Abstract
Recurrent aphthous stomatitis (RAS) is the most common idiopathic intraoral ulcerative disease in the USA. Aphthae typically occur in apparently healthy individuals, although an association with certain systemic diseases has been reported. Despite the unclear etiopathogenesis, new drug trials are continuously conducted in an attempt to reduce pain and dysfunction.
We investigated four controversial topics: (1) Is complex aphthosis a mild form of Behçet’s disease (BD)? (2) Is periodic fever, aphthous stomatitis, pharyngitis and adenitis (PFAPA) syndrome a distinct medical entity? (3) Is RAS associated with other systemic diseases (e.g., celiac disease and B12 deficiency)? (4) Are there any new RAS treatments?
Results from extensive literature searches, including a systematic review of RAS trials, suggested that: (1) Complex aphthosis is not a mild form of BD in North America or Western Europe; (2) Diagnostic criteria for PFAPA have low specificity and the characteristics of the oral ulcers warrant further studies; (3) Oral ulcers may be associated with celiac disease; however, these ulcers may not be RAS; RAS is rarely associated with B12 deficiency; nevertheless, B12 treatment may be beneficial, via mechanisms that warrant further study; (4) Thirty-three controlled trials published in the past 6 years reported some effectiveness, though potential for bias was high.
Keywords: aphthous stomatitis, Behçet syndrome, pharyngitis, celiac disease, vitamin B 12, therapy
INTRODUCTION
In this chapter of the Urban Legends series on controversial topics in Oral Medicine we focused on four questions about recurrent aphthous stomatitis (RAS): (1) Is complex aphthosis a mild form of Behçet’s Disease (BD)? (2) Is periodic fever, aphthous stomatitis, pharyngitis and adenitis (PFAPA) syndrome a distinct medical entity? (3) Is RAS associated with other systemic diseases? (focusing here on celiac disease and vitamin B12 deficiency) (4) Are there any new RAS treatments?
SECTION 1: Is complex aphthosis a mild form of Behçet’s Disease (BD)?
To address this question, one must understand the difference between simple and complex aphthosis. Recurrent aphthous stomatitis (RAS) is a disease state characterized by the development of oral aphthae (aphthosis). RAS has 2 presentation forms and 3 morphological types. The 2 forms are simple and complex aphthosis, and the 3 morphological types are minor, major and herpetiform aphthous ulcers, though not everyone agrees that herpetiform ulcers are RAS. All 3 forms can occur in simple and in complex aphthosis patients. RAS lesions occur in the context of Behçet’s Disease (BD). Table 1 presented by Rogers at the International Conference on Behçet’s Disease (ICBD) in 2003 illustrates the difference between simple, the most common, and complex, the much less common presentation of RAS (Rogers, 2003). The answer to this question in much of Asia and the Middle East would be a qualified YES because many patients who present with recurrent oral ulcerations develop BD, as reported in a prospective series from Korea (Bang et al., 1995) or in Anatolia (Turkey), which has the highest prevalence of BD in the world (370 patients/100,000 inhabitants) (Zouboulis, 2001). On the other hand, the answer to this question would be a definite NO in Western Europe or North America (Ghate & Jorizzo, 1999, Jorizzo et al., 1985, Jurge et al., 2006, Mc Carty & Jorizzo, 2003, Rogers, 2003).
Table 1.
Classification of recurrent aphthous stomatitis
| Simple Aphthosis | Complex Aphthosis |
|---|---|
| Common | Uncommon |
| Episodic | Episodic or continuous |
| Short-lived lesions | Persistent lesions |
| Few lesions | Few to many lesions |
| Three-six episodes/year | Frequent or continuous ulcerations |
| Prompt healing | Slow healing |
| Pain | Marked pain |
| Little disability | Disabling |
| Limited to oral cavity | May have genital lesions |
The term complex aphthosis was coined by Jorizzo and colleagues (Jorizzo et al., 1985) to describe patients suffering three or more almost constantly present oral aphthae, or oral and genital aphthae, in the absence of BD. This construct was used to describe patients referred to Professor Jorizzo with a possible diagnosis of BD, when most of them did not have BD. McCarty and Jorizzo (Mc Carty & Jorizzo, 2003) described 81 patients with possible BD. Of this cohort, 11 (13.6%) had simple aphthosis, 6 (7.4%) did not have aphthosis at all, leaving 64 (79%) patients with complex aphthosis. Ten of the 64 patients with complex aphthosis did have BD (15.6 %).
At the 2003 ICBD conference, Rogers described his experience with 244 Mayo patients with complex aphthosis. These patients suffered oral aphthous ulcerations approximately 50% of the time and/or had continuous oral aphthous ulcerations and/or had oral and genital aphthous ulcerations and/or suffered major disability from aphthosis. Patients with simple aphthosis were excluded from this cohort. In addition, twenty-five patients with complex aphthosis due to BD were excluded from his cohort. Thus, true BD occurred in only 25/269 or 9.3% of these patients with complex aphthosis (Rogers, 2003). The work by Jorizzo and Rogers highlights, therefore, that the vast majority of patients with complex aphthosis do not have, nor will they develop, BD. Their disease appears quite distinct in its manifestations, natural history and prognosis. Hence, this should not be considered an attenuated form of BD, but rather a discrete, more benign entity with similar mucosal manifestations. This can be very reassuring to patients with complex aphthosis.
Jurge et al. (Jurge et al., 2006), in their excellent review of recurrent aphthous stomatitis (RAS) in this journal emphasized that, in contradistinction to patients with RAS, patients with BD had a multisystem disease affecting oral, ocular, and anogenital mucosal surfaces as well as vascular, neurological and rheumatological systems. Moreover, they emphasized that RAS does not have a notable geographic distribution and does not share the HLA associations of BD.
Complex aphthosis can be divided into primary and secondary groups (Mc Carty & Jorizzo, 2003, Rogers, 2003). Primary complex aphthosis has no identifiable underlying cause and remains idiopathic. Causes of secondary complex aphthosis (Jurge et al., 2006, Mc Carty & Jorizzo, 2003, Rogers, 2003) include: hematinic deficiencies (iron, vitamins B1, B2, B6, B12, folic acid and zinc), cyclic neutropenia, benign familial neutropenia, primary and secondary immunodeficiencies including HIV disease, MAGIC syndrome, Sweet’s syndrome, PFAPA, gluten-sensitive enteropathy, inflammatory bowel disease including ulcerative colitis and Crohn’s disease, drug reactions to nonsteroidal anti-inflammatory agents (NSAIDs) and nicorandil and, of course, BD.
Evaluation of patients with complex aphthosis depends upon the identification of secondary factors and the exclusion of BD. Managing these secondary factors (“correctable causes”) can reduce the severity of the disease. Treatments with anti-inflammatory medications including both systemic corticosteroids and NSAIDs utilizing the therapeutic ladder emphasized by Jorizzo and colleagues (Ghate & Jorizzo, 1999, Jorizzo et al., 1985, Mc Carty & Jorizzo, 2003), and affirmed by the study of Lynde et al. (Lynde et al., 2009), offer the clinician an opportunity to achieve an excellent level of disease control or even remission in many of these patients with this chronic, disabling condition.
In summary, in western Europe and North America some patients with complex aphthosis do arguably have a forme fruste of BD, perhaps 10%, while the vast majority have a condition which merits a careful evaluation, seeking “correctable causes” and is responsive to treatment with an excellent prognosis for control or remission. However, along the “Silk Road” in the Middle East and much of Asia, complex aphthosis is more likely to signify the subsequent development of full-blown BD.
SECTION 2: Is Periodic Fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome a distinct medical entity?
First described in 1987 (Marshall et al., 1987), PFAPA syndrome is a clinical entity characterized by recurrent episodes of fevers without an identifiable source of infection. Despite better understanding of some the basic aspects of the disease, the etiology of PFAPA syndrome is still unknown and the question remains as to whether PFAPA syndrome is or not a distinct medical entity.
The diagnosis is established on the basis of clinical criteria that require the presence of a recurrent fever of early onset (<5 years) with a clockwork periodicity (usual interval <4 weeks) and ≥1 of the 3 associated symptoms (aphthosis, cervical adenitis, and pharyngitis), in the absence of upper respiratory tract infections and cyclic neutropenia (Marshall et al., 1989). The primary complaint is the periodic fever rather than the stomatitis. There is a level of uncertainty about the pattern of intraoral ulcers in PFAPA, but they are generally described as few to several, non-clustered, small (<5 mm), shallow ulcers that heal over 5 to 10 days (Long, 1999). However, it has been suggested that those ulcers could just mimic typical aphthae and the term aphthous-like ulcers has been consequentially used (Femiano et al., 2008). In 1999, the diagnostic criteria were partially modified to exclude leukocytosis and elevated sedimentation rate as these frequently accompany febrile illnesses and do not add specificity for the diagnosis (Thomas et al., 1999).
By definition, the diagnosis of PFAPA syndrome requires exclusion of other monogenic periodic fevers, which are hereditary conditions and include Familial Mediterranean Fever (FMF), the spectrum of mevalonate kinase deficiencies (MKD) (such as Hyper Ig-D syndrome and mevalonate aciduria), and tumor necrosis factor-associated periodic syndrome (TRAPS) amongst others (Scully et al., 2008), each characterized by a specific genetic mutation involving the mediterranean fever (MEFV), mevalonate kinase (MVK) and tumor necrosis factor receptor superfamily, member 1A (TNFRSF1A) gene, respectively. However, genetic tests have been only sporadically used to support the PFAPA diagnosis despite the fact that current PFAPA syndrome diagnostic criteria have very low specificity.
