Summary
Background
Many investigators have suggested that malaria infection predisposes individuals to bacteraemia. We tested this hypothesis with mendelian randomisation studies of children with the malaria-protective phenotype of sickle-cell trait (HbAS).
Methods
This study was done in a defined area around Kilifi District Hospital, Kilifi, Kenya. We did a matched case-control study to identify risk factors for invasive bacterial disease, in which cases were children aged 3 months to 13 years who were admitted to hospital with bacteraemia between Sept 16, 1999, and July 31, 2002. We aimed to match two controls, by age, sex, location, and time of recruitment, for every case. We then did a longitudinal case-control study to assess the relation between HbAS and invasive bacterial disease as malaria incidence decreased. Cases were children aged 0–13 years who were admitted to hospital with bacteraemia between Jan 1, 1999, and Dec 31, 2007. Controls were born in the study area between Jan 1, 2006, and June 23, 2009. Finally, we modelled the annual incidence of bacteraemia against the community prevalence of malaria during 9 years with Poisson regression.
Results
In the matched case-control study, we recruited 292 cases—we recruited two controls for 236, and one for the remaining 56. Sickle-cell disease, HIV, leucocyte haemozoin pigment, and undernutrition were positively associated with bacteraemia and HbAS was strongly negatively associated with bacteraemia (odds ratio 0·36; 95% CI 0·20–0·65). In the longitudinal case-control study, we assessed data from 1454 cases and 10 749 controls. During the study period, the incidence of admission to hospital with malaria per 1000 child-years decreased from 28·5 to 3·45, with a reduction in protection afforded by HbAS against bacteraemia occurring in parallel (p=0·0008). The incidence of hospital admissions for bacteraemia per 1000 child-years also decreased from 2·59 to 1·45. The bacteraemia incidence rate ratio associated with malaria parasitaemia was 6·69 (95% CI 1·31–34·3) and, at a community parasite prevalence of 29% in 1999, 62% (8·2–91) of bacteraemia cases were attributable to malaria.
Interpretation
Malaria infection strongly predisposes individuals to bacteraemia and can account for more than half of all cases of bacteraemia in malaria-endemic areas. Interventions to control malaria will have a major additional benefit by reducing the burden of invasive bacterial disease.
Funding
Wellcome Trust.
Introduction
Invasive bacterial diseases are a major cause of childhood illness and death in sub-Saharan Africa, and are sustained by the high prevalence of contributory risk factors such as HIV infection, malnutrition, and sickle-cell disease.1,2 Similarity in the geographical and seasonal variations of malaria and invasive bacterial diseases, especially those caused by non-typhi Salmonella species, and the occasional detection of malaria and bacterial infections in the same patient has led researchers to suggest that malaria might also be a risk factor for invasive bacterial disease.3–5 Ascertainment of the cause of such an association is difficult, except with a randomised study of a malaria control intervention. In this study, however, we make use of the random population distribution of sickle-cell trait (HbAS), a phenotype that is highly protective against malaria,6,7 to investigate this association in an unbiased manner.
Methods
Study design
We did an individually matched, population-based, case-control study of children admitted to hospital for invasive bacterial disease between 1999 and 2002. We recorded an association between HbAS and protection against bacteraemia after adjustment for major confounders. We speculated that this protection could be mediated by the known protection of HbAS against malaria, which would imply that malaria is a cause of invasive bacterial disease. An alternative explanation was that the recorded protection could be a direct genetic effect, independent of malaria. Because the incidence of malaria in our study setting was decreasing steadily, we were able to distinguish between these two hypotheses by doing a longitudinal case-control study, repeated yearly throughout a 9-year study period. Finally, to show the public health importance of malaria as a risk factor for invasive bacterial disease we estimated the magnitude of the association between the two diseases in a longitudinal study of disease trends.
Study population
The studies were done at Kilifi District Hospital (KDH), Kilifi, Kenya. The hospital admits about 4900 children per year. Since 1998, routine clinical and laboratory data, including assessment of blood films for malaria, have been systematically recorded. All children had blood cultures done on admission to hospital, apart from those admitted for elective procedures or for observation after minor accidents,1 and a sample of blood had been stored at −80°C for a range of study-specific genetic tests.
In September, 2000, the Kilifi Health and Demographic Surveillance System (HDSS) was established in an 891 km2 area around KDH.8 All analyses presented here are restricted to residents of this area. In this system, all births, deaths, and migration events were captured at home visits occurring every 4–6 months. During the study period, insecticide-treated bednets were introduced progressively into the district, and effective combination antimalarial treatment became widely available.9 Haemophilus influenzae type b (Hib) conjugate vaccine was first introduced to the childhood immunisation schedule in November, 2001, and coverage for three doses of vaccine at 12 months was 88% when estimated in 2004.10 In routine antenatal screening at KDH between August, 2004, and December, 2007, HIV prevalence was 4·9% with no evidence of a temporal trend (χ2 test for trend, p=0·3). In 2004, no child received co-trimoxazole or antiretroviral treatment, but, in 2007, 499 children received co-trimoxazole and 144 received antiretroviral treatment. By linear regression, there was no change in mean weight-for-age Z scores by year for all inpatients aged 1–59 months (p=0·5).
