The combination of a candidate gene approach, column chromatography, and mass spectrometry identifies several fibroblast-derived proteins essential for endothelial cell sprouting and lumen formation. Furthermore, proteins responsible for EC lumen formation increase matrix stiffness, which correlates with EC lumenogenesis.
Abstract
A role for fibroblasts in physiological and pathological angiogenesis is now well recognized; however, the precise mechanisms underlying their action have not been determined. Using an in vitro angiogenesis model in combination with a candidate gene approach, column chromatography, and mass spectrometry, we identify two classes of fibroblast-derived factors—one that supports vessel sprouting but not lumen formation, and one that promotes lumen formation. In the absence of fibroblasts a combination of angiopoietin-1, angiogenin, hepatocyte growth factor, transforming growth factor-α, and tumor necrosis factor drives robust endothelial cell (EC) sprouting; however, lumens fail to form. Subsequent addition of fibroblast-conditioned medium restores lumenogenesis. Using small interfering RNA–mediated knockdown, we show that five genes expressed in fibroblasts—collagen I, procollagen C endopeptidase enhancer 1, secreted protein acidic and rich in cysteine, transforming growth factor-β–induced protein ig-h3, and insulin growth factor–binding protein 7—are necessary for lumen formation. Moreover, lumen formation can be rescued by addition of purified protein to knockdown cultures. Finally, using rheology, we demonstrate that the presence of these matricellular proteins results in significantly stiffer gels, which correlates with enhanced lumen formation. These findings highlight the critical role that fibroblast-derived extracellular matrix components play in EC lumen formation and provide potential insight into the role of fibroblasts in the tumor microenvironment.
INTRODUCTION
Angiogenesis is the formation of new blood vessels from the existing vasculature. This process occurs in both physiological conditions, such as embryonic development and wound healing (Karamysheva, 2008), and pathological conditions, such as tumor growth (Folkman, 1975). On binding of growth factors such as vascular endothelial growth factor (VEGF), endothelial cells (ECs) proliferate, migrate, and differentiate to make new blood vessels. Much research has focused on the underlying genetic changes within the ECs during the course of angiogenesis, but it is becoming increasingly clear that stromal cells such as fibroblasts also play a significant role (Bhowmick et al., 2004; Orimo et al., 2005).
Fibroblasts are largely defined by their location and by what they are not: non–smooth muscle cells, non–endothelial cells, non–epithelial cells of the stroma (Hughes, 2008). No reliable marker for fibroblasts exists, and one gene analysis study revealed that fibroblasts are quite different cells, depending on their tissue of origin (Chang et al., 2002), although their primary function in all cases is the synthesis and maintenance of the extracellular matrix (ECM). In response to wounding and during tumor growth, fibroblasts become activated, secreting various collagens, fibronectin, heparan sulfate proteoglycans, secreted protein acidic and rich in cysteine (SPARC), tenascin, and connective tissue growth factor, among others (Chang et al., 2004; Sato et al., 2004).
Apart from, and in conjunction with, their role as synthesizers and modifiers of the ECM, fibroblasts play a key role in angiogenesis. Numerous studies have shown that these cells secrete soluble angiogenic growth factors such as VEGF (Fukumura et al., 1998; Kellouche et al., 2007), transforming growth factor-β (TGF-β; Paunescu et al., 2011), and platelet-derived growth factor (PDGF; Antoniades et al., 1991), to name a few. It is also evident that fibroblasts—more specifically, carcinoma-associated fibroblasts (CAFs)—promote tumor growth partially through promotion of angiogenesis. One study showed that inhibition of stromal PDGF receptors reduced tumor angiogenesis in part by suppressing the expression of fibroblast growth factor-2 (FGF-2) and FGF-7 in CAF (Pietras et al., 2008).
A number of in vitro studies examined the role of fibroblasts in EC tubulogenesis (Montesano et al., 1993; Nakatsu et al., 2003; Berthod et al., 2006). All of these studies showed promotion of EC sprouting and lumen formation in their presence. In particular, reducing fibroblast ECM synthesis by removing ascorbate from the medium reduced EC tube formation (Berthod et al., 2006). Another study reported that EC tube formation in three-dimensional (3D) fibrin gels is much more strictly dependent on the distance between the EC and the fibroblasts than on the distance between the EC and the media (Griffith et al., 2005). The implication is that the fibroblast-derived factors required for EC tubulogenesis are poorly diffusible, perhaps because they are large or have strong interactions with the matrix.
Although a number of fibroblast-derived factors important for angiogenesis have been reported, such as those listed earlier, the specific contributions of fibroblasts in the process of angiogenesis remain largely unknown. In the study presented here, we aimed to identify fibroblast-derived factors that impart an angiogenic phenotype in EC. Using an in vitro model of angiogenesis in which fibroblasts and ECs are cocultured in 3D fibrin matrices, we identify a combination of fibroblast-derived proteins that promotes EC sprouting and is necessary for EC lumen formation. Furthermore, we show that the fibroblast-derived proteins necessary for EC lumen formation increase the stiffness of the matrix, shedding light on the possible mechanism of EC lumen formation.
RESULTS
Fibroblasts secrete soluble proteins that support EC sprouting and lumen formation in three-dimensional cocultures
We and others have shown that stromal cells, such as fibroblasts and pericytes, synergize with VEGF to support EC vessel formation in several angiogenic models (Montesano et al., 1993; Tille and Pepper, 2002; Velazquez et al., 2002; Nakatsu et al., 2003; Stratman et al., 2009). Using our previously described model (Nakatsu et al., 2003) in which ECs sprout into fibrin gels in response to VEGF and fibroblast-derived factors, we investigated the nature of the fibroblast signal.