Several Authors have indeed expressed concern about the diagnostic accuracy of the revised criteria (Brown et al., 2010, Hofer, 2008, Lierl, 2007). When comparing the clinical manifestations of published PFAPA cohorts (Table 2), the heterogeneity is evident, particularly regarding the presence of aphthae sometimes just reported in a minority of the cases. This is possibly due to selective referral patterns as most of the cohorts are seen by pediatric, ENT or rheumatologic groups.
Table 2.
Distribution of main clinical manifestations associated with fever episodes in different cohorts of patients with PFAPA
| Author, year | N | Symptoms (%) |
|||||
|---|---|---|---|---|---|---|---|
| Fever | Pharyngitis | Cervical adenopathy |
Aphthous stomatitis |
Headache | Abdominal pain |
||
| Marshall et al., 1987 | 12 | 100 | 75 | 67 | 75 | NA | NA |
| Thomas et al., 1999 | 66 | 100 | 65 | 77 | 67 | 65 | 45 |
| Padeh et al., 1999 | 28 | 100 | 100 | 100 | 68 | 18 | 18 |
| Galanakis et al., 2002 | 15 | 100 | 100 | 87 | 33 | NA | NA |
| Tasher et al., 2006 | 54 | NA | 96 | 61 | 39 | 46 | 65 |
| Renko et al., 2007 | 35 | 100 | 29 | 21 | 21 | NA | NA |
| Padeh et al., 2008 | 15 | 100 | 100 | 100 | 40 | 20 | 20 |
| Pignataro et al., 2009 | 18 | 100 | 100 | 89 | 89 | 33 | 44 |
| Gattorno et al., 2009 | 130 | 100 | 84 | 84 | 58 | 41 | 53 |
| Garavello et al., 2009 | 39 | 100 | 97 | 85 | 59 | NA | NA |
| Kovacs et al., 2010 | 14 | 100 | 93 | 86 | 21 | NA | NA |
| De Cunto et al., 2010 | 12 | 100 | 100 | 83 | 67 | NA | 42 |
| Brown et al., 2010 | 10 | 100 | 100 | 90 | 90 | NA | 40 |
| Feder & Salazar, 2010 | 105 | 100 | 85 | 62 | 38 | 44 | 41 |
| Dagan et al., 2010 | 57 | 100 | NA | 44 | 33 | NA | 35 |
NA: not available
A relevant number of patients with monogenic periodic fevers also meet the diagnostic criteria for PFAPA syndrome (Gattorno et al., 2008). In a preliminary experience, 83% of patients with MKD, 57% of patients with TRAPS, and 8% of patients with FMF satisfied the criteria for PFAPA syndrome, which shows that the criteria have limited utility in differentiating PFAPA syndrome from monogenic periodic fevers. Importantly, oral aphthosis was found to be independently associated with a positive genetic test result indirectly suggesting the possible lack of specificity of this clinical feature for PFAPA diagnosis (Gattorno et al., 2008).
Moreover, a recent large multicenter multinational study employing genetic tests to distinguish PFAPA from other inherited periodic fevers clearly confirmed that PFAPA syndrome criteria are not able to distinguish genetically positive patients (i.e. patients likely without PFAPA but with a PFAPA-like phenotype) from genetically negative patients (the likely PFAPA affected). In this case-control study of 210 children that met the clinical criteria for PFAPA syndrome, 38% were genetically positive for either MKD, FMF, TRAPS or displayed low penetrance or incomplete mutations, and 62% had negative genetic testing profiles (Gattorno et al., 2009). Among the genetically positive individuals, the frequency of diarrhea, vomiting, abdominal pain, rash and arthralgias was higher, whereas exudative pharyngitis was more common in genetically negative patients. Cardinal features of the PFAPA syndrome, such as oral aphthosis and enlargement of cervical lymph nodes, were observed with similar frequencies in genetically negative patients and in subjects positive for MKD and FMF.
The authors then applied the Gaslini diagnostic score (Gattorno et al., 2008), which takes into account several clinical features to predict the likelihood that a patient would have positive genetic markers. This score identified 91% of the genetically positive individuals and those at risk for carrying genes associated with monogenic periodic fevers. The authors concluded that low-risk patients can be diagnosed as having PFAPA syndrome without genetic testing, conversely, those at high-risk should be diagnosed with PFAPA syndrome only in light of negative genetic markers as they would likely evolve into monogenic periodic fevers (Gattorno et al., 2009).
PFAPA syndrome may not be as sporadic as initially thought. With the aid of a European registry encompassing 14 rheumatological centers in 8 countries Cochard et al. noted that many PFAPA patients had a positive family history (FH+) of periodic fever. The authors recruited 84 PFAPA patients and 47 healthy children and found 45% of PFAPA patients FH+ for recurrent fever, with the affected family member being a sibling or parent in 76% of the cases. The recurrent fever was indeed PFAPA in 26% of FH+. All healthy children had a negative family history of PFAPA or recurrent fever (Cochard et al., 2010). However, in a previously cited large study (Gattorno et al., 2008) only 14% of PFAPA patients were FH+.
In addition to genetic testing, the measure of procalcitonin has been recently proposed as a marker for PFAPA syndrome, the hypothesis being that elevated procalcitonin levels would rule out PFAPA syndrome since procalcitonin is reported to be a sensitive marker for systemic bacterial infection, which by definition should be absent in PFAPA (Yoshihara et al., 2007).
Lastly, the mainstay of treatment for PFAPA syndrome is the systemic administration of corticosteroids, just one or two doses of either prednisone or prednisolone (1–2 mg/kg/dose) normally result in rapid resolution of fever and the associated symptoms (Thomas et al., 1999). Early studies have also examined the therapeutic use of cimetidine and found that it was efficacious in both treating the condition and inducing remission (Feder, 1992). In recent years, however, a role for tonsillectomy for treatment of this syndrome has been suggested (Garavello et al., 2009, Wong et al., 2008). Nonetheless, since prednisone is an effective, inexpensive and relatively safe medication and since patients typically outgrow the condition by age 10–11 without further recurrence or sequelae, the role of tonsillectomy is still a matter of controversy (Hofer, 2008, Leong et al., 2006).
In conclusion, it seems that the question that needs to be addressed is not “Is PFAPA syndrome a distinct medical entity?”, but rather, “How to differentiate PFAPA from other similar diseases causing recurring fever?”. In that regard, the importance of oral aphthous ulcers in PFAPA is still questionable and further specific studies are clearly warranted to better describe oral ulcerations in PFAPA patients and at large to set up specific and reliable diagnostic criteria.
SECTION 3: Is RAS associated with other systemic diseases?
A number of systemic conditions have been associated with RAS. These include celiac disease (CD), vitamin B12 deficiency, iron deficiency anemia, HIV/AIDS, cyclic neutropenia, Reiter’s syndrome etc. However, it is controversial whether the oral ulcerations associated with these systemic conditions are truly RAS or just oral ulcers similar to RAS. Here, we address this question by examining the literature on two conditions commonly associated with RAS: CD and vitamin B12 deficiency. It is worth noting that the answer to this question may not be all or none, i.e. some systemic conditions may truly be associated with RAS while others with non-RAS oral ulceration.
Celiac disease (CD)
An association between RAS and CD has been extensively studied in the literature. Studies examining the presence of CD in patients with RAS (Table 3) provide a wide range of prevalence of CD in patients with RAS (ranging from 4 to 40%). Case reports were excluded when examining prevalence ranges but are included in the tables for completeness of the literature review. Similarly, studies examining the prevalence of RAS in patients with CD (Table 4) indicate that the number of CD patients who have RAS ranges from 3–61%, excluding case reports. This can be compared against an approximately 37% lifetime prevalence of RAS in the general population (Kleinman et al., 1994). A few controlled studies suggested a higher prevalence of recurrent oral ulcers in CD patients than in comparable control groups. Interestingly, in some cases, oral ulcers can be the first sign of CD. Several authors have reported cases where patients presenting with recurrent oral ulceration were subsequently diagnosed with CD (Jokinen et al., 1998; Olszewska et al., 2006; Veloso and Saleiro, 1987; da Silva et al., 2008). The features of oral ulcers associated with CD have been described as being characteristic of minor RAS, with an average size of 5 mm and a typical mucosal distribution (Ferguson et al., 1980). Another study described the oral ulcers in CD patients as purpuric, papular, or erosive in nature, often surrounded by erythematous margins (Lahteenoja et al., 1998). Most of the studies reporting associations between RAS and CD did not report any well-defined criteria for RAS diagnosis, while the diagnosis of CD was usually well-supported by biopsy and/or antibody tests.
Table 3.