Written informed consent was obtained from the parents of all study participants. The studies were approved by the Kenya Medical Research Institute (KEMRI) National Ethical Review Committee.
Study participants and sampling methods
In the matched case-control study, we identified risk factors for invasive bacterial disease. Cases were children, aged 3 months to 13 years, admitted to KDH between Sept 16, 1999, and July 31, 2002, whose blood cultures grew pathogenic bacteria and who had not previously been enrolled. To exclude hospital-associated infections, children who had been discharged from hospital within 14 days before readmission were excluded. We were unable to interview parents of children who died before their blood culture yielded a pathogen and did not recruit these children.
Controls were healthy children individually matched to cases on age, sex, and residential location, and were recruited within 2 weeks of their matched case being identified. For each case, two controls were sought at home in the community. After visiting the household of a case, a study fieldworker spun a pencil on the ground and proceeded in the direction of the pencil point, inquiring at every house in turn until two suitably matched controls were identified. Fieldworkers then used a questionnaire to find out the following information about the controls: age, sex, ethnic group, and level of parents’ education—splenomegaly, BCG scar, foot oedema, anthropometry, and skin or hair changes caused by malnutrition were ascertained later by physical examination at the hospital.
In the longitudinal case-control study, we assessed the relation between HbAS and invasive bacterial disease as malaria incidence decreased. Cases were bacteraemic patients aged 13 years or younger who were admitted to KDH between Jan 1, 1999, and Dec 31, 2007. Children were excluded if they were readmitted within 6 months of a previous episode. Children who died before blood cultures became positive were not excluded. Controls were children born within the study area between Jan 1, 2006, and June 23, 2009, who were sampled systematically during home visits at age 3–11 months as part of a cohort study of genetic susceptibility to infectious disease.2
Laboratory methods
Blood cultures were consistently processed in BACTEC Peds Plus bottles with a BACTEC 9050 automated blood-culture instrument (Becton Dickinson, Oxford, UK). Positive samples were subcultured on standard media by routine microbiological techniques.1 Quality assurance was provided by the UK National External Quality Assessment Service. Blood was examined for malaria parasites by microscopy of Giemsa-stained thick and thin films. Parasite densities were calculated as parasite counts per 500 red blood cells (or per 100 white blood cells) multiplied by the relevant blood-cell concentration measured by Coulter counter (Beckman Coulter, High Wycombe, UK).
In the matched case-control study, sickle haemoglobin (HbS) phenotype was established by electrophoresis. Two ELISA tests were used to identify HIV infections, and discordant results were resolved by PCR, as were positive results from children younger than 18 months. Plasma concentrations of the malaria antigen histidine-rich protein 2 (HRP2) were measured by sandwich ELISA against a known concentration standard;11 HRP2 is secreted by red blood cells infected by Plasmodium falciparum and is detectable in the plasma of adult patients with uncomplicated malaria for 2 or more weeks.12 Leucocyte haemozoin pigment—a specific marker of malaria disease and a stronger predictor of outcome in malaria than parasitaemia13—was detected on malaria blood-films.
In the longitudinal case-control study, DNA from cases was extracted retrospectively from frozen blood samples obtained on admission with Qiagen DNA blood mini kits (Qiagen, Crawley, UK) and typed for HbS by PCR. For controls, capillary blood samples were phenotyped for HbS by high-performance liquid chromatography (Variant Analyzer, BioRad, Hercules, CA) with the β-thalassaemia Short Program (BioRad).
Statistical analysis
The univariate analysis of the matched case-control study was undertaken with classical methods for matched data with variable controls per case.14 In the multivariable model, malaria and malnutrition measures were first analysed as groups, in a hierarchical design, then all variables significant at p<0·1 in first-level analyses were included in a backward stepwise conditional logistic regression.
We analysed data from the longitudinal case-control study with logistic regression, taking account of potential confounders—sex, ethnic group, and geographical division of residence in five strata. The analysis was done separately for each year of clinical surveillance. All the controls were used as the comparison group in each of these yearly analyses. Although this analysis was not adjusted for age, such adjustment would be unlikely to alter the odds ratios (OR) because the prevalence of HbAS does not vary much throughout childhood.
We estimated the yearly incidence of admission with bacteraemia or malaria by dividing the number of cases resident in the Kilifi HDSS study area by the mid-year population. The population size in 1999 was estimated by log linear extrapolation from count data obtained in years 2000–03. Linear trends in the incidence of admission with malaria or bacteraemia were tested with Poisson regression.