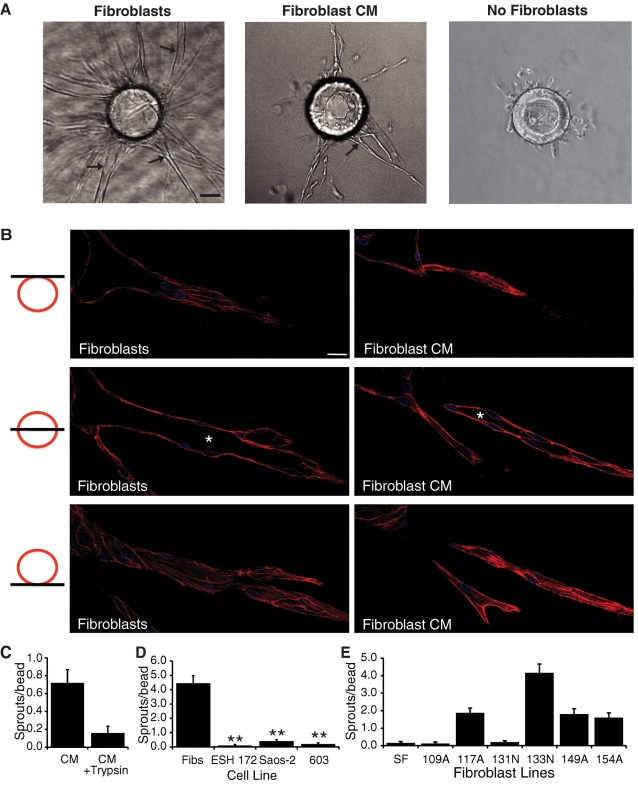
In the presence of cocultured fibroblasts, EC sprouting is robust, and most sprouts form intercellular lumens within 4–5 d (Figure 1, A and B). In contrast, if fibroblasts are not present, single ECs migrate off the beads and do not form vessels, and most cells die after 4–5 d (Figure 1A). Of importance, although fibroblasts in these assays secrete 5–10 pg/ml VEGF (unpublished data), the presence of VEGF does not substitute for fibroblasts, as the medium contains severalfold higher concentrations. Substituting fibroblasts with fibroblast-conditioned medium (CM) in this assay does promote EC sprouting and lumen formation, although at reduced levels (Figure 1, A and B). To examine EC lumens at higher resolution, cultures were stained with 4′,6-diamidino-2-phenylindole (DAPI) and Texas Red-X phalloidin, and z-stack images were taken of single vessels using confocal microscopy. In these images, a lumenal space can clearly be seen (Figure 1B). On the basis of these results, we reasoned that fibroblasts secrete a factor(s) other than VEGF that induces an angiogenic phenotype in the ECs. Preliminary studies suggested that the necessary factor was also not FGF-2 (unpublished data). Incubation of fibroblast-CM with immobilized trypsin almost completely inhibited its ability to support angiogenesis (Figure 1C), indicating that the fibroblast-derived factor(s) is a secreted protein(s).
FIGURE 1:
Fibroblasts and fibroblast-CM support EC sprouting and lumen formation, but tumor cells do not. (A) Representative images of fibrin gel bead assays in the presence or absence of fibroblasts and in the presence of fibroblast-CM. Arrows, EC lumens. Scale bar, 50 μm. (B) Confocal images of EC sprouts in the presence of fibroblasts or fibroblast-CM. Three images in three different z-planes for a single sprout are shown. Asterisks indicate EC lumenal space. Scale bar, 10 μm. (C) Quantification of EC sprouting in the fibrin gel bead assay in the presence of fibroblast-CM or fibroblast-CM treated with trypsin. (D) Quantification of ECs sprouting in the fibrin gel bead assay in the presence of fibroblasts or various tumor cell lines. (E) Quantification of EC sprouting in the fibrin gel bead assay in the presence of various fibroblast lines. Data are shown as mean number of sprouts/bead ± standard error of the mean (SEM; n = 20). *p < 0.05; **p < 0.005.
Stromal cells are present in the tumor microenvironment in vivo (Albini et al., 2010), and it is well known that angiogenesis plays an important role in the growth of large tumors (Folkman, 2002). However, the contribution of stromal cells in promoting angiogenesis and tumor growth is still poorly defined. Of interest, we found that various tumor cell lines, including ESH 172, Saos-2, and HT1080/603, supported little to no EC sprouting or lumen formation (Figure 1D), even though the tumor cells made significantly more VEGF than the fibroblasts and generated strongly angiogenic tumors in vivo (Fujimoto et al., 2004). For example, fibroblasts secrete ∼5 pg/ml VEGF, whereas HT1080/603 cells made greater than 800 pg/ml. A combination of tumor cells and fibroblasts did not enhance the angiogenic response of ECs beyond that seen with fibroblasts alone (unpublished data), likely due to the presence of exogenous VEGF in the medium. Substituting fibroblasts with smooth muscle cells leads to induction of EC sprouting and lumen formation to a similar extent as fibroblasts (A. C. Newman and C. W. Hughes, unpublished data). Finally, we noted that different fibroblast lines varied in their ability to support angiogenesis (Figure 1E).
A combination of angiopoietin-1, angiogenin, hepatocyte growth factor, transforming growth factor-α, and tumor necrosis factor supports EC sprouting but not lumen formation
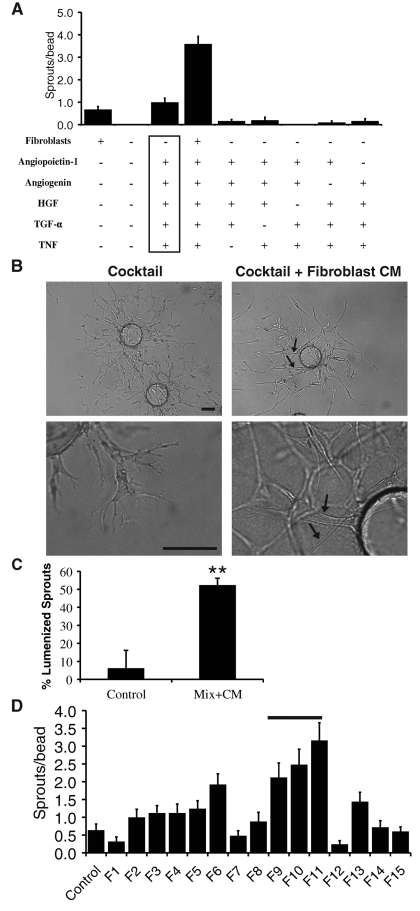
To identify proteins secreted by fibroblasts that induce EC sprouting and lumen formation, we first used a candidate gene approach. To this end, we focused on proteins that were shown to be proangiogenic in previous studies and are known to be expressed by fibroblasts (Supplemental Table S1). Each of these proteins was tested alone and in various combinations. It should be noted that proteins were added to EGM-2. No protein alone could substitute for having fibroblasts present (unpublished data). Combinations of proteins varied in their ability to support EC sprouting, with a combination of angiopoietin-1 (ANG-1), angiogenin, hepatocyte growth factor (HGF), transforming growth factor-α (TGF-α), and tumor necrosis factor (TNF) inducing EC sprouting to the same extent as having fibroblasts present (Figure 2A). There was a greater-than-additive effect when both mix and fibroblasts were present. If any one protein from this combination—referred to hereafter as the angiogenic cocktail—was taken out, sprouting was reduced (Figure 2A). Despite the high level of sprouting, EC sprouts that formed in the presence of the angiogenic cocktail were disorganized, and sprouts failed to form lumens (Figure 2B, left).
