Abstract
Fyn kinase phosphorylates tau and exacerbates Aβ-mediated synaptic dysfunction. However, Fyn also increases the non-pathological cleavage of amyloid precursor protein (APP), suggesting opposing roles for Fyn in the pathogenesis of Alzheimer’s disease (AD). To determine the effect of Fyn on both Aβ and tau pathologies, we crossed homozygous AD triple transgenic (3xTg) mice harboring mutations in APP, presenilin-1, and tau with wild-type or Fyn knock-out mice to generate Fyn+/+3xTg+/− or Fyn+/−3xTg+/− mice. We found that Fyn+/−3xTg+/− mice had increased soluble and intracellular Aβ, and these changes were accompanied by impaired performance on the Morris water maze at 18 months. Fyn+/−3xTg+/− mice had decreased phosphorylated tau at 15–18 months (as did Fyn knock-out mice), but Fyn+/−3xTg+/− mice had increased phosphorylated tau by 24 months. In addition, we observed that Fyn+/−3xTg+/− males were delayed in developing Aβ pathology compared to females, and displayed better spatial learning performance at 18 months. Overall, these findings suggest that loss of Fyn at early stages of disease increases soluble A accumulation and worsens spatial learning in the absence of changes in tau phosphorylation.
Keywords: Fyn, APP, Aβ, tau, phosphorylation
1. Introduction
The pathogenesis of AD is defined by the presence of two neuropathological lesions—amyloid plaques and neurofibrillary tangles (Duyckaerts et al., 2009). Plaques are extracellular aggregates of the Aβ peptide, a fragment of the β-amyloid precursor protein (APP) (Nunan and Small, 2000). Processing of APP to Aβ is altered by extracellular interactions (Hoe and Rebeck, 2008), intracellular adaptor proteins (King and Turner, 2004), and covalent alterations (Suzuki and Nakaya, 2008). The accumulation of neurofibrillary tangles occurs when the microtubule protein tau is hyper-phosphorylated under pathological conditions and dissociates from microtubules, forming highly insoluble paired helical filaments which aggregate to form tangles (Duyckaerts et al., 2009). Several kinases have been shown to phosphorylate the serine and threonine residues of tau which are thought to underlie the tangle formation observed in AD (Trojanowski and Lee, 1994), including cyclin-dependent kinase 5 (Cdk5), glycogen synthase kinase 3 (Gsk3α and β), mitogen-activated protein kinase (MAPK), protein kinase A (PKA), and calcium/calmodulin activated kinase II (CaMKII). However, it is unclear which kinases are responsible for initiating the cascade of pathological tau phosphorylation.
Fyn tyrosine kinase is hypothesized to play a dual role in both the Aβ and tau pathologies observed in AD. Fyn co-localizes with pathological serine/threonine phosphorylated forms of tau in AD (Shirazi and Wood, 1993), and interacts directly with tau (Lee et al., 1998), phosphorylating it at tyrosine 18 (Lee et al., 2004). Tyrosine 18-phosphorylated tau is found in neurofibrillary tangles of AD brains and AD transgenic mice (Bhaskar et al., 2010; Lee et al., 2004), and Fyn expression increases with increased degree of pathology (Ho et al., 2005). Fyn-tau complexes have been hypothesized to localize to post-synaptic densities to affect glutamate receptor function (Ittner et al., 2010). Fyn also plays a role in Aβ-mediated pathological events, including Aβ-induced disruption of hippocampal network activity (Pena et al., 2010) and impairment of synaptic transmission and plasticity (Venkitaramani et al., 2007). In vivo models show that Fyn exacerbates Aβ-induced neuronal and behavioral deficits, and these effects are blocked by the genetic ablation of Fyn (Chin et al., 2005; Chin et al., 2004). However, our group has found that Fyn causes decreased Aβ production in vitro and Fyn knock-out mice have decreased α-secretase APP products (Hoe et al., 2008). These findings suggest antagonistic roles for Fyn in increasing tau phosphorylation and Aβ-mediated neurotoxicity and in decreasing Aβ production, leading to the question of whether Fyn inhibition will ultimately prove beneficial or detrimental for the treatment of AD.
In order to investigate this question, we utilized a triple transgenic model of AD (3xTg) harboring mutations in human APP, presenilin-1, and tau, which recapitulates both the Aβ and tau pathologies of AD, to determine the effect of Fyn on each (Oddo et al., 2003a; Oddo et al., 2003b). We employed a genetic approach by breeding 3xTg mice with either wild-type or Fyn knock-out mice to generate wild-type or heterozygous Fyn mice on a heterozygous 3xTg background (Fyn+/+3xTg+/− and Fyn+/−3xTg+/−). We found that knock-down of Fyn resulted in both increased Aβ levels and decreased tau phosphorylation accompanied by deficits in spatial learning on the Morris water maze. These findings implicate a greater role for Aβ, and not tau, pathology in mediating cognitive performance at early disease stages in the triple transgenic model of AD. Taken together, our study implicates a harmful effect of long-term reduction of Fyn kinase on Aβ production and cognitive performance.
2. Materials and methods
2.1 Animals and breeding
Fyn knock-out mice were obtained from Jackson Laboratories (Bar Harbor, ME) at 3 months of age for analysis of phospho-tau. Wild-type B6129SF2/J controls were also purchased from Jackson Laboratories. We crossed male Fyn knock-out and wild-type mice with female 3xTg AD mice originally generated by co-microinjection of human APP (K670M/N671L) and tau (P301L) transgenes under the control of the Thy 1.2 promoter into mutant PS-1 (M146V) knock-in mice (Oddo et al., 2003b). Female and male Fyn+/+3xTg+/− or Fyn+/−3xTg+/− mice were generated and euthanized by rapid cervical dislocation (to eliminate anesthesia-mediated tau phosphorylation (Planel et al., 2007)) at 15, 18, 21, and 24 months of age for females and 18, 21, and 24 months of age for males. Brains were quickly isolated, and hemi-brains were either snap-frozen in dry ice for biochemical analyses or immersion fixed in 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4, for histochemical analyses.