The bacteraemia incidence rate ratio (IRR) associated with malaria parasitaemia was estimated by Poisson regression of the yearly incidence of bacteraemia against the yearly prevalence of malaria parasitaemia across 9 years after adjustment by calendar year. The yearly parasite prevalence was estimated in children admitted to hospital with trauma, as previously described.15 The population attributable risk fraction for a given malaria parasite prevalence (P) was calculated for each year as P(IRR – 1) / P(IRR – 1) + 1. Children with Hib bacteraemia were excluded from analyses of incidence trends, Poisson regression, and population attributable risk fraction, but not from the case-control studies.
Role of the funding sources
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Of 340 patients enrolled, we included 292 in the matched case-control analysis (figure 1; webappendix p 5). We enrolled two matched community controls for 236 patients, but could identify and enrol only one matched control for the remaining 56 patients. The mean difference in age between the patients and their matched controls for individuals aged 2–11 months was 1·10 (95% CI 0·4 to 1·79), 12–23 months was 0·62 (−0·18 to 1·43), 24–59 months was −1·22 (−2·78 to 0·34), and ≥60 months was −8·16 (−11·55 to −4·76).
Figure 1.
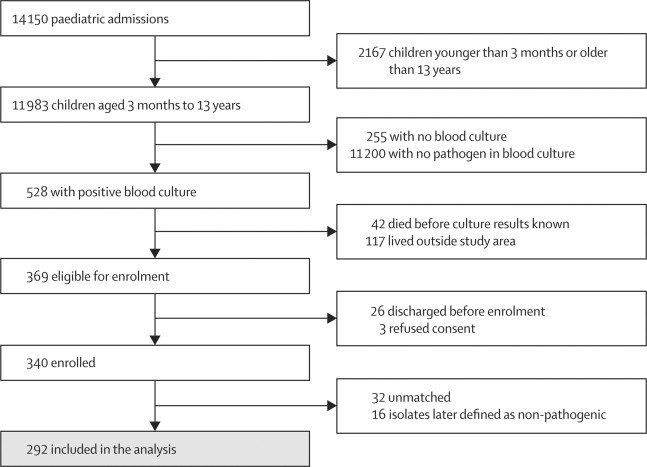
Matched case-control study profile, 1999–2002
The prevalence of HbAS in controls was 17·8% (93 of 522 children). In univariate analyses, HbAS was negatively associated with bacteraemia (table). In the multivariable model, bacteraemia was positively associated with homozygous sickle-cell anaemia (HbSS), HIV, and leucocyte haemozoin pigment (table) and negatively associated with increasing mid-upper arm circumference. After adjustment for these potential confounders, the negative association between HbAS and bacteraemia increased (table). Further adjustment with any of the markers of malaria (any parasitaemia, >10 000 parasites per μL, >50 000 parasites per μL, presence of HRP2, HRP2 concentration >20 ng/mL, HRP2 concentration >44 ng/mL, splenomegaly, or leucocyte haemozoin pigment) did not reduce the OR for HbAS by more than 15% and after inclusion of the strongest malaria marker, leucocyte haemozoin pigment, none of the others made a statistically significant contribution to the final model (webappendix p 9).
Table.
Risk factors for admission to hospital with bacteraemia, 1999–2002
|
Case-control pairs (n) |
Univariate analysis (odds ratio [95% CI]) | Adjusted analysis (odds ratio [95% CI]) | |||
|---|---|---|---|---|---|
| Total | Discordant | ||||
| Sickle-cell phenotype | |||||
| HbSS | 383 | 20 | 15·0 (2·30–97·9) | 22·9 (2·47–211) | |
| HbAS | 483 | 111 | 0·47 (0·29–0·77) | 0·36 (0·20–0·65) | |
| HIV status (positive) | 527 | 132 | 14·6 (7·10–29·8) | 9·03 (3·59–22·7) | |
| Malaria | |||||
| Any parasitaemia | 528 | 219 | 0·53 (0·38–0·76) | .. | |
| Parasitaemia >10 000/μL | 528 | 78 | 0·75 (0·43–1·31) | .. | |
| Parasitaemia >50 000/μL | 528 | 36 | 2·89 (1·25–6·70) | .. | |
| Leucocyte haemozoin pigment | 524 | 111 | 3·28 (2·02–5·31) | 3·52 (1·92–6·47) | |
| Histidine-rich protein-2 >0 ng/mL | 254 | 52 | 3·03 (1·52–6·07) | .. | |
| Histidine-rich protein-2 >20 ng/mL | 251 | 12 | 4·80 (0·99–23·2) | .. | |
| Palpable spleen | 528 | 194 | 2·01 (1·43–2·81) | .. | |
| Malnutrition | |||||
| Weight-for-age Z score ≥−1·8 | 528 | 257 | 0·39 (0·28–0·54) | .. | |
| Height-for-age Z score ≥−1·6 | 528 | 241 | 0·71 (0·52–0·96) | .. | |
| Weight-for-height Z score ≥−1·1 | 528 | 252 | 0·43 (0·31–0·59) | .. | |
| Skin or hair changes caused by malnutrition | 528 | 106 | 5·56 (3·15–9·81) | .. | |
| Mid-upper arm circumference (cm)* | 512 | 243 | 0·54 (0·48–0·62) | 0·57 (0·49–0·66) | |
No other exposure variable had a significant association with bacteraemia in the univariate analysis—these included pedal oedema, BCG scar, maternal ethnic group, paternal ethnic group, and maternal and paternal education level.
