Abstract
Purpose
Fatigue is one of the most disturbing complaints of cancer patients and often is the reason for discontinuing treatment. This randomized controlled study tested the hypothesis that increased morning bright light, compared to dim light, would result in less fatigue in women with breast cancer undergoing chemotherapy.
Methods
39 women newly diagnosed with Stage I-III breast cancer were randomized to either bright white light (BWL) or dim red light (DRL) treatment and were instructed to use the light box for 30 minutes every morning throughout the first 4 cycles of chemotherapy. The Multidimensional Fatigue Symptom Inventory was administered prior to the start of chemotherapy (baseline), during the chemotherapy treatment week of cycle 1 (C1TW), the last week (recovery week) of cycle 1 (C1RW), chemotherapy treatment week of cycle 4 (C4TW), the last week (recovery week) of cycle 4 (C4RW).
Results
The DRL group reported increased fatigue at C1TW (p=0.003) and C4TW (p<0.001) compared to baseline while there was no significant change from baseline in the BWL group. A secondary analysis showed that the increases in fatigue levels in the DRL group were not mediated through associated with changes in sleep or in circadian rhythms as measured with wrist actigraphy.
Conclusions
The results of this study suggest that morning bright light treatment may prevent overall fatigue from worsening during chemotherapy. Although our hypothesis that overall fatigue would improve with bright light treatment was not supported, the lack of deterioration in total fatigue scores suggests that bright morning light may be a useful intervention during chemotherapy for breast cancer.
Keywords: fatigue, breast cancer, chemotherapy, light treatment
Fatigue is one of the most frequent and most disturbing complaints of cancer patients.[1] Studies have shown that over 75% of patients undergoing radiation or chemotherapy report feeling tired or weak.[2] Fatigue interferes with daily activities, reduces quality of life,[1] and often is the reason for discontinuing treatment.[2] The fatigue of cancer is different from general fatigue. Cancer related fatigue (CRF) has been defined as a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.[3] CRF is characterized by feeling tired even after rest, a reduced capacity to carry out normal daily activities, slow physical recovery from tasks, diminished concentration, and is more severe and distressing than typical fatigue.[4, 5]
Factors contributing to fatigue include post-operative pain, emotional distress (depression, anxiety), anemia, sleep disturbance, nutritional deficits, decreased physical fitness and activity levels, endocrine dysfunction, inflammatory cytokines and/or disrupted circadian rhythms.[6, 7, 8, 9] There is some evidence that the amount of fatigue might be affected by chemotherapy.[10] In our previous work however, we found that patients are already fatigued even before they began chemotherapy, and that fatigue worsened during treatment.[8] Other clinical studies have indicated that a large proportion of breast cancer patients continue to experience fatigue for months after therapy is completed.[11], [12]
On the other hand, fatigue is also a common complaint in patients with other disorders, such as depression, jet lag, shift work disorder, circadian rhythm disturbances (e.g., delayed or advanced sleep phase) and sleep disorders. These disorders are commonly treated with bright light therapy.[13, 14] In addition to improving depression, sleep and shifting circadian rhythms,[15, 16, 17, 13, 18, 19] light is known to have an alerting effect. Phipps-Nelson et al. found that increasing bright light exposure as compared to dim light, decreased subjective sleepiness and improved performance.[20] Other investigators found similar results.[21, 22, 23] Reviews and meta-analyses have confirmed the efficacy of light treatment for these disorders.[24] Although fatigue among breast cancer patients has been found to be related to disrupted circadian rhythms;[25, 26] and it is known that bright light improves circadian rhythms, the effect of light treatment on fatigue has not been investigated.
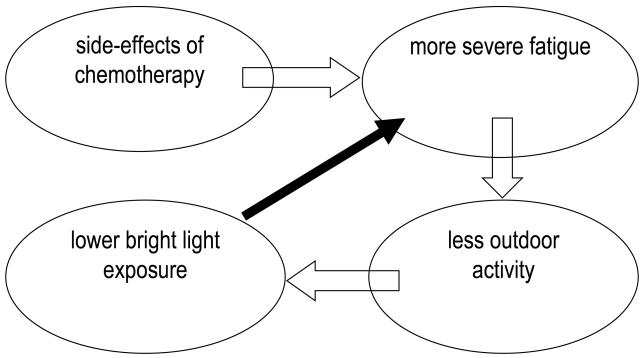
Liu et al. examined the relationship between ambient light exposure and fatigue in women with breast cancer prior to and during chemotherapy.[27, 28] Prior to chemotherapy, women were exposed to 54 minutes of bright light with a mean of 468 lux exposure per day. (As a point of references, social lighting is <200 lux, a bright office <500 lux, outdoors on a cloudy day 1500-5000 lux, and outdoors on a sunny day 10,000 to 50,000 lux or more.) During chemotherapy, particularly during week 1 of cycle 1 and week 1 of cycle 4, the amount of time exposed to bright light was reduced to about 35 minutes with a mean of only 300 lux. In addition, greater fatigue was associated with lower light intensity and less time exposed to bright light.[28] This association between fatigue and light exposure may suggest the existence of a vicious cycle, with side-effects of chemotherapy causing more severe fatigue, more severe fatigue causing less outdoor activity, less time spent outdoors causing lower bright light exposure, and lower light exposure then further exacerbating the fatigue (see Fig 1a). Increased light exposure may break this vicious cycle and mitigate the fatigue (see Fig 1b). As many studies have shown that light treatment is more effective when administered in the morning than in the evening,[29, 30] this randomized controlled study tested the hypothesis that increased morning bright light, compared to dim light exposure, would result in less fatigue during chemotherapy.
