Abstract
Purpose
Insulin-like growth factors (IGF) regulate a wide range of biological functions including cell proliferation, differentiation, and apoptosis through paracrine and autocrine mechanisms. Accordingly, the present study analyzed polymorphisms of IGF genes and their impact on the prognosis for patients with colorectal cancer.
Materials and Methods
Four hundred and two consecutive patients with curatively resected colorectal adenocarcinoma were enrolled in the present study. The genomic DNA was extracted from fresh colorectal tissue and 8 polymorphisms of IGF genes determined using a real-time polymerase chain reaction genotyping assay.
Results
Pathologic stages after surgery were as follows: stage 0/I (n=85, 21.1%), stage II (n=147, 36.6%), stage III (n=145, 36.1%), and stage IV (n=25, 6.2%). Multivariate survival analysis including stage, age, site of disease, and carcinoembryonic antigen level showed that the progression-free survival for patients with the IGF2 +1280 GG genotype was slightly better than for the patients with the combined IGF2 +1280 AA and AG genotype (p=0.056), although there was no significant difference in the overall survival. However, the other polymorphisms were not associated with survival.
Conclusion
None of the 8 IGF1 or IGF2 gene polymorphisms investigated in this study were found to be independent prognostic markers for Korean patients with surgically resected colorectal cancer.
Keywords: Colorectal neoplasms, Insulin-like growth factor, Genetic polymorphism, Prognosis
Introduction
Anatomic and pathologic staging is still the most accurate predictor of clinical outcomes in patients with colorectal cancer, enabling physicians to evaluate the benefit of adjuvant chemotherapy for individual patients. However, supplementing standard clinical and pathologic staging with molecular markers would allow a more precise identification of those patients with the highest or lowest risk of relapse following colon cancer surgery. One of the most promising molecular markers that have been investigated in relation to colorectal cancer is the presence of tumor microsatellite instability [1].
In addition to their classical role as endocrine hormones, insulin-like growth factors (IGF) regulate a wide range of biological functions, such as cell proliferation, differentiation, and apoptosis, through paracrine and autocrine mechanisms [2]. Also, the IGF1 receptor-mediated initiation of signal transduction activates important intracellular signal pathways, including the Ras/Raf/mitogen-activated protein kinase and phosphoinositide 3-kinase pathway [3]. IGF1 is a polypeptide that has previously been associated with sporadic colorectal cancer. Numerous in vitro and animal studies of colorectal cancer have implicated IGF1 in cell transformation, tumor growth, metastasis, and poor prognosis [4-7]. In addition, epidemiologic studies have indicated that high plasma IGF1 plays a role in energy balance, which has also been shown to influence risk for colorectal cancer [4,5].
Single nucleotide polymorphisms (SNPs) have already been widely implicated in cancer development, prognosis, and treatment response, yet similar evidence is lacking for IGF genes. Although IGF1 tag SNPs have been associated with circulating IGF1 levels [8], functional polymorphisms that may be mediating these associations have not been identified. A cytosine-adenosine dinucleotide repeat sequence (CA15-22) that resides in the promoter region has been inconsistently associated with serum levels and with risk of colorectal cancer. However, a recent study by Wong et al. [9] reported that a putative regulatory IGF1 in the promoter region is associated with reduced colorectal cancer risk. Furthermore, Zecevic et al. [10] also demonstrated that IGF1 variant genotypes modify risk of a hereditary nonpolyposis colorectal cancer. For pancreatic cancer, IGF1 haplotype and the IGF2 Ex4 -233 C>T polymorphism was also found to be significantly associated with risk of pancreatic cancer [11]. Therefore, given these results, it is possible that SNPs in the IGF genes may play an important role in cancer development and prognosis.
However, no published study has yet investigated SNPs in IGF genes and their relationship to the clinical outcomes of colorectal cancer. Hence, the present study analyzed 8 SNPs of IGF genes and their impact on prognosis for patients with colorectal cancer.
Materials and Methods
1. Study population
All the tissues investigated in this study were obtained from 402 consecutive Korean patients who had undergone a surgical resection between January, 2003 and August, 2006 at Kyungpook National University Hospital (Daegu, Korea). Written informed consent for gene expression analyses was received from all the patients before surgery, and the study approved by the Institutional Research Board at Kyungpook National University Hospital. The diagnosis and staging of the colorectal cancer was assessed according to the World Health Organization (WHO) classifications [12] and tumor, node and metastasis (TNM) classifications set out by the American Joint Committee on Cancer [13].