Studies of celiac disease (CD) in recurrent aphthous stomatitis (RAS) patients
| Author, Year | Number of RAS patients with CD / Total RAS patients (%) | Comments |
|---|---|---|
| Ferguson et al., 1975 | 7/35 (20%) | CD diagnosed based on jejunal biopsies. |
| Wray et al., 1975 | 5/130 (4%) | |
| Ferguson et al., 1976 | 8/33 (24%) | CD diagnosed based on jejunal biopsies. Use of a gluten-free diet resulted in complete remission of RAS in these 8 patients. |
| Wray et al., 1978 | 20/330 (6%) | Extension of above study. CD was present in 6 of 10 patients who had iron deficiency, 12 of 15 patients with folic acid deficiency and 2 of 11 patients with vitamin B12 deficiency. |
| Rose et al., 1978 | 1/26 (4%) | CD diagnosed based on jejunal biopsies. A gluten-free diet did not result in any change in this patient’s mouth ulcers. |
| Ferguson et al., 1980 | 2/50 (4%) | |
| Veloso & Saleiro, 1987 | 4/24 (17%) | In the 4 patients with RAS and biopsy-proven CD, RAS resolved completely upon gluten withdrawal. 0 of 19 non-RAS controls had CD, based on jejunal biopsy. |
| O’Farrelly et al., 1991 | 4/10 (40%) | Of 10 RAS patients with normal intestinal biopsies, 4 patients had increased levels of antibodies to gliadin. In 3 of these 4 patients, RAS resolved on a gluten-free diet and recurred on re-challenge. |
| Jokinen et al., 1998 | 9/27 (33%) | Of 27 patients suffering from “recurrent oral ulcerations”, 6 had anti-gliadin antibodies and 3 were diagnosed with CD by biopsy. |
| Biel et al., 2000 | 1/1 (100%) | Case Report. A 51 year old woman with a 30 year history of painful refractory RAS was diagnosed with CD by duodenal biopsy. Treatment with a gluten-free diet resulted in complete resolution of RAS lesions and no recurrences in a 3 year follow-up. |
| Nowak et al., 2002 | 1/20 (5%) | Diagnosis based on anti-endomysial antibodies in the one patient. |
| Aydemir et al., 2004 | 2/41 (5%) | The 2 CD patients had positive duodenal biopsy and were both positive for antibodies to gliadin and endomysium. None of 49 (0%) controls were diagnosed with CD. |
| Olszewska et al., 2006 | 2/42 (5%) | 2 RAS patients were diagnosed with CD based on anti-endomysial antibodies and duodenal biopsies vs. 0 of 42 controls (not statistically significant) In the 2 patients with both RAS and CD, a gluten-free diet resulted in complete resolution of RAS lesions. |
| da Silva et al., 2008 | 1/1 (100%) | Case Report. CD was confirmed by small intestine biopsy and circulating antigliadin and anti-endomysium antibodies in a woman with frequent RAS episodes. Topical dexamethasone rinse was effective in resolving RAS lesions; the patient was then put on a gluten-free diet. |
Table 4.
Studies of recurrent aphthous stomatitis (RAS) in patients with celiac disease (CD)
| Author, Year | Number of CD patients with RAS / Total CD patients (%) | Comments |
|---|---|---|
| Stevens, 1980 | 88/144 (61%) | |
| Andersson-Wenckert et al., 1984 | 6/17 (35%) | “Recurrent oral ulcerations” were reported by 6 of 17 children with CD and 5 of 19 controls |
| Majorana et al., 1992 | 19/113 (17%) | In the 19 patients with both CD and RAS, a significant association was found between DRw10 and DQw1 HLA antigens and the two diseases. |
| Meini et al., 1993 | 20/113 (18%) | Follow-up to the above series by Majorana et al., 1992. In all 20 cases, a marked improvement in RAS was noted within 1 year of starting a gluten-free diet. Re- challenge with a gluten-containing diet resulted in relapse of RAS in 9 of 10 cases. |
| Corazza et al., 1993 | 36/226 (16%) | Chart review of 226 patients diagnosed with CD revealed that 1 of 22 CD patients (4.5%) were diagnosed with RAS in 1972–1977; 8 of 63 (12.7%) in 1978–1973; and 27 of 141 (19.1%) in 1984–1989. |
| Srinivasan et al., 1998 | 1/1 (100%) | Case report of a 14 year old boy with a history of oral ulcers since the age of 3 years with increased gliadin antibody levels and normal duodenal biopsy. Use of a gluten- free diet caused resolution of the oral ulcers and re-challenge with a regular diet resulted in recurrence. The patient presented again at age 20 with severe oral ulcers on a regular diet, a duodenal biopsy confirmed celiac disease. Once again, a gluten-free diet resulted in resolution of the oral ulcers |
| Lahteenoja et al., 1998 | 4/128 (3%) | Presence of RAS was found in 4 of 128 CD patients on a gluten-free diet compared to 0 of 30 healthy controls (p = 0.327) and 0 of 8 patients with newly diagnosed (untreated) CD. |
| Sedghizadeh et al., 2002 | 25/61 (41%) | Presence or history of RAS in 25 of 61 (41%) CD patients vs. 17 of 62 (27%) age- and gender- matched healthy controls (p = 0.11). |
| de Freitas et al., 2002 | 15/48 (31%) | Reviewed records for 48 adult CD patients and found a history of oral aphthae in 15. |
| Sood et al., 2003 | 19/96 (20%) | RAS history based on medical records. |
| Bucci et al., 2006 | 24/72 (33%) | Found RAS to be present in 24 of 72 (33.3%) CD patients and 38 of 162 (23.4%) healthy controls (p > 0.05).Among the 24 patients with both RAS and CD, in 5 patients RAS resolved with a gluten-free diet, in 1 patient it improved, and in 10 patients RAS persisted even with a gluten-free diet. The remaining 8 patients continued gluten intake and RAS persisted in all of them. |
| Procaccini et al., 2007 | 18/50 (36%) | Reported that history, records or clinical signs of RAS were present in 18 of 50 (36%) CD patients vs. 6 of 50 (12%) age- and gender-matched controls (p = 0.009). |
| Campisi et al., 2007 | 37/197 (19%) | Screened 197 CD patients and found the clinical presence (34 patients) or history (3 patients) of RAS in a total of 37 cases. In comparison, RAS was found in 3 of 413 healthy controls (p < 0.0001). After a year on a gluten-free diet, 33 of the 37 cases reported complete resolution of RAS. The other 4 cases had persistently elevated serum antibodies to tissue transglutaminase, indicating non-compliance with a gluten-free diet. |
| Campisi et al., 2007 | 61/269 (23%) | Extended their previous series and found the presence or history of “aphthous-like ulcers” was present in 61 of 269 (22.7%) CD patients vs. 41 of 575 (7.1%) healthy controls (p < 0.0001).53 of the 61 patients presented for a one year follow-up. Of the 53 patients, 46 adhered strictly to a gluten-free diet: 33 of them reported complete remission of RAS, 4 reported improvement and the remaining 9 reported no change; 7 of the 53 patients did not comply with the gluten-free diet: 6 of them reported no change. |
| Cheng et al., 2010 | 28/67 (42%) | History of RAS in 42.4% of 67 CD patients vs. 23.2% of 69 controls (p = 0.02). |
Thus, the literature reviewed does support an association between oral ulcers and CD; however, these oral ulcers may not be RAS. Multiple reports of a proportion of CD-associated oral ulcers responding to a gluten-free diet support this conclusion. The oral ulcers that are a manifestation of CD would respond to a gluten-free diet, while classical RAS would not.
Vitamin B12 deficiency
A large number of studies have examined the prevalence of vitamin B12 deficiency in RAS patients (Table 5). Collectively, these studies indicate that 0–42% of RAS patients may have a deficiency of vitamin B12 (excluding case reports). This variation may be attributable to geographical and temporal variations in diet and food supplementation. A study compared the dietary intake of 100 RAS patients to age- and gender-matched nutrient intake data from 9033 subjects from the US National Health and Nutrition Examination Survey (NHANES). Interestingly, RAS patients were found to have significantly lower daily intake of vitamin B12 as compared to controls (p < 0.0002) (Kozlak et al., 2010).
Table 5.