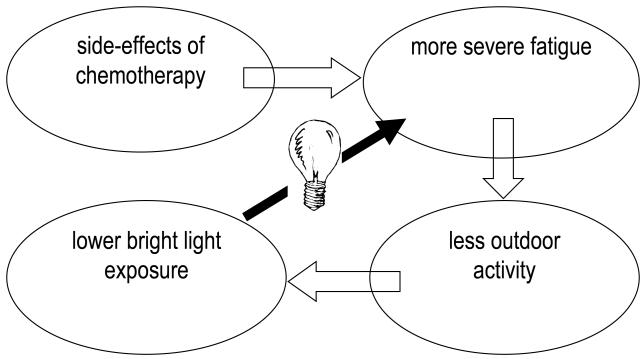
Figure 1a and b.
Model of a negative feedback loop of chemotherapy leading to fatigue, which leads to less time spent outdoors, which leads to less bright light exposure, which could lead to increased fatigue (Fig 1a). We hypothesized that the addition of morning bright light would break this cycle (Fig. 1b).
Methods
Subjects
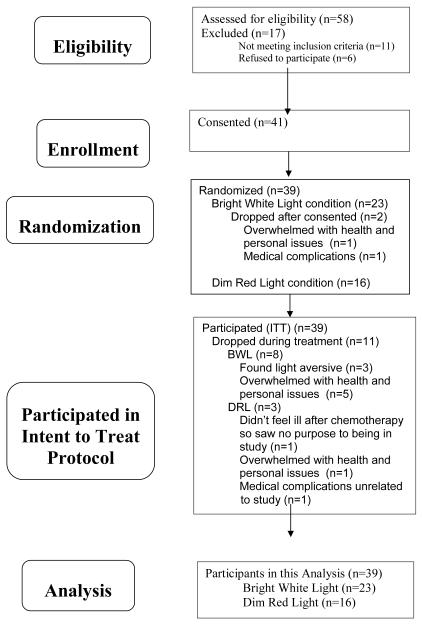
Fifty-eight women were referred by medical oncologists in the San Diego community or from the Moores UCSD Cancer Center. All women were newly diagnosed with State I-III breast cancer, as determined by their oncologist using the American Joint Committee on Cancer Staging Manual 6th Edition,[31] and each was scheduled to receive at least 4 cycles of chemotherapy. As shown in Fig 2, of the 58 women screened, 41 (71%) were consented and randomized, and two dropped out after randomization but before the start of light treatment. The mean age of the remaining 39 women (67% of the original sample) was 53.95 yrs (SD=9.1, range=32-70 years).
Figure 2.
Consort table of participant recruitment and retention
Exclusion criteria included being pregnant, having metastatic or stage IIIB (including inflammatory) breast cancer, significant pre-existing anemia or confounding underlying medical illnesses or any other physiological or psychological impairments that would have limited participation.
The study was approved by the University of California Committee on the Protection of Human Subjects.
Procedure
Once informed consent was signed, the women were randomized based on a randomization table, to either the bright white light group (BWL; n=23) or the dim red light group (DRL; n=16) with a larger proportion randomized to BWL to provide a larger sample treated with this noninvasive, potentially beneficial treatment. Rather than being told that the DRL was a placebo, participants were told that “It is unclear if certain frequencies of light are better than others and so both white and red lights are being tested.”
Participants met with research staff either at their homes or at the UCSD General Clinical Research Center where questionnaires were administered prior to the start of chemotherapy (baseline, BL), during the chemotherapy treatment week of cycle 1 (C1TW), the last week (recovery week) of cycle 1 (C1RW), chemotherapy treatment week of cycle 4 (C4TW), and the last week (recovery week) of cycle 4 (C4RW). All but two women received 3-week cycles of chemotherapy while the two receiving two-week cycles. Only data from the primary outcome of fatigue are presented.