FIGURE 2:
A protein mixture supports EC sprouting but not lumen formation. (A) Quantification of EC sprouting with various combinations of fibroblasts and proteins. Angiogenic cocktail is indicated by the box. Data are shown as mean number of EC sprouts/bead ± SEM (n = 20). (B) Representative images of fibrin gel bead assay. Arrow, EC lumen. Scale bar, 100 μm. (C) Quantification of EC lumen formation in the fibrin gel bead assay. Data are shown as mean percentage of lumenized sprouts/bead ± SEM (n = 20). **p > 0.005. (D) Quantification of EC sprouting in the presence of different fibroblast-CM fractions. Bar, fractions that were pooled for active fraction.
We attempted to rescue lumen formation in EC sprouts induced by the angiogenic cocktail by subsequently adding fibroblast-CM. Fibroblast-CM added 3 d after stimulation with the angiogenic cocktail was indeed able to rescue lumen formation (Figure 2, B and C), indicating that sprouts induced by the angiogenic cocktail still retained their ability to form lumens. Thus a combination of fibroblast-derived proteins has been identified that supports robust sprouting but not lumen formation.
Identification of proangiogenic fractions through cation-exchange and size exclusion high-pressure liquid chromatography of fibroblast-CM
The second approach used to identify fibroblast-derived proteins is high-pressure liquid chromatography/mass spectrometry (HPLC/MS). Fibroblast-CM was separated into different fractions on a cation-exchange column and eluted with concentrated salt buffer. From 15 to 20 fractions were collected, dialyzed/concentrated, and tested in the fibrin gel bead assay to identify fractions that retained angiogenic activity in the presence of a suboptimal number of fibroblasts. A number of peaks of proangiogenic activity were observed (Figure 2D).
Fractions F9, F10, and F11 were pooled (Figure 2D, bar), and proteins were identified in these fractions by nanoflow liquid chromatography and tandem mass spectrometry (nanoLC-MS/MS) analysis and compared with fractions that did not exhibit high angiogenic activity. Table 1 lists several proteins that were identified in the active fractions only.
TABLE 1:
Proteins identified in high-angiogenic fractions.
| Name | Number of unique peptides | Molecular weight |
|---|---|---|
| Collagen α-1 (I) chain precursor | 19 | 138.9 |
| Sulfhydryl oxidase 1 precursor | 9 | 82.6 |
| Fibronectin precursor | 6 | 262.6 |
| Transforming growth factor-β–induced protein ig-h3 precursor | 9 | 74.7 |
| Collagen α-2 (I) chain precursor | 6 | 129.2 |
| Procollagen C-endopeptidase enhancer 1 precursor | 4 | 48.0 |
| Insulin-like growth factor–binding protein 7 precursor | 4 | 29.1 |
| Laminin subunit gamma-1 precursor | 3 | 177.6 |
| Annexin A2 | 5 | 38.6 |
| Secreted protein acidic and rich in cysteine | 4 | 34.6 |
Fibroblast-derived matrix proteins are necessary for EC lumen formation in the fibrin gel bead assay
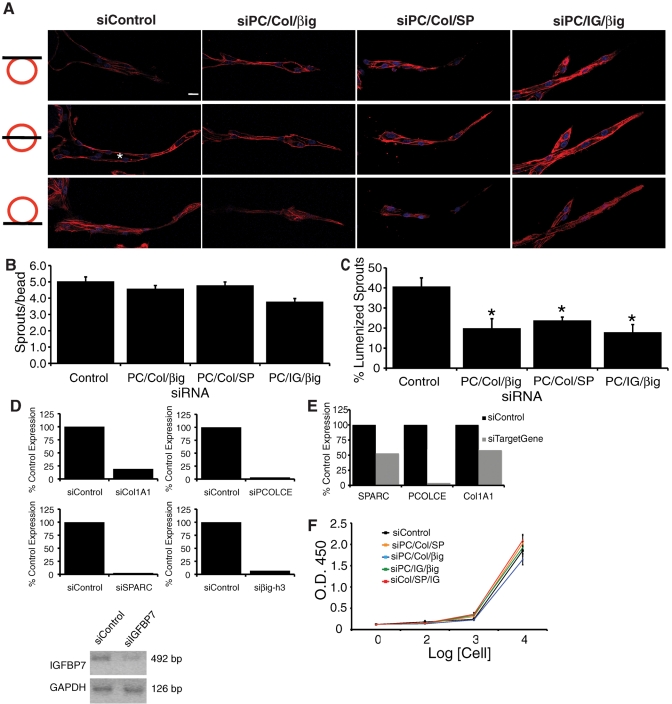
Collagen alpha-1 (I) (Col1A1), procollagen C-endopeptidase enhancer 1 (PCOLCE), transforming growth factor-β–induced protein ig-h3 (βig-h3), insulin-like growth factor–binding protein 7 (IGFBP7), and secreted protein acidic and rich in cysteine (SPARC) were all identified by nanoLC-MS/MS as being present in active CM fractions only (Table 1) and were selected for further analysis. To test whether these proteins were necessary for EC sprout and lumen formation, we knocked down their expression in fibroblasts using small interfering RNA (siRNA) and monitored microvessel formation in the fibrin gel bead assay. Of interest, knocking down various combinations of three of these proteins at the same time in fibroblasts, including PCOLCE/Col1A1/βig-h3, PCOLCE/Col1A1/SPARC, or PCOLCE/IGFBP7/βig-h3, had little to no effect on EC sprouting (Figure 3, A and B) but significantly inhibited the ability of ECs to form lumens (Figure 3, A and C). When cultures are stained with DAPI and Texas Red-X phalloidin, it is clear that ECs in the presence of triple-knockdown fibroblasts lack lumenal spaces and instead exist in a single plane of migrating ECs (Figure 3A). Knockdown of each gene was confirmed by quantitative real-time PCR (RT-PCR) or semiquantitative PCR, in cells treated with either a single siRNA or siRNA to three genes. When targeted individually, knockdown was greater than 80% (Figure 3D). Even when three genes were targeted with siRNAs in the same fibroblasts, knockdown was near 50% for each gene (Figure 3E), in line with an ∼50% decrease in lumen formation in triple-knockdown cultures. Fibroblast cell viability was unchanged by any of the combinations of siRNAs, used as determined by a tetrazolium hydroxide (XTT) assay (Figure 3F).
