Abstract
STEP61 is a protein tyrosine phosphatase recently implicated in the pathophysiology of Alzheimer’s disease (AD). STEP61 is elevated in human AD prefrontal cortex and in the cortex of several AD mouse models. The elevated levels of active STEP61 down-regulate surface expression of GluN1/GluN2B (formerly NR1/NR2B) receptor complexes, while genetically reducing STEP levels rescues both the biochemical and cognitive deficits in a triple transgenic AD mouse model (3xTg-AD). Here we show that increased STEP61 also plays a role in Aβ-mediated internalization of the AMPA receptor (AMPAR) subunits GluA1/GluA2 (formerly GluR1/GluR2). We purified Aβ oligomers and determined that oligomers, but not monomers, lead to endocytosis of GluA1/GluA2 receptors in cortical cultures. The decrease in GluA1/GluA2 receptors is reversed in the progeny of STEP KO mice crossed with Tg2576 mice, despite elevated levels of Aβ. These results provide strong support for the hypothesis that STEP61 is required for Aβ-mediated internalization of GluA1/GluA2 receptors.
Keywords: Alzheimer’s disease, beta amyloid, glutamate receptor trafficking, protein tyrosine phosphatase, Striatal enriched protein tyrosine phosphatase, AMPA receptor
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that predominantly afflicts individuals over 65 years of age. The neuropathological hallmarks of AD are the appearance of amyloid plaques and neurofibrillary tangles. The main constituents of amyloid plaques are Aβ peptides that are derived from successive cleavage of amyloid precursor protein (APP) by the action of β- and γ-secretases. Previous studies suggest that beta amyloid (Aβ) peptides affect synaptic function early in the disease process (Selkoe, 2002), even before the appearance of amyloid plaques (Hsiao et al., 1996; Jacobsen et al., 2006). For example, exogenous application of either soluble or oligomeric Aβ peptides to cultures and slices disrupts spine morphology and blocks long-term potentiation (LTP), while in vivo administration impairs cognitive function in rodent models (Walsh et al., 2002; Lacor et al., 2007; Shankar et al., 2008). Decreased surface expression of NMDA receptors (NMDAR) (Snyder et al., 2005; Dewachter et al., 2009; Kurup et al., 2010; Zhang et al., 2010) and AMPAR (Almeida et al., 2005; Gu et al., 2009, Zheng et al., 2010) complexes are likely to underlie some of the biochemical, electrophysiological and behavioral defects seen in AD.
STEP61 (STriatal-Enriched protein tyrosine Phosphatase of MW 61 kDa) is a brain-specific tyrosine phosphatase implicated in the pathophysiology of AD (Zhang et al., 2010; Snyder et al., 2005). Elevated levels of STEP61 are found in the prefrontal cortex of AD patients and in cortical tissue from three transgenic mouse models (J20, Tg2576, and 3xTg-AD mice; Chin et al., 2005; Kurup et al., 2010b; Zhang et al., 2010). STEP61 dephosphorylates GluN2B (formerly NR2B) at a regulatory tyrosine (Tyr1472) (Snyder et al., 2005), and resulting in internalization of NMDARs by clathrin-mediated endocytosis (Roche et al., 2001; Lavezzari et al., 2003). Exogenous application of Aβ-enriched 7PA2 conditioned medium increased STEP61 levels and reduced surface GluN1/GluN2B receptors, whereas this effect of Aβ was absent in STEP KO cultures (Kurup et al., 2010a; Kurup et al., 2010b). Finally, genetically reducing STEP levels in a triple transgenic mouse model of AD (3xTg-AD) restored both the surface levels of GluN1/GluN2B receptors and the cognitive deficits in 6-month old 3xTg-AD mice (Zhang et al., 2010).
We purified soluble Aβ oligomers and monomers and tested their effects on glutamate receptor internalization in cortical cultures. We also determined whether reducing STEP61 levels might reverse the loss of surface GluA1/GluA2 (formerly GluR1 and GluR2) receptors caused by Aβ. To address this question, we crossed STEP KO mice with APP transgenic mice (Tg2576), and used western blotting and biotinylation experiments to analyze cortical tissue and neuronal cultures of the progeny. Our results show that STEP regulates GluA1/GluA2 endocytosis and suggests an important role in AD pathophysiology in mouse models.
Experimental procedures
Materials
YM-3 Centriprep filters were from Millipore (Bedford, MA). Superdex 75 10/300 columns were from Amersham Biosciences (Piscataway, NJ) and10–20% Tris-Tricine gradient acrylamide gels from Bio-Rad (Hercules, CA). All primary and secondary antibodies and their dilutions are listed in Table 1.
Table 1.