Actigraphy
Wrist actigraphs were used to estimate sleep/wake activity and circadian rhythm data. Activity was recorded with the Actiwatch-Light® (Mini Mitter/Phillips/Respironics) for three consecutive days (i.e., 72 hours) at each time point: pre-chemotherapy and during the treatment and recovery weeks of cycle 1 and cycle 4. The Actiwatch-Light® uses a piezoelectric linear accelerometer (sensitivity <.01 g-force) with a sampling rate of 32Hz to measure and record wrist movement. Calculating wrist activity over time allowed for an objective measure of duration and disruption of sleep and circadian activity rhythms. The recorded actigraphy data were analyzed using Actiware® sleep and activity monitoring software program (version 5, by Mini Mitter∣Respironics/Philips). Women kept a sleep log with bed time and up time for each of the three days at each time point and the sleep log was used to edit the actigraphy data.
Light Treatment
Light Treatment: Light was administered via a Litebook 1.2 (Litebook®, Ltd. Medicine Hat, Canada). The Litebook® is a small (6” ×5” ×1”) and lightweight (8 oz.) box designed to be placed on a table about 18” from the patient’s head and within 45° of the midline of the visual field. The Litebook® utilizes 60 white light emitting diode (LED) lights with a distribution of energy particularly concentrated in the middle and long wavelengths.[32] For purposes of safety, the Litebook® emits no ultraviolet (UV) light. An identical-appearing device utilizing red LEDs emitting dim red light at less than 50 lux was used for the placebo group.
The appropriate light box was given to each woman. All women were instructed to place the light box on a table or countertop while seated with the distance between the eyes and the device of approximately 18” and to self-administer the light treatment for 30 minutes every morning upon awakening throughout the first 4 cycles of chemotherapy.
The Litebooks® used for this study were modified to include an integrated meter which allowed for compliance monitoring by recording time and duration the light box was on each day. Partial compliance data were available for 30 patients (BWL n=17; DRL n=13), with some data missing for some weeks. The compliance meter failed to record usage for the remainder of the patients.
Fatigue
The Short Form of the Multidimensional Fatigue Symptom Inventory (MFSI-sf) was used to measure fatigue. The MFSI-sf is a 30-item scale with five subscales: General, Physical, Emotional, and Mental Fatigue, and Vigor. Each subscale includes 6 items and each item is rated on a 5-point scale indicating how true the statement was during the last week (0=not at all, 4=extremely). The sum of General, Physical, Emotional, and Mental subscale scores minus the Vigor subscale score generates a total score. Range of possible score for each subscale is 0 to 24, and the range for total score is −24 to 96, with higher score indicating more severe fatigue, except for the Vigor subscale, where larger score indicates less fatigue. The mean of the normal controls in the original validation study was 0.85.[33, 34]
Data Analysis
Descriptive statistics were calculated both for the entire group and separately for each treatment group. T-tests and Fisher’s exact tests were used to assess group differences at baseline for possible confounders (i.e., demographic variables, clinical characteristics, and chemotherapy regimen). Variables that significantly differed between the treatment groups were to be controlled for in the inferential analysis, however there were no significant differences between groups so no confounders were controlled for.
Linear mixed-effects models and restricted maximum likelihood methods were used for comparing the progression of fatigue during chemotherapy for each group.[35] Contrasts were included in the models to compare fatigue levels during chemotherapy regimen (i.e., C1TW, C1RW, C4TW, and C4RW) to pre-treatment baseline values for each treatment group.
Mixed models allow for partial data where the number of measures per person could vary and therefore women with missing data at some time-points could be included in the analysis.[35] Mixed models rely on the “missing at random” assumption. Furthermore, mixed models decrease the chance of bias from analyzing only completing subjects.
Results
Demographics
The women were primarily Caucasian, married with high school or some college education and a yearly family income of less than $100,000 (Table 1). Their cancer history is presented in Table 2 which shows the percent of women in each stage of breast cancer, menopausal status at study entry, surgery status and chemotherapy regimen. All but two women had chemotherapy regimens that lasted three weeks per cycle. There were no significant differences between randomized groups in any of these variables.
Table 1.