Studies of vitamin B12 deficiency in patients with recurrent aphthous stomatitis (RAS)
| Author, Year | Number of RAS patients with B12 deficiency / Total RAS patients (%) | Comments |
|---|---|---|
| Walker, 1973 | 4/4 (100%) | Case series. In all 4 cases, complete resolution of RAS was achieved by vitamin B12 supplementation. |
| Nally & Blake, 1975 | 15/36 (42%) | Therapy with vitamin B12 resulted in complete resolution of RAS in 77% of cases. |
| Wray et al., 1975 | 5/130 (14%) | 5 of the RAS patients were deficient in vitamin B12, while only 1 of 130 age- and sex-matched controls was B12 deficient (due to latent Addisonian pernicious anemia). When treated with 1000 µg hydroxocobalamin IM followed by 1000 µg every two months, 4 of the 5 RAS patients were promptly relieved of symptoms and remained disease-free for at least one year, and the 5th subject showed marked improvement. |
| Challacombe et al., 1977 | 3/193 (2%) | Reported B12 deficiency in 3 of 193 RAS patients, 0 of 80 patients with other oral ulcers, 4 of 204 patients with non-ulcerative oral diseases, and 0 of 100 healthy controls (p > 0.05). |
| Wray et al., 1978 | 11/330 (3%) | Extension of previous series to 330 RAS patients, of which 11 were deficient in vitamin B12. 3 of these 11 patients were also deficient in iron, 1 in folate and 1 in both iron and folate, in addition to B12. Of the 11 RAS patients deficient in vitamin B12, 8 had Addisonian pernicious anemia, 2 had CD and 1 had Crohn’s disease. |
| Olson et al., 1982 | 0/90 (0%) | Screened 90 RAS patients and 23 healthy controls. There were no cases of B12 deficiency in either group. |
| Tyldesley, 1983 | 4/102 (4 %) | Patients had “recurrent oral ulceration”, not specifically stated to be RAS. |
| Rogers & Hutton, 1986 | 0/102 (0%) | Patients with a history of malabsorption syndromes or inflammatory bowel disease were excluded. Although several patients were deficient in iron and/or folate, none were B12 deficient. |
| Field et al., 1987 | 0/100 (0%) | All subjects were children. |
| Porter et al., 1988 | Not reported/69 (3%) | Low levels of serum vitamin B12 occurred in 3.2% of both patients with RAS and disease control subjects. |
| Palopoli & Waxman, 1990 | 1/1 (100%) | Case report of a patient with pernicious anemia and frequent RAS recurrences for 4.5 years, which completely resolved with vitamin B12 therapy. |
| Weusten & van de Wiel, 1998 | 3/3 (100%) | Case reports. The causes of B12 deficiency were pernicious anemia in 1 case, CD in 1 case, and unknown in 1 case. In all 3 cases, restoration of B12 levels to normal was accompanied by complete resolution of RAS lesions. |
| Piskin et al., 2002 | 8/35 (23%) | 8 of 35 RAS patients had B12 deficiency vs. 0 of 26 healthy controls (p < 0.05). Mean B12 levels were significantly lower in the RAS group (p = 0.005). |
| Thongprasom et al., 2002 | 0/23 (0%) | Serum B12 levels were normal in 23 RAS patients and 19 controls. |
| Volkov et al., 2005 | 3/3 (100%) | Case reports. All 3 patients had complete resolution of RAS on replacement therapy with vitamin B12. |
| Koybasi et al., 2006 | 12/34 (35%) | Twelve of 34 RAS patients had deficient serum B12 levels vs. 0 of 32 controls. B12 levels were associated with occurrence of RAS lesions (p = 0.028). |
A number of case reports indicate that some cases of RAS in patients with vitamin B12 deficiency are successfully treated with vitamin B12 supplementation. Further, treatment with vitamin B12 may be of benefit even in the absence of vitamin B12 deficiency. Submucous injection of vitamin B12 and hydrocortisone, in 22 RAS cases, resulted in reduced frequency, more rapid healing and diminution of lesions in 36% of cases (Biedowa & Knychalska-Karwan, 1983). A randomized, double-blind, placebo-controlled trial examined the use of once daily sublingual vitamin B12 for RAS (Volkov et al., 2009). After 6 months of treatment, 20 of 31 (74.1%) RAS patients in the active intervention group were free of ulceration, as compared to 8 of 27 (32%) RAS patients in the placebo group (p < 0.01). This significant response to vitamin B12 was independent of initial blood B12 level. It has been pointed out that this trial had some shortcomings; however, the findings support further investigation to confirm the effectiveness of vitamin B12 in the treatment of RAS (Carrozzo, 2009). Thus, although RAS may only rarely be associated with low blood levels of vitamin B12; treatment with vitamin B12 may nevertheless be of benefit in RAS, via mechanisms that warrant further study.
SECTION 4: Are there any new RAS treatments?
Introduction
Recurrent aphthous stomatitis (RAS) is a widespread disease affecting over 100 million Americans. New treatments are tested each year in an attempt to reduce its associated pain and dysfunction. The diversity and multitude of published studies of RAS treatments is staggering. In this section we answered the question: “Are there any new RAS treatments?” by conducting a systematic review of RAS trials published in the past 6 years.
Methods
We searched PubMed using the keyword “Aphth*”, limiting the search to human clinical trials published in any language from May 15, 2005 to the date of submission, May 30, 2011. We also searched the Cochrane Central Register of Controlled Trials (CENTRAL/CCRCT; www.cochrane.org) using the keyword “Aphth*” in “All text”, with “2005–2011” as date range. We excluded letters, abstracts of meetings and full text studies without a comparison to a control group or not targeted at RAS. We also excluded vitamin B12 trials, covered above. The remaining trials were thoroughly reviewed and assigned an OCEBM level of evidence (Richards, 2009) by a single reviewer (L.B.).
Results
From 76 unique studies we excluded 33 (Figure 1): 11 studies that did not test RAS treatments (Akman et al., 2007, Arvola et al., 2006, Lebranchu et al., 2009, Sunitha & Shanmugam, 2006, van der Hilst et al., 2008, Yao et al., 2008, Yoshihara et al., 2007, Chams-Davatchi et al., 2010, Davatchi et al., 2010, Erkalp et al., 2010, Perico et al., 2010), 11 studies without a statistical comparison to a control arm (Karaca et al., 2008, Kaufman et al., 2005, Lee et al., 2008, Mimura et al., 2009, Nanke et al., 2008, Sharma et al., 2007, Akhionbare & Ojehanon, 2007, Hello et al., 2010, Ciancio et al., 2010, Yasui et al., 2010, Sharquie & Hayani, 2005) and 9 other studies (Brignone et al., 2007, Passarini et al., 2007, Pignatello et al., 2009, Renko et al., 2007, Moezzi et al., 2005, Chuang & Langone, 2007, Garavello et al., 2009, Koray et al., 2009, Volkov et al., 2009, Burgess, 2009 Budde et al., 2011).
Figure 1.
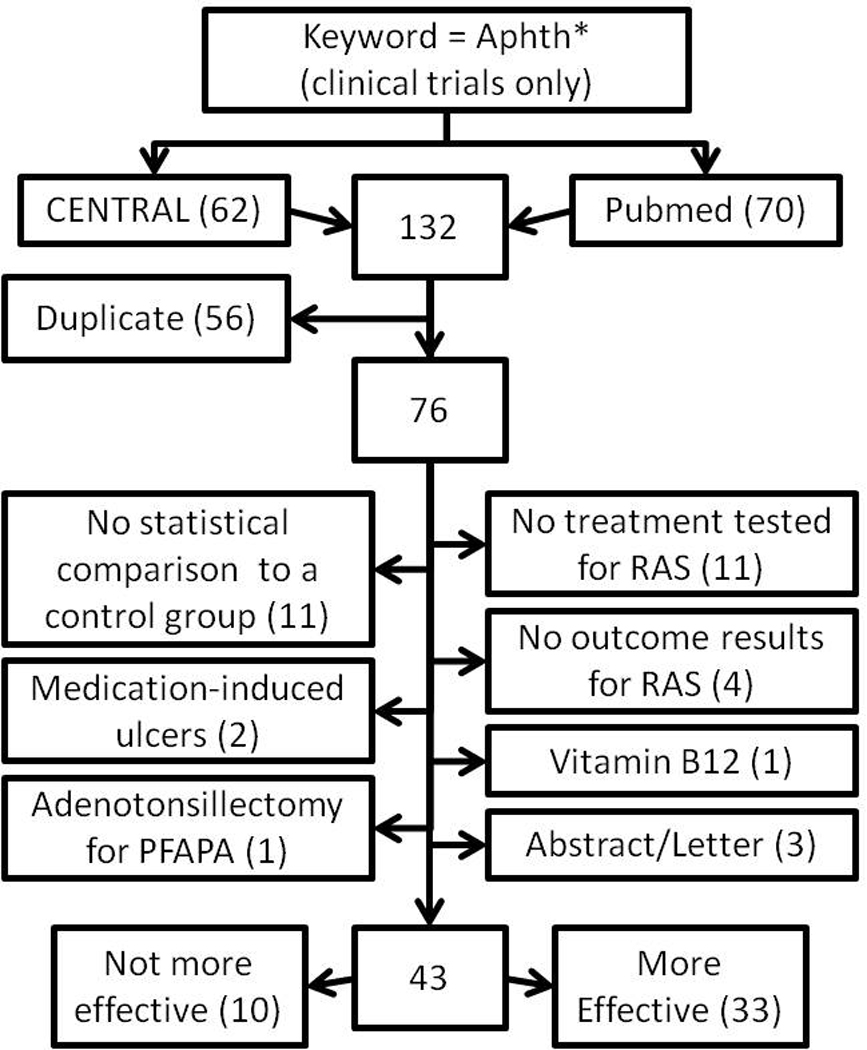
Study selection process for systematic review of recurrent aphthous stomatitis (RAS) treatments. Number of studies is indicated in parentheses.
Of the remaining 43 trials, 10 did not report significant differences in effectiveness between treatment arms (Table 6), and 33 reported significant effects of various topical or local (23 studies, Table 7) and systemic (10 studies, Table 8) treatments. All trials were conducted in idiopathic RAS patients with no known systemic diseases associated with RAS, except for 2 studies of Behçet’s disease (BD) patients (Arabaci et al., 2009, Davatchi et al., 2009). The types of ulcers included in individual trials (based on size, number, frequency of occurrence, or response to prior treatment) varied and were not always reported.
Table 6.