Primary and secondary antibodies used in western blots
| Antibody | Format | Immunogen | Host | Dilution | Source |
|---|---|---|---|---|---|
| anti-ERK2 | whole IgG, unconjugated | C-terminus of rat sequence | rabbit | 1:5000 | Santa Cruz Biotechnology, Santa Cruz, CA |
| anti-Aβ (6E10) | Ascites (IgG1) | Peptide corresponding to 3–8 amino acid of beta amyloid | mouse | 1:1000 | Covance, Berkeley, CA |
| anti-GluN2A | whole IgG, unconjugated | Amino acids 1265–1464 of mouse (GluN2A) | rabbit | 1:1000 | Millipore Billerica, MA |
| anti-GluN2B | whole IgG, unconjugated | C-terminal of mouse NR2B | rabbit | 1:1000 | Millipore |
| anti-GABAA (β2/3) | IgG1, unconjugated | extracellular domain of β2/3 | mouse | 1:1000 | Millipore |
| anti-GluA1 | whole IgG, unconjugated | C-terminus | mouse | 1:600 | Millipore |
| anti-STEP | IgG1, unconjugated | rat synthetic peptide | mouse | 1:1000 | Boulanger et al., 1995 |
| anti-pSTEP | whole IgG, unconjugated | synthetic phosphopeptide | rabbit | 1:1000 | Snyder et al., 2005 |
| anti-GluA1 | whole IgG, unconjugated | rat synthetic peptide | rabbit | 1:1000 | Millipore |
| anti-GluA2 | IgG1, unconjugated | recombinant human GluR2 | mouse | 1:1000 | Millipore |
| anti-PSD-95 | IgG2a, unconjugated | recombinant rat PSD-95 | mouse | 1:5000 | Millipore |
| anti-syntaxin | IgG1, unconjugated | recombinant rat syntaxin | mouse | 1:1000 | Millipore |
| anti-rabbit | whole IgG peroxidase-conjugated | rabbit Fc | donkey | 1:10,000 | Amersham Biosciences, Piscataway, NJ |
| anti-mouse | whole IgG peroxidase-conjugated | mouse Fc | sheep | 1:10,000 | Amersham Biosciences |
Purification of Aβ monomers and oligomers
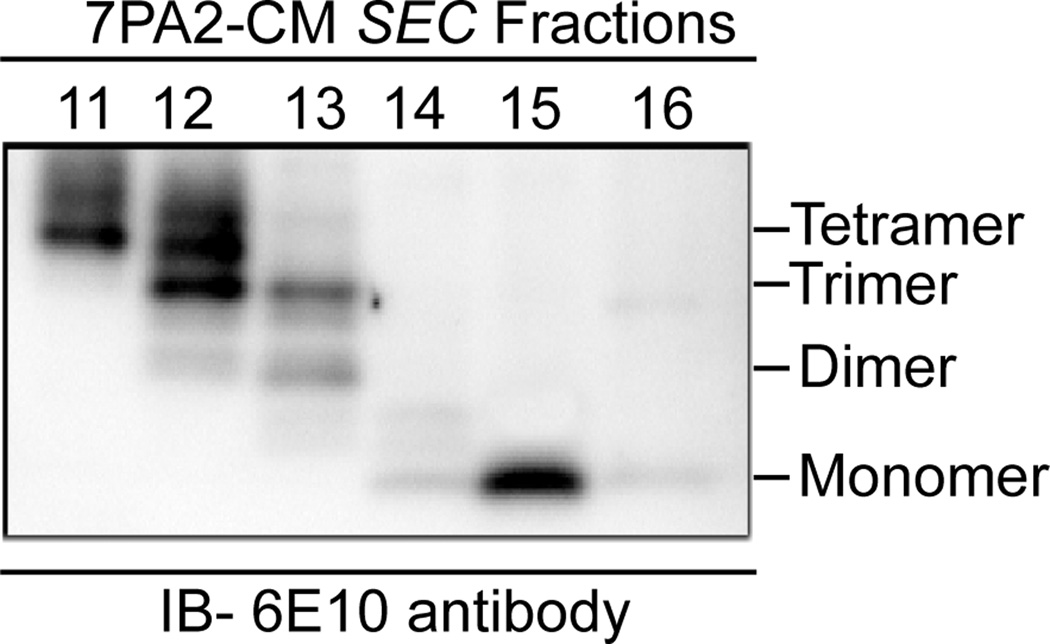
7PA2 cells or control Chinese Hamster Ovary (CHO) cells were grown in DMEM without serum for 16 h. Medium was collected and centrifuged at 200×g for 10 min to remove cell debris, and concentrated 15-fold using YM-3 Centriprep (Millipore) filters. Size exclusion chromatography was used to purify Aβ monomers and oligomers from the 7PA2 conditioned medium as described (Shankar et al., 2007). In brief, the concentrated conditioned medium (0.90 ml) was injected onto a Superdex 75 10/300 GL column (10×300 mm), and eluted with 1 ml/min 0.05 M ammonium acetate, and collected in 1 ml fractions. An aliquot (850 µl) from each fraction was lyophilized and stored at −80°C. The remaining 150 µl was lyophilized and loaded on 4–20% Tris-Tricine gradient gels to determine purity and sizes of the Aβ oligomers. Dimers, trimers and tetramers eluted in fraction 12, while monomers eluted in fraction 15 (Fig. 1).
Figure 1. Aβ oligomers purified from Aβ expressing 7PA2 conditioned medium (7PA2-CM) using size-exclusion chromatography.
Aβ oligomers and monomers in 7PA2-CM were fractionated by size-exclusion chromatography (SEC) and samples were analyzed by SDS-PAGE and immunoblotting with 6E10 antibody. Tetramers, trimers and dimers are present in fraction 12, while monomers were present in fraction 15.