Demographic and medical characteristics at baseline for the two randomized groups
| Bright White Light (n=23) |
Dim Red Light (n=16) |
|
|---|---|---|
| Age (mean years [SD]) | 54.3 (9.3) | 53.5 (9.0) |
| Marital Status (n [%]) | ||
| Never Married | 1 (4.4) | 1 (6.3) |
| Divorced | 7 (30.3) | 3 (18.7) |
| Widowed | 2 (8.7) | 1 (6.3) |
| Married | 13 (56.6) | 11 (68.7) |
| Ethnicity/Race (n [%]) | ||
| Caucasian | 15 (65.2) | 13 (81.3) |
| African American Black | 4 (17.4) | 2 (12.4) |
| Asian | 2 (8.7) | 1 (6.3) |
| Other | 2 (8.7) | |
| Education (n [%]) | ||
| Some High School or Less | 1 (4.4) | 0 |
| Completed High School | 1 (4.4) | 0 |
| Some College | 6 (26.1) | 6 (37.5) |
| College Degree | 8 (34.7) | 4 (25) |
| Graduate Degree | 7 (30.4) | 6 (37.5) |
| Annual Family Income (n [%]) | ||
| ≤ $15,000 | 5 (21.7) | 3 (18.8) |
| ≤ $30,000 | 6 (26.1) | 0 |
| ≤ $50,000 | 1 (4.4) | 2 (12.5) |
| ≤ $100,000 | 4 (17.4) | 2 (12.5) |
| > $100,000 | 5 (21.7) | 6 (37.4) |
| Did not Answer | 2 (8.7) | 3 (18.8) |
Table 2.
Cancer History at baseline for the two randomized groups, n (%)
| Bright White Light (n=23) |
Dim Red Light (n=16) |
|
|---|---|---|
| Menopausal Status Pre-chemotherapy | ||
| Premenopausal | 5 (21.7) | 4 (25.0) |
| Perimenopausal | 3 (13.0) | 2 (12.5) |
| Postmenopausal | 8 (34.8) | 7 (43.8) |
| Post-Hysterectomy | 6 (26.1) | 3 (18.8) |
| Unknown | 1 (4.4) | 0 |
| Cancer Stage | ||
| Stage I | 4 (17.4) | 5 (31.3) |
| Stage II | 10 (43.5) | 6 (37.5) |
| Stage III | 4 (17.4) | 2 (12.5) |
| Unknown | 5 (21.7) | 3 (18.7) |
| Surgery per chemotherapy | ||
| Lumpectomy | 7 (30.4) | 8 (50.0) |
| Mastectomy | 9 (39.1) | 6 (37.5) |
| Double Mastectomy | 4 (17.4) | 1 (6.3) |
| Pre-op Chemotherapy | 2 (8.7) | 1 (6.3) |
| Unknown | 1 (4.4) | 0 |
| Chemotherapy Regimen | ||
| Exactly 4 cycles of AC | 3 (13.0) | 3 (18.8) |
| Exactly 4 cycles of AC + Taxotere | 5 (21.7) | 0 |
| Exactly 4 cycles of AC + Taxol | 6 (26.1) | 2 (12.5) |
| 6 cycles of TAC | 2 (8.7) | 4 (25.0) |
| Other regimen | 7 (30.4) | 7 (43.7) |
| Prior Use of Hormone Replacement Therapy | ||
| Yes | 2 (8.7) | 4 (25.0) |
| No | 13 (56.5) | 10 (62.5) |
| Unknown | 8 (34.8) | 2 (12.5) |
Compliance with Treatment
In the BWL group, the 17 patients for whom compliance data were available used their light boxes for a mean of 32 days (mean of 46.7% of the days) for an average of 31.5 (SD=9.89) minutes per day. In the DRL group, the 13 patients for which compliance data were available used their light boxes for a mean of 36 days (mean of 48.7% of the days) for an average of 33.92 (SD=10.93) minutes per day. There were no significant differences in compliance between the two groups.
Fatigue – MFSI-sf Total Score
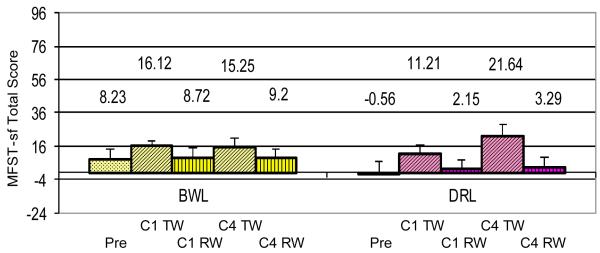
Fig 3 shows the levels of the total MFSI-sf scores for each group for each time point. There was no statistical difference between groups at baseline prior to the start of chemotherapy. There was a group × time interaction at C4TW (p=0.022) suggesting that the change from baseline in the DRL group was significantly different than the change in the BWL group. The DRL group fatigue scores significantly increased 11.7 points from baseline to C1TW (p=0.003), and 22.2 points from baseline to C4TW (p<0.001). There were no significant changes from baseline to recovery weeks in cycle 1 or cycle 4 in the DRL group. On the other hand, the fatigue scores for the BWL group did not significantly change at any time point compared to baseline.