2.2 Chemicals and antibodies
We used antibody 6E10 (Signet, Dedham, MA) to detect Aβ, antibodies AT8, AT180, and AT270 (Pierce, Rockford, IL) for phospho-tau epitopes, and Tau46 (Sigma, St. Louis, MO) for total tau. Polyclonal Fyn antibody was purchased from Millipore (Billerica, MA). Antibodies 1A10 (for Aβ1–40), 1C3 (for Aβ1–42), and 82E1 (human-specific Aβ antibody) for Aβ ELISAs were obtained from IBL (Gunma, Japan).
2.3 Tissue preparation
Mouse brains were homogenized in a 10-fold volume of 50 mM Tris–HCl buffer, pH 7.6, containing 250 mM sucrose and protease inhibitor cocktail (Sigma). Soluble APP and Aβ were extracted in 0.4% diethylamine (DEA), as previously described (Nishitomi et al., 2006). Briefly, crude 10% brain homogenate was mixed with an equal volume of 0.4% DEA, sonicated, and ultracentrifuged for 1 hr at 100,000 × g. The supernatant was collected and neutralized with 10% 0.5M Tris base, pH 6.8. The resulting DEA fraction was used for soluble Aβ ELISA analyses. Insoluble Aβ was extracted from the pellet after ultracentrifugation in formic acid (FA), sonicated, and ultracentrifuged for 1 hr at 100,000 × g. The supernatant was collected and neutralized with 1M Tris base and 0.5M Na2HPO4, and the resulting FA fraction was used for insoluble Aβ ELISA analyses.
2.4 Western blot
Proteins were extracted from brain homogenates with radioimmunoprecipitation assay (RIPA) buffer containing 50mM Tris-HCl, pH 7.4, 150mM NaCl, 0.25% deoxycholic acid, 1% NP-40, and 1mM EDTA (Millipore), and probed for phosphorylated tau using antibodies AT8 (Ser202/Thr205), AT180 (Thr231), or AT270 (Thr181) (Hirata-Fukae et al., 2008). Total tau was detected with antibody Tau46 as a band at 60 kDa, and all phosphorylated tau values were normalized to total tau. Proteins were separated by SDS-PAGE on 10% polyacrylamide gels, transferred onto a polyvinylidene fluoride (PVDF) membrane, and blocked with 5% nonfat dry milk. The blots were incubated with antibodies at room temperature overnight. Horseradish peroxidase-conjugated secondary antibodies were visualized using an enhanced chemiluminescence detection system and exposed to film. For quantification of Fyn levels, one sample was run on every gel to allow for normalization across age, sex, and genotype. Bands were quantified using BioRad QuantityOne software. For quantification of phospho-tau levels, either AT8 or AT270, which produce similar bands, was used to Western blot for each cohort (i.e. 15 month old Fyn+/+3xTg+/− and Fyn+/−3xTg+/− females).
2.5 Aβ ELISAs
Levels of human Aβ1–40 from brain DEA fractions were quantified using sandwich ELISA as previously described (Horikoshi et al., 2004). Briefly, a 96-well plate (Maxisorp) was coated with an anti-Aβ40 antibody, clone 1A10, overnight at 4°C. After blocking for 2 hrs, standards (synthetic human Aβ40 peptide) and samples were loaded and incubated overnight at 4°C. The plate was incubated with HRP-coupled detection antibody, 82E1, and visualized using a 3,3',5,5'-tetra methyl benzidine (TMB) substrate. For analysis of human Aβ1–42, antibody 1C3 was used as the coating antibody and 82E1 as the detection antibody, using synthetic human Aβ42 peptide as standard.
2.6 Immunohistochemistry and stereology
Brains were immersion fixed and sections were prepared as previously described (Matsuoka et al., 2001). Sections were sagitally sliced at 50µm for stereological counting, and sections were incubated in 0.3% H2O2 for 30 min, blocked with 1% FBS, and incubated with primary antibody 6E10 against Aβ/APP in 100mM phosphate buffered saline consisting of 0.3% Triton X-100 overnight. Primary antibodies were used at 1µg IgG/mL, detected by a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) and visualized using the ABC method (Vectastain, Vector Laboratories) and DAB substrate.
Following immunostaining, 10 sections at evenly spaced intervals were selected from the sections encompassing the hippocampal region. 6E10-positive cells within the CA1 and subiculum regions of the hippocampus were stereologically counted from Fyn+/+3xTg+/− or Fyn+/− 3xTg+/− mice using Stereologer (Stereology Resource Center, Chester, MD).
2.7 Morris water maze
We used the Morris water maze (MWM) (Morris et al., 1982) as a test for spatial learning and memory, which measures the time required (latency) to find a hidden platform in a pool. A large white circular pool was filled with white-colored water (using Crayola Premier Tempera white paint; Crayola, Easton, PA), and the water temperature was maintained at 24°C. Visual cues were placed around the pool, and a hidden platform (15cm in diameter) was placed at a fixed location. Two daily training sessions, 120 seconds each, were performed on 5 consecutive days. On the sixth day, the platform was removed from the pool, and the time spent in the quadrant where the platform was previously located was recorded for a period of 60 seconds (probe test). The number of entries into the target quadrant and the number of crossings over the target platform were also recorded. Immediately following the probe test, the platform was returned to the pool and made visible, and latency to reach the visible platform was recorded as a measure of visual acuity. MWM analysis was performed using video-tracking software (Actimetrics, Wilmette, IL).
2.8 Statistical analysis
Experiments were repeated a minimum of three times unless otherwise noted. All data were analyzed using ANOVA with Graphpad Prism 5 software, using Tukey's multiple comparison test for post hoc analyses with significance determined as p<0.05. Descriptive statistics are displayed as mean ± SEM.