2. SNP selection
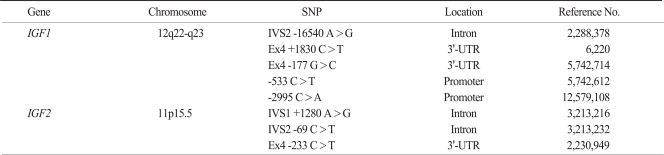
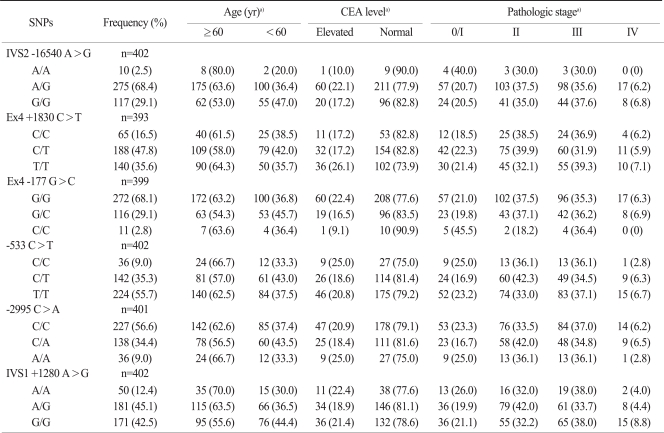
Due to the huge number of SNPs in the human genome, the efficient selection of the SNPs most likely to contribute to phenotypic effects was the first challenge. Thus, a prioritizing strategy was created using public databases that provide diverse information on the potential phenotypic risks of SNPs. Finally, 8 potential functional polymorphisms from the 3'-untranslated region (UTR) and the intron regions were identified (IGF1 -16540 A>G, IGF1 +1830 C>T, IGF1 -177 G>C, IGF1 -533 C>T, IGF1 -2995 C>A, IGF2 +1280 A>G, IGF2 -69 C>T, IGF2 -233 C>T) (Table 1).
Table 1.
Characteristics of examined SNPs
SNP, single nucleotide polymorphism; IGF, insulin-like growth factors; 3'-UTR, 3'-untranslated region.
3. Genotyping of polymorphisms in IGF genes
Genomic DNA was extracted from fresh colorectal mucosal tissue at the time of surgery using the Wizard genomic DNA purification kit (Promega, Madison, WI). The 8 selected polymorphisms of the IGF genes were then determined using a real-time polymerase chain reaction (PCR) genotyping assay. For quality control, genotyping analysis was performed blind. The selected PCR-amplified DNA samples (n=2, for each genotype) were also examined by DNA sequencing to confirm the genotyping results.
4. Statistical analysis
The genotypes for each SNP were analyzed as a three-group categorical variable (referent model), and also grouped according to the dominant and recessive model. The survival estimates were calculated using the Kaplan-Meier method. The differences in overall survival (OS), disease specific survival (DSS) or progression-free survival (PFS) according to the SNPs in the IGF genes were compared using log-rank tests. Cox's proportional hazard regression model was used for the multivariate survival analyses, and the analyses were always adjusted for age (< 60 years vs.≥60 years), site of disease (colon vs. rectum), preoperative carcinoembryonic antigen (CEA) level (normal vs. elevated), and stage (0 to IV). The hazard ratio (HR) and 95% confidence interval (CI) were also estimated. A cut-off p-value of 0.05 was adopted for all the statistical analyses. The statistical data was obtained using an SPSS ver. 11.5 (SPSS Inc., Chicago, IL) or SAS Genetic software (SAS Institute, Cary, NC).