Preparation of cortical neurons
Cortical cultures from rat (E18) or mouse (E15) embryos were grown as previously described (Xu et al., 2009; Zhang et al., 2008). In some experiments, TAT-STEP proteins (2 µM) were introduced into STEP WT or KO cultures for 45 min followed by Aβ oligomers for 1 h. The basic 11 amino acid TAT peptide is cell permeable and is used to transduce proteins into cell cultures or slices. TAT-STEP proteins were purified as previously described (Zhang et al., 2008) and were shown to enter cells with high efficiency within 30 minutes of application (Xu et al., 2009).
Subcellular fractionation and immunoblot analysis
Subcellular fractionations were obtained as described (Dunah et al., 2001; Xu et al., 2009). In brief, mouse brain homogenates were prepared in homogenization buffer (in mM): 10 Tris-HCl, pH 7.6, 320 sucrose, 150 NaCl, 5 EDTA, 5 EGTA, 50 NaF, 50 Na3VO4, and protease inhibitors. Homogenates were centrifuged at 800×g to remove nuclei and large debris (P1); S1 was centrifuged at 9200×g for 15 min to produce the P2 fraction. The P2 fraction was re-suspended in homogenization buffer containing 35.6 mM sucrose and centrifuged at 25000×g for 20 min to produce LP1. The purity of LP1 fraction was verified by marker proteins (Supplementary Fig. 2)
Proteins (30–50 µg) were loaded on 8% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% (w/v) nonfat dry milk and incubated with primary antibodies overnight at 4°C followed by incubation with secondary antibody. Bands were visualized using a G:BOX with a GeneSnap image program and quantified using Image J 1.33 (NIH).
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of Yale University. Two strains of mice were used in this study: Tg2576 mice (Taconic, Hudson, NY; Hsiao et al., 1996) and STEP KO mouse (Venkitaramani et al., 2009). Heterozygous STEP mice with and without the APP transgene were bred to produce progeny with or without the Tg2576 transgene, and STEP KOs with and without the APP transgene (Zhang et al., 2010). For biochemistry experiments, male 9 months old animals were used.
Surface biotinylation assay
Primary cortical neurons were treated with Aβ oligomers or monomers for 1 hr, and incubated in PBS containing 1.5 mg/ml sulfo-NHS-LC-biotin (Pierce, Rockford, IL) for 20 min at 4°C. Neurons were then processed to obtain surface and total receptors as described previously (Kurup et al., 2010a).
Aβ assay and western blot
Aβ1–42 concentrations were measured using a human Aβ (1–42) ELISA kit (Invitrogen). For Aβ western blots, mouse hemibrains (Tg2576, male: 3, 6, and 9 months old) were processed as described (Zhang et al., 2010).
Synthetic Aβ (1–42) was purchased from EMD biosciences. Aβ oligomers were prepared by dissolving the peptide in 1X PBS (phosphate buffered saline, pH 9.0) and incubating at 37°C for 48 hrs. For surface biotinylation experiments, the stock was diluted to 1 µM of Aβ (1–42) in Neurobasal medium and applied on cortical cultures for 1 hr.
In vitro phosphorylation
Two µg of purified GST-GluN2B (1361–1482aa) or GST-GluA2 (834–883aa) was phosphorylated using 50 ng of active Fyn (Upstate Biotechnology) in kinase assay buffer (in mM): 50 Tris-HCl, pH 7.5, 0.1 EGTA, 10 MgCl2, 500 µM ATP for 30 min at 30° C. Total reaction volume of kinase assay was 30 ml. The phosphorylation reaction was stopped by adding EDTA/ EGTA mix to a final concentration of 5 mM. This phosphorylation reaction mix was used as a source of pGluN2B or pGluA2 substrate for the in vitro dephosphorylation assay.
In vitro phosphatase assay
For in vitro phosphatase assays, 7PA2-CM derived monomer and oligomer treated cortical cultures were lysed in buffer containing (in mM): 50 Tris-HCl, pH 7.4, 150 NaCl, 0.5 EGTA, 2 EDTA, 1% Triton-X-100 and complete protease inhibitors (Roche). The samples were precleared with protein G-sepharose beads (GE Health sciences) and mixed with anti-STEP antibody (2 mg; 23E5) for 2 h at 4 °C. The antibody-bound complex was immunoprecipitated by adding protein G-sepharose (50 ml) and incubated for 2 h at 4 °C. The beads were washed three times with Tris buffer and a final wash with phosphatase assay buffer (in mM): 25 HEPES pH 7.3, 5 EDTA, 10 DTT. Beads were re-suspended in fresh phosphatase assay buffer (100 µl) and used as enzyme source for in vitro phosphatase assay. The phosphatase assay reaction was initiated by adding 20 µl of beads to Fyn phosphorylated GST-GluN2B or GST-GluA2 substrate and incubated for 30 mins at 30°C. The reaction was stopped by adding 2X SDS sample buffer and subjected to western blotting.
Statistics
All data are presented as means ± S.E. Differences among multiple groups were evaluated by ANOVA with Tukey’s post hoc test, and p values of 0.05 or less were considered significant.