3. Results
3.1 Endogenous tau phosphorylation is decreased in Fyn knock-out mice
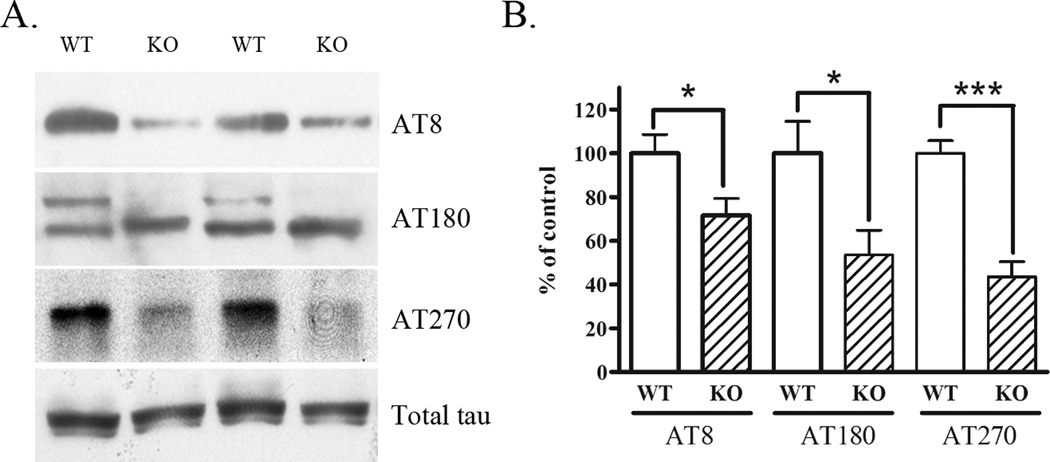
Fyn phosphorylates tau on tyrosine 18 (Lee et al., 2004), and associates with phospho-tau (AT8) positive cells (Ho et al., 2005). However, whether knock-down of Fyn results in an alteration in the phosphorylation of tau, is unknown. Therefore, we tested whether Fyn knock-out mice (3 months of age) exhibited alterations in serine and threonine phosphorylation of endogenous murine tau (detected by antibodies AT8 (Ser202/Thr205), AT180 (Thr231), or AT270 (Thr181)) by Western blots. Fyn knock-out mice had significantly lower levels of phospho-tau epitopes compared to wild-type mice (Fig. 1A). Total tau levels were not altered in Fyn knock-out mice (Fig. 1A, bottom panel). Quantification revealed significant 36% (p<0.05), 47% (p<0.05), and 56% (p<0.001) reductions in AT8, AT180, and AT270, respectively (Fig. 1B). These results suggest that Fyn plays a role in promoting serine/threonine phosphorylation of tau in vivo, and reductions in Fyn could reduce the pathological phosphorylation of tau.
Figure 1. Endogenous tau phosphorylation is decreased in Fyn knock-out mice.
A. Brain lysates from wild-type or Fyn homozygous knock-out mice were Western blotted for phosphorylated tau epitopes Ser202/Thr205 (AT8), Thr231 (AT180), or Thr181 (AT270) (top 3 panels) or total tau (bottom panel). B. Quantification of data in A. shows Fyn knock-out mice had significantly decreased tau phosphorylation as detected by AT8 (36%, p<0.05), AT180 (quantification of both bands: 47%, p<0.05), and AT270 (56%, p<0.001).
3.2 Soluble Aβ1–40 is increased in Fyn+/−3xTg+/− mice
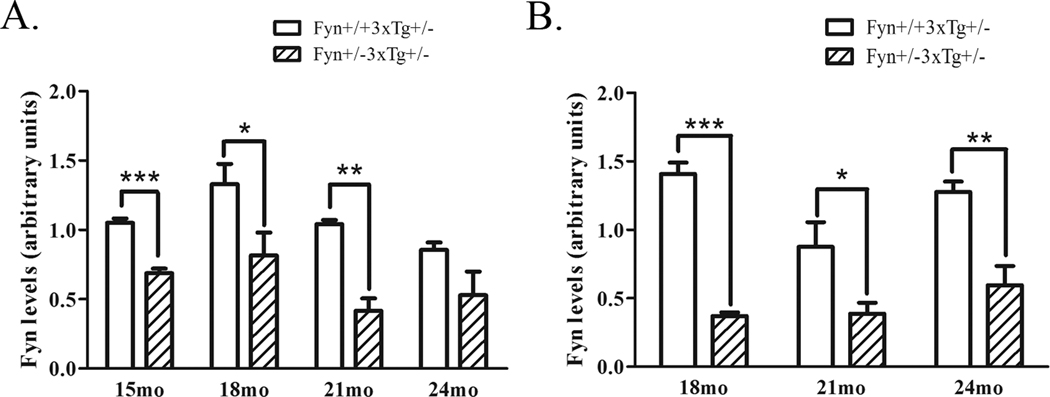
We have been interested in whether altering Fyn levels could affect AD-associated pathological changes in vivo. Our previous studies demonstrated that overexpression of Fyn decreased Aβ production in vitro and Fyn knock-out mice exhibited decreased α-secretase processing of APP in vivo (Hoe et al., 2008). To determine whether Fyn played a role in APP processing under pathological conditions, we crossed wild-type or Fyn knock-out mice with AD triple transgenic mice (3xTg) to generate Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice. We examined mice over the course of two years, starting at 15 months of age. We chose older mice, since Aβ accumulation did not begin until at least 15 months in homozygous females in our colony, and even later in the male 3xTg mice (Hirata-Fukae et al., 2008). Therefore, we analyzed female mice at 15, 18, 21, and 24 months and male mice at 18, 21, and 24 months of age. We determined the level of Fyn knock-down at each age, and observed a 34% decrease at 15 months (p<0.001), a 39% decrease at 18 months (p<0.05), a 60% decrease at 21 months (p<0.01) and a non-significant decrease in Fyn levels at 24 months in female mice (Fig. 2A). Male Fyn+/−3xTg+/− mice showed a 74% decrease in Fyn levels at 18 months (p<0.001), a 56% decrease at 21 months (p<0.05), and a 55% decrease at 24 months (p<0.01) (Fig. 2B).
Figure 2. Fyn levels are reduced in Fyn+/−3xTg+/− mice.
A. Brain lysates from 15, 18, 21, and 24 month old female Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice were Western blotted for Fyn. Female Fyn+/−3xTg+/− mice showed a 34% decrease at 15 months (p<0.001), a 39% decrease at 18 months (p<0.05), a 60% decrease at 21 months (p<0.01), and a non-significant decrease at 24 months. B. 18, 21, and 24 month old male Fyn+/+3xTg+/− and Fyn+/− 3xTg+/− mice were Western blotted for Fyn. Fyn+/−3xTg+/− mice had a 74% decrease in Fyn levels at 18 months (p<0.001), a 56% decrease at 21 months (p<0.05), and a 55% decrease at 24 months (p<0.01).