The univariate odds ratio for mid-upper arm circumference as a continuous variable was analysed by conditional logistic regression. The distribution of concordant and discordant cells used in the classic analysis are given in the webappendix p 6.
In a repetition of the final model, restricted to cases of non-typhi salmonella and paired controls, the OR for leucocyte-haemozoin pigment was 16·5 (95% CI 3·44–79·3; webappendix p 7). To examine interactions by bacterial species, we stratified cases into four groups: those with Streptococcus pneumoniae (111 children), other Gram-positive bacteraemias (23 children), non-typhi salmonella (61 children), and other Gram-negative bacteraemias (97 children). The prevalence of leucocyte-haemozoin pigment differed between groups; it was 11·7% in children with S pneumoniae, 8·7% in children with other Gram-positive bacteraemias, 32·8% in children with non-typhi salmonella, and 15·5% in children with other Gram-positive bacteraemias (p=0·003). The prevalence of HbAS did not vary significantly between groups (p=0·49, webappendix p 7).
Figure 2 shows the study profile for the longitudinal case-control study and surveillance. A pathogenic bacterium was isolated in 1791 children (webappendix p 8)—116 of which were Hib. 76 210 children aged 13 years or younger lived within the Kilifi HDSS boundary in 1999, increasing to 111 054 in 2007. Between 1999 and 2007, the incidence of admission to hospital with a positive blood-film result per 1000 child-years of observation decreased from 28·5 to 3·45, and admissions with bacteraemia per 1000 child-years of observation decreased from 2·59 to 1·45 (figure 3; webappendix p 8). IRRs per year of surveillance were 0·80 (95% CI 0·79–0·80) for malaria, 0·92 (0·91–0·94) for bacteraemia, 0·89 (0·86–0·91) for Gram-negative bacteraemia, 0·96 (0·94–0·98) for Gram-positive bacteraemia, 0·78 (0·74–0·82) for non-typhi salmonella bacteraemia, and 0·94 (0·91–0·97) for S pneumoniae bacteraemia.
Figure 2.
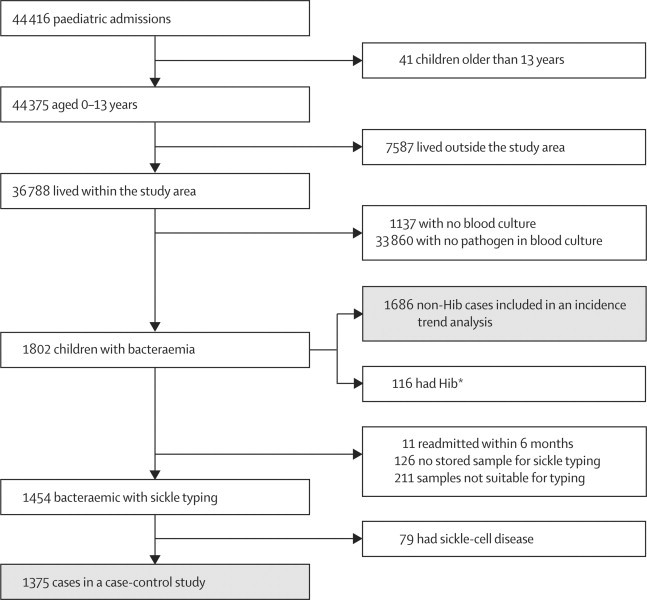
Longitudinal case-control and surveillance profile, 1999–2007
*Cases with Haemophilus influenzae type b (Hib) bacteraemia were excluded from the incidence trend and Poisson regression analyses but included in the longitudinal case-control study.
Figure 3.