Results
Aβ oligomers but not monomers lead to GluA1/GluA2 endocytosis
The addition of synthetic Aβ or Aβ-enriched 7PA2 conditioned medium (CM) to cortical neurons leads to NMDAR endocytosis through activation of STEP (Snyder et al., 2005; Kurup et al., 2010b). These earlier studies did not address whether endocytosis of AMPAR subunits was also regulated by STEP, or whether specific species of Aβ were involved in this process. Using size-exclusion chromatography, we purified oligomers (fraction containing dimers, trimers and tetramers) and monomers from Aβ-enriched-CM (Fig. 1) and tested their effects on endocytosis of glutamate receptors in rat cortical cultures by using surface biotinylation. Treatment with oligomers (fraction 12; 100 pM) for 1 hr resulted in a significant reduction in surface GluN1/GluN2B and GluA1/GluA2 subunits compared to cultures treated with the monomeric fraction (fraction 15; 100 pM) or control cultures (Fig. 2a–d, GluA1: 73.2 ± 5.7%; GluA2: 60.5 ± 6.3%; GluN1: 71.3 ± 6.2%; GluN2B: 63.8 ± 5.1%, p < 0.01, n = 4). Surface expression of GluN2A and GABAAβ2/3 were not changed (Fig. 2e and f, p > 0.05, n = 4). To rule out the possibility of contaminants in the 7PA2-CM oligomeric fraction, we tested the effect of synthetic oligomers on the internalization of GluA1, GluA2, GluN1, GluN2B receptors compared with synthetic monomers by using surface biotinylation in cortical cultures. The results showed the internalization of glutamate receptors (Sup. Fig. 1 a–d, GluA1: 77.23 ± 6.67, p<0.01; GluA2: 68.78 ± 6.02, p<0.01; GluN1: 77.43 ± 8.03, p<0.01; GluN2B: 67.80 ± 7.73, p<0.01) are specific to Aβ oligomers and independent of the preparation procedure. Similarly, the surface expression of GluN2A and GABAAβ2/3 were unchanged with synthetic oligomers (Sup. Fig. 1e – f, GluN2A: 96.63 ± 5.61, p>0.05; GABAAβ2/3: 102.55 ± 6.32, p>0.05, n = 4). These results indicate that either 7PA2-CM derived or synthetic Aβ oligomeric forms lead to the internalization of GluA1/GluA2 and GluN1/GluN2B receptors.
Figure 2. 7PA2-CM derived oligomers but not monomers result in AMPAR and NMDAR endocytosis.
Surface proteins of cortical cultures were biotinylated and processed for immunoblots. Representative western blots of surface and total (a), GluA1; (b), GluA2; (c), GluN1; (d), GluN2B; (e), GluN2A; and (f), GABAAβ2/3 levels after treatment with Aβ monomers and oligomers from 7PA2-CM and control CHO-CM fractions. Histograms (lower panels) show quantification of surface receptors normalized to total receptor levels. Histograms show oligomers but not monomers lead to significant loss of surface NMDA and AMPA receptors (**p < 0.01; n = 4)
Aβ-induced GluA1/GluA2 receptor endocytosis is reduced in STEP KO cultures
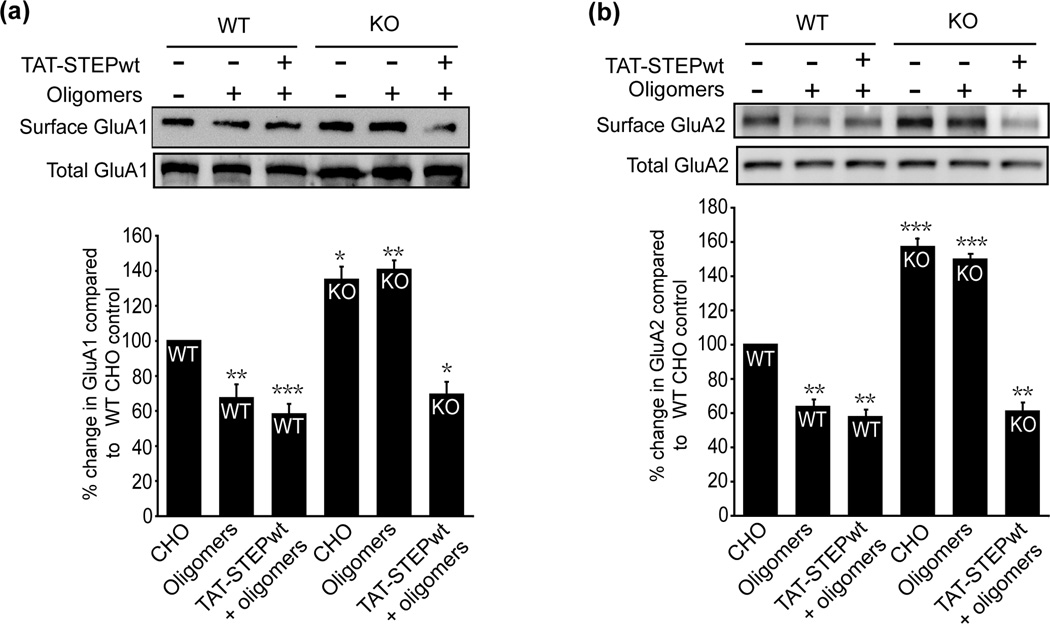
We next determined whether STEP regulates Aβ-induced GluA1/GluA2 receptor endocytosis by using biotinylation experiments. Aβ oligomers decreased the surface expression of GluA1 and GluA2 receptors in wild type (WT) mouse cortical cultures (Fig. 3a and b, GluA1: 67.3 ± 7.3%; GluA2: 63.6 ± 4.0%, p < 0.01, n = 5). In contrast, we observed no significant Aβ-induced decrease in the surface expression of these receptors in STEP KO cultures (GluA1: 143.6 ± 4.7%; GluA2: 149.2 ± 3.2%, p > 0.05 compared to STEP KO control levels). Higher baseline surface expression of GluA1 and GluA2 was detected in STEP KO cultures, consistent with the role of STEP in mediating the internalization of these receptors (Zhang et al., 2008) (GluA1: 134.5 ± 7.3%, p < 0.05; GluA2: 156.8 ± 4.4%, p < 0.001).