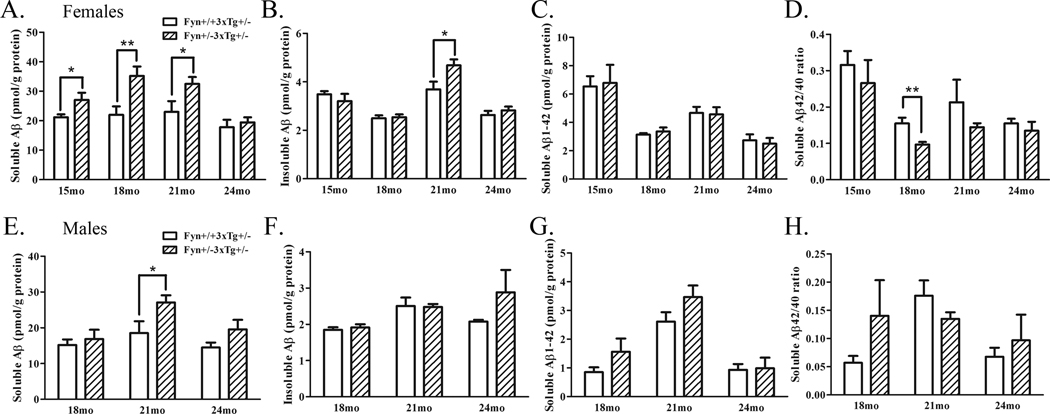
We then examined Aβ levels in these mice, testing 8–10 male and female mice at each age. Soluble Aβ1–40 was significantly increased in female Fyn+/−3xTg+/− mice compared to controls at three ages: 15, 18, and 21 months by 28% (p<0.05), 60% (p<0.01), and 39% (p<0.05), respectively (Fig. 3A); no changes were observed at 24 months. Insoluble Aβ1–40 was also increased slightly in Fyn+/−3xTg+/− mice at 21 months (by 15%, p<0.05), but not at other ages (Fig. 3B). No significant changes were observed in Aβ1–42 levels at any age (Fig. 3C). Soluble Aβ42/40 levels were calculated and were different only at 18 months (Fig. 3D), the age at which we observed the highest increase in Aβ40 levels. Male Fyn+/−3xTg+/− mice also exhibited increased soluble Aβ1–40 levels, but at an older age, 21 months (46%, p<0.05), with a trend towards increased soluble Aβ1–40 at 24 months (35%, p=0.19) (Fig. 3E). Male Fyn+/−3xTg+/− mice exhibited a trend towards increased insoluble Aβ1–40 at 24 months (46%, p=0.12) (Fig. 3F). Again, no changes to Aβ1–42 levels were observed at any age (Fig. 3G), and no change in Aβ42/40 ratio was observed at any age in male Fyn+/−3xTg+/− mice (Fig. 3H). These data demonstrate that Fyn heterozygotes show increased levels of soluble Aβ1–40, although not at the oldest ages.
Figure 3. Soluble Aβ1–40 is increased in Fyn+/−3xTg+/− mice.
A. Soluble Aβ1–40 was extracted from 15, 18, 21, and 24 month old female Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mouse brain homogenates and measured by ELISA. Soluble Aβ1–40 was significantly increased in Fyn+/−3xTg+/− mice at 15 (28%, p<0.05), 18 (60%, p<0.01), and 21 months (39%, p<0.05), but not at 24 months. B. Insoluble Aβ1–40 was extracted with formic acid (FA), and was significantly increased in 21 month old Fyn+/−3xTg+/− mice by 46% (p<0.05). C. Soluble Aβ 1–42 levels were not significantly different at any age. D. The ratio of soluble Aβ42/40 was calculated, and was unchanged at all ages except at 18 months, when it was significantly decreased (37%, p<0.01). E. Soluble Aβ1–40 was measured from 18, 21, and 24 month old male Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice. 21 month old Fyn+/−3xTg+/− mice had significantly increased soluble Aβ1–40 by 46% (p<0.05). 24 month old Fyn+/−3xTg+/− mice had a trend towards increased soluble (35%, p=0.19) Aβ1–40. F. Insoluble (FA) Aβ1–40 was measured from 18, 21, and 24 month male Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice. There were no significant increases in insoluble Aβ1–40 at any age, but a trend towards increased insoluble Aβ1–40 at 24 months (46%, p=0.12). G. Soluble Aβ1–42 levels were measured from male mice and not found to be significantly different at any age. H. Aβ42/40 ratios were calculated and found to be not significantly different at any age.
3.3 6E10 positive cells are increased in female Fyn+/−3xTg+/− mice