Clinical trials for recurrent aphthous stomatitis (RAS) reporting no difference in effectiveness between the two treatment arms
| Author, Year | Active treatment | Control |
|---|---|---|
| Kolseth et al., 2005 | Norwegian LongoVital | Placebo |
| Bratel et al., 2005 | Swedish LongoVital | Placebo |
| Arikan et al., 2006 | Cryotherapy | Hydrogel with cellulose |
| Nolan et al., 2006 | Hyaluronic acid gel | Placebo gel |
| Hamazaki et al., 2006 | Perilla oil | Soybean oil |
| Rodriguez et al., 2007* | Amlexanox paste | Clobetasol paste |
| Ersoy et al., 2007† | H. Pylori eradication | No eradication |
| Weckx et al., 2009 | Levamisole | Placebo |
| Moghadamnia et al., 2009 | Licorice patch | Patch only |
| Pakfetrat et al., 2010* | Prednisolone | Colchicine |
Trial compared two active treatment arms.
Trial enrolled only BD patients.
Table 7.
Controlled trials reporting effectiveness of active topical or local treatment for recurrent aphthous stomatitis (RAS).*
| Author, Year | Therapy and Regimen | Treatment Arms (n)† |
Mean age‡ |
Trial Duration |
Outcome/Adverse Events (AE) | OCEBM Quality rating - Sources of bias |
|---|---|---|---|---|---|---|
| de Armas et al., 2005 | Liquid R. Mangle (RMABE; RM) vs. placebo (water and RMABE excipients), QD | RM (17) PL (15) | 39–41 | 18 days | Faster healing (including pain and erythema) with RM (p<0.0001). No AE. | 2B – Single (patient) blind (or unblinded, if PL tasted/looked differently), randomization method unknown. |
| Murray et al., 2005 | Amlexanox 5% paste QID at prodromal (PR) vs. onset (ON) of ulcerative stage and vs. no treatment (NT) | NT/PR (17)§ NT/ON (29)§ | 37 | 10 days | Mean maximum ulcer size was 84% lower in PR and 59% lower in ON vs. NT (p<0.05). Nine mild AE. | 2B – Open label, only 81% of randomized patients entered the treatment phase, possible period effect (NT preceded PR/ON). |
| Alidaee et al., 2005 | Cauterization with silver nitrate (CX) vs. placebo (melted sugar on a wire) once | CX (52-5) PL (45-7) | 25–26 | 7 days | Less pain with CX starting at day 1 (p<0.001), but no difference in ulcer healing. No AE. | 2B – Patient and examiner blind only, PL may have tasted differently, 12% total attrition, per protocol analysis. |
| Murray et al., 2006 | Amlexanox 2 mg patch vs. vehicle patch, QID | AX (26-0) PL (26-0) | 32–40 | 4 days | Smaller thermographically active area at day 4 with AX (p<0.05), but similar size and erythema. Mostly mild 74 AE in both arms. | 2B – Patches had different color, PL arm younger, 15% of participants had missing data |
| Sharquie et al., 2006 | Lactic acid 5% mouthwash (LA) vs. placebo (distilled water) TID | LA (40-4) PL (40-6) | 30 | 7 days | Less ulcers and pain at day 3 and 7 with LA (p<0.05). Two mild AE (irritation) in LA arm. | 2B –Patient blind (or unblinded, if water placebo tasted differently), 12.5% attrition, non-randomized, per protocol analysis. |
| Liu et al., 2006 | Amlexanox 2 mg adhesive tablet vs. vehicle tablet QID for 5 days | AX (107-3) PL (108-0) | 34 | 6 days | Smaller size and less pain at days 4 and 6 with AX (p<0.001). One mild AE in each arm. | 2B – Unknown if PL tasted differently, non- parallel design, per protocol analysis, unknown how compliance was monitored, center effects unknown.¶ |
| Amanlou et al., 2007 | S. Khuzistanica extract (SE) vs. S. Khuzistanica essential oil (SO) vs. placebo (ethanol/water), 5 drops QID | SE (20)§ SO (20)§ PL (20)§ | 28 | 7 days | Faster healing with SE and SO vs. PL (p<0.001). Two mild AE in SO arm. | 2B - Unknown if placebo looked/tasted the same as SE/SO, 8% total attrition, randomization method unknown, baseline data incomplete, per protocol analysis. |
| Gorsky et al., 2007 | Minocycline 0.2% (MN) vs. Tetracycline 0.25% rinse (TE), 5 ml QID | MN (16)/ TE (17)§ | 39 | 10 days then cross over | Lower pain starting at day 2 with MN (p<0.05). No AE. | 2B - Blinding unknown, no PL, only self- reports, per protocol analysis, 48% total attrition. |
| Gorsky et al., 2008 | Minocycline rinse 0.2% (MN) vs. placebo, 5 ml QID | MN (18)§ PL (15)§ | 37–38 | 10 days | Less pain starting at day 2 with MN (p<0.05). No AE. | 2B – 39% total attrition, unknown who was blinded, only self-reports, PL may have tasted differently, per protocol analysis. |
| Shemer et al., 2008 | Patch with citrus oils and Mg salts (PA) QD vs. Benzocaine 20% with benzoin tincture oral solution (BZ) TID | PA (26-3) BZ (22-4) | 41–48 | 10 days | Less pain at 12 and 24 hr, and faster pain relief with PA (p<0.05). Less local AE with PA. | 2B – Open label, per protocol analysis, only self-reported outcomes, inconsistent statistical results, 15% attrition. |
| Motallebnejad et al., 2008 | Hypericum mouthwash 0.5% in water QID (HY) vs. placebo (pure water) QID vs. no treatment (NT) | NT/HY/PL (30)§ 3 episodes/ patient | 25 | 7 days then cross over | Less pain at day 2–7 with HY vs. PL/NT (p<0.05), but no difference in ulcer size. AE unknown. | 2B – Unknown if water placebo looked/tasted the same as HY; NT phase before HY and PL, crossover design and timing unclear, randomization unclear. |
| Martin et al., 2008 | Licorice patch (16 hours/day; LP) vs. star anise patch (AP) vs. no treatment (NT) | LP (23) AP (23) NT (23) | Not stated (≥18) | 8–10 days | Smaller size after 7 days and less pain after 3 days with LP vs. NT (p<0.05). P-value for LP vs. AP unknown. No AE. | 2B – Examiner blind only (or unblinded, because NT recruited later), randomization method not described, incomplete baseline results. No inactive placebo used. |
| Al-Na’mah et al., 2009 | Dexamucobase 0.1 g/100 g (DM) vs. Triamcinolone acetonide in Orabase (TR) QID | DM (53)§ TR (37)§ | 28–30 | 14 days | Faster healing with DM (p<0.001). Mild AE in both arms. | 2B – Open label, no randomization, attrition unknown. |
| Meng et al., 2009 | Amlexanox 5% pellicle vs. placebo pellicle, QID for 5 days | AX (109-1) PL (107-2) | 30 | 6 days | Smaller size and less pain at days 4 and 6 with AX (p<0.04). No AE. | 2B – Unknown if PL tasted/looked differently, larger baseline ulcer size in PL arm, per protocol analysis, unknown how compliance was monitored, center effects unknown.¶ |
| Zand et al., 2009 | CO2 Laser (LS) vs. placebo (inactive laser) once | LS/PL (15) 2 RAS/patient | 38 | 4 days | Less pain with LS starting immediately until 96 h (p<0.001). | 2B – Attempted to blind patient, attrition unknown, only self-reports. |
| Arabaci et al., 2009‖ | Nd:YAG Laser(LS) once vs. Triamcinolone acetonide 0.1% in Orabase (TR) TID | LS (14-0) TR (14-0) | 30 | 7 days | Less exudation with LS (p<0.001), but similar erythema. Less pain immediately and at days 4 and 7 with LS (p<0.001). No AE. | 2B – Unblinded. |
| Tezel et al., 2009 | Nd:YAG Laser(LS) once vs. Triamcinolone acetonide 0.1% in Orabase (TR) TID | LS (10-0) TR (10-0) | 32 | 7 days | Less exudation with LS (p<0.05) at the end, but similar erythema. Less pain immediately and at days 4 and 7 with LS (p<0.05). No AE. | 2B – Unblinded. |
| Skulason et al., 2009 | Doxycycline gel 1.5 mg/g QID (DX) vs. placebo (PL) | DX (28-3) PL (28-4) | Not stated (allowed range 18- 65) | 3 days | Shorter duration with DX (p<0.005, 1-sided). Four AE (transient burning/pain). | 2B - Unknown if PL tasted/looked differently, 12.5% attrition, per protocol analysis, incomplete baseline results, only self-reports (diaries). |
| Porter et al., 2009 | HybenX cauterization (once; HX) vs. Salicept patches as needed (SL) | HX (32-8) SL (31-2) | Not stated (median 23–27) | 8 days | Less pain with HX at days 1–2 (p<0.02), but no difference in ulcer healing. Nine AE, likely unrelated. | 2B –Examiner blind only, randomization method unknown, multiple comparisons, per protocol analysis, 16% attrition with more HX patients withdrawn for developing new RAS, significantly different baseline pain levels. |
| Yang & Jang, 2009 | Botulinum Toxin A (BT) vs. placebo (saline) injection | BT (35-2) PL (35-2) | 26–29 | 6 days (6- month f/u) | Less pain with BT at 6 days (p<0.001) and no recurrence at 6 months in the same location (vs. 5 recurrences in PL). No AE. | 2B – Patient blind only, 6% attrition, per protocol analysis. |
| Babaee et al., 2010 | Myrtle oral paste 5% (MT) vs. placebo paste, QID | MT/PL (45- 5) | 30 | 6 days then cross over | Less pain (p<0.05) and smaller size (p<0.001) with MT. No AE. | 2B – Unknown if PL tasted/looked differently, period and center effects unknown, compliance unknown, 11% attrition.¶ |
| Zhou et al., 2010 | Penicillin G potassium troche 50 mg QID (PN) vs. placebo QID (PL) vs. no treatment (NT) | PN (88-2) PL (90-2) NT (85-1) | 36 | 4 days plus 2 days follow-up | >50% smaller size and less pain in PN vs. PL/NT at day 3 (p<0.001) and 4–6. Two mild AE in each PN/PL arm. | 2B – Unknown if PL had same color. Randomization method, compliance and center effects unknown. Ulcer selection not random, no sample size justification, per protocol analysis.¶ |
| Hamdy & Ibrahem, 2010 | Quercetin cream (QC) vs. Benzydamine hydrochloride (BZ) rinse TID | QC (20)§ BZ (20)§ | 25 | 10 days | Smaller size (p<0.004) and less pain (p<0.01) at day 10 with QC. AE unknown. | 2B - Randomization method, dosage, and attrition unknown, unblinded, no placebo, compliance unknown. |
All clinical trials were single center and they were based on a randomized, double-blind, placebo-controlled, parallel arm design including intent to treat (ITT) analysis of at least one objective outcome (i.e. confirmed by examination) unless otherwise specified. Trials that did not report presence or absence of attrition also did not mention ITT analysis.
