Abstract
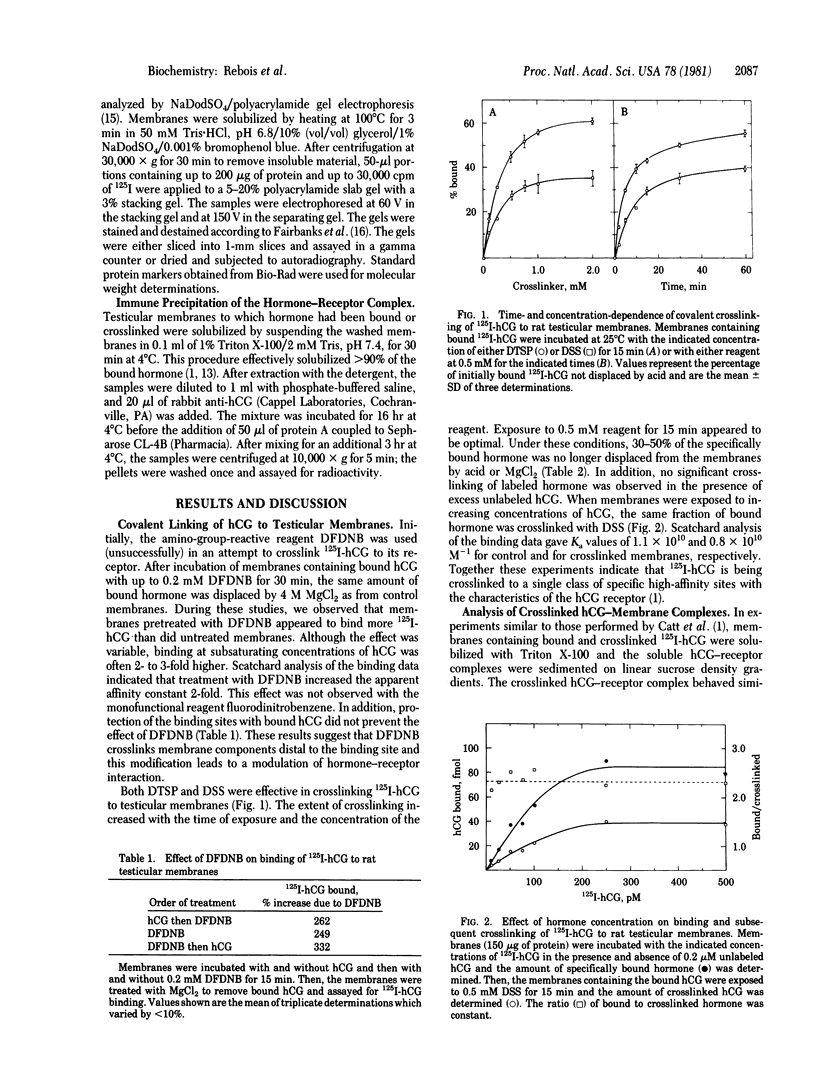
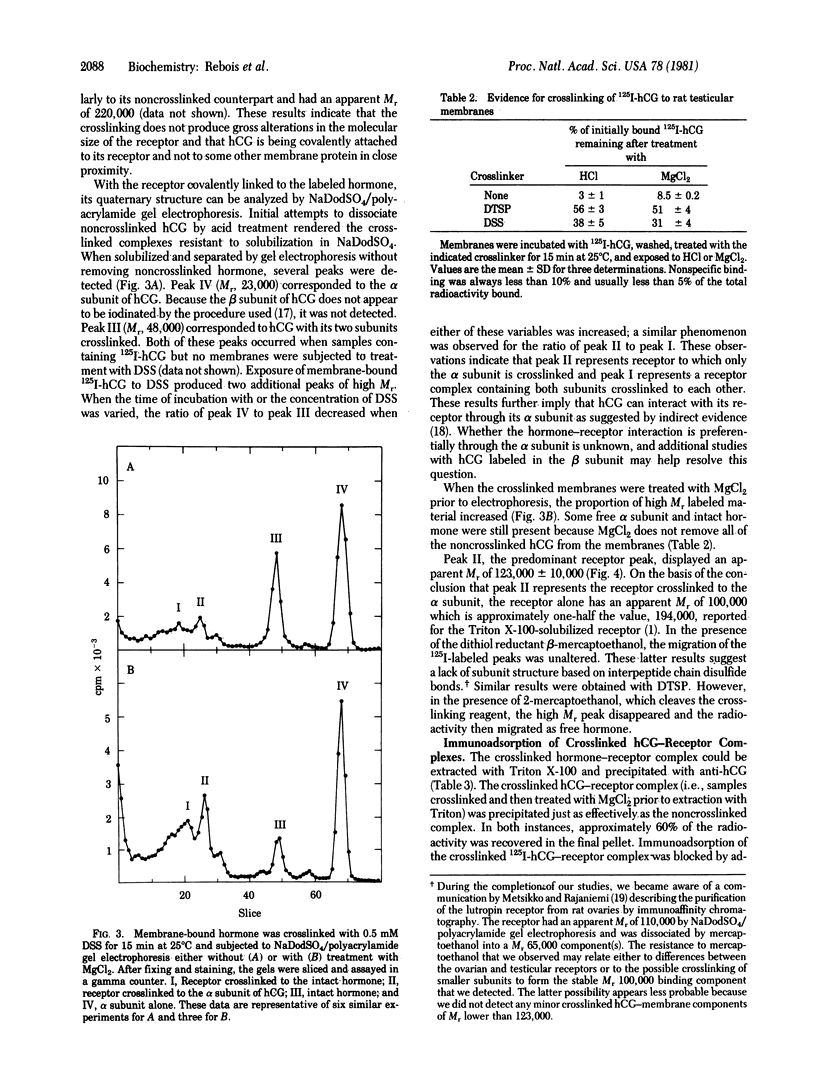
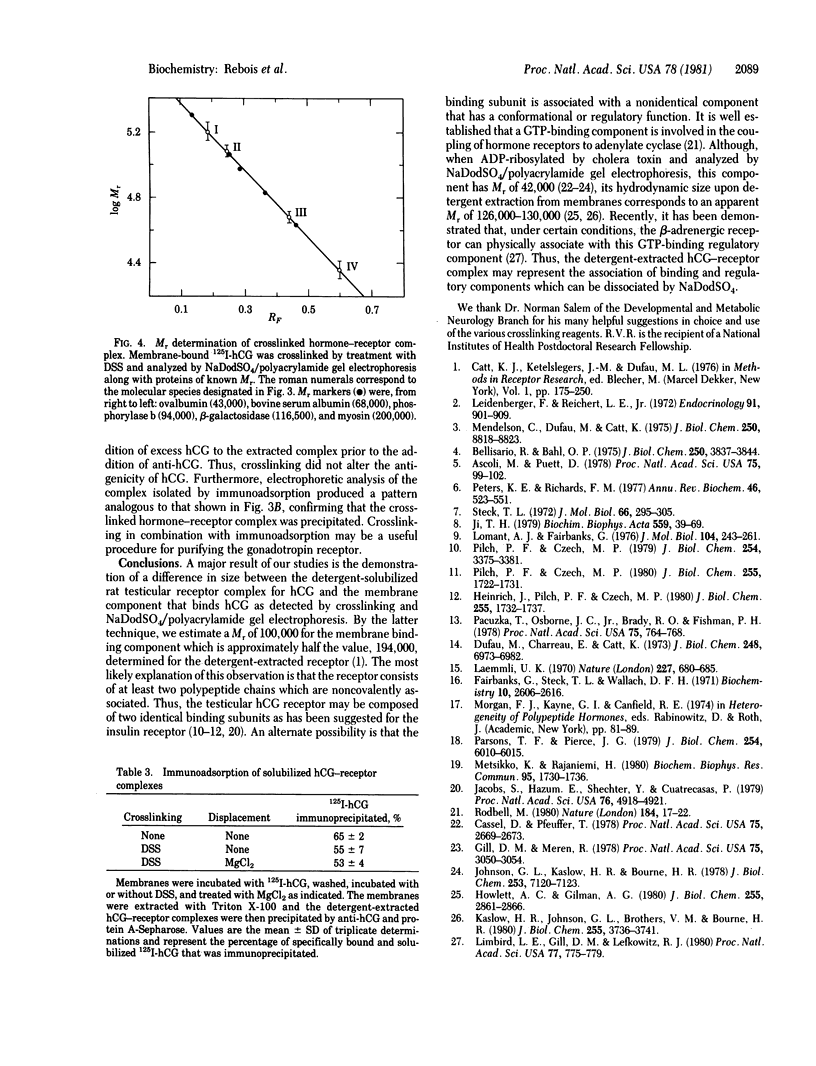
The bifunctional crosslinking reagents disuccinimidyl suberate and dithiobis(succinimidyl propionate) were used to attach 125I-labeled human chorionic gonadotropin (125I-hCG) covalently to rat testicular membranes. The extent of crosslinking was dependent on time and concentration; routinely, 30% of the specifically bound hormone was covalently linked to the membranes in the presence of 0.5 mM crosslinking reagent when incubated at 25 degrees C for 15 min. Excess unlabeled hCG blocked the crosslinking of 125I-hCG to the membranes. When solubilized with Triton X-100 and analyzed by sucrose density gradient centrifugation, both the native and the crosslinked hormone-receptor complex sedimented with an apparent Mr of 220,000. Thus, the receptor itself would have Mr 180,000. When the crosslinked complex was analyzed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis, the predominant species had a Mr of 123,000 and appeared to represent the labeled alpha subunit of hCG covalently linked to a membrane component. The Mr of this receptor component would be 100,000, a value approximately half that of the Triton X-100-solubilized receptor. Thus, the membrane receptor for hCG may consist of a dimer of two binding subunits or a binding subunit associated with one or more additional subunits that might play a coupling or regulatory function.