Figure 3. STEP KO cultures show no decrease in surface AMPAR after Aβ oligomers treatment.
Surface proteins of WT and STEP KO cortical cultures were biotinylated and processed for immunoblots. Representative western blots of (a), GluA1 and (b), GluA2 after treatment with control CHO fraction 12, 7PA2 fraction 12 (oligomers), and wild type TAT-STEP + 7PA2 fraction 12. Histograms (lower panels) show quantification of surface receptors normalized to total receptor levels. STEP KO cultures showed higher levels of AMPA receptors (GluA1: *p < 0.05; GluA2: ***p < 0.001; n = 5). Oligomers led to a significant decrease of these receptors in STEP WT cultures (**p < 0.01; n = 5), but no decrease in STEP KO cultures (p > 0.05; n = 5). Addition of wild type TAT-STEP to STEP KO cultures rescued GluA1 and GluA2 endocytosis (GluA1: *p < 0.05; GluA2: **p < 0.01; n = 5).
The addition of TAT-STEP restored Aβ-mediated glutamate receptor endocytosis (Fig. 3a and b, GluA1: 69.1 ± 6.5%; GluA2: 60.2 ± 4.3%, p < 0.001). These results indicate that replacing STEP is sufficient to rescue the Aβ-induced GluA1/GluA2 receptor endocytosis in STEP KO cultures.
Aβ-induced GluA1/GluA2 endocytosis is reduced in STEP KO mice
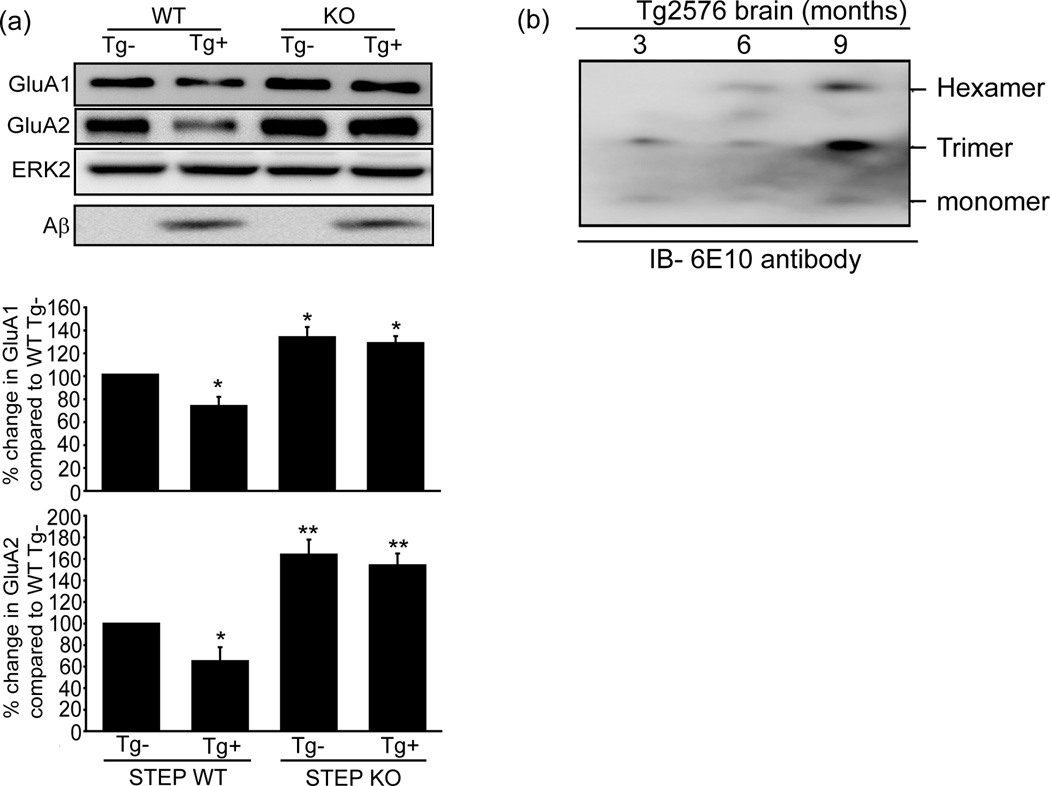
We next tested whether crossing STEP KO mice with Tg2576 mice would produce progeny that no longer exhibit reduced glutamate receptor surface expression in the presence of elevated Aβ. We tested 9-month old mice because at this age Tg2576 mice have elevated levels of Aβ and learning deficits (Hsiao et al., 1996). Basal surface expression of GluA1/GluA2 subunits was higher in cortical synaptosomal fractions (LP1) derived from STEP KOs compared to WT mice (Fig. 4a, GluA1: 132.6 ± 8.7%, p < 0.05; GluA2: 164.1 ± 12.8%, p < 0.01, n = 6). Mice with the APP transgene and WT levels of STEP61 showed a significant decrease in surface GluA1/GluA2 subunits in LP1 fractions (Fig. 4a, GluA1: 78.5 ± 7.1%, p < 0.05; GluA2: 64.7 ± 12.8%, p < 0.05). In contrast, mice with the Aβ transgene and null for STEP did not exhibit a significant decrease in the surface expression of GluA1/GluA2 subunits (Fig. 4a, GluA1: 134.1 ± 7.6%; GluA2: 153.8 ± 9.6%, p > 0.05 compared to STEP KO mice without the transgene, n = 6). Immunoblot for Aβ confirmed that 9-month old Tg2576 mice had higher Aβ oligomers than 3-month old mice (Fig. 4b). These findings indicate that despite high Aβ levels, GluA1/GluA2 endocytosis was significantly reduced in the absence of STEP.