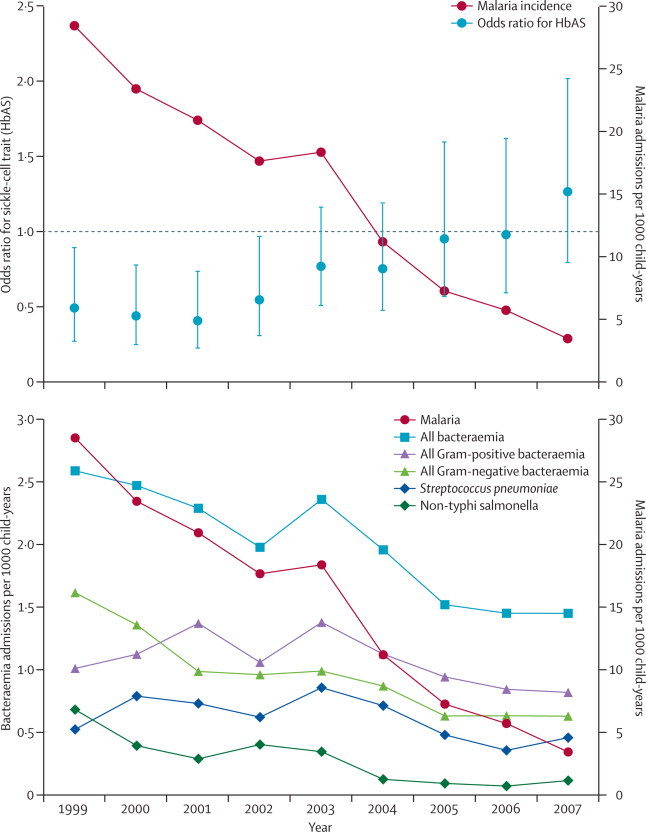
Admissions to Kilifi District Hospital for malaria in children younger than 14 years and (A) odds ratios for HbAS in admissions for bacteraemia and matched community controls or (B) admission to hospital for bacteraemia, 1999–2007
Error bars are 95% CIs.
1454 children were successfully typed for HbS, of whom 154 (11%) had HbAS and 79 (5%) had HbSS (figure 2). Inpatient mortality was much the same between normal (HbAA) bacteraemic children (264 [22%] of 1220) and bacteraemic children with HbAS (39 [25%] of 154; p=0·30). Of 26 351 infants born in the Kilifi HDSS area in the study period, we visited the parents of 18 403 at their homes during the first year of the infant's life—the parents of 10 884 (59%) consented for their infants to be screened; 10 749 were successfully typed for HbS, of whom 1604 (15%) had HbAS and 95 (1%) had HbSS. With these controls as a constant representation of the background prevalence of HbAS, we calculated ORs for HbAS in cases versus controls in each of the years 1999–2007 (figure 3). The OR for HbAS during the period overlapping the matched case-control study (1999–2002) was 0·47 (95% CI 0·35–0·63), which is identical to the unadjusted OR in the matched study. However, in parallel with the decreasing incidence of malaria, the prevalence of HbAS in cases increased significantly by calendar year (χ2 test for trend: p=0·0008), reducing the OR for HbAS to unity.
We explored the pathogen specificity of the association between HbAS and bacteraemia in 12 species groups (figure 4). These analyses were restricted to the period 1999–2005, after which malaria incidence decreased by more than 75% and any association is likely to be diluted. We recorded significant protective associations between HbAS and bacteraemia caused by five gram-negative groups (figure 4). The OR for HbAS and pneumococcal bacteraemia was 0·92 (95% CI 0·67–1·28). However, compared with the background prevalence of HbAS in controls (14·9%), the prevalence in patients with pneumococcal bacteraemia increased from 8·9% (12 of 135 individuals) during 1999–2002 to 19·3% (33 of 138 individuals) during 2004–07 (p=0·01).
Figure 4.
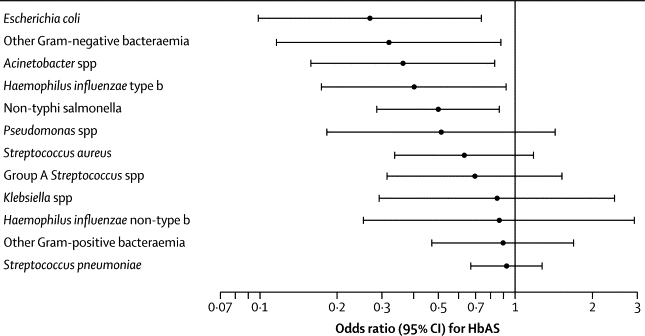
Odds ratio for sickle-cell trait in cases with bacteraemia compared with community controls in the longitudinal case-control study, by type of bacteraemic pathogen (1999–2005)
Children infected with more than one species of bacteraemic pathogen were included in analysis for each species or group.
The IRR for bacteraemia in populations with or without malaria parasitaemia was 6·69 (95% CI 1·31–34·3). This ecological analysis provides an estimate of effect size but does not take account of variation in other risk factors for bacteraemia during the study period. The population attributable risk fraction for malaria as a cause of bacteraemia was 62% (95% CI 8·2–91) in 1999 when the prevalence of parasitaemia in the study population was 29%.