Results
1. Patient characteristics and survival analysis
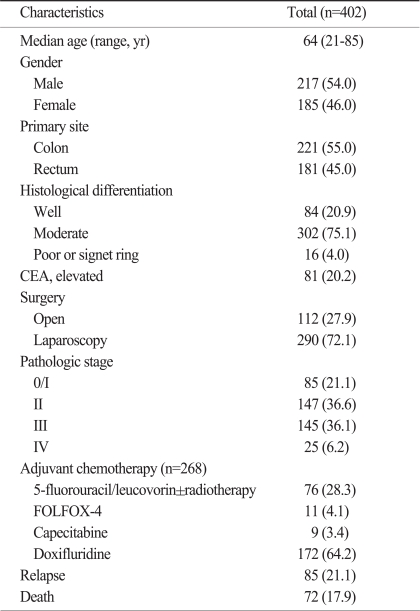
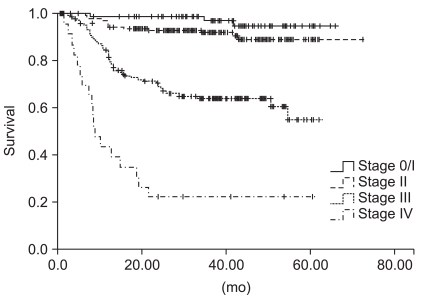
The median age of the patients was 64 years (range, 21 to 85 years), and 217 (54.0%) patients were male. Two hundred and twenty-one (55.0%) patients had colon cancer, whereas the others had rectal cancer. The pathologic stages after surgical resection were as follows: stage 0/I (n=85, 21.1%), stage II (n=147, 36.6%), stage III (n=145, 36.1%), and stage IV (n=25, 6.2%). Among the 291 patients with stage II or III diseases, 268 (92.1%) patients received adjuvant chemotherapy with 6 cycles of 5-fluorouracil/leucovorin±radiotherapy (n=76), 12 cycles of 5-FU/LV+oxaliplatin (FOLFOX) (n=11), 8 cycles of capecitabine (n=9), or doxifluridine for 1 year (n=171) (Table 2). At the time of last analysis, 85 patients had experienced a disease relapse and 72 patients had died. However, the deaths of 11 patients were not related to colorectal cancer. At the median follow-up duration of 37.0 months (range, 0.7 to 65.7 months), the estimated 5-year OS and PFS for all the patients was 70.9±3.7% and 74.4±3.0%, respectively, and the survival rate differed according to the disease stage (p<0.001) (Fig. 1).
Table 2.
Patient characteristics
Values are presented as number (%). CEA, carcinoembryonic antigen; FOLFOX, 5-FU/LV+oxaliplatin.
Fig. 1.
Progression-free survival curves for all patients according to stage (p<0.001).
2. Genotype frequency and effects on survival
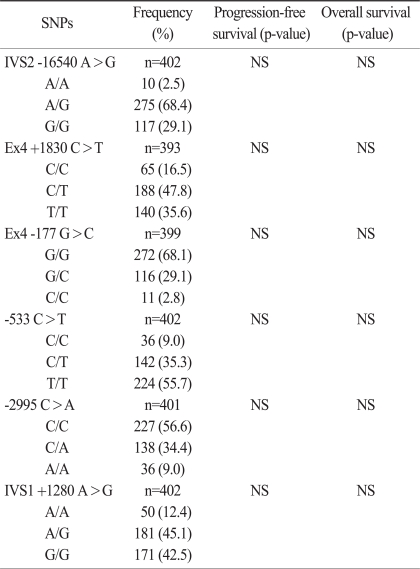
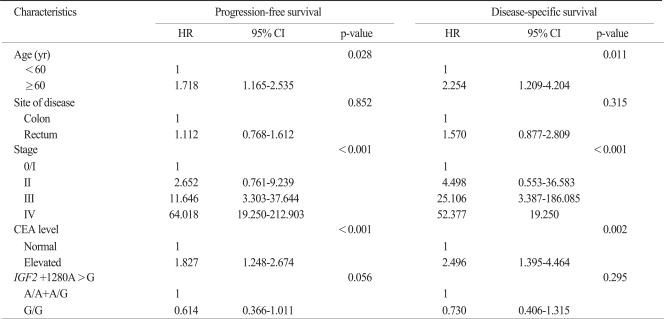
The 8 SNPs of the IGF genes were successfully amplified in more than 95% of the patients. The IGF2 -69C>T and IGF2 -233C>T SNPs were excluded from analysis because their minor allele frequencies were less than 5%. The frequencies of each genotype also conformed to a Hardy-Weinberg equilibrium (p>0.05). There were no sexual differences in relation to any genotype and allele. No correlation was observed between any frequency of the genotype or allele and the T, N, or M stage. In a univariate analysis, PFS or OS was not different according to the SNPs of the IGF genes (Table 3). Multivariate survival analysis including stage, age, site of disease, and CEA level showed that PFS for patients with the IGF2 +1280 GG genotype was slightly better than for patients with combined IGF2 +1280 AA and AG genotype (HR, 0.614; 95% CI, 0.366 to 1.011; p=0.056), although there was no significant difference in the DSS or OS. However, the other polymorphisms were not associated with survival.