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli M., Puett D. Gonadotropin binding and stimulation of steroidogenesis in Leydig tumor cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):99–102. doi: 10.1073/pnas.75.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellisario R., Bahl O. P. Human chorionic gonadotropin. V. Tissue specificity of binding and partial characterization of soluble human chorionic gonadotropin-receptor complexes. J Biol Chem. 1975 May 25;250(10):3837–3844. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Charreau E. H., Catt K. J. Characteristics of a soluble gonadotropin receptor from the rat testis. J Biol Chem. 1973 Oct 25;248(20):6973–6982. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich J., Pilch P. F., Czech M. P. Purification of the adipocyte insulin receptor by immunoaffinity chromatography. J Biol Chem. 1980 Feb 25;255(4):1732–1737. [PubMed] [Google Scholar]
- Howlett A. C., Gilman A. G. Hydrodynamic properties of the regulatory component of adenylate cyclase. J Biol Chem. 1980 Apr 10;255(7):2861–2866. [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T. H. The application of chemical crosslinking for studies on cell membranes and the identification of surface reporters. Biochim Biophys Acta. 1979 Apr 23;559(1):39–69. doi: 10.1016/0304-4157(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Kaslow H. R., Johnson G. L., Brothers V. M., Bourne H. R. A regulatory component of adenylate cyclase from human erythrocyte membranes. J Biol Chem. 1980 Apr 25;255(8):3736–3741. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leidenberger F., Reichert L. E., Jr Evaluation of a rat testis homogenate radioligand receptor assay for human pituitary LH. Endocrinology. 1972 Oct;91(4):901–909. doi: 10.1210/endo-91-4-901. [DOI] [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Lefkowitz R. J. Agonist-promoted coupling of the beta-adrenergic receptor with the guanine nucleotide regulatory protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1980 Feb;77(2):775–779. doi: 10.1073/pnas.77.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3':5'-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975 Nov 25;250(22):8818–8823. [PubMed] [Google Scholar]
- Metsikkö K., Rajaniemi H. Purification of luteinizing hormone receptor and its subunit structure. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1730–1736. doi: 10.1016/s0006-291x(80)80098-2. [DOI] [PubMed] [Google Scholar]
- Pacuszka T., Osborne J. C., Jr, Brady R. O., Fishman P. H. Interaction of human chorionic gonadotropin with membrane components of rat testes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):764–768. doi: 10.1073/pnas.75.2.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. F., Pierce J. G. Biologically active covalently cross-linked glycoprotein hormones and the effects of modification of the COOH-terminal region of their alpha subunits. J Biol Chem. 1979 Jul 10;254(13):6010–6015. [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]