Discussion
The results of our two case-control studies show that HbAS protects against invasive bacterial disease, that this protection is mediated by the known protection of HbAS against malaria, and therefore that malaria is a cause of invasive bacterial disease.
The βs mutation of the β-globin gene is a classic example of a balanced polymorphism in human populations. Children who are homozygous for the βs mutation (HbSS) have sickle-cell anaemia, a disorder associated with childhood mortality of more than 90% in much of Africa.2,16 Conversely, heterozygotes with HbAS have 50% protection against mild malaria and 90% protection against severe malaria.6,7 The βs mutation has therefore been maintained at high frequencies in malaria-endemic areas through a balancing survival advantage.17 Children with HbSS have a pronounced risk of invasive bacterial disease,18 a finding we substantiated in our matched case-control study of children admitted to hospital with bacteraemia. In most studies,19 children with HbSS are compared with all other participants combined, but in our study we differentiated the heterozygous state, HbAS, and noted that HbAS was associated with significant protection against invasive bacterial disease (table).
In a study of bacterial infections in children from the 1960s, Eeckels and colleagues20 noted that the prevalence of HbAS (26 of 196 [13%]) in children presenting with invasive bacterial disease was substantially lower than it was in the local population (23%). They concluded that “although the difference between observed and expected frequencies is not statistically significant, the possibility that sickle-cell trait is linked with a lowered sensitivity to bacterial infections must be considered”.20
Our substantiation of this finding suggested two potential hypotheses. First, that HbAS is independently protective against invasive bacterial disease through genetic pleiotropy or linkage disequilibrium. Second, that the effect of HbAS on such bacterial disease is mediated by protection against malaria and, therefore, that malaria itself is a risk factor for invasive bacterial disease. We originally planned to distinguish between these two hypotheses by repeating the case-control study in a population that had a similar prevalence of HbAS but no malaria. However, because the incidence of hospital admissions for malaria was decreasing sharply on the Kenyan coast, we were able to repeat the study at the same site.
In the longitudinal case-control study we recorded that, in parallel with the decreasing incidence of malaria, the prevalence of HbAS in patients with invasive bacterial disease increased significantly by calendar year, reducing the OR for HbAS to unity. This finding effectively eliminates pleiotropy or linkage disequilibrium as alternative explanations for the protective association between HbAS and invasive bacterial disease. Although the possibility of confounding by factors that we have not considered remains, any factor that could explain this trend would have to satisfy three criteria: it must have increased or decreased steadily between 1999 and 2007, it must be associated with HbAS, and it must be independently associated with the incidence of bacteraemia. Malaria clearly satisfies the first two criteria; previous studies4,21,22 and biological theory suggest that it also satisfies the third.
A plausible biological link between malaria and invasive bacterial disease has previously been suggested, although most work has focused on salmonellosis. First, malaria causes haemolytic anaemia, a mechanism believed to be important in the increased susceptibility to invasive salmonellosis in children with bartonellosis or sickle-cell disease.4,21 Experimentally induced haemolysis also increases susceptibility to salmonellosis in mice.23 Second, accumulation of haemozoin pigment in monocytes impairs diverse macrophage functions, inhibiting expression of intercellular adhesion molecule-1, integrin-CD11c, and MHC class II antigens, delaying differentiation into functional antigen-presenting cells, and stimulating proinflammatory cytokines and chemokines.24 Each of these functions has a role in antibacterial immunity. Third, severe malaria leads to bacterial seeding of the bloodstream because of microvascular parasite sequestration in gut mucosa.25 As noted elsewhere,26 this finding suggests a broader susceptibility to bacteria that colonise the gut, rather than to Salmonella spp in particular.
We explored the effect of malaria on the risk of invasive bacterial disease caused by 12 different bacterial species in the longitudinal case-control study. We recorded significant associations between only gram-negative bacteraemia and HbAS. Use of HbAS as a simple marker of malaria protection might not be valid for pneumococcal bacteraemia because in the absence of malaria HbAS is weakly associated with the risk of pneumococcal disease.27 In children with pneumococcal bacteraemia, we recorded a significant variation in the prevalence of HbAS during periods of high and low malaria parasitaemia. In the absence of malaria, children with HbAS seem to have some of the direct susceptibility to pneumococcal bacteraemia associated with HbSS, but in the presence of malaria this risk is small in relation to their indirect protection against pneumococcal disease because of their reduced susceptibility to malaria.
We used Poisson regression to estimate the magnitude of the association between HbAS and bacteraemia in an ecological study design. Although this analysis is susceptible to secular trends in confounding variables, little variation occurred during the study period in the prevalence of major known confounders, such as HIV and malnutrition. Furthermore, introduction of co-trimoxazole and antiretroviral drugs for children with HIV infection did not occur until after 2004 and were available to only a small proportion of the HIV-infected population.