FIGURE 3:
Collagen 1, PCOLCE, SPARC, IGFBP7, and βig-h3 are required for EC lumen formation. (A) Three confocal images in three different z-planes for a single sprout. Asterisks, EC lumenal space. Scale bar, 10 μm. Quantification of EC sprouting (B) and lumen formation (C) in the fibrin gel bead assay in the presence of fibroblasts treated with control siRNA or siRNA targeted to the indicated genes. (D) Relative mRNA levels of targeted gene in fibroblasts treated with the indicated siRNA. (E) Relative mRNA levels of the indicated gene in fibroblasts treated with siRNAs to Col1A1, PCOLCE, and SPARC. (F) XTT cell viability assay. Results in B and C are shown as mean number of EC sprouts/bead or mean percentage of lumenized sprouts/bead, as indicated, ± SEM (n = 60). *p < 0.05.
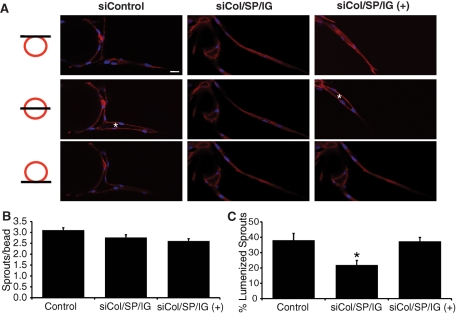
Clearly these genes are working in combination, as knockdown of each gene individually had no effect on EC sprouting or lumen formation (unpublished data). Knockdown of PCOLCE, Col1A1, SPARC, IGFBP7, and βig-h3 in pairs again had little to no effect on EC sprouting, but the double-siRNA combinations of SPARC/Col1A1, IGFBP7/PCOLCE, IGFBP7/βig-h3, and PCOLCE/βig-h3 all significantly reduced EC lumen formation (Supplemental Figure S1). Together, these results indicate that fibroblast-derived PCOLCE, Col1A1, SPARC, IGFBP7, and βig-h3 are required for EC lumen formation in the fibrin gel bead assay.
Inhibition of EC lumen formation is rescued by addition of exogenous proteins
To confirm a role for these proteins in EC lumen formation, we attempted to rescue lumen formation in cultures containing triple-siRNA–treated fibroblasts by adding back the missing proteins. As expected, fibroblasts deficient in the production of Col1A1, SPARC, and IGFBP7 induced significantly less EC lumen formation while having no effect on EC sprouting (Figure 4). When purified collagen 1, SPARC, and IGFBP7 were added to a subset of wells containing Col1A1/SPARC/IGFBP7–deficient fibroblasts at concentrations of 100 μg/ml, 500 ng/ml, and 50 ng/ml, respectively, we saw rescue of EC lumen formation (Figure 4, A and C). It is important to note that these concentrations are similar to what is seen in vivo and what was used in previous in vitro studies (Fligiel et al., 2003; Sato et al., 2003; Kutsukake et al., 2008). Thus the absence of EC lumen formation seen in the presence of knockdown fibroblasts is due to the absence of the target proteins collagen 1, SPARC, and IGFBP7 and not to off-target effects.
FIGURE 4:
Addition of exogenous proteins rescues EC lumen formation. (A) Representative confocal images of EC sprouts and lumens in the presence of fibroblasts treated with control siRNA or siRNAs targeted to the indicated genes are shown in the presence or absence of exogenous proteins. Three images in three different z-planes for a single sprout are shown. Asterisks, EC lumenal space. Scale bar, 10 μm. Quantification of EC sprouting (B) and lumen formation (C). Data are shown as mean number of EC sprouts/bead or mean percentage of lumenized sprouts/bead, as indicated, ± SEM (n = 60). *p < 0.05.
Fibroblast-derived proteins increase the stiffness of the matrix
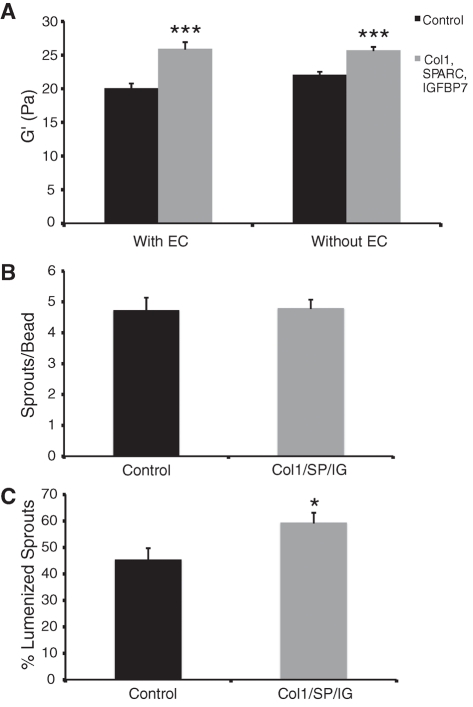
Because the proteins identified as being required for EC lumen formation are all components or modifiers of the ECM, we reasoned that the presence or absence of these proteins might be altering the physical properties of the matrix. To test this, we measured fibrin gel stiffness using rheology. Fibrin gels (2.5 mg/ml) were made in the presence or absence of ECs. Purified collagen 1 (100 μg/ml), SPARC (500 ng/ml), and IGFBP7 (50 ng/ml) were added to a subset of wells, the concentrations being chosen on the basis of known in vivo concentrations. An equal concentration of bovine serum albumin (BSA) was added to control wells to keep total protein concentration consistent. In the presence of collagen 1, SPARC, and IGFBP7, gel stiffness, as measured by shear storage modulus (G′), was increased regardless of whether ECs were present (Figure 5A). Shear loss modulus (G′′) was unchanged between conditions (Supplemental Figure S2A).