Figure 3.
MFSI-sf total fatigue scores for the BWL treatment group and the DRL treatment group at five time points (BL = pre-chemotherapy baseline; C1TW = cycle 1 chemotherapy treatment week; C1RW = cycle 1 recovery week; C4TW = cycle 4 chemotherapy treatment week; C4RW = cycle 4 recovery week). There was a group × time interaction at C4TW (p=0.022) suggesting that the change from baseline in the DRL group was significantly different than the change in the BWL group. The DRL group reported increased fatigue at C1 TW (p=0.003) and C4 TW (p<0.001) compared to baseline while there was no significant change from baseline in the BWL group.
A secondary mixed-model analysis was conducted to examine whether these changes in fatigue levels were associated with concomitant changes in sleep or circadian activity rhythms. The results indicated that the changes in fatigue were not significantly associated with changes in sleep or in circadian rhythms, and hence the effect of light treatment on fatigue was not mediated through either of these other variables.
Fatigue – MFSI-sf Subscales
Analyses were conducted on the MFSI-sf subscales showing different results for the different subscales. Table 3 shows the means and standard errors for each group for each subscale.
Table 3.
Means (standard error) for MFSI-sf subscales
| Baseline | Cycle 1 Treatment Week | Cycle 1 Recovery Week | Cycle 4 Treatment Week | Cycle 4 Recovery Week | |
|---|---|---|---|---|---|
| General | |||||
| Bright white light | 6.59 (1.67) | 9.59 (2.00) | 8.06 (1.52) | 10.69 (1.66) | 7.6 (1.26) |
| Dim red light | 4.44 (1.12) | 9.50 (1.94) | 7.46 (1.73) | 13.71 (1.94) | 6.64 (1.79) |
| Physical | |||||
| BWL | 3.09 (0.83) | 5.59 (1.54) | 4.61(1.34) | 5.13 (1.15) | 3.33 (1.12) |
| DRL | 2.25 (0.78) | 4.93 (1.35) | 2.15 (0.82) | 6.43 (1.75) | 3.21 (1.03) |
| Mental | |||||
| BWL | 4.68 (1.23) | 5.76 (1.75) | 5.28 (1.53) | 6.31 (1.11) | 7.20 (1.49) |
| DRL | 2.75 (0.65) | 3.57 (1.12) | 3.00 (1.06) | 6.14 (1.61) | 4.07 (1.28) |
| Emotional | |||||
| BWL | 6.59 (1.48) | 5.47 (1.26) | 4.06 (1.37) | 4.56 (1.19) | 3.80 (0.86) |
| DRL | 3.06 (0.75) | 3.42 (1.33) | 2.47 (0.81) | 5.00 (1.50) | 2.43 (0.77) |
| Vigor | |||||
| BWL | 12.73 (1.16) | 10.29 (1.38) | 13.28 (1.12) | 11.44 (1.54) | 12.73 (1.36) |
| DRL | 13.06 (1.15) | 10.21 (1.70) | 12.92 (1.85) | 9.64 (1.95) | 13.07 (2.03) |
Note: Lower scores are better except for vigor, where higher scores are better.
See text for significance levels
There were no group × time interactions for the general or physical, or mental sub-scales. On the general MFSI-sf subscale, both groups had worse scores compared to baseline during C1TW (BWL p=0.033; DRL p=0.0006) and during C4TW (BWL p=0.002; DRL p<0.0001). There was a similar pattern on the physical subscale, with both groups again having worse scores compared to baseline during C1TW (BWL p=0.035; DRL p=0.026) and C4TW (BWL p=0.048; DRL p=0.0008). On the mental subscale, the two groups showed a decrement compared to baseline but at different time points, with the BWL group showing a decrement during C4RW (p=0.0019) and the DRL showing a decrement during C4TW (p=0.022).
However, on the emotional subscale, there was a group effect (p=0.014) indicating that the groups differed at baseline, and a group × time effect (p=0.03) suggesting that the change in emotional subscale scores from baseline in the BWL group was significantly different than the change in the DRL group. The BWL showed an improvement compared to baseline during C1RW (p=0.006), C4TW (p=0.053) and C4RW (p=0.058) while there were no significant changes in the DRL group.
There were no significant changes in the BWL group and no group × time interaction on the vigor subscale, however, the DRL group showed decreased vigor compared to baseline during C1TW (p=0.0092) and C4TW (p=0.0135).