Table 3.
Genotype frequencies and univariate survival analysis
SNP, single nucleotide polymorphism; NS, not significant.
In haplotype analysis for IGF1 or IGF2 gene, none of haplotypes associated with prognosis of colorectal cancer. For the clinicopathologic parameters, the age, CEA level, and TNM stage were all significant prognostic factors in a Cox model for PFS or DSS (Tables 4 and 5).
Table 4.
Genotype frequencies and clinicopathologic characteristics
SNP, single nucleotide polymorphism; CEA, carcinoembryonic antigen. a)p-value, not significant.
Table 5.
Multivariate survival analysis
p-values correspond to multivariate Cox model adjusted for age, site of disease, CEA level, and stage. HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; IGF2, insulin-like growth factors 2.
Discussion
The prognostic impact of 8 SNPs of IGF genes was investigated in a large population of patients with surgically resected colorectal adenocarcinoma. However, no association was observed between the polymorphisms in the IGF1 or IGF2 genes and survival in these patients. Given the homogenous ethnic background of Korean patients, any potential confounding effect due to ethnicity is likely to be small in the present study.
Since extensive studies, both in vitro and in vivo, have suggested that IGF promotes cancer growth, prevent apoptosis, and increase metastasis [14-16], it is possible that germ line polymorphisms in IGF genes might alter serum IGF levels, thereby affecting an individual's cancer risk or prognosis. In previous studies, polymorphic variants of IGF1 and elevated serum levels of IGF1 protein have been associated with an increased risk of common cancers including prostate, colorectal, and breast cancers [8,9,17-19], while information on IGF2 polymorphisms and their correlation with cancer risk or prognosis is scarce, and the results that have been published are similarly inconsistent [20,21]. For example, significant associations between the SNPs in the IGF1 promoter region (IGF1 -2995 G>A) and the risk of cancer were found in 298 Chinese patients with colorectal cancer and 1,142 controls [9], suggesting that IGF1 plays a role in colonic carcinogenesis and genetically inherited variation in IGF1 expression influences risk of colorectal cancer. Tsuchiya et al. [22] also reported that IGF1 (CA) repeat polymorphism in the promoter region was associated with prognosis in 111 prostate cancer patients with bone metastasis at the diagnosis. However, these polymorphisms were not found to have any prognostic significance in the survival of the patients with colorectal cancer in the current study. Furthermore, Patel et al. [23] have demonstrated that several genetic variations in IGF1 and IGF binding protein 3 (IGFBP3) predicts circulating levels of IGF1 and IGFBP3, respectively, but no associations between these variations and breast cancer risk. It is thus unlikely that these polymorphisms and their associated hormone levels substantially affect breast cancer risk.
The present study also evaluated SNPs of IGF2 gene, yet none was found to have a significant influence on the prognosis of colorectal cancer. In a previous study by Suzuki et al. [11] that compared the frequency of 6 SNPs of IGF1 and IGF2 in a large-scale case control study to determine whether genetic variations of IGF modify pancreatic cancer risk, the IGF2 3'-UTR Ex4 -233T/T genotype was significantly associated with a reduced risk of pancreatic cancer. In contrast, Lai et al. [20] reported that the polymorphism of IGF2 gene is not likely to contribute to the pathogenesis of prostate cancer or be involved in tumor progression, although the expression of IGF2 and androgen receptors in the prostate suggested that IGF2 plays a role in regulating androgen receptor expression in prostate cancer cells. Thus, given these results, a better understanding of the distinct polymorphisms in IGF genes and protein expression regulation in different cancers will be a critical step toward the clinical utilization of this new subclass of genetic variations in cancer management.