FIGURE 5:
Addition of exogenous collagen 1, SPARC, and IGFBP7 increase matrix stiffness. (A) Shear storage modulus of 2.5 mg/ml gels with collagen 1, SPARC, and IGFBP7 or BSA added. Data shown as mean ± SEM (n = 8). ***p < 0.0005. Quantification of EC sprouting (B) and lumen formation (C) in the fibrin gel bead assay in the presence of collagen 1, SPARC, and IGFBP7 or BSA. Data shown as mean ± SEM (n = 30). *p < 0.05.
To determine what effect the addition of collagen 1, SPARC, and IGFBP7 might have on EC sprouting and lumen formation, the fibrin gel bead assay was performed in the presence of these proteins or BSA at the same concentrations as were used for rheology testing. As expected, EC sprouting was unchanged by the addition of these proteins (Figure 5B); however, EC lumen formation was significantly increased in the presence of collagen 1, SPARC, and IGFBP7 (Figure 5C). Thus increased EC lumen formation correlates with increased matrix stiffness.
It is well known that fibrillar collagen can increase matrix stiffness and both PCOLCE and SPARC can aid in collagen 1 processing. Therefore we asked whether the addition of high concentrations of collagen 1 could recover EC lumen formation in the presence of fibroblasts treated with combinations of the siRNAs used in previous studies. We saw no consistent recovery of EC lumen formation even at concentrations of collagen 1 as high as 1.0 mg/ml (Supplemental Figure S2B).
DISCUSSION
In this study, we identified a combination of fibroblast-derived proteins that induce EC sprouting and are necessary for EC lumen formation. Our studies also suggest that collagen 1, PCOLCE, SPARC, IGFBP7, and βig-h3 induce EC-lumen formation in part by increasing the stiffness of the ECM.
We showed that substituting fibroblasts with an angiogenic cocktail consisting of ANG-1, angiogenin, HGF, TGF-α, and TNF induces robust EC sprouting. However, sprouting is disorganized, and the sprouts fail to form lumens. It is our hypothesis that the lack of EC lumen formation seen under these conditions is due to the absence of a complex, stiff ECM when fibroblasts are not present in the cultures. This hypothesis is supported by the fact that replacing the angiogenic cocktail with fibroblast-CM after 3 d in the fibrin gel bead assay rescues EC lumen formation. Furthermore, we showed that collagen 1, PCOLCE, SPARC, βig-h3, and IGFBP7 are required for EC lumen formation in EC-fibroblast cocultures. Collagen 1, PCOLCE, SPARC, IGFBP7, and βig-h3 are all components or modifiers of the ECM, which is recognized to provide more than structural support for cells (Davis and Senger, 2008; Hynes, 2009), and we showed that addition of collagen 1, SPARC, and IGFBP7 increases the stiffness of the matrix. In line with our findings, previous studies showed that increased matrix stiffness, as measured by shear storage modulus, correlates with increased vessel diameter (Critser et al., 2010). Our studies indicate that matrix stiffness is important for the formation of EC lumens.
Many cell types, including ECs, have been shown to respond to changes in matrix stiffness in both 2D and 3D matrices. Recently, matrix stiffness has been shown to regulate EC branching morphogenesis (Myers et al., 2011) and epithelial cell proliferation (Kim and Asthagiri, 2011). ECs integrate signals from the ECM by membrane-bound integrins. A number of signaling molecules downstream of integrins are known to be essential for EC lumen formation, including Src and FAK, and Rho GTPases such as Cdc42 and Rac1 (Ilic et al., 2003; Liu and Senger, 2004; Bryan and D'Amore, 2007; Koh et al., 2008). Indeed, integrin signaling was shown to be altered by substrate stiffness (Friedland et al., 2009).
The exact mechanism by which these proteins alter matrix stiffness remains unclear. Collagens are synthesized and secreted as highly soluble precursors that require processing by procollagen C-endopeptidase in order to form self-assembling collagen monomers (Li et al., 1996). The main role of PCOLCE, as its name indicates, is to aid in the processing of collagen fibrils, most likely by binding to and presenting procollagen as a substrate to procollagen C-endopeptidase (Kronenberg et al., 2009). Subsequent cross-linking of processed collagen increases insoluble matrix deposition and matrix stiffness (Payne et al., 2007).
Like PCOLCE, SPARC is implicated in ECM assembly and, more specifically, collagen processing and deposition (Bradshaw, 2009). SPARC binds to collagen 1 (Hohenester et al., 2008), and the skin of SPARC-null mice has roughly half the amount of collagen in comparison to wild-type skin (Bradshaw et al., 2003). It is possible that fibroblasts deficient in the production of both collagen 1 and SPARC will have considerable reductions in fibrillar collagen deposition, significantly reducing the stiffness of the ECM.
The role of βig-h3 and IGFBP7 in matrix assembly is less clear. βig-h3 binds to collagen 1 (Hashimoto et al., 1997), and this interaction could alter the interactions of collagen 1 with other components of the ECM. IGFBP7 is highly up-regulated in tumor-associated endothelium relative to normal blood vessels, although the mechanism is poorly understood (St Croix et al., 2000; Pen et al., 2007). Our data indicate that up-regulation of IGFBP7 in tumors (either in the ECs themselves or in associated fibroblasts) might be a critical step in the induction of EC lumenogenesis in tumor vasculature.