Our findings provide convincing evidence that malaria predisposes children to the development of invasive bacterial disease (panel), and we estimate the magnitude of this association as an increase in rate of 6·7 times. In Kilifi, bacteraemia is a serious disease with a mortality of 22% despite prompt treatment with intravenous antibiotics and supportive treatment. Mortality associated with invasive bacterial disease is likely to accentuate the survival advantage of the HbAS phenotype and sustain the frequency of the βs mutation in Africa at levels much greater than would have occurred without a high background incidence of invasive bacterial disease. Several lines of evidence already lend support to the idea that malaria indirectly causes deaths normally attributed to other causes. Elimination of malaria from Guyana and Sri Lanka, and control of malaria in Bioko Island, Equatorial Guinea, led to much greater reductions in mortality than were predicted from estimates of malaria-related deaths before the interventions.22,28,29 A similar occurrence was recorded in a randomised controlled trial of insecticide-treated bednets.30 Mathematical models of the magnitude of the survival advantage of HbAS needed to match the recorded gene frequencies of the βs mutation in Africa predict much greater malaria mortality than that directly measured,31 and models of malaria mortality fit epidemiological data from Africa only when indirect mortality is accounted for.32
Panel. Research in context.
Systematic review
We systematically searched PubMed up to June 8, 2011, with the search terms “bacterial infections”[MeSH] and “malaria, falciparum”[MeSH], which we supplemented with secondary citations and our personal collections. We did not identify any studies of the quantitative relation between malaria and invasive infections caused by specific bacterial pathogens.
Interpretation
A link is widely speculated to exist between malaria and invasive bacterial disease, but it has never been proven epidemiologically. In this 9-year study, we investigated this association through a novel mendelian randomisation approach with the malaria-protective phenotype of sickle-cell trait. Our findings show that the sickle-cell trait gave strong protection against all-cause bacteraemia at a time when malaria was common, but that this association decreased in parallel with the falling incidence of malaria. We conclude that the protective effect of sickle-cell trait against bacteraemia is mediated by its ability to confer resistance to malaria, confirming a causal link between malaria and bacteraemia. In modelling this relation, we estimate that, when the prevalence of Plasmodium falciparum infection was 29% in our study population, more than half of all bacteraemic infections were attributable to malaria. Strategies that focus on the prevention of childhood malaria will therefore have a disproportionately large effect on all-cause childhood mortality.
We have previously reported from Kilifi—during a period of high malaria parasite prevalence—that invasive bacterial disease caused at least as many inpatient deaths as did malaria.1 If, as we estimate here, more than half of these same cases of invasive bacterial disease are attributable to infection with P falciparum, then the control of malaria in tropical Africa will lead to substantial additional benefits through an indirect reduction of childhood mortality from invasive bacterial diseases.
Acknowledgments
Acknowledgments
This study was supported by the Kenya Medical Research Institute (KEMRI) and the Wellcome Trust of Great Britain. JAGS (grant number 081835), JAB (083579), and TNW (076934) are supported by research fellowships from the Wellcome Trust. We thank the patients who took part in this study, and the many staff at Kilifi District Hospital and the KEMRI Centre for Geographic Medicine Research-Coast who contributed to this study including clinical and nursing staff and the staff of the microbiology and genetic laboratories. This paper was published with the permission of the Director of KEMRI.
Contributors
JAGS and KM designed the matched case-control study, which was done by IM and JAB and analysed by JAGS. Bacteraemia surveillance was established by JAB and bacteriology was run by BSL and SM. LO did the HRP2 assays. JAGS, TNW, and EB set up the Kilifi HDSS and analysed the incidence rate changes. TNW set up longitudinal surveillance of sickle phenotypes among inpatients and established the control group for the longitudinal case-control study. JAGS and TNW did the remaining analyses and wrote the paper. All authors discussed the results and commented on the paper.
Conflicts of interests
We declare that we have no conflicts of interest.