Conclusion
None of the 8 SNPs of the IGF genes investigated in this study was found to be an independent prognostic marker for Korean patients with surgically resected colorectal cancer. However, since genetic polymorphisms often vary between different ethnic groups, further studies are warranted to clarify the association between IGF1 or IGF2 gene polymorphisms and the prognosis of colorectal cancer in diverse ethnic populations.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 2.Fürstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 3.Werner H, Le Roith D. New concepts in regulation and function of the insulin-like growth factors: implications for understanding normal growth and neoplasia. Cell Mol Life Sci. 2000;57:932–942. doi: 10.1007/PL00000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11 Suppl):3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 5.Kaaks R. Nutrition, energy balance and colon cancer risk: the role of insulin and insulin-like growth factor-I. IARC Sci Publ. 2002;156:289–293. [PubMed] [Google Scholar]
- 6.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 7.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 8.Al-Zahrani A, Sandhu MS, Luben RN, Thompson D, Baynes C, Pooley KA, et al. IGF1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF1, IGFBP3 and risk of breast cancer. Hum Mol Genet. 2006;15:1–10. doi: 10.1093/hmg/ddi398. [DOI] [PubMed] [Google Scholar]
- 9.Wong HL, Koh WP, Probst-Hensch NM, Van den, Yu MC, Ingles SA. Insulin-like growth factor-1 promoter polymorphisms and colorectal cancer: a functional genomics approach. Gut. 2008;57:1090–1096. doi: 10.1136/gut.2007.140855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zecevic M, Amos CI, Gu X, Campos IM, Jones JS, Lynch PM, et al. IGF1 gene polymorphism and risk for hereditary nonpolyposis colorectal cancer. J Natl Cancer Inst. 2006;98:139–143. doi: 10.1093/jnci/djj016. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Li Y, Dong X, Hassan MM, Abbruzzese JL, Li D. Effect of insulin-like growth factor gene polymorphisms alone or in interaction with diabetes on the risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3467–3473. doi: 10.1158/1055-9965.EPI-08-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton SR, Aaltonen LA. World Health Organization classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 14.Tricoli JV, Rall LB, Karakousis CP, Herrera L, Petrelli NJ, Bell GI, et al. Enhanced levels of insulin-like growth factor messenger RNA in human colon carcinomas and liposarcomas. Cancer Res. 1986;46(12 Pt 1):6169–6173. [PubMed] [Google Scholar]
- 15.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–1035. [PubMed] [Google Scholar]
- 16.Björndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng I, Stram DO, Penney KL, Pike M, Le Marchand L, Kolonel LN, et al. Common genetic variation in IGF1 and prostate cancer risk in the Multiethnic Cohort. J Natl Cancer Inst. 2006;98:123–134. doi: 10.1093/jnci/djj013. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 19.Canzian F, McKay JD, Cleveland RJ, Dossus L, Biessy C, Rinaldi S, et al. Polymorphisms of genes coding for insulin-like growth factor 1 and its major binding proteins, circulating levels of IGF-I and IGFBP-3 and breast cancer risk: results from the EPIC study. Br J Cancer. 2006;94:299–307. doi: 10.1038/sj.bjc.6602936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai MT, Chen RH, Tsai FJ, Wan L, Chen WC. Glutathione S-transferase M1 gene but not insulin-like growth factor-2 gene or epidermal growth factor gene is associated with prostate cancer. Urol Oncol. 2005;23:225–229. doi: 10.1016/j.urolonc.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Kim YJ, Yoon JH, Kim CY, Kim LH, Park BL, Shin HD, et al. IGF2 polymorphisms are associated with hepatitis B virus clearance and hepatocellular carcinoma. Biochem Biophys Res Commun. 2006;346:38–44. doi: 10.1016/j.bbrc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya N, Wang L, Suzuki H, Segawa T, Fukuda H, Narita S, et al. Impact of IGF-I and CYP19 gene polymorphisms on the survival of patients with metastatic prostate cancer. J Clin Oncol. 2006;24:1982–1989. doi: 10.1200/JCO.2005.02.9439. [DOI] [PubMed] [Google Scholar]
- 23.Patel AV, Cheng I, Canzian F, Le Marchand L, Thun MJ, Berg CD, et al. IGF-1, IGFBP-1, and IGFBP-3 polymorphisms predict circulating IGF levels but not breast cancer risk: findings from the Breast and Prostate Cancer Cohort Consortium (BPC3) PLoS One. 2008;3:e2578. doi: 10.1371/journal.pone.0002578. [DOI] [PMC free article] [PubMed] [Google Scholar]