Numbers in parenthesis indicate initial sample size minus number lost to follow-up, if known.
In years. All trials included patients of both genders with idiopathic RAS unless otherwise specified.
Assumed to be the final sample size after attrition (attrition was either not reported or was not reported by each arm).
BD patients.
Multicenter study.
Table 8.
Controlled trials reporting effectiveness of systemic treatments for recurrent aphthous stomatitis (RAS).*
| Author, Year | Therapy and Regimen | Treatment Arms (n)† |
Mean age‡ |
Trial Duration |
Outcome/Adverse Events (AE) | OCEBM Quality rating - Sources of bias |
|---|---|---|---|---|---|---|
| Thornhill et al., 2007 | Pentoxifylline (400 mg TID; PX) vs. Placebo TID | PX (14-3) PL (12-4) | 33–34 | 2 months (plus 2 pre/post) | Reduced ulcer size with PX (p=0.05) but minimal benefits seen for other parameters. AE in both arms. | 2B – 27% total attrition, small sample size, only self-reported outcomes (diaries). |
| Preshaw et al., 2007 | Doxycycline (20 mg BID; DX) vs. placebo BID | DX (25)§ PL (25)§ | 37–43 | 3-month | More days with no new RAS with DX (p=0.04). Other parameters tended to improve, though not significantly. AE unknown. | 2B – Attrition and compliance unknown, only self-reported outcomes (diaries). |
| Samet et al., 2007 | Bee propolis (500 mg QD; BP) vs. Placebo (calcium-based supplement QD; PL) | BP (10-0) PL (9-2) | Not stated (≥18) | 6–13 months | Higher % of patients on BP with ≥50% reduction in # of outbreaks (p=0.06) and improved quality of life (QoL; p=0.03). AE unknown. | 2B – Only self-reports, PL may not be inert, small sample, incomplete baseline results, 10.5% attrition, randomization method unknown, QoL data collection not standardized. |
| Yazdanpanah et al., 2008 | Poliovirus vaccine (4 drops at baseline; PV) vs. Placebo (PL) | PV (20)§ PL (28)§ | 29–30 | 3 months | Fewer symptoms with PV (p<0.01). No AE. | 2B – PL and blinding not described, attrition unknown, only self-reported outcomes, some PV contained antibiotics. |
| Sharquie et al., 2008 | Zinc sulfate (150 mg BID; ZN) vs. Dapsone (50 mg BID; DP) vs. Placebo (glucose, 250 mg BID) | ZN (15)§ DP (15)§ PL (15)§ | 31 | 3 months | Significantly smaller size and fewer manifestations with ZN or DP vs. PL at weeks 4–12. No AE. | 2B – PL may not be inert, no randomization reported, small sample, multiple comparisons, attrition unknown. |
| Davatchi et al., 2009‖ | Colchicine (1 mg QD; CO) vs. Placebo (PL) | CO/PL (169) | 32 | 4 months, then cross over | Fewer ulcers with CO (p<0.005). AE in both groups, with increased liver enzymes in 2 patients on CO. | 2B – Unclear design (sample size, attrition, PL description, randomization method) and statistics. |
| de Abreu et al., 2009 | Clofazimine (100 mg QD for 30 days, then QOD; CL) vs. Colchicine (0.5 mg QID) vs. Placebo BID | CL (23)§ CO (23)§ PL (20)§ | 34–45 | 6 months | Improved number and duration, but not size, with CL vs. CO/PL (p<0.05). CO not better than PL. Gastrointestinal (CO) and cutaneous (CL) AE. | 2B – Incomplete blinding, PL regimen different from CO/CL, attrition unknown, PL older. |
| Mousavi et al., 2009 | Homeopathic (multiple treatments; HM) vs. Placebo (PL) | HM (50-0) PL (50-0) | 38 | 6 days | Less pain and smaller size at day 4 and 6 with HM (p<0.05). No dropouts due to AE. | 2B – Single (patient) blind, PL may have tasted differently, individual treatment effectiveness unknown, short trial. |
| Femiano et al., 2010 | Prednisone (0–25 mg; PN) vs. Montelukast (0- 10 mg, MK) vs. Cellulose placebo (0–100 mg, PL) QD | PN (20-0) MK (20-0) PL (20-0) | 27 | 2 months plus 2 months follow-up | Fewer RAS with PN/MK vs. PL at 1–4 months (p<0.01). Less time to healing and pain free with PN vs. MK and PN/MK vs. PL (p<0.0001). Two mild AE in each MK/PL arm, 6 AE with PN. | 2B – PN regimen different than MK/PL, initial sample size unclear, full blinding unclear, unknown if PL pill different from MK/PN, more females in PL arm, inconsistencies in results. |
| Pourahmad et al., 2010 | Camel thorn distillate (CT) vs. distilled water (PL), rinse+swallow QID | CT (49)§ PL (44)§ | 27–32 | 14 days | Less time to pain resolution in CT arm. Less size and pain with CT at days 3–7 (p<0.001) and 10 (p<0.02). AE unknown | 2B – Randomization method, dosage and attrition unknown, PL arm had larger RAS at baseline, PL may have tasted/looked differently |
All clinical trials were single center and they were based on a randomized, double-blind, placebo-controlled, parallel arm design including intent to treat (ITT) analysis of at least one objective outcome (i.e. confirmed by examination) unless otherwise specified. Trials that did not report presence or absence of attrition also did not mention ITT analysis.
Numbers in parenthesis indicate initial sample size minus number lost to follow-up, if known.
In years. All trials included both genders and were conducted in idiopathic RAS patients unless otherwise specified.
Assumed to be the final sample size after attrition (attrition was either not reported or was not reported by each arm).
BD patients.
Doxycycline, minocycline, amlexanox, triamcinolone acetonide in Orabase, colchicine and laser therapy were tested more than once, although not always as the same formulation or in the same patient group.
Four studies were in general agreement that synthetic tetracyclines may be useful for RAS. Low dose doxycyline seemed effective, particularly as a topical gel (Preshaw et al., 2007, Skulason et al., 2009). Minocycline rinses (0.2%) also seemed safe and more effective than either placebo or tetracycline rinses (Gorsky et al., 2007, Gorsky et al., 2008).
Four studies tested various formulations of topical amlexanox (paste, disc, pellicle or tablet) and reported improvement of some outcomes, especially for treatment initiated during the prodromal phase. A fifth study reported similar responses to 5% amlexanox and 0.05% clobetasol pastes (Rodriguez et al., 2007).
Systemic colchicine produced inconsistent results. The drug appeared to be effective in BD-related oral ulcers, but not in otherwise healthy patients with frequent RAS unresponsive to topical treatments (Davatchi et al., 2009, de Abreu et al., 2009).
Two unblinded studies by the same team showed greater pain reduction with Nd:YAG laser versus triamcinolone acetonide in Orabase (Arabaci et al., 2009, Tezel et al., 2009).
Various compounds and plant extracts with anti-inflammatory, analgesic or antiseptic properties showed some effectiveness as topical treatments (Amanlou et al., 2007, de Armas et al., 2005, Martin et al., 2008, Babaee et al., 2010, Motallebnejad et al., 2008). However, most trials were not fully blinded.
Overall, different types of systemic and topical treatments were reported to be effective in regard to at least one of the outcomes studied. However, the quality of evidence of these trials was low (OCEBM rating 2B), due to moderate to high potential for bias (Tables 7–8). We did not find systematic reviews of randomized clinical trials published in the past 6 years (OCEBM rating 1A).
Discussion
The most commonly studied treatments in the past six years were doxycycline, minocycline, amlexanox, colchicine, triamcinolone acetonide in Orabase and laser therapy.
Low dose synthetic tetracyclines (doxycycline and minocycline), particularly as gel or rinse, appeared to reduce RAS pain and duration, possibly through local inhibition of collagenases or immunomodulatory effects. However, long-term adverse events (AE) in the general population are unknown and may include bacterial resistance, fungal overgrowth and fetal harm.