Figure 4. Progeny from crosses between Tg2576 and STEP KO mice restores surface AMPAR levels.
Crosses were made between Tg2576 and STEP KO mice, cortical tissues were analyzed for AMPAR levels in synaptosome fractions (LP1). (a), GluA1, GluA2: Representative western blots from 9-month old mice. Histograms of results shown in lower panel. Tg2576 mice brains with high levels of Aβ showed significantly lower levels of GluA1/GluA2 (GluA1, GluA2: *p < 0.05; n = 6). Progeny with no STEP protein had significantly higher levels of GluA1/GluA2 (GluA1: *p < 0.05; GluA2: **p < 0.01; n = 6). STEP KO mice no longer showed detectable endocytosis of GluA1/GluA2 even with high levels of Aβ (p > 0.05; n = 6). ERK2 immunoreactivity was the loading control. (b), Homogenates of hemibrains (3-, 6-, 9-months Tg2576 mice) were analyzed by western blot and show an increased levels of oligomers occur in Tg2576 mice brains with aging.
Aβ oligomer treatment of cortical cultures leads to increased STEP61 activity
Previous studies reported that Aβ oligomer leads to increase in STEP activity by two mechanisms, (i) a calcineurin-mediated dephosphorylation of the regulatory PKA site on STEP, (ii) an increase in total STEP levels due to decreased degradation by the proteasome system (Snyder et al., 2005; Kurup et al., 2010a). To explore whether oligomeric Aβ treatment led to activation of STEP61, we immunoprecipitated STEP61 from cortical cell lysate treated with Aβ monomers or oligomers. Immunoprecipitated STEP61 was incubated with either p-tyr1472 GST-GluN2B or p-tyr GST-GluA2, both of which were previously phosphorylated by Fyn. STEP61 immunoprecipitated from cultures treated with oligomers significantly decreased p-tyr1472 GluN2B (Fig. 5a, pGluN2B: 61.8 ± 9.1%, p < 0.01, n = 3). Tyrosine phosphorylation of GluA2 was also significantly decreased as measured by an anti-p-tyr antibody (Fig. 5b, pGluA2: 59.6 ± 7.6%, p < 0.01, n = 3). Monomer treatment did not alter STEP-mediated dephosphorylation of the receptors. These results demonstrated that STEP61 was activated by Aβ oligomers and led to the tyrosine dephosphorylation of GluN2B and GluA2 in vitro. We also found the activation of STEP by oligomers involves dephosphorylation of STEP at its regulatory PKA site and accumulation of total STEP levels (Fig. 5c).
Figure 5. STEP activity is required for Aβ induced NMDAR and AMPAR endocytosis.
(a), STEP protein was immunoprecipitated from cortical cultures treated with Aβ oligomers or monomers, and assayed in vitro using GST-phospho-GluN2B as substrate. Dephosphorylation of pGluN2B was assessed with a p-tyr1472-specific antibody (**p < 0.01; n = 3). (b), STEP protein was immunoprecipitated from cortical cultures treated with Aβ oligomers or monomers, and assayed in vitro using GST-phospho-GluA2 as substrate. Dephosphorylation of GluA2 was assessed with a tyrosine phosphorylation antibody (**p < 0.01; n = 3). (c), 7PA2-CM derived monomer and oligomer fractions treated cortical cultures were immunoprecipitated with STEP antibody and analyzed for pSTEP and STEP immunoreactivity. The oligomer treated samples show increased STEP levels and decreased pSTEP levels compared to monomer treated samples.
Discussion
The data reported here demonstrate that STEP is involved in Aβ-induced ionotrophic glutamate receptor internalization. STEP is elevated in the cortex of AD mouse models and human AD patients and contributes to the internalization of surface GluN1/GluN2B receptors (Kurup et al., 2010a, b). We recently showed that genetic reduction of STEP restores surface GluN1/GluN2B subunits and attenuates cognitive deficits in 3xTg-AD mice (Zhang et al., 2010). The data reported here expand on these findings by demonstrating that STEP is also involved in regulating surface expression of GluA1/GluA2 receptors.
We first demonstrate that treatment with Aβ oligomers derived either from 7PA2-CM or synthetic Aβ are equally capable of internalizing GluA1/GluA2 and GluN1/GluN2B receptor complexes. Aβ oligomers leads to GluA1/GluA2 endocytosis in wild type cortical cultures, but not in cultures derived from STEP KO mice. The addition of wild type TAT-STEP to the KO cultures rescues the endocytosis, demonstrating a role of STEP in this process. In addition, Tg2576 mice expressing elevated Aβ levels have reduced levels of GluA1/GluA2 receptors in synaptic membrane fractions, whereas these receptors were restored in progeny of STEP KO and Tg2576 crosses.