Our studies do not rule out the possibility that these proteins directly interact with the ECs. There is much evidence that collagen 1 is an excellent scaffold for angiogenesis (Montesano et al., 1983; Nicosia and Ottinetti, 1990; Davis and Camarillo, 1996), and in line with these findings, a blocking antibody to α2β1-integrin inhibited EC lumen formation in the fibrin gel bead assay in the absence of exogenous collagen 1 (Supplemental Figure S3). βig-h3 binds to αvβ3 integrin on the surface of ECs (Nam et al., 2003), and αvβ3-integrin is critical for angiogenesis (Brooks et al., 1994). Of interest, one study showed that inhibiting the function of αvβ3-integrin disrupted vasculogenesis in quail embryos by inhibiting lumen formation (Drake et al., 1995), whereas a second study showed that inhibition of αvβ3-integrin inhibited EC lumen formation in 3D fibrin gels (Bayless et al., 2000). Further studies need to be done to determine whether interaction of these proteins with EC is important for lumen formation.
Previous studies in our lab, using a short-term (24 h) collagen gel assay in the absence of fibroblasts, showed that knockdown of βig-h3 in ECs reduced their ability to form tubes (Aitkenhead et al., 2002). In contrast, knockdown of EC-expressed βig-h3 in the fibrin gel bead assay did not reduce lumen formation (unpublished data). Our interpretation is that the overall amount of βig-h3 made by EC in collagen gels is sufficient to support some tube formation over the short term, but in long-term cultures in fibrin gels, fibroblast-derived βig-h3 is essential.
It is worth noting that the expression level of each of these proteins has been tested in EC-CM as well as fibroblast-CM, and all are generally expressed at much higher levels in fibroblasts than in EC (A. C. Newman and C. W. Hughes, unpublished data). It is also apparent that these proteins are acting in concert, as knockdown of each gene alone had no effect on the angiogenic response of the ECs. Moreover, addition of collagen 1 alone, even at concentrations as high as 1.0 mg/ml, was not able to consistently recover EC lumen formation in the presence of fibroblasts treated with the combinations of siRNAs tested in this study (Supplemental Figure S2B). Therefore, although many of these proteins participate in the processing of collagen 1, it appears that their effects on EC lumen formation are not mediated solely by collagen 1 processing.
It seems likely that we have not identified the complete set of fibroblast-derived proteins that play an important role in angiogenesis, as combinations of the proteins identified here are not sufficient for induction of EC lumen formation in the absence of fibroblasts (unpublished data). Indeed, fractions from the CE columns were initially screened for those that induced sprouts; however, the proteins in these fractions that we have pursued regulate lumen formation and not sprouting. We therefore suspect that there are other proteins yet to be identified in these fractions that promote sprouting, and we are working to identify these.
Finally, our data provide an explanation for the necessary role of fibroblasts in the promotion of angiogenesis in the tumor microenvironment. A critical role for CAF in tumor angiogenesis was shown in a number of studies (Orimo et al., 2005; Maeda et al., 2006; Guo et al., 2008), but it remains challenging to tease out the specific contributions these cells make. Our data suggest that a critical role of fibroblasts in the tumor microenvironment is to condition the ECM in a manner that allows ECs, when stimulated by growth factors derived from both the stromal and tumor cells present in the tumor, to form functional lumens. This is partly accomplished by the secretion of collagen 1, PCOLCE, SPARC, βig-h3, and IGFBP7.
MATERIALS AND METHODS
Cell lines and tissue culture
Primary human umbilical vein ECs (HUVECs) were isolated from umbilical cords obtained from local hospitals under University of California, Irvine, Institutional Review Board approval. HUVECs were cultured in M199 (GIBCO, Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS) and endothelial growth supplement (BD Biosciences, San Diego, CA). Normal human lung fibroblasts (NHLFs) were purchased from Lonza (Basel, Switzerland) and cultured in M199 with 10% FBS. The fibroblast lines SF, 109A, 117A, 131N, 133N, 149A, and 154A were a kind gift from Cory Hogaboam (University of Michigan, Ann Arbor). The breast cancer cell line ESH 172 was a kind gift from Randy Holcomb (University of California, Irvine). The osteosarcoma cell line Soas-2 was a kind gift from Bang Hoang (University of California, Irvine). The fibrosarcoma cell line HT-1080/603 was a kind gift from Eric Stanbridge (University of California, Irvine).
Fibrin gel bead assay
The 3D in vitro model of angiogenesis was performed as described previously (Nakatsu and Hughes, 2008). In brief, p3 HUVEC were cultured on collagen-coated Cytodex microcarrier beads (Sigma-Aldrich, St. Louis, MO). For studies involving protein mixtures instead of NHLFs, proteins were mixed at indicated concentrations (Supplemental Table S1) in EGM-2, which contains VEGF and other proangiogenic factors, and added to fibrin gels every other day of the assay. For studies using fibroblast-CM, CM was mixed at a 1:1 ratio with EGM-2 and added every day. For studies involving collagen 1 addition, collagen 1 was added to the fibrin gel at the indicated concentrations before clotting. The α2β1 antibody was purchased from Chemicon (Temecula, CA) and diluted in EGM-2; it was added to the media at day 4 of the assay and every day thereafter at a concentration of 5 μg/ml.
For quantification of sprouting, only sprouts whose lengths were greater than or equal to the diameter of the bead were counted. Lumen formation was quantified by counting the number of sprouts that had formed lumens.
Preparation of fibroblast-CM
NHLFs in M199 containing 10% FBS were allowed to grow to 80% confluence. Medium was replaced with EGM-2 for 1 d and then replaced with serum-free EGM-2. CM was harvested and filtered through a 0.22-μm filter 2 d later.
Cation-exchange HPLC
A 200-ml amount of CM from NHLF was applied to a 1-ml SP Sepharose FF cation-exchange column preequilibrated with 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 5.6. After complete loading, the column was washed with equilibration buffer, and then proteins were eluted with a 2 M NaCl gradient. Twenty 1-ml fractions were collected. Fractions were then dialyzed against Hank's balanced salt solution 1× media (Mediatech, Manassas, VA) and filtered.
MS analysis
In-solution trypsin digestion and dimethyl labeling.