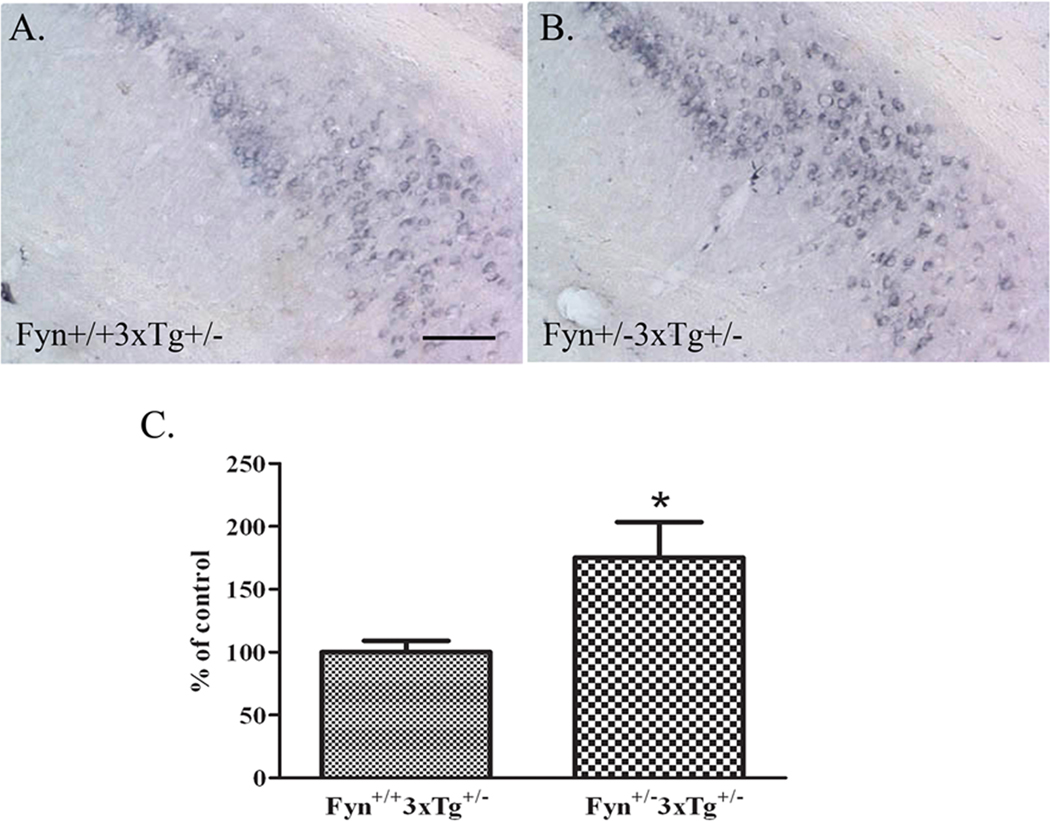
To determine whether intracellular Aβ, one of the earlier hallmarks of disease in the 3xTg model of AD (Oddo et al., 2003b), was also increased in Fyn+/−3xTg+/− mice, we immunostained hippocampal sections from 18 month-old Fyn+/+3xTg+/− or Fyn+/−3xTg+/− female mice with antibody 6E10 (which recognizes both APP and Aβ). As reported (Hirata-Fukae et al., 2008), we found that 6E10 immunostaining specifically accumulated with age in neurons of the CA1 and subiculum regions of the hippocampus as well as in the cortex. Consistent with Aβ ELISAs, Fyn+/−3xTg+/− mice had greater 6E10 immunoreactivity compared to control mice (Fig. 4A–B). To determine whether numbers of 6E10 positive cells were increased in these mice, we systematically counted 6E10 positive cells by stereology in the hippocampal regions (CA1 and subiculum). Fyn+/−3xTg+/− mice had higher numbers of 6E10 positive cells in the hippocampus compared to controls, a 75% increase (beyond the 95% confidence limit) (Fig. 4C). Total levels of APP in Fyn+/−3xTg+/− mice were not different by Western blot (data not shown).
Figure 4. 6E10 positive cells are increased in female Fyn+/−3xTg+/− mice.
A–B. Brain sections from 18 month old female Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice were stained with antibody 6E10 to detect APP/Aβ. Fyn+/−3xTg+/− mice had greater intracellular 6E10 immunoreactivity in the hippocampus compared to controls. Scale bar= 20µm. C. Stereological quantification of 6E10 positive cells in the CA1 and subiculum regions of the hippocampus in Fyn+/+3xTg+/− (n=2) and Fyn+/−3xTg+/− (n=3) mice. Fyn+/−3xTg+/− mice had a significant increase in 6E10 positive cells by 75% (p<0.05).
3.4 Tau phosphorylation is decreased in Fyn+/−3xTg+/− mice at 15 and 18 months
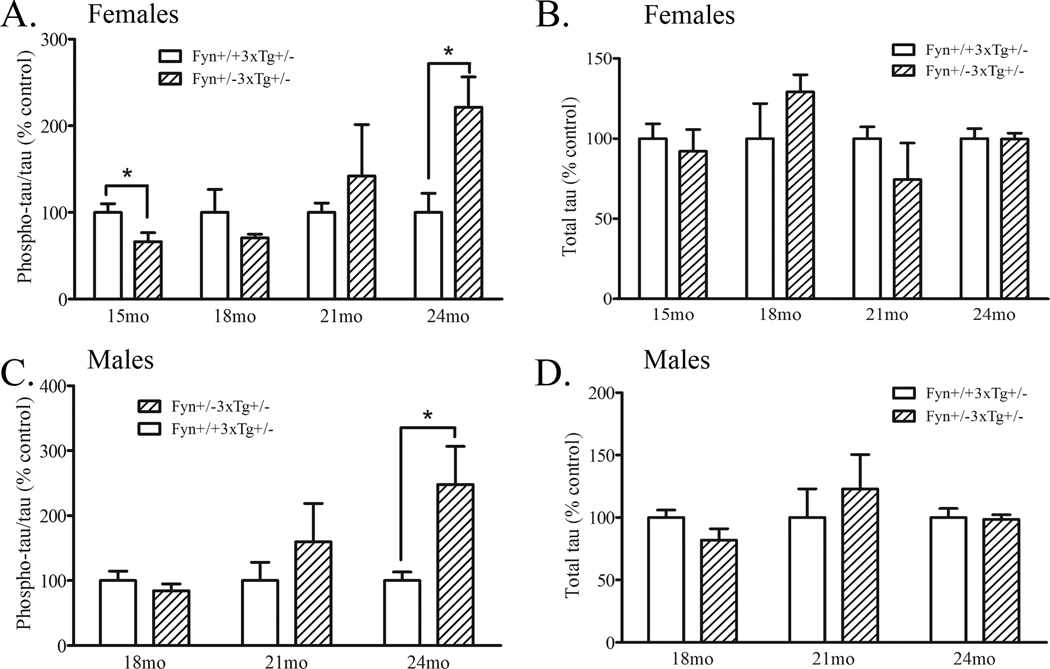
We demonstrated above that Fyn knock-out mice had decreased levels of serine/threonine phosphorylated murine tau (Fig. 1). To determine whether knock-down of Fyn in an AD model also resulted in a decrease in human tau phosphorylation, we measured levels of phosphorylated and total tau by Western blots. We found that female Fyn+/−3xTg+/− mice had significantly decreased phospho-tau at 15 months by 34% (p<0.05) and a trend towards decreased phosphotau at 18 months (29%, p=0.26), with no changes to total tau levels (Fig. 5A–B). We found that male Fyn+/−3xTg+/− mice did not exhibit any changes to phospho-tau at 18 months and had no changes in total tau levels (Fig. 5C–D). With increasing age (and increasing Aβ pathology), the effect of Fyn knock-down on tau phosphorylation was either lost or reversed at 21 and 24 months, in both female and male Fyn+/−3xTg+/− mice (Fig. 5A, C). There was a significant 122% increase in phospho-tau in 24 month-old Fyn+/−3xTg+/− female mice (p<0.05) and a similar increase in phospho-tau in 24 month-old Fyn+/−3xTg+/− male mice (148%, p<0.05) (Fig. 5A, C).