The finding that GluA1/GluA2 receptors are internalized by STEP is consistent with several studies that demonstrate a reduction of AMPAR subunits in AD mouse model brains (Almeida et al., 2005; Gu et al., 2009), in neuronal cultures treated with Aβ (Hsieh et al., 2006; Parameshwaran et al., 2007), as well as in human AD brains (Armstrong et al., 1994; Thorns et al., 1997). Our results suggest that STEP contributes to the disruption of synapses caused by Aβ oligomers by promoting internalization of both AMPARs and NMDARs.
The mechanism by which STEP regulates GluA1/GluA2 internalization remains unclear. One study showed that stimulation of the metabotrophic glutamate receptor mGluR5 leads to a STEP-mediated tyrosine dephosphorylation of GluA2 and internalization of GluA1/GluA2 (Zhang et al., 2008), although the tyrosine residue on GluA2 that is dephosphorylated by STEP remains unidentified. A second report shed some light on one molecular mechanism that might regulate Aβ-mediated AMPAR endocytosis (Scholz et al., 2010). GluA2 was found to directly interact with BRAG2, a synaptic protein that is a guanine-exchange factor (GEF) for the GTPase Arf6. When Arf6 is activated by BRAG2, it recruits the adaptor protein AP2 and clathrin to synaptic membranes, thereby promoting internalization of GluA1/GluA2 receptor complexes (Krauss et al., 2003; Scholz et al., 2010). Activation of Arf6 requires the dephosphorylation of GluA2 Tyr876 by an unknown tyrosine phosphatase. Whether STEP is the PTP that dephosphorylates GluA2 Tyr876 is the subject of current investigation. Our in vitro dephosphorylation assay with a Tyr-phosphorylated C-terminal sequence GluA2(834–883) suggests that GluA2 is a substrate of STEP. STEP immunoprecipitated from oligomer treated samples significantly decreases the tyrosine phosphorylation of GluA2 compared to STEP immunoprecipitated from monomer treated samples. The current data also demonstrate that oligomer-treated cortical culture samples show increased total STEP levels and increased catalytic activity, consistent with earlier findings that Aβ inhibits the proteasome-mediated degradation of STEP (Kurup et al., 2010). Our findings are also consistent with the finding that proteasome activity is significantly inhibited by Aβ oligomers, but not by Aβ monomers (Tseng et al., 2008).
Additional STEP substrates include members of the mitogen activated protein kinase family extracellular-signal regulated kinase 1/2 (ERK1/2), p38 (Paul et al., 2003; Munoz et al., 2003), and Fyn, a member of the Src kinase family (Nguyen et al., 2002). STEP dephosphorylates ERK1/2, p38, and Fyn at a regulatory tyrosine residue in their activation loop, thereby inactivating these enzymes. The emergent model of STEP function therefore suggests that STEP normally opposes the development of synaptic strengthening by regulating the activity of these key signaling proteins (reviewed by Gobel-Goody et al., in press). One prediction of this model is that increases in activated STEP disrupt synaptic plasticity in two ways: through a direct dephosphorylation of glutamate receptor subunits and through the indirect dephosphorylation and inactivation of tyrosine kinases required for glutamate receptor stabilization in synaptosomal membranes.
The present findings support this model of STEP function. We demonstrate that Aβ oligomers, and not monomers, are responsible for the internalization of two glutamate receptor subtypes, AMPARs and NMDARs. We purified a fraction from 7PA2 conditioned medium consisting of dimers, trimers, and tetramers, and used this sample at picomolar concentrations. We did not use higher molecular weight oligomers in this study, and cannot address whether they might also induce AMPAR/ NMDAR endocytosis. Nonetheless, our findings are consistent with the earlier evidence suggesting the involvement of oligomers in the pathophysiology of AD (Lesné et al., 2006). Aβ dimers from human Alzheimer's brains impair memory consolidation (Shankar et al., 2008), whereas Aβ trimers isolated from 7PA2 medium inhibit LTP in hippocampal slices (Townsend et al., 2006).
In summary, we demonstrate that Aβ oligomers lead to the internalization of GluA1 and GluA2 receptors. STEP KO cultures treated with Aβ are resistant to Aβ–mediated internalization of GluA1/GluA2 receptors, but adding wild type STEP into these cultures restored endocytosis of these receptors. A similar pattern emerged when we examined progeny of STEP KO and Tg2576 mice. Crosses between mice with the APP mutation and mice null for STEP produced progeny that no longer showed increased internalization of GluA1/GluA2 receptors, despite elevated levels of Aβ. The work extends earlier studies demonstrating that STEP mediates the internalization of NMDAR complexes (Kurup et al., 2010a, b), and that genetic removal of STEP is sufficient to restore cognitive deficits in 6-month old 3xTg-AD mice (Zhang et al., 2010). As later stages of AD are characterized by increased excitotoxicity and neuronal degeneration, the increased glutamate receptor levels in the absence of STEP are likely to have detrimental effects in more advanced AD, a hypothesis that needs to be experimentally tested in future studies. Taken together, this body of work validates STEP as a candidate for drug discovery in an effort to find STEP inhibitors as potential therapeutic agents for the treatment of AD.