Web Extra Material
References
- 1.Berkley JA, Lowe BS, Mwangi I. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 2.Williams TN, Uyoga S, Macharia A. Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. Lancet. 2009;374:1364–1370. doi: 10.1016/S0140-6736(09)61374-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giglioli G. Paratyphoid C an endemic disease of British Guiana: a clinical and pathological outline. B paratyphosum C as a pyogenic organism: case reports. Proc R Soc Med. 1929;23:165–177. [PMC free article] [PubMed] [Google Scholar]
- 4.Mabey DC, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987;155:1319–1321. doi: 10.1093/infdis/155.6.1319. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie G, Ceesay SJ, Hill PC. A decline in the incidence of invasive non-typhoidal Salmonella infection in The Gambia temporally associated with a decline in malaria infection. PLoS One. 2010;5:e10568. doi: 10.1371/journal.pone.0010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill AV, Allsopp CE, Kwiatkowski D. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 7.Williams TN, Mwangi TW, Wambua S. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–186. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowgill KD, Ndiritu M, Nyiro J. Effectiveness of Haemophilus influenzae type b conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA. 2006;296:671–678. doi: 10.1001/jama.296.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okiro EA, Hay SI, Gikandi PW. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndiritu M, Cowgill KD, Ismail A. Immunization coverage and risk factors for failure to immunize within the expanded programme on immunization in Kenya after introduction of new Haemophilus influenzae type b and hepatitis b virus antigens. BMC Public Health. 2006;6:132. doi: 10.1186/1471-2458-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochola LB, Marsh K, Lowe B, Gal S, Pluschke G, Smith T. Estimation of the sequestered parasite load in severe malaria patients using both host and parasite markers. Parasitology. 2005;131:449–458. doi: 10.1017/S0031182005008085. [DOI] [PubMed] [Google Scholar]
- 12.Mayxay M, Pukrittayakamee S, Chotivanich K, Looareesuwan S, White NJ. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:179–182. doi: 10.1016/s0035-9203(01)90156-7. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen PH, Day N, Pram TD, Ferguson DJ, White NJ. Intraleucocytic malaria pigment and prognosis in severe malaria. Trans R Soc Trop Med Hyg. 1995;89:200–204. doi: 10.1016/0035-9203(95)90496-4. [DOI] [PubMed] [Google Scholar]
- 14.Breslow NE, Day NE. vol 1. International Agency for Research on Cancer; Lyon: 1980. Statistical methods in cancer research. (The analysis of case-control studies). [Google Scholar]
- 15.O’Meara WP, Bejon P, Mwangi TW. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–1562. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allison AC. Polymorphism and natural selection in human polymorphisms. Cold Spring Harb Symp Quant Biol. 1964;29:137–149. doi: 10.1101/sqb.1964.029.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Aidoo M, Terlouw DJ, Kolczak MS. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–1312. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 18.Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine (Baltimore) 1971;50:97–112. [PubMed] [Google Scholar]
- 19.Ramakrishnan M, Moisi JC, Klugman KP. Increased risk of invasive bacterial infections in African people with sickle-cell disease: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:329–337. doi: 10.1016/S1473-3099(10)70055-4. [DOI] [PubMed] [Google Scholar]
- 20.Eeckels R, Gatti F, Renoirte AM. Abnormal distribution of haemoglobin genotypes in Negro children with severe bacterial infections. Nature. 1967;216:382. doi: 10.1038/216382a0. [DOI] [PubMed] [Google Scholar]
- 21.Bronzan RN, Taylor TE, Mwenechanya J. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 22.Giglioli G. Changes in the pattern of mortality following the eradication of hyperendemic malaria from a highly susceptible community. Bull World Health Organ. 1972;46:181–202. [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye D, Hook EW. The influence of hemolysis or blood loss on susceptibility to infection. J Immunol. 1963;91:65–75. [PubMed] [Google Scholar]
- 24.Schwarzer E, Skorokhod OA, Barrera V, Arese P. Hemozoin and the human monocyte—a brief review of their interactions. Parassitologia. 2008;50:143–145. [PubMed] [Google Scholar]
- 25.Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- 26.Prada J, Alabi SA, Bienzle U. Bacterial strains isolated from blood cultures of Nigerian children with cerebral malaria. Lancet. 1993;342:1114. doi: 10.1016/0140-6736(93)92096-c. [DOI] [PubMed] [Google Scholar]
- 27.Poehling K, Light L, Rhodes M. Sickle cell trait, hemoglobin C trait, and invasive pneumococcal disease. Epidemiology. 2010;21:340–346. doi: 10.1097/EDE.0b013e3181d61af8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleinschmidt I, Schwabe C, Benavente L. Marked increase in child survival after four years of intensive malaria control. Am J Trop Med Hyg. 2009;80:882–888. [PMC free article] [PubMed] [Google Scholar]
- 29.Molineaux L. Malaria and mortality: some epidemiological considerations. Ann Trop Med Parasitol. 1997;91:811–825. doi: 10.1080/00034989760572. [DOI] [PubMed] [Google Scholar]
- 30.Alonso PL, Lindsay SW, Armstrong JR. The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet. 1991;337:1499–1502. doi: 10.1016/0140-6736(91)93194-e. [DOI] [PubMed] [Google Scholar]
- 31.Molineaux L. The impact of parasitic disease and their control, with an emphasis on malaria and Africa. In: Vallin J, Lopez AD, editors. Health policy, social policy and mortality prospects. Ordina Editions; Liége: 1985. pp. 13–44. [Google Scholar]
- 32.Ross A, Maire N, Molineaux L, Smith T. An epidemiologic model of severe morbidity and mortality caused by Plasmodium falciparum. Am J Trop Med Hyg. 2006;75(suppl 2):63–73. doi: 10.4269/ajtmh.2006.75.63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