Figure 5. Tau phosphorylation is decreased in Fyn+/−3xTg+/− mice at 15 months and increased at 24 months.
A. Brain homogenates from 15, 18, 21, and 24 month old female Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice were Western blotted for phosphorylated tau by antibody AT8 or AT270. Fyn+/−3xTg+/− mice showed significantly decreased phospho-tau at 15 months (34%, p<0.05), no significant change at 18 or 21 months, and significantly increased phospho-tau at 24 months (122%, p<0.05). All values were normalized to total tau levels. B. Brain homogenates from 15, 18, 21, and 24 month old female Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice were Western blotted for total tau. There were no significant differences in total tau at any age. C. Brain homogenates from 18, 21, and 24 month old male Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice were Western blotted for phosphorylated tau with antibody AT8 or AT270. 24 month old Fyn+/−3xTg+/− mice had significantly increased phospho-tau by 148% (p<0.05). D. Brain homogenates from 18, 21, and 24 month old male Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice were Western blotted for total tau. There were no differences in total tau at any age.
3.5 Fyn+/−3xTg+/− mice perform worse on the Morris water maze
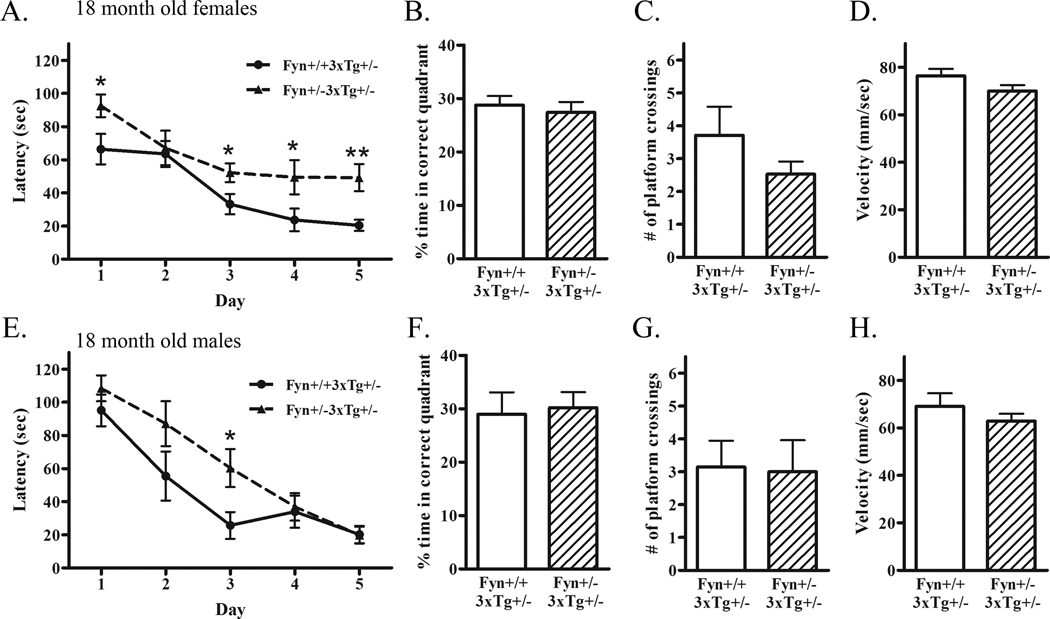
Knock-down of Fyn increases Aβ and decreases tau phosphorylation at early ages (Fig. 3–5). To examine cognitive performance in these animals, we subjected 18 month-old female and male Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice to the Morris water maze as a test of spatial learning and memory. Female Fyn+/−3xTg+/− mice were even more impaired in latency to find the hidden platform compared to Fyn+/+3xTg+/− mice on days 3, 4, and 5 (Fig. 6A). Although the percent time spent in the target quadrant on the probe trial was not different (Fig. 6B), there was a trend towards fewer number of platform crosses in Fyn+/−3xTg+/− mice (32% decrease, p=0.19) (Fig. 6C). The average swim velocity was not significantly different between groups (Fig. 6D). Male Fyn+/−3xTg+/− mice were impaired in latency to find the hidden platform on the third day of training; however, by the fifth day, were performing similar to controls (Fig. 6E). Neither performance on the probe trial nor average swim velocity were different between groups of male mice (Fig. 6F–H). Overall, female 18 month-old Fyn+/−3xTg+/− mice had increased Aβ and showed deficits in spatial learning, while male Fyn+/−3xTg+/− mice, which did not exhibit a significant change in Aβ, performed similarly to control mice. There was no relationship between behavioral changes and phospho-tau levels, since both female and male Fyn+/−3xTg+/− mice showed a trend towards decreased phospho-tau levels at 18 months.
Figure 6. Fyn+/−3xTg+/− mice are impaired in spatial learning in the Morris water maze.
A. 18 month old female Fyn+/+3xTg+/− (n=15) and Fyn+/−3xTg+/− (n=17) mice were tested in the Morris water maze. Animals were trained on 5 consecutive days with 2 120-second trials per day. The average latencies to find the hidden platform of the two trials per day for each animal in each group are depicted above. Fyn+/−3xTg+/− mice were significantly impaired compared to controls on day 3 (p<0.05), 4 (p<0.05), and 5 (p<0.01) of learning. B. Percent time spent in the target quadrant during the probe trial was not statistically different. C. The number of target platform crossings during the probe trial was not statistically significant, but showed a trend towards decreased number of crossings in Fyn+/−3xTg+/− mice. D. Average swim velocity was calculated for each animal and averaged per group. Swim speeds were not significantly different between Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice. E. 18 month old male Fyn+/+3xTg+/− (n=10) and Fyn+/−3xTg+/− (n=8) mice were subjected to the Morris water maze. Fyn+/−3xTg+/− mice were significantly impaired compared to controls on day 3 of learning (p<0.05); however, were not significantly different on days 4 or 5. F. Percent time spent in the target quadrant during the probe trial was not statistically different. G. The number of target platform crossings during the probe trial was not statistically significant. H. Average swim velocity was not different between Fyn+/+3xTg+/− and Fyn+/−3xTg+/− mice.