Supplementary Material
Surface proteins of cortical cultures were biotinylated and processed for immunoblots. Representative western blots of surface and total (a), GluA1; (b), GluA2; (c), GluN1; (d), GluN2B; (e), GluN2A; and (f), GABAAβ2/3 levels after treatment with synthetic Aβ monomers and oligomers. Histograms (lower panels) show quantification of surface receptors normalized to total receptor levels. Histograms show oligomers but not monomers lead to significant loss of surface NMDARs and AMPARs (**p < 0.01; n = 3).
(a), Schematic representation of subcellular fractionation procedure.
(b), Characterization of subcellular compartments for marker proteins (PSD-95; Syntaxin; STEP) with respective antibodies. Note that PSD-95, syntaxin and STEP are present in the LP1 fraction.
Acknowledgements
We thank laboratory members and Dr. Susan G Goody for helpful discussions and critical reading of the manuscript. We thank Dr. Deepa Venkitramani for helpful suggestions on mouse crosses. This work was funded by The American Health Assistance Foundation, NIH grants MH01527 and MH052711 to PJL, AG09464 to PG and ACN, and The Fisher Center for Alzheimer’s Research Foundation and Cure Alzheimer’s Fund to PG.
Abbreviations used
- STEP
Striatal Enriched tyrosine Phosphatase
- KO
Knock-out
- AD
Alzheimer’s disease
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-Methyl-D-aspartate
- Aβ
amyloid beta
- APP
amyloid precursor protein
- LTP
long-term potentiation
- CHO
chinese hamster ovary
- TAT
trans-activator of transcription
- PBS
phosphate buffered saline
Footnotes
The authors declare no conflict of interest regarding the work reported here.
References
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Ikonomovic MD, Sheffield R, Wenthold RJ. AMPA-selective glutamate receptor subtype immunoreactivity in the entorhinal cortex of non-demented elderly and patients with Alzheimer's disease. Brain Res. 1994;639:207–216. doi: 10.1016/0006-8993(94)91732-9. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: one STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewachter I, Filipkowski RK, Priller C, Ris L, Neyton J, Croes S, Terwel D, Gysemans M, Devijver H, Borghgraef P, Godaux E, Kaczmarek L, Herms J, Van Leuven F. Deregulation of NMDA-receptor function and down-stream signaling in APP [V717I] transgenic mice. Neurobiol Aging. 2009;30:241–256. doi: 10.1016/j.neurobiolaging.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. 2001 doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Baum ML, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for STriatal-Enriched protein tyrosine Phosphatase (STEP) in neuropsychiatric disorders. Pharmacological Reviews. doi: 10.1124/pr.110.003053. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Liu W, Yan Z. {beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem. 2009;284:10639–10649. doi: 10.1074/jbc.M806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JS, Wu CC, Redwine JM, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Venkitaramani DV, Xu J, Lombroso PJ. The role of STEP in Alzheimer's disease. Channels (Austin) 2010a;4:347–350. doi: 10.4161/chan.4.5.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010b;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharm. 2003;45:729–737. doi: 10.1016/s0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Munoz JJ, Tarrega C, Blanco-Aparicio C, Pulido R. Differential interaction of the tyrosine phosphatases PTP-SL, STEP and HePTP with the mitogen-activated protein kinases ERK1/2 and p38alpha is determined by a kinase specificity sequence and influenced by reducing agents. Biochem J. 2003;372:193–-201. doi: 10.1042/BJ20021941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Parameshwaran K, Sims C, Kanju P, Vaithianathan T, Shonesy BC, Dhanasekaran M, Bahr BA, Suppiramaniam V. Amyloid beta-peptide Abeta(1–42) but not Abeta(1–40) attenuates synaptic AMPA receptor function. Synapse. 2007;61:367–374. doi: 10.1002/syn.20386. [DOI] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Thorns V, Mallory M, Hansen L, Masliah E. Alterations in glutamate receptor 2/3 subunits and amyloid precursor protein expression during the course of Alzheimer's disease and Lewy body variant. Acta Neuropathol. 1997;94:539–548. doi: 10.1007/s004010050748. [DOI] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BP, Green KN, Chan JL, Blurton-Jones M, LaFerla FM. Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol Aging. 2008;29:1607–1618. doi: 10.1016/j.neurobiolaging.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding L, Tressler L, Allen M, Sacca R, Picciotto MR, Lombroso PJ. Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63:69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, Lombroso PJ. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model. Proc Natl Acad Sci USA. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surface proteins of cortical cultures were biotinylated and processed for immunoblots. Representative western blots of surface and total (a), GluA1; (b), GluA2; (c), GluN1; (d), GluN2B; (e), GluN2A; and (f), GABAAβ2/3 levels after treatment with synthetic Aβ monomers and oligomers. Histograms (lower panels) show quantification of surface receptors normalized to total receptor levels. Histograms show oligomers but not monomers lead to significant loss of surface NMDARs and AMPARs (**p < 0.01; n = 3).
(a), Schematic representation of subcellular fractionation procedure.
(b), Characterization of subcellular compartments for marker proteins (PSD-95; Syntaxin; STEP) with respective antibodies. Note that PSD-95, syntaxin and STEP are present in the LP1 fraction.