Adverse Effects
There were no adverse effects reported in either the BWL or DRL group. Three women, all in the BWL group, dropped out of the study (one during C1TW and two at the end of C1TW) because they found the light uncomfortable.
Discussion
The results of this study suggest that morning bright light treatment may prevent overall fatigue from worsening during chemotherapy. Although our hypothesis that overall fatigue would improve with bright light treatment was not supported, the lack of deterioration in total fatigue scores is very encouraging. Women with breast cancer experience increasing fatigue during chemotherapy. Minimizing an increase in fatigue could have a substantial positive impact on these women.
Analyses of the subscales showed that the group receiving bright white light showed improvement on the emotional subscale while the dim red light group showed no change, and the dim red light group had less vigor while the bright white light group reported no change compared to baseline. Both groups showed deterioration on the general, physical and mental subscales.
The mechanism by which light is related to fatigue is not clear. Analyses examining whether changes in fatigue were related to changes in sleep or in circadian rhythms, as measured by wrist actigraphy, showed no such association so it is likely that changes in fatigue were not mediated through these other factors. However, it is also possible that statistical power was insufficient to demonstrate any relationship. It is also possible that the addition of morning bright light broke the cycle of low light levels contributing to increased fatigue (Fig 1a and 1b). But whether it was the alerting effect of light or some other mechanism that led to the lack of deterioration of fatigue can not be determined from this study.
Although the mechanism is unclear, other investigators have also found light therapy to be alerting and alertness could be construed to be the opposite of fatigue. Daurat et al.[23] found that bright light vs. dim light counteracted the effects of sleep deprivation on alertness and performance. In a study of simulated shift work, increased bright light exposure was associated with an amelioration of sleepiness and increased alertness.[36] Campbell et al.,[37] in a consensus report on the alerting and activating effects of bright light, concluded that data from studies of bright light support the notion that bright light is associated with reports of alertness as well as with objective physiological measures.
To date, few behavioral treatments have been tested in cancer. One small study found that progressive muscle relaxation training improved sleep quality (measured with questionnaire) and CRF in women with breast cancer undergoing adjuvant chemotherapy compared to no treatment.[38] The one behavioral treatment most studied however, has been exercise.[39, 40, 41, 42] In one meta-analysis of 44 studies of survivors of various cancers, exercise was effective in reducing CRF, particularly when the exercise was of moderate-intensity and in older cancer survivors.[40] In another meta-analysis of 18 studies (12 in breast cancer), Velthuis et al.[42] found that during breast cancer treatment, home-based exercise programs showed no significant reductions in fatigue while supervised aerobic programs resulted in a medium and significant reduction compared to no exercise. Adherence ranged from 39% of the patients in the supervised programs and 100% completion of a home-based walking program. Adverse events were reported in 67% of the studies. While the adherence rate was better in the home based exercise program than in our light study, there was no effect on CRF and there were more adverse events reported. In addition, none of the exercise studies controlled for the amount of light exposure, although many took place outdoors and so the amount of contribution of light vs. exercise is yet to be determined. Studies are needed to determine if increased light combined with increased exercise might lead to the most effective treatment of CRF.
There were some limitations to this study. The sample size was small, yet even with the small sample size our data support lack of worsening of fatigue and a protective effect of bright light therapy. While the compliance with treatment did not differ between groups and most women used their light box for the full 30 minutes, use was only for about half of the days. We know of no other study that measured compliance to light treatment, so we can not estimate if this compliance was better or worse than studies in other disorders. Nevertheless, even with partial compliance, BWL helped protect these women from increased fatigue. In this study, light was administered in the morning as for most other conditions, morning light is most effective. Future studies might compare the effect or morning vs. evening light on fatigue. Future studies should also examine the effect of bright light on fatigue in other cancer patients.
In summary, fatigue is a major complaint in women with breast cancer undergoing chemotherapy. There is no standard treatment for fatigue and so women suffer from this debilitating symptom, often discontinuing their chemotherapy treatment. Oncologists may want to council their breast cancer patients to increase their light exposure by either spending more time outdoors in the morning hours or buy using a bright light box. Morning bright light is an easy, non-invasive, non-harmful behavioral treatment that may prevent worsening of fatigue in the women with breast cancer.
Acknowledgement
The authors would like to thank Sherella Johnson, Feng He and Lee Cohen for their help with aspects of the study, the participants who volunteered their time, and Drs. Andrew Hampshire, Peter Reissman and William Stanton of Internal Medicine Associates, Dr. Sabina Wallach of Scripps/Ximed Medical Group, Hillcrest Internal Medicine, Inc, the Moores UCSD Cancer Center, Oncology Associates of San Diego, Pacific Oncology and Hematology Group, San Diego Pacific Oncology & Hematology Associates, for helping recruitment by informing their patients about the research protocol.