4. Discussion
In these studies, we tested whether knock-down of Fyn kinase resulted in alterations to Aβ or phospho-tau levels by generating Fyn heterozygous mice on a 3xTg heterozygous background which accumulate both Aβ and phospho-tau (Fyn+/−3xTg+/− and control Fyn+/+3xTg+/− mice). We first demonstrated that Fyn+/−3xTg+/− mice had increased Aβ utilizing two independent assays, biochemically measuring Aβ and stereologically counting 6E10-positive hippocampal cells (Fig. 34). In contrast to the increased Aβ in Fyn+/−3xTg+/− mice, we observed significantly decreased phospho-tau in these mice at 15 months and a trend toward decreased phospho-tau at 18 months (Fig. 5). When we tested whether these changes affected spatial learning and memory, we found that 18 month-old female Fyn+/−3xTg+/− mice, which had increased Aβ, had deficits in the Morris water maze, but 18 month-old male Fyn+/−3xTg+/− mice, which had unchanged Aβ levels, did not demonstrate deficits in the Morris water maze (Fig. 6).
Previous studies have implicated roles for Fyn in increasing Aβ-mediated neurotoxicity (Pena et al., 2010; Venkitaramani et al., 2007) and decreasing amyloidogenic APP processing (Hoe et al., 2008). We now show an in vivo effect of Fyn knock-down on increasing Aβ production in AD 3xTg mice. Therefore, it is likely that Fyn serves a dual role in mediating both APP processing to reduce Aβ production, and in exacerbating Aβ-mediated toxicity when high levels of Aβ are already present. It is noteworthy that Fyn knockout in a high APP-expressing mouse model (J20 mouse with Swedish (K670N, M671L) and Indiana (V717F) familial AD mutations (hAPP770 numbering) directed by the platelet-derived growth factor (PDGF) β chain promoter) resulted in no changes to Aβ1-x or Aβ1–42 levels (Chin et al., 2004). Our genetic studies, which allowed us to examine the effect of chronic Fyn inhibition on APP processing in a low level Aβ environment (APP Swedish mutation and tau (P301L) mutation both driven by the Thy1.2 regulatory element in a PS1(M146V) knock-in mouse), support the hypothesis that Fyn inhibition increases Aβ production in AD 3xTg mice. In addition, our present study suggests that altering Aβ40 production alone, with no change in Aβ42, is sufficient to induce cognitive deficits. The 3xTg+/− mouse model produces higher levels of Aβ40 compared to Aβ42, and consequently has a much lower ratio of Aβ42/40 compared to other APP models (Chin et al., 2004). Therefore, it is difficult to determine whether Fyn may also have an effect on Aβ42 levels due to its low level of detection in this particular model. Furthermore, at the time points we studied, we did not observe significant levels of insoluble Aβ, indicating that our model may recapitulate an early AD disease state. These data suggest that the levels and isoforms of Aβ present, and the corresponding severity of disease, may influence the effect of Fyn on Aβ production, and highlights the importance of considering disease burden when planning treatment options.
One interesting observation from our study is the reversal of Fyn knock-down effect on tau phosphorylation. We report that serine/threonine phosphorylation of endogenous tau is reduced in Fyn knock-out mice, and we see a similar effect in young Fyn+/−3xTg+/− mice. However, at 21 and 24 months, tau phosphorylation is increased in Fyn+/−3xTg+/− mice. This effect may be a secondary consequence of increasing Aβ levels, or Fyn may be differentially regulating the phosphorylation of tau at different ages/disease states. Although Fyn is known to colocalize with phospho-tau in AD brains (Ho et al., 2005), it is unknown how tyrosine phosphorylation by Fyn leads to pathological serine/threonine phosphorylation of tau. Fyn may regulate the activity of tau kinases such as Gsk3β and Cdk5, which phosphorylate a number of serine/threonine sites, some of which are recognized by common commercial antibodies such as AT8, AT180, and AT270. Fyn directly phosphorylates Gsk3β, leading to its activation (Hughes et al., 1993), but also phosphorylates Akt, which phosphorylates Gsk3β on Ser9, leading to its inactivation (Stambolic and Woodgett, 1994). Thus, it may be a balance between tyrosine and serine phosphorylations of Gsk3β that regulates its activity. We found that 15 month-old Fyn+/− 3xTg+/− mice, which had decreased tau phosphorylation, also had decreased tyrosine phosphorylation of Gsk3β, and 24 month-old Fyn+/−3xTg+/− mice, which had increased tau phosphorylation, had decreased serine phosphorylation of Gsk3β (data not shown). These findings suggest that the regulation of Gsk3β may be age or pathology dependent, where Fyn initially tyrosine phosphorylates Gsk3β, but with aging or increased pathology, increases its phosphorylation of Akt. Our studies stress the importance of studying animal models of disease at various time points, because the biochemical and pathological markers of the disease can be dramatically altered as the animal ages.
There exists conflicting evidence regarding the relative contributions of Aβ and tau pathology to deficits in behavior and cognitive outcome. Studies demonstrate that mice singly transgenic for APP exhibit alterations in spatial learning and memory (King et al., 1999; Morgan, 2003), supporting the hypothesis that amyloid pathology alone is sufficient to induce cognitive deficits. However, studies in humans have demonstrated that the presence of neurofibrillary tangles correlate better with behavioral outcome than plaques (Bierer et al., 1995; Gomez-Isla et al., 1997). In the 3xTg model of AD, reducing both Aβ and tau pathology was necessary for improved cognitive function compared to reducing Aβ alone (Oddo et al., 2006). A recent study of Fyn overexpressing mice on an APP background with or without tau found that endogenous tau was necessary for the behavioral deficits observed in APP/Fyn mice (Roberson et al., 2011). Here, we demonstrate that an elevation in soluble Aβ40 in the absence of hyperphosphorylated tau impairs spatial learning during training but not memory on the probe trial. However, because neither group performed better than chance, it is possible that the memory deficit in 3xTg+/− mice is already too significant to observe an even greater deficit. Overall, our data support the model that at early stages of disease, Aβ alone is sufficient to trigger the onset of cognitive impairment; however, at later stages after the accumulation of secondary tau pathology, it may be necessary to reduce both Aβ and phospho-tau to improve cognitive function.