A 0.5-ml aliquot of each sample was adjusted to 50 mM of triethylammonium bicarbonate (Sigma-Aldrich) and 0.5% sodium deoxycholate, 5 mM Tris 2-carboxyethyl phosphine (5 mM; Thermo Pierce, Rockford, IL) and then incubated at room temperature for 20 min. After treatment with iodoacetamide (10 mM final concentration; Sigma-Aldrich) in the dark at room temperature for 20 min, samples were supplemented with trypsin (Sigma-Aldrich) at a substrate-to-enzyme ratio of 1:50 (wt/wt) and then incubated at 37°C for 4 h. After addition of a second, equivalent aliquot of trypsin, samples were incubated overnight at 37°C. Samples were subjected to phase transfer (Masuda et al., 2008). The resulting peptides were fractionated as described (Ishihama et al., 2006), eluting strong cation exchange with seven salt cuts (20, 35, 50, 70, 100, 500, and 750 mM).
NanoLC-MS/MS.
Salt fractions were analyzed via nanoLC-MS/MS using an LTQ Mass Analyzer (Thermo Fisher Scientific, Rockford, IL) coupled to a Waters 600E HPLC Pump (Waters, Milford, MA) and Famos Autosampler (Dionex, Sunnyvale, CA). C18 materials (MaC-MOD, Chadds Ford, PA) were pressure packed into a laser-pulled nanospray tip (15 cm × 75 μm inner diameter). Mobile phases were 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B) with a flow rate of ∼50 nl/min and an active gradient portion running from 2 to 35% B in A over 220 min. MS data were acquired in profile mode, with the top three precursor ions selected for MS/MS.
Database search and quantitation.
Spectral peak processing used MASCOT Distiller, version 2.3.2.0 (Matrix Science, London, United Kingdom), with LCQ_plus_zoom.opt parameters and a precursor tolerance of 1.2 Da. Searches were against SwissProt (57.1; human taxonomy) with (fixed mod) cysteine carbamiodomethylation and (variable mods) methionine oxidation, along with heavy, intermediate, and light dimethyl label selected for both N-terminus and lysine (Boersema et al., 2009). Homology threshold (expected, 0.05) was applied for protein ID. Spectral quantitation used MASCOT Distiller with simple ratio selected and elution time shift set to 15 s.
Transfection of NHLFs with siRNAs
siRNAs designed to SPARC, IGFBP7, PCOLCE, Col1A1, and TGFBI were obtained from Ambion (Austin, TX). Transfection was performed using Lipofectamine (Invitrogen) following the manufacturer's recommended protocol. Fibroblast viability was measured using an XTT assay.
Quantitative RT-PCR
RNA was isolated at 48 h posttransfection from NHLF using TRIzol (Invitrogen) and the manufacturer's recommended protocol. A total of 3 μg of RNA was used for cDNA synthesis using the iScript cDNA Synthesis kit (Bio-Rad, Madison, WI). All mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase. Primers were synthesized by IDT (San Diego, CA), and sequences can found in Supplemental Table S2.
Mechanical analysis of three-dimensional fibrin gels
Rheology was performed on acellular and cellular (2 × 104 cells/gel) gels using an AR-G2 Rheometer (TA Instruments, New Castle, DE) with a 20-mm-diameter, parallel-plate configuration. The gels were tested at an oscillation frequency of 10 to 0.1.
Microscopy/imaging and statistical analysis
Visualization of fibrin gel bead assays was performed using bright-field images collected on an Olympus (Center Valley, PA) IX70 inverted microscope with a SPOT Idea 3.0-megapixel color mosaic camera and SPOT software (SPOT Imaging Solutions, Sterling Heights, MI). Confocal images were collected using an Olympus FluoView FV1000 confocal microscope. Images were processed in Photoshop (Adobe, San Jose, CA) to adjust contrast and color balance. All images in a given experiment were treated similarly.
Analysis of HUVEC sprouting and lumen formation in fibrin gel bead assays was performed by observers blinded to the experimental conditions. The differences between experimental groups of equal variance were analyzed using Student's t test.
Supplementary Material
Acknowledgments
We thank Cory Hogaboam, Randy Holcomb, Bang Hoang, and Eric Stanbridge for various cell lines used in this study. We also thank Claire Robertson for help with gel mechanical testing. This study was funded by National Institutes of Health Grant RO1 HL60067.
Abbreviations used:
- βig-h3
transforming growth factor-β–induced protein ig-h3
- CAF
carcinoma-associated fibroblast
- CM
conditioned medium
- EC
endothelial cell
- IGFBP7
insulin-like growth factor–binding protein 7
- PCOLCE
procollagen C endopeptidase enhancer 1
- SPARC
secreted protein acidic and rich in cysteine
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-05-0393) on August 24, 2011.