Supported by California Breast Cancer Research Program11IB-0034, a grant from Litebook, Inc, NCI CA112035, NIH M01 RR00827, P60MD00220, The Rebecca and John Moores UCSD Cancer Center (NCI P30 CA23100).
Footnotes
Parts of this study were presented at the 2008 Annual Sleep Meetings, Baltimore, Md.
References
- 1.Richardson A. Fatigue in cancer patients: a review of the literature. European Journal of Cancer Care. 1995;4:20–32. doi: 10.1111/j.1365-2354.1995.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 2.Winningham ML, Nail LM, Burke MB, Brophy L, Cimprich B, Jones LS, Pickard-Holley S, Rhodes V, St.Pierre B, Beck S, Glass EC, Mock VL, Mooney KH, Piper B. Fatigue and the cancer experience; the state of the knowledge. Oncology Nursing Forum. 1994;21:23–36. [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network . Cancer-related fatigue: NCCN clinical practice guidelines in oncology. NCCN; 2008. [Google Scholar]
- 4.Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98:1786–1801. doi: 10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- 5.Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, Donnelly J, Eisenberger MA, Escalante C, Hinds P, Jacobsen PB, Kaldor P, Knight SJ, Peterman A, Piper BF, Rugo H, Sabbatini P, Stahl C. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology (Williston.Park) 2000;14:151–161. [PubMed] [Google Scholar]
- 6.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills PJ, Parker BA, Dimsdale JE, Sadler GR, Ancoli-Israel S. The relationship between fatigue, quality of life and inflammation during anthracycline-based chemotherapy In breast cancer. Biological Psychology. 2005;69:85–96. doi: 10.1016/j.biopsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Liu L, Marler M, Parker BA, Jones V, Sadler G Robins, Dimsdale JE, Cohen-Zion M, Fiorentino L. Fatigue, sleep and circadian rhythms prior to chemotherapy for breast cancer. Support.Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Fiorentino L, Natarajan L, Parker BA, Mills PJ, Sadler GR, Dimsdale JE, Rissling M, He F, Ancoli-Israel S. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psycho-oncology. 2009;18:187–194. doi: 10.1002/pon.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haes Jd, Raategever J, Van der Berg M, Hamersma E, Neijt J. Evaluation of the quality of life of patients with advanced ovarian cancer treated with combination chemotherapy. In: Aaronson N, Beckmann J, editors. Quality of Life of Cancer Patients. Raven Press; New York: 1987. pp. 215–226. [Google Scholar]
- 11.Ganz P, Coscarelli A, Fred C, et al. Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 12.Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and Psychosocial Recovery in the Year After Primary Treatment of Breast Cancer. Journal of Clinical Oncology. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden RN, Gaynes BN, Ekstrom RD. The Efficacy of Light Therapy in the Treatment of Mood Disorders: A Review and Meta-Analysis of the Evidence. Am J Psychiatry. 2005;162:656–62. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- 14.Chesson A, Littner M, Davila DG, Anderson WM, Grigg-Damberger M, Hartse KM, Johnson S, Wise M. Practice parameters for the use of light therapy in the treatment of sleep disorders. Sleep. 1999;22:641–660. doi: 10.1093/sleep/22.5.641. [DOI] [PubMed] [Google Scholar]
- 15.Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. Journal of the American Geriatrics Society. 2002;50:282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk DJ, Eastman CI. Light Treatment for Sleep Disorders: Consensus report. VII. Jet lag. Journal of Biological Rhythms. 1995;10:167–176. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- 17.Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk DJ, Lewy AJ. Light Treatment for Sleep Disorders: Consensus report. VI. Shift work. Journal of Biological Rhythms. 1995;10:157–164. doi: 10.1177/074873049501000208. [DOI] [PubMed] [Google Scholar]
- 18.Klerman EB, Duffy JF, Dijk DJ, Czeisler CA. Circadian phase resetting in older people by ocular bright light exposure. Journal of Investigative Medicine. 2001;49:30–40. doi: 10.2310/6650.2001.34088. [DOI] [PubMed] [Google Scholar]
- 19.Lack L, Wright H, Kemp K, Gibbon S. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005;28:616–623. doi: 10.1093/sleep/28.5.616. [DOI] [PubMed] [Google Scholar]