The present study is the first to show the effect of Fyn reduction on both Aβ and tau pathology in the 3xTg model of Alzheimer’s disease. We demonstrate that Fyn knock-down does not rescue AD-related deficits as previously hypothesized, but in contrast, increases Aβ and worsens spatial learning. These results parallel those of a recent study using the same 3xTg model in which striatal-enriched phosphatase (STEP), which is involved in the deactivation of Fyn, was genetically removed, and reversed the cognitive deficits seen in 3xTg mice (Zhang et al., 2010). Overall, these findings contribute to our understanding of Fyn-mediated alterations to APP processing and tau phosphorylation, and provide evidence against the pharmacological inhibition of Fyn as a therapeutic approach, at least at early stages of disease progression.
Acknowledgements
This work was supported by NIH NS059178 (SSM) and NIH AG014473 (GWR). We acknowledge Dr. Frank LaFerla for providing the 3xTg mice, Dr. Kebreten Manaye for advice regarding stereological analysis of brain tissue, and Dr. Scott Turner for valuable insights and discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors declare that they have no competing interests. Appropriate approval and procedures were used concerning animal subjects.
References
- Bhaskar K, Hobbs GA, Yen SH, Lee G. Tyrosine phosphorylation of tau accompanies disease progression in transgenic mouse models of tauopathy. Neuropathol Appl Neurobiol. 2010;36:462–477. doi: 10.1111/j.1365-2990.2010.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, Perl DP. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol. 1995;52:81–88. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Yu GQ, Kojima N, Masliah E, Mucke L. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J Neurosci. 2004;24:4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Hirata-Fukae C, Li HF, Hoe HS, Gray AJ, Minami SS, Hamada K, Niikura T, Hua F, Tsukagoshi-Nagai H, Horikoshi-Sakuraba Y, Mughal M, Rebeck GW, LaFerla FM, Mattson MP, Iwata N, Saido TC, Klein WL, Duff KE, Aisen PS, Matsuoka Y. Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 2008;1216:92–103. doi: 10.1016/j.brainres.2008.03.079. [DOI] [PubMed] [Google Scholar]
- Ho GJ, Hashimoto M, Adame A, Izu M, Alford MF, Thal LJ, Hansen LA, Maslish E. Altered p59Fyn kinase expression accompanies disease progression in Alzheimer's disease: implications for its functional role. Neurobiol Aging. 2005;26:625–635. doi: 10.1016/j.neurobiolaging.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Minami SS, Makarova A, Lee J, Hyman BT, Matsuoka Y, Rebeck GW. Fyn modulation of Dab1 effects on amyloid precursor protein and ApoE receptor 2 processing. J Biol Chem. 2008;283:6288–6299. doi: 10.1074/jbc.M704140200. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Rebeck GW. Functional interactions of APP with the apoE receptor family. J Neurochem. 2008;106:2263–2271. doi: 10.1111/j.1471-4159.2008.05517.x. [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Sakaguchi G, Becker AG, Gray AJ, Duff K, Aisen PS, Yamaguchi H, Maeda M, Kinoshita N, Matsuoka Y. Development of Abeta terminal endspecific antibodies and sensitive ELISA for Abeta variant. Biochem Biophys Res Commun. 2004;319:733–737. doi: 10.1016/j.bbrc.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J. Dendritic Function of Tau Mediates Amyloid-beta Toxicity in Alzheimer's Disease Mouse Models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ. Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. Behav Brain Res. 1999;103:145–162. doi: 10.1016/s0166-4328(99)00037-6. [DOI] [PubMed] [Google Scholar]
- King GD, Turner RS. Adaptor protein interactions: modulators of amyloid precursor protein metabolism and Alzheimer's disease risk? Exp Neurol. 2004;185:208–219. doi: 10.1016/j.expneurol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998;111(Pt 21):3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. Phosphorylation of tau by fyn: implications for Alzheimer's disease. J Neurosci. 2004;24:2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Picciano M, Malester B, LaFrancois J, Zehr C, Daeschner JM, Olschowka JA, Fonseca MI, O'Banion MK, Tenner AJ, Lemere CA, Duff K. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer's disease. Am J Pathol. 2001;158:1345–1354. doi: 10.1016/S0002-9440(10)64085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. Learning and memory deficits in APP transgenic mouse models of amyloid deposition. Neurochem Res. 2003;28:1029–1034. doi: 10.1023/a:1023255106106. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nishitomi K, Sakaguchi G, Horikoshi Y, Gray AJ, Maeda M, Hirata-Fukae C, Becker AG, Hosono M, Sakaguchi I, Minami SS, Nakajima Y, Li HF, Takeyama C, Kihara T, Ota A, Wong PC, Aisen PS, Kato A, Kinoshita N, Matsuoka Y. BACE1 inhibition reduces endogenous Abeta and alters APP processing in wild-type mice. J Neurochem. 2006;99:1555–1563. doi: 10.1111/j.1471-4159.2006.04178.x. [DOI] [PubMed] [Google Scholar]
- Nunan J, Small DH. Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 2000;483:6–10. doi: 10.1016/s0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003a;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003b;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Pena F, Ordaz B, Balleza-Tapia H, Bernal-Pedraza R, Marquez-Ramos A, Carmona-Aparicio L, Giordano M. Beta-amyloid protein (25–35) disrupts hippocampal network activity: role of Fyn-kinase. Hippocampus. 2010;20:78–96. doi: 10.1002/hipo.20592. [DOI] [PubMed] [Google Scholar]
- Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, Krishnamurthy P, Herman M, Wang L, Schachter JB, Nelson RB, Lau LF, Duff KE. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci. 2007;27:3090–3097. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu GQ, Palop JJ, Noebels JL, Mucke L. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi SK, Wood JG. The protein tyrosine kinase, fyn, in Alzheimer's disease pathology. Neuroreport. 1993;4:435–437. doi: 10.1097/00001756-199304000-00024. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303(Pt 3):701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Nakaya T. Regulation of amyloid beta-protein precursor by phosphorylation and protein interactions. J Biol Chem. 2008;283:29633–29637. doi: 10.1074/jbc.R800003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. Paired helical filament tau in Alzheimer's disease. The kinase connection. Am J Pathol. 1994;144:449–453. [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Chin J, Netzer WJ, Gouras GK, Lesne S, Malinow R, Lombroso PJ. Beta-amyloid modulation of synaptic transmission and plasticity. J Neurosci. 2007;27:11832–11837. doi: 10.1523/JNEUROSCI.3478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, Lombroso PJ. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]