REFERENCES
- Aitkenhead M, Wang SJ, Nakatsu MN, Mestas J, Heard C, Hughes CC. Identification of endothelial cell genes expressed in an in vitro model of angiogenesis: induction of ESM-1, (beta)ig-h3, and NrCAM. Microvasc Res. 2002;63:159–171. doi: 10.1006/mvre.2001.2380. [DOI] [PubMed] [Google Scholar]
- Albini A, Magnani E, Noonan DM. The tumor microenvironment: biology of a complex cellular and tissue society. Q J Nucl Med Mol Imaging. 2010;54:244–248. [PubMed] [Google Scholar]
- Antoniades HN, Galanopoulos T, Neville-Golden J, Kiritsy CP, Lynch SE. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci USA. 1991;88:565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless KJ, Salazar R, Davis GE. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am J Pathol. 2000;156:1673–1683. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthod F, Germain L, Tremblay N, Auger FA. Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol. 2006;207:491–498. doi: 10.1002/jcp.20584. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJ. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat Protoc. 2009;4:484–494. doi: 10.1038/nprot.2009.21. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009;3:239–246. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol. 2003;120:949–955. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Bryan BA, D'Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–2065. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res. 2010;80:23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Cheresh DA, Little CD. An antagonist of integrin alpha v beta 3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci. 1995;108:2655–2661. doi: 10.1242/jcs.108.7.2655. [DOI] [PubMed] [Google Scholar]
- Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: a possible control point in tumor growth. Ann Intern Med. 1975;82:96–100. doi: 10.7326/0003-4819-82-1-96. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Fujimoto A, Onodera H, Mori A, Isobe N, Yasuda S, Oe H, Yonenaga Y, Tachibana T, Imamura M. Vascular endothelial growth factor reduces mural cell coverage of endothelial cells and induces sprouting rather than luminal division in an HT1080 tumour angiogenesis model. Int J Exp Pathol. 2004;85:355–364. doi: 10.1111/j.0959-9673.2004.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC, George SC. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11:257–266. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283:19864–19871. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, et al. Characterization of a cartilage-derived 66-kDa protein (RGD-CAP/beta ig-h3) that binds to collagen. Biochim Biophys Acta. 1997;1355:303–314. doi: 10.1016/s0167-4889(96)00147-4. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Sasaki T, Giudici C, Farndale RW, Bachinger HP. Structural basis of sequence-specific collagen recognition by SPARC. Proc Natl Acad Sci USA. 2008;105:18273–18277. doi: 10.1073/pnas.0808452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CC. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol. 2008;15:204–209. doi: 10.1097/MOH.0b013e3282f97dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Kovacic B, McDonagh S, Jin F, Baumbusch C, Gardner DG, Damsky CH. Focal adhesion kinase is required for blood vessel morphogenesis. Circ Res. 2003;92:300–307. doi: 10.1161/01.res.0000055016.36679.23. [DOI] [PubMed] [Google Scholar]
- Ishihama Y, Rappsilber J, Mann M. Modular stop and go extraction tips with stacked disks for parallel and multidimensional peptide fractionation in proteomics. J Proteome Res. 2006;5:988–994. doi: 10.1021/pr050385q. [DOI] [PubMed] [Google Scholar]
- Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc) 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- Kellouche S, Mourah S, Bonnefoy A, Schoevaert D, Podgorniak MP, Calvo F, Hoylaerts MF, Legrand C, Dosquet C. Platelets, thrombospondin-1 and human dermal fibroblasts cooperate for stimulation of endothelial cell tubulogenesis through VEGF and PAI-1 regulation. Exp Cell Res. 2007;313:486–499. doi: 10.1016/j.yexcr.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Kim JH, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J Cell Sci. 2011;124:1280–1287. doi: 10.1242/jcs.078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- Kronenberg D, Vadon-Le Goff S, Bourhis JM, Font B, Eichenberger D, Hulmes DJ, Moali C. Strong cooperativity and loose geometry between CUB domains are the basis for procollagen C-proteinase enhancer activity. J Biol Chem. 2009;284:33437–33446. doi: 10.1074/jbc.M109.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake M, et al. Circulating IGF-binding protein 7 (IGFBP7) levels are elevated in patients with endometriosis or undergoing diabetic hemodialysis. Reprod Biol Endocrinol. 2008;6:54. doi: 10.1186/1477-7827-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc Natl Acad Sci USA. 1996;93:5127–5130. doi: 10.1073/pnas.93.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Senger DR. Matrix-specific activation of Src and Rho initiates capillary morphogenesis of endothelial cells. FASEB J. 2004;18:457–468. doi: 10.1096/fj.03-0948com. [DOI] [PubMed] [Google Scholar]
- Maeda T, Desouky J, Friedl A. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene. 2006;25:1408–1412. doi: 10.1038/sj.onc.1209168. [DOI] [PubMed] [Google Scholar]
- Masuda T, Tomita M, Ishihama Y. Phase transfer surfactant-aided trypsin digestion for membrane proteome analysis. J Proteome Res. 2008;7:731–740. doi: 10.1021/pr700658q. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983;97:1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Pepper MS, Orci L. Paracrine induction of angiogenesis in vitro by Swiss 3T3 fibroblasts. J Cell Sci. 1993;105((Pt 4)):1013–1024. doi: 10.1242/jcs.105.4.1013. [DOI] [PubMed] [Google Scholar]
- Myers KA, Applegate KT, Danuser G, Fischer RS, Waterman CM. Distinct ECM mechanosensing pathways regulate microtubule dynamics to control endothelial cell branching morphogenesis. J Cell Biol. 2011;192:321–334. doi: 10.1083/jcb.201006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu MN, Hughes CC. An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods Enzymol. 2008;443:65–82. doi: 10.1016/S0076-6879(08)02004-1. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, Carpenter PM, Hughes CC. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and angiopoietin-1. Microvasc Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Nam JO, Kim JE, Jeong HW, Lee SJ, Lee BH, Choi JY, Park RW, Park JY, Kim IS. Identification of the alphavbeta3 integrin-interacting motif of betaig-h3 and its anti-angiogenic effect. J Biol Chem. 2003;278:25902–25909. doi: 10.1074/jbc.M300358200. [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Paunescu V, et al. Tumor-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med. 2011;15:635–646. doi: 10.1111/j.1582-4934.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- Pen A, Moreno MJ, Martin J, Stanimirovic DB. Molecular markers of extracellular matrix remodeling in glioblastoma vessels: microarray study of laser-captured glioblastoma vessels. Glia. 2007;55:559–572. doi: 10.1002/glia.20481. [DOI] [PubMed] [Google Scholar]
- Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su GH, Hruban RH, Goggins M. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene. 2003;22:5021–5030. doi: 10.1038/sj.onc.1206807. [DOI] [PubMed] [Google Scholar]
- Sato N, Maehara N, Goggins M. Gene expression profiling of tumor-stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 2004;64:6950–6956. doi: 10.1158/0008-5472.CAN-04-0677. [DOI] [PubMed] [Google Scholar]
- St Croix B, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tille JC, Pepper MS. Mesenchymal cells potentiate vascular endothelial growth factor-induced angiogenesis in vitro. Exp Cell Res. 2002;280:179–191. doi: 10.1006/excr.2002.5635. [DOI] [PubMed] [Google Scholar]
- Velazquez OC, Snyder R, Liu ZJ, Fairman RM, Herlyn M. Fibroblast-dependent differentiation of human microvascular endothelial cells into capillary-like 3-dimensional networks. FASEB J. 2002;16:1316–1318. doi: 10.1096/fj.01-1011fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.