- 20.Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26:695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- 21.Lafrance C, Dumont M, Lesperance P, Lambert C. Daytime vigilance after morning bright light exposure in volunteers subjected to sleep restriction. Physiol Behav. 1998;63:803–810. doi: 10.1016/s0031-9384(97)00538-6. [DOI] [PubMed] [Google Scholar]
- 22.Akerstedt T, Landstrom U, Bystrom M, Nordstrom B, Wibom R. Bright light as a sleepiness prophylactic: a laboratory study of subjective ratings and EEG. Perceptual.&.Motor Skills. 2003;97:811–819. doi: 10.2466/pms.2003.97.3.811. [DOI] [PubMed] [Google Scholar]
- 23.Daurat A, Aguirre A, Foret J, Gonnet P, Keromes A, Benoit O. Bright light affects alertness and performance rhythms during a 24-h constant routine. Physiol Behav. 1993;53:929–929. doi: 10.1016/0031-9384(93)90271-g. [DOI] [PubMed] [Google Scholar]
- 24.Terman M, Terman JS, Quitkin FM, McGrath PJ, Stewart JW, Rafferty B. Light therapy for seasonal affective disorder: A review of efficacy. Neuropsychopharmacology. 1989;2(1):1–22. doi: 10.1016/0893-133x(89)90002-x. [DOI] [PubMed] [Google Scholar]
- 25.Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncology Nursing Forum. 1999;26:1663–1671. [PubMed] [Google Scholar]
- 26.Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, Andrews PLR. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Supportive Care in Cancer. 2002;10:329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Johnson SS, Fiorentino L, Parker BA, Sadler GR, Cohen-Zion M, Marler M, Ancoli-Israel S. Light exposure and reported sleep, mood, functional performance and quality of life in breast cancer. Sleep. 2004;27:A323. [Google Scholar]
- 28.Liu L, Marler M, Parker BA, Jones V, Johnson S, Cohen-Zion M, Fiorentino L, Sadler GR, Ancoli-Israel S. The relationship between fatigue and light exposure during chemotherapy. Support.Care Cancer. 2005;13:1010–1017. doi: 10.1007/s00520-005-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewy AJ, Bauer VK, Cutler NL, Sack RL, Ahmed S, Thomas K, Blood ML, Jackson JM. Morning vs evening light treatment of patients with winter depression. Arch Gen.Psychiat. 1998;55:875–882. doi: 10.1001/archpsyc.55.10.890. [DOI] [PubMed] [Google Scholar]
- 30.Terman M, Terman JS, Ross DC. A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen.Psychiat. 1998;55:875–882. doi: 10.1001/archpsyc.55.10.875. [DOI] [PubMed] [Google Scholar]
- 31.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6th Edition 2002. [Google Scholar]
- 32.Desan PH, Weinstein AJ, Michalak EE, Tam EM, Meesters Y, Ruiter MJ, Horn E, Telner J, Iskandar H, Boivin DB, Lam RW. A controlled trial of the Litebook light-emitting diode (LED) light therapy device for treatment of Seasonal Affective Disorder (SAD) BMC.Psychiatry. 2007;7:38. doi: 10.1186/1471-244X-7-38. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Practice. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 34.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. 1996. pp. 1–254.
- 36.Dawson D, Campbell SS. Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep. 1991;14(6):511–516. doi: 10.1093/sleep/14.6.511. [DOI] [PubMed] [Google Scholar]
- 37.Campbell SS, Dijk DJ, Boulos Z, Eastman CI, Lewy AJ, Terman M. Light Treatment for Sleep Disorders: Consensus report. III. Alerting and activating effects. Journal of Biological Rhythms. 1995;10:129–132. doi: 10.1177/074873049501000205. [DOI] [PubMed] [Google Scholar]
- 38.Demiralp M, Oflaz F, Komurcu S. Effects of relaxation training on sleep quality and fatigue in patients with breast cancer undergoing adjuvant chemotherapy. Journal of Clinical Nursing. 2010;19:1073–1083. doi: 10.1111/j.1365-2702.2009.03037.x. [DOI] [PubMed] [Google Scholar]
- 39.Litterini AJ, Jette DU. Exercise for Managing Cancer-Related Fatigue. Physical Therapy. 2011;91:301. doi: 10.2522/ptj.20100273. [DOI] [PubMed] [Google Scholar]
- 40.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of Exercise Interventions in Modulating Cancer-Related Fatigue among Adult Cancer Survivors: A Meta-Analysis. Cancer Epidemiology Biomarkers & Prevention. 2011;20:123. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 41.Brem S, Kumar NB. Management of Treatment-Related Symptoms in Patients With Breast Cancer. Clinical Journal of Oncology Nursing. 2011;15:63–71. doi: 10.1188/11.CJON.63-71. [DOI] [PubMed] [Google Scholar]
- 42.Velthuis MJ, gasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol.(R.Coll.Radiol.) 2010;22:208–221. doi: 10.1016/j.clon.2009.12.005. [DOI] [PubMed] [Google Scholar]