Amlexanox showed some effectiveness short-term, particularly when used during the prodromal phase. Its exact mechanism of action on RAS is unknown, although it is an anti-inflammatory drug. The number of AE reported during amlexanox trials varied by research team (ranging from 0 to 74), suggesting possible differences in reporting standards.
Systemic colchicine lacked effectiveness in frequent idiopathic RAS and was associated with gastrointestinal AE. More severe AE have also been reported in the literature. We could not find double-blind, randomized, placebo-controlled trials of colchicine for idiopathic RAS before 2005. Thus, this drug should be used with caution until further evidence.
Lasers or chemical cauterization may provide fairly rapid pain relief, attributed to disruption of local nerve endings or reduction in inflammatory mediators. However, some of these therapies require repeated dental visits, which are not feasible long-term or for frequent RAS.
Triamcinolone acetonide in Orabase was used in three trials as the active control and was less effective than Nd:YAG laser (for immediate pain relief) or dexamucobase (for accelerating healing).
Other topical therapies were tested with some success. However, incomplete blinding due to differences in taste, texture or appearance between products was likely.
When selecting treatments, the patient’s clinical status and preferences should be considered, such as potential fetal harm in pregnant women and accidental ingestion in children. Most studies were conducted in adults, limiting generalizability to all ages. This is a consideration, because RAS is common in teenagers and differences in compliance in younger patients can alter drug’s effectiveness.
Although statistical significance was achieved in many studies, results may have been affected by underlying study bias. Studies testing multiple outcomes were more susceptible to false positive results.
Most trials reported generally mild or no AE. However, these studies were also short and small. Thus, less common AE or AE from long-term use cannot be excluded. Safety could not be assumed for studies failing to report presence or absence of AE.
Our review included studies published in the past six years and listed in two major databases. Grey literature, additional databases or earlier studies were not included. Thus, this is not an exhaustive review of all RAS trials. For example, topical and systemic corticosteroids, widely used in practice for RAS treatment, were infrequently studied in recent trials, although positive effects of prednisone were noted in one study (Femiano et al., 2010). A single reviewer performed the review, which may have resulted in more selection and rating bias than in the case of multiple reviewers.
When rating the evidence, assessment of potential bias was often limited by lack of study details. For example, diagnostic criteria for RAS were universally not specified or incompletely specified. It was sometimes unclear how outcome data were measured.
Studies rarely described the randomization method or allocation concealment strategy. Post-randomization loss to follow-up was often unclear or, if attrition had occurred, intent-to-treat analyses were not always conducted. The degree of item non-response (e.g. incomplete data collection from a patient) was almost universally never reported. Measurement of treatment compliance was almost never described.
A number of studies reported the use of a placebo, although not all studies actually used a placebo in its strict definition or fully described potential differences with the active drug. Strong placebo effects were frequent in RAS patients. Thus, studies of treatment effectiveness based solely on before and after differences in RAS outcome without a control group provide a very low level of evidence and were excluded from this review.
Conclusions
Until RAS etiology is discovered, treatment options will remain few and only partially effective. Recent trials have focused primarily on local and topical treatments. These therapies in general carry lower risks of systemic adverse effects and should be considered as the first line of treatment. Improved design, analysis and standardized reporting of clinical trials are needed to maximize study quality, disclose potential sources of bias, and ensure complete assessment of product safety and effectiveness. Thus, future research should focus on identifying RAS etiology, developing standardized diagnostic criteria for RAS, and improving the design and reporting of clinical trials. Trials should be carefully planned by clinician-statistician teams and reported using universal guidelines, such as the Consolidated Standards of Reporting Trials (CONSORT; http://www.consort-statement.org/).
Acknowledgments
FUNDING
This work was funded in part by NIH grants #R21DE018714 (to Dr. Baccaglini) and K23DE016946 (to Dr. Lalla).
REFERENCES
- Akhionbare O, Ojehanon PI. The Palliative Effects of Lidocaine with Adrenaline on Recurrent Aphthous Stomatitis (RAS) J Med Sci. 2007;7:860–864. [Google Scholar]
- Akman A, Kacaroglu H, Donmez L, Bacanli A, Alpsoy E. Relationship between periodontal findings and Behcet’s disease: a controlled study. Journal of clinical periodontology. 2007;34:485–491. doi: 10.1111/j.1600-051X.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- Al-Na’mah ZM, Carson R, Thanoon IA. Dexamucobase: a novel treatment for oral aphthous ulceration. Quintessence Int. 2009;40:399–404. [PubMed] [Google Scholar]
- Alidaee MR, Taheri A, Mansoori P, Ghodsi SZ. Silver nitrate cautery in aphthous stomatitis: a randomized controlled trial. The British journal of dermatology. 2005;153:521–525. doi: 10.1111/j.1365-2133.2005.06490.x. [DOI] [PubMed] [Google Scholar]
- Amanlou M, Babaee N, Saheb-Jamee M, Salehnia A, Farsam H, Tohidast Akrad Z. Efficacy of Satureja khuzistanica extract and its essential oil preparations in the management of recurrent aphthous stomatitis. DARU. 2007;15:231–235. [Google Scholar]
- Andersson-Wenckert I, Blomquist HK, Fredrikzon B. Oral health in coeliac disease and cow’s milk protein intolerance. Swedish dental journal. 1984;8:9–14. [PubMed] [Google Scholar]
- Arabaci T, Kara C, Cicek Y. Relationship between periodontal parameters and Behcet’s disease and evaluation of different treatments for oral recurrent aphthous stomatitis. Journal of periodontal research. 2009;44:718–725. doi: 10.1111/j.1600-0765.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- Arikan OK, Birol A, Tuncez F, Erkek E, Koc C. A prospective randomized controlled trial to determine if cryotherapy can reduce the pain of patients with minor form of recurrent aphthous stomatitis. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2006;101:e1–e5. doi: 10.1016/j.tripleo.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Arvola T, Ruuska T, Keranen J, Hyoty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics. 2006;117:e760–e768. doi: 10.1542/peds.2005-1069. [DOI] [PubMed] [Google Scholar]
- Aydemir S, Tekin NS, Aktunc E, Numanoglu G, Ustundag Y. Celiac disease in patients having recurrent aphthous stomatitis. Turk J Gastroenterol. 2004;15:192–195. [PubMed] [Google Scholar]
- Babaee N, Mansourian A, Momen-Heravi F, Moghadamnia A, Momen-Beitollahi J. The efficacy of a paste containing Myrtus communis (Myrtle) in the management of recurrent aphthous stomatitis: a randomized controlled trial. Clinical oral investigations. 2010;14:65–70. doi: 10.1007/s00784-009-0267-3. [DOI] [PubMed] [Google Scholar]
- Bang D, Hur W, Lee ES, Lee S. Prognosis and clinical relevance of recurrent oral ulceration in Behcet’s disease. The Journal of dermatology. 1995;22:926–929. doi: 10.1111/j.1346-8138.1995.tb03947.x. [DOI] [PubMed] [Google Scholar]
- Biedowa J, Knychalska-Karwan Z. [Submucous injections of vitamin B12 and hydrocortisone in cases of recurrent aphthae] Czasopismo stomatologiczne. 1983;36:565–567. [PubMed] [Google Scholar]
- Biel K, Bohm M, Luger TA, Bonsmann G. Long-standing oral aphthae - a clue to the diagnosis of coeliac disease. Dermatology. 2000;200:340. doi: 10.1159/000018404. [DOI] [PubMed] [Google Scholar]
- Bratel J, Hakeberg M, Jontell M. The effect of LongoVital on recurrent aphthous stomatitis in a controlled clinical trial. Oral health & preventive dentistry. 2005;3:3–8. [PubMed] [Google Scholar]
- Brignone C, Grygar C, Marcu M, Perrin G, Triebel F. IMP321 (sLAG-3), an immunopotentiator for T cell responses against a HBsAg antigen in healthy adults: a single blind randomised controlled phase I study. Journal of immune based therapies and vaccines. 2007;5:5. doi: 10.1186/1476-8518-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KL, Wekell P, Osla V, Sundqvist M, Savman K, Fasth A, Karlsson A, Berg S. Profile of blood cells and inflammatory mediators in periodic fever, aphthous stomatitis, pharyngitis and adenitis (PFAPA) syndrome. BMC pediatrics. 2010;10:65. doi: 10.1186/1471-2431-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci P, Carile F, Sangianantoni A, D’Angio F, Santarelli A, Lo Muzio L. Oral aphthous ulcers and dental enamel defects in children with coeliac disease. Acta Paediatr. 2006;95:203–207. doi: 10.1080/08035250500355022. [DOI] [PubMed] [Google Scholar]
- Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, Kramer S, Fischer W, Gschaidmeier H, Pietruck F. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet. 2011;377:837–847. doi: 10.1016/S0140-6736(10)62318-5. [DOI] [PubMed] [Google Scholar]
- Burgess J. Re: Effectiveness of vitamin B(12) in treating recurrent aphthous stomatitis: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Med. 2009;22:590–591. doi: 10.3122/jabfm.2009.05.090083. author reply 591. [DOI] [PubMed] [Google Scholar]
- Campisi G, Di Liberto C, Iacono G, Compilato D, Di Prima L, Calvino F, Di Marco V, Lo Muzio L, Sferrazza C, Scalici C, Craxi A, Carroccio A. Oral pathology in untreated coeliac [corrected] disease. Alimentary pharmacology & therapeutics. 2007;26:1529–1536. doi: 10.1111/j.1365-2036.2007.03535.x. [DOI] [PubMed] [Google Scholar]
- Carrozzo M. Vitamin B12 for the treatment of recurrent aphthous stomatitis. Evid Based Dent. 2009;10:114–115. doi: 10.1038/sj.ebd.6400688. [DOI] [PubMed] [Google Scholar]
- Challacombe SJ, Barkhan P, Lehner T. Haematological features and differentiation of recurrent oral ulceration. The British journal of oral surgery. 1977;15:37–48. doi: 10.1016/0007-117x(77)90006-3. [DOI] [PubMed] [Google Scholar]
- Chams-Davatchi C, Barikbin B, Shahram F, Nadji A, Moghaddassi M, Yousefi M, Davatchi F. Pimecrolimus versus placebo in genital aphthous ulcers of Behcet’s disease: a randomized double-blind controlled trial. International journal of rheumatic diseases. 2010;13:253–258. doi: 10.1111/j.1756-185X.2010.01531.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Malahias T, Brar P, Minaya MT, Green PH. The association between celiac disease, dental enamel defects, and aphthous ulcers in a United States cohort. Journal of clinical gastroenterology. 2010;44:191–194. doi: 10.1097/MCG.0b013e3181ac9942. [DOI] [PubMed] [Google Scholar]
- Chuang P, Langone AJ. Clobetasol ameliorates aphthous ulceration in renal transplant patients on sirolimus. Am J Transplant. 2007;7:714–717. doi: 10.1111/j.1600-6143.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- Ciancio G, Colina M, La Corte R, Lo Monaco A, De Leonardis F, Trotta F, Govoni M. Nicotine-patch therapy on mucocutaneous lesions of Behcet’s disease: a case series. Rheumatology (Oxford, England) 2010;49:501–504. doi: 10.1093/rheumatology/kep401. [DOI] [PubMed] [Google Scholar]
- Cochard M, Clet J, Le L, Pillet P, Onrubia X, Gueron T, Faouzi M, Hofer M. PFAPA syndrome is not a sporadic disease. Rheumatology (Oxford, England) 2010;49:1984–1987. doi: 10.1093/rheumatology/keq187. [DOI] [PubMed] [Google Scholar]
- Corazza GR, Frisoni M, Treggiari EA, Valentini RA, Filipponi C, Volta U, Gasbarrini G. Subclinical celiac sprue. Increasing occurrence and clues to its diagnosis. Journal of clinical gastroenterology. 1993;16:16–21. [PubMed] [Google Scholar]
- da Silva CA, Dourado I, Dahia SR, Harzheim E, Rutherford GW. Oral manifestations of HIV infection in patients receiving highly active antiretroviral therapy (HAART) in Bahia, Brazil. Journal of public health dentistry. 2008;68:178–181. doi: 10.1111/j.1752-7325.2007.00071.x. [DOI] [PubMed] [Google Scholar]
- Dagan E, Gershoni-Baruch R, Khatib I, Mori A, Brik R. MEFV, TNF1rA, CARD15 and NLRP3 mutation analysis in PFAPA. Rheumatology international. 2010;30:633–636. doi: 10.1007/s00296-009-1037-x. [DOI] [PubMed] [Google Scholar]
- Davatchi F, Sadeghi Abdollahi B, Tehrani Banihashemi A, Shahram F, Nadji A, Shams H, Chams-Davatchi C. Colchicine versus placebo in Behcet’s disease: randomized, double-blind, controlled crossover trial. Modern rheumatology / the Japan Rheumatism Association. 2009;19:542–549. doi: 10.1007/s10165-009-0200-2. [DOI] [PubMed] [Google Scholar]
- Davatchi F, Shahram F, Chams-Davatchi C, Shams H, Nadji A, Akhlaghi M, Faezi T, Sadeghi Abdollahi B. How to deal with Behcet’s disease in daily practice. International journal of rheumatic diseases. 2010;13:105–116. doi: 10.1111/j.1756-185X.2010.01462.x. [DOI] [PubMed] [Google Scholar]
- de Abreu MA, Hirata CH, Pimentel DR, Weckx LL. Treatment of recurrent aphthous stomatitis with clofazimine. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2009;108:714–721. doi: 10.1016/j.tripleo.2009.05.009. [DOI] [PubMed] [Google Scholar]
- de Armas E, Sarracent Y, Marrero E, Fernandez O, Branford-White C. Efficacy of Rhizophora mangle aqueous bark extract (RMABE) in the treatment of aphthous ulcers: a pilot study. Current medical research and opinion. 2005;21:1711–1715. doi: 10.1185/030079905X65493. [DOI] [PubMed] [Google Scholar]
- De Cunto C, Britos M, Eymann A, Deltetto N, Liberatore D. [Periodic fever: a description of twelve patients with periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis (PFAPA)] Archivos argentinos de pediatria. 2010;108:445–458. doi: 10.1590/S0325-00752010000500011. [DOI] [PubMed] [Google Scholar]
- de Freitas IN, Sipahi AM, Damiao AO, de Brito T, Cancado EL, Leser PG, Laudanna AA. Celiac disease in Brazilian adults. Journal of clinical gastroenterology. 2002;34:430–434. doi: 10.1097/00004836-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Erkalp K, Korkut YA, Meric A, Kahya V, Gedikli O, Su OK, Saitoglu L. Pharyngeal packing is a predisposing factor for postoperative aphthous stomatitis in nasal surgery. Otolaryngol Head Neck Surg. 2010;142:672–676. doi: 10.1016/j.otohns.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Ersoy O, Ersoy R, Yayar O, Demirci H, Tatlican S. H pylori infection in patients with Behcet’s disease. World J Gastroenterol. 2007;13:2983–2985. doi: 10.3748/wjg.v13.i21.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder HM, Salazar JC. A clinical review of 105 patients with PFAPA (a periodic fever syndrome) Acta Paediatr. 2010;99:178–184. doi: 10.1111/j.1651-2227.2009.01554.x. [DOI] [PubMed] [Google Scholar]
- Feder HM., Jr Cimetidine treatment for periodic fever associated with aphthous stomatitis, pharyngitis and cervical adenitis. The Pediatric infectious disease journal. 1992;11:318–321. doi: 10.1097/00006454-199204000-00011. [DOI] [PubMed] [Google Scholar]
- Femiano F, Buonaiuto C, Gombos F, Lanza A, Cirillo N. Pilot study on recurrent aphthous stomatitis (RAS): a randomized placebo-controlled trial for the comparative therapeutic effects of systemic prednisone and systemic montelukast in subjects unresponsive to topical therapy. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2010;109:402–407. doi: 10.1016/j.tripleo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Femiano F, Lanza A, Buonaiuto C, Gombos F, Cirillo N. Oral aphthous-like lesions, PFAPA syndrome: a review. J Oral Pathol Med. 2008;37:319–323. doi: 10.1111/j.1600-0714.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- Ferguson MM, Wray D, Carmichael HA, Russell RI, Lee FD. Coeliac disease associated with recurrent aphthae. Gut. 1980;21:223–226. doi: 10.1136/gut.21.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R, Basu MJ, Asquith P, Cooke WT. Proceedings: Recurrent aphthous ulceration and its association with coeliac disease. Gut. 1975;16:393. [PubMed] [Google Scholar]
- Ferguson R, Basu MK, Asquith P, Cooke WT. Jejunal mucosal abnormalities in patients with recurrent aphthous ulceration. British medical journal. 1976;1:11–13. doi: 10.1136/bmj.1.6000.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field EA, Rotter E, Speechley JA, Tyldesley WR. Clinical and haematological assessment of children with recurrent aphthous ulceration. British dental journal. 1987;163:19–122. doi: 10.1038/sj.bdj.4806174. [DOI] [PubMed] [Google Scholar]
- Galanakis E, Papadakis CE, Giannoussi E, Karatzanis AD, Bitsori M, Helidonis ES. PFAPA syndrome in children evaluated for tonsillectomy. Archives of disease in childhood. 2002;86:434–435. doi: 10.1136/adc.86.6.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavello W, Romagnoli M, Gaini RM. Effectiveness of adenotonsillectomy in PFAPA syndrome: a randomized study. The Journal of pediatrics. 2009;155:250–253. doi: 10.1016/j.jpeds.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Gattorno M, Caorsi R, Meini A, Cattalini M, Federici S, Zulian F, Cortis E, Calcagno G, Tommasini A, Consolini R, Simonini G, Pelagatti MA, Baldi M, Ceccherini I, Plebani A, Frenkel J, Sormani MP, Martini A. Differentiating PFAPA syndrome from monogenic periodic fevers. Pediatrics. 2009;124:e721–e728. doi: 10.1542/peds.2009-0088. [DOI] [PubMed] [Google Scholar]
- Gattorno M, Sormani MP, D’Osualdo A, Pelagatti MA, Caroli F, Federici S, Cecconi M, Solari N, Meini A, Zulian F, Obici L, Breda L, Martino S, Tommasini A, Bossi G, Govers A, Touitou I, Woo P, Frenkel J, Kone-Paut I, Baldi M, Ceccherini I, Martini A. A diagnostic score for molecular analysis of hereditary autoinflammatory syndromes with periodic fever in children. