 shows that the influences of the BDNF gene were significant in both Groups 1 and 2 in the splenium of the corpus callosum and the left optic radiation. These two regions coincide the white matter regions where the strongest BDNF genotype effects (up to 15% FA reduction associated with the Val allele) were detected in n = 455 subjects (see Fig. 1). Positive associations between the additive Val allele effect and FA (βw > 0) were not significant.
Fig. 1. BDNF Val66Met polymorphism effects on white matter architecture, estimated based on all 455 subjects.
The upper row shows white matter regions where the Val-BDNF allele is associated with reduced FA (βw < 0). Reduction in FA at each voxel is displayed as the percentage difference between those people with w > 0 (an excess of the Val genetic variant) and those with w < 0 (an excess of the Met genetic variant), relative to the average FA across all 455 subjects. Only the voxel clusters that passed the topological FDR ≤ 0.05 threshold are displayed. The lower row shows the voxelwise P-value for βw < 0, displayed on a log10 scale. L: left.
Fig. 2. Influence of the BDNF Val66Met polymorphism on FA compared across different subject groups.
The upper and middle rows show the percentage reduction in average FA for those people with an excess of the Val genetic variant relative to those with an excess of the Met genetic variant (as displayed in Fig. 1), for Groups 1 and 2 respectively. To better visualize the regions where the BDNF-FA association is significant in both subject groups, we conjoined the two significance maps for βw < 0 by taking the larger P-value at every voxel that belongs to the intersection of the significant clusters (as shown in the upper two rows) of the two groups. The conjunction P-maps are displayed on a log10 scale (lower row). MNI coordinates (mm) of the slices, in radiological orientation, are shown at the top. L: left.
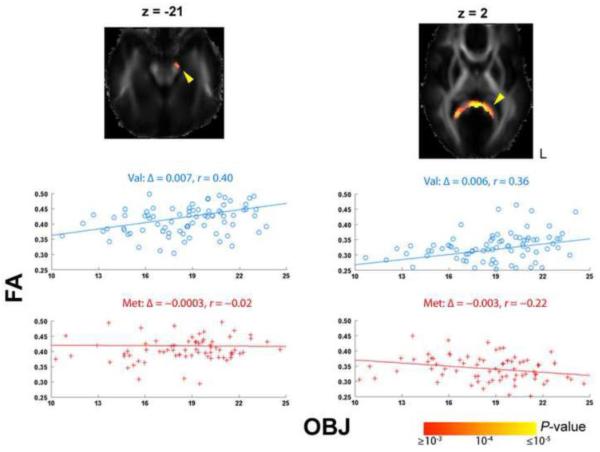
The BDNF polymorphism was not directly associated with intellectual performance, at least when measured using FIQ, VIQ, PIQ, or OBJ scores. However, the BDNF polymorphism may indirectly influence intellectual performance in specific brain regions by modulating the association between white matter anisotropy and IQ. Figure 3 shows this; the slope for the OBJ score regressed against FA in subjects with w > 0 (Val excess) was greater than the corresponding slope in subjects with w < 0 (i.e. βint > 0), in the pons on the left (z = −21) and the splenium of the corpus callosum (z = 2). The interaction effects of the BDNF polymorphism on the association between FA and FIQ, VIQ, or PIQ were not significant.
Fig. 3. Interaction effect of the BDNF polymorphism on the correlation between FA and object assembly (OBJ) sub-scales.
The probability maps (log10 scale; radiological orientation) display significant clusters (that passed the topological FDR threshold) for βint > 0. Scatter plots show differences between those subjects with w > 0 (Val; blue circles) and w < 0 (Met; red crosses), in the slope (Δ) and Pearson’s correlation coefficient (r) of the regression for OBJ versus the average FA of the representative cluster (yellow arrow). L: left.
4. Discussion
In this paper, we mapped the influences of the BDNF Val66Met polymorphism on brain white matter architecture, extending genetic association analyses to 3D images. The Val allele was found to be associated with up to 15% reduction in FA in major fiber tracts, especially in the splenium of the corpus callosum and the optic radiation on the left, the left IFO/ILF, and superior corona radiata. Moreover, associations between BDNF and FA were consistent in the two sub-groups of the subjects in the splenium of the corpus callosum and the optic radiation on the left. Although these two sub-groups were not truly independent of each other as all subjects were recruited from the same twin registry and their brain images acquired using the same MR scanner, testing BDNF-FA associations in these two sub-groups might still help to support the consistency and robustness of the associations, assuming that the genetic determinants for white matter architecture are independent across families (Van Steen et al., 2005). In other words, the results are robust to subsampling within the same cohort, but further analysis of new cohorts that were independently assessed from the outset will be valuable as more large DTI datasets become available. Findings in this paper are consistent with those in our previous study, where genetic influences accounted for around 80% of the variance in FA in these regions (Chiang et al., 2009). This suggests that BDNF is a key candidate gene that influences white matter integrity.
In all brain regions affected by the BDNF polymorphism, the additive effect of the Val allele was associated with a lower FA. Supporting the direction and validity of this effect, a recent conference abstract (Alam et al., 2010) reported significant BDNF genotype-dependent differences in FA, largely restricted to the body of the corpus callosum, where the Val allele was associated with a lower FA. In that study, DTI scans were collected from 85 young healthy volunteers (mean age: 33.5±9.6 years; 46 men/39 women) – a sample of around one fifth the size of that analyzed here. FA is usually considered as a measure of fiber myelination and organization (Beaulieu, 2002). Higher FA reflects greater myelination, and a directional coherence in the myelinated fiber tracts (Beaulieu, 2002), and has generally been linked with better functional performance (Chiang et al., 2009). Even so, lower FA does not necessarily always imply lower white matter integrity, and may reflect (1) larger axonal diameter, which results in increased intracellular water content (Takahashi et al., 2002), (2) greater fiber branching, or (3) higher intravoxel fiber crossing in, e.g., where the optic radiation and corpus callosum fibers intermix (see Fig. 1) (Tuch et al., 2005). The above hypotheses may be tested by comparing the T1-weighted and DT images in the same subjects using voxel-based morphometry (Ashburner and Friston, 2000), where larger axonal diameter or more extensive fiber branching reflected by lower FA may also be detectable as reduced white matter density. Tuch et al. (2005) showed that lower FA, at the junction of the optic radiation and corpus callosum, was associated with a faster reaction time to visual stimuli. Schmithorst and Wilke (2002) found that FA in the corona radiata and internal capsule was lower in musicians than non-musicians. They attributed this to the rearrangement of neural representations for motor control after intensive musical training. The association between lower FA and the Val BDNF allele in this study may provide another exception to the “higher FA is always better” hypothesis.
We found that BDNF variants significantly modulated the association between FA and the OBJ sub-scale in the splenium of the corpus callosum, where the Val allele was associated with a positive FA-OBJ correlation, whereas the Met allele was associated with no correlation or with a negative correlation. The callosal splenium interconnects bilateral primary visual and visual association areas of the parietal and occipital cortex, and is relevant for visuospatial memory (Rudge and Warrington, 1991). Positive correlation between FA and IQ may support the theory that optimal intellectual performance typically requires high processing speed and short reaction times, which are influenced by axonal myelination levels (Arbuthnott et al., 1980). Furthermore, the myelination of white matter fibers may itself be enhanced by neuronal activity (Fields, 2005). On the other hand, negative correlation between FA and IQ may reflect greater fiber crossing or branching that lowers FA, as in a broadly connected cognitive network (Tuch et al., 2005). Reduced FA in one brain region may also indicate that during the maturation or improvement of cognitive function, myelination may be greater in other brain regions to optimize processing efficiency (Schmithorst and Wilke, 2002). All these mechanisms may contribute to the association between white matter architecture and intellectual performance, and the causal pathways are influenced by genetic factors, including the BDNF gene. The interaction of the BDNF gene with FA and OBJ provides an example that the cross-trait correlation between FA and OBJ in major white matter regions is genetically mediated (Chiang et al., 2009).
Given that the Val-BDNF allele was previously associated with superior cognitive performance compared to the Met allele (Hansell et al., 2007), and that in our previous study FA was found to be in positive genetic correlation with IQ, especially with the OBJ subscale (Chiang et al., 2009), the association between the Val allele and a lower FA we discovered here may appear somewhat counterintuitive. The surprising direction of the relationship may be explainable by considering the empirical plots of the modulatory effect of the BDNF gene on FA and IQ. For example, in the splenium of the corpus callosum where the modulatory effect of the BDNF gene on FA and IQ was significant (the right column in Fig. 3), the score for OBJ at the intersection of the two FA-OBJ regression lines for the Val (blue line) and the Met (red line) allele was higher than the average OBJ score across all the subjects (21.3 vs. 18.0). This may explain why the Val allele mediated a positive correlation between FA and OBJ in that region, but was associated with a lower FA than the Met allele. Genetic influences on FA and IQ may result from an overall combination of effects of positive and negative modulations on FA-IQ correlation by many cognition-related genes, including the BDNF gene here. Based on the currently available data, it seems like the net effect of all these genes results in a positive genetic correlation between FA and IQ, as found in our previous study. Nonetheless a genome-wide association study (GWAS) that examines the modulatory effects of all SNPs across the genome will be warranted to test this hypothesis. We have begun to examine GWAS in DTI (Hibar et al., 2010), but the sheer number of tests across the genome makes it relatively underpowered without a meta-analytic approach of many large samples, or without a restricted set of prior hypotheses. Currently, the number of available large samples with both DTI and GWAS is limited. This will change in the future, perhaps facilitated by the Enigma consortium (http://enigma.loni.ucla.edu) and by other meta-analytic approaches to imaging genetics.
Our findings need to be interpreted with caution, because: (1) We did not find significant BDNF interactions with FA and FIQ, VIQ, or PIQ. This may be due to a much higher genetic correlation between FA and OBJ in most white matter regions (Chiang et al., 2009), so that with the current sample size, the modulatory effect of the BDNF gene for FA and OBJ may be easier to detect than the other IQ scales. (2) Given that interaction effects tend to be much weaker than direct associations, even larger samples may be required to replicate the modulatory effect of the BDNF gene for FA and OBJ, which was detected here in the pooled sample of 455 subjects, but not when the samples were split. (3) The subjects were scanned on average 7 years after their intellectual performance was evaluated, so we cannot rule out that some environmental factors, e.g., school education or work experiences, may confound the associations between gene effects, IQ, and white matter integrity, or that changes in IQ may have occurred between the time of the cognitive evaluation and the scan.
The BDNF allelic effects we detected here (up to 15%) are larger than those reported in genome-wide association studies (GWAS) of other complex traits (Visscher, 2008). Nevertheless, there has been a strong hypothesis for many years that as we dissect complex traits into more and more basic endophenotypes, gene effects will become larger, and some recent work supports this (Benyamin et al., 2009). We suggest that the phenotypes we report here are just such fundamental elements and that our findings of relatively large gene effects are not surprising. Even so, the BDNF gene affects cortical gray matter and intellectual performance (Egan et al., 2003; Hariri et al., 2003; Pezawas et al., 2004), so it unquestionably plays a critical role in aspects of white matter microstructure that are relevant for intellectual performance.
Research Highlight.
The commonly-carried BDNF Val66Met polymorphism affects the integrity of cerebral white matter.
The Val allele was associated with up to 15% reduction in FA in major fiber tracts.
BDNF modulates the association between FA and the object assembly IQ sub-scale, showing how a common variants in a growth factor gene can influence cognition.
Acknowledgments
This study was supported by the National Institute of Child Health and Human Development, USA, and the National Health and Medical Research Council, Australia. IQ data collection and zygosity typing was supported by the Australian Research Council. Additional support for algorithm development was provided by the NIA, NIBIB, and the National Center for Research Resources. We are grateful to the twins for participating, to the radiographer, Matt Meredith, Centre for Magnetic Resonance, University of Queensland, for image acquisition, and research nurses, Marlene Grace and Ann Eldridge, Queensland Institute of Medical Research, for twin recruitment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66:279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam T, Tost H, Geramita M, Rebsch C, Bhaskar K, Lemaitre H, Barnett A, Dickinson D, Weinberger D, Marenco S. Impact of the BDNF Val66Met polymorphism on white matter tract integrity in healthy humans. 16th Annual Meeting of the Organization of Human Brain Mapping; Barcelona, Spain. June 6-10.2010. [Google Scholar]
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance. IEEE Trans Med Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Arbuthnott ER, Boyd IA, Kalu KU. Ultrastructural dimensions of myelinated peripheral nerve fibres in the cat and their relation to conduction velocity. J Physiol. 1980;308:125–157. doi: 10.1113/jphysiol.1980.sp013465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsigny V, Fillard P, Pennec X, Ayache N. Fast and simple calculus on tensors in the log-Euclidean framework. Int Conf Med Image Comput Comput Assist Interv (MICCAI) 2005;8:115–122. doi: 10.1007/11566465_15. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- Benyamin B, McRae AF, Zhu G, Gordon S, Henders AK, Palotie A, Peltonen L, Martin NG, Montgomery GW, Whitfield JB, Visscher PM. Variants in TF and HFE explain approximately 40% of genetic variation in serum-transferrin levels. Am J Hum Genet. 2009;84:60–65. doi: 10.1016/j.ajhg.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Dutton RA, Hayashi KM, Lopez OL, Aizenstein HJ, Toga AW, Becker JT, Thompson PM. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. Neuroimage. 2010;49:3057–3064. doi: 10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worseley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Hansell NK, James MR, Duffy DL, Birley AJ, Luciano M, Geffen GM, Wright MJ, Montgomery GW, Martin NG. Effect of the BDNF V166M polymorphism on working memory in healthy adolescents. Genes Brain Behav. 2007;6:260–268. doi: 10.1111/j.1601-183X.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman JK, Elston RC. The investigation of linkage between a quantitative trait and a marker locus. Behav Genet. 1972;2:3–19. doi: 10.1007/BF01066731. [DOI] [PubMed] [Google Scholar]
- Hibar D, Stein JL, Jahanshad N, Barysheva M, Feng A, Kogachi S, McMahon KL, de Zubicaray GI, Hansell NK, Martin NG, Wright MJ, Toga AW, Thompson P. Voxelwise genome-wide association of Diffusion Tensor Images identifies putative novel variants influencing white matter integrity in 467 related young adults. 40th annual meeting of the Society for Neuroscience; San Diego, CA. November 13-17.2010. [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Jackson DN. MAB, multidimensional aptitude battery: manual. Research Psychologists Press; Port Hurton, Michigan: 1984. [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Li J, Yu C, Li Y, Liu B, Liu Y, Shu N, Song M, Zhou Y, Zhu W, Li K, Jiang T. COMT val158met modulates association between brain white matter architecture and IQ. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:375–380. doi: 10.1002/ajmg.b.30825. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Rudge P, Warrington EK. Selective impairment of memory and visual perception in splenial tumours. Brain. 1991;114(Pt 1B):349–360. doi: 10.1093/brain/114.1.349. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M. Differences in white matter architecture between musicians and non-musicians: a diffusion tensor imaging study. Neurosci Lett. 2002;321:57–60. doi: 10.1016/s0304-3940(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. 2004;126B:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Slinker BK, Glantz SA. Multiple regression for physiological data analysis: the problem of multicollinearity. Am J Physiol. 1985;249:R1–12. doi: 10.1152/ajpregu.1985.249.1.R1. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Hackney DB, Zhang G, Wehrli SL, Wright AC, O’Brien WT, Uematsu H, Wehrli FW, Selzer ME. Magnetic resonance microimaging of intraaxonal water diffusion in live excised lamprey spinal cord. Proc Natl Acad Sci U S A. 2002;99:16192–16196. doi: 10.1073/pnas.252249999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steen K, McQueen MB, Herbert A, Raby B, Lyon H, Demeo DL, Murphy A, Su J, Datta S, Rosenow C, Christman M, Silverman EK, Laird NM, Weiss ST, Lange C. Genomic screening and replication using the same data set in family-based association testing. Nat Genet. 2005;37:683–691. doi: 10.1038/ng1582. [DOI] [PubMed] [Google Scholar]
- Visscher PM. Sizing up human height variation. Nat Genet. 2008;40:489–490. doi: 10.1038/ng0508-489. [DOI] [PubMed] [Google Scholar]
- Vonesh EF, Carter RL. Mixed-effects nonlinear regression for unbalanced repeated measures. Biometrics. 1992;48:1–17. [PubMed] [Google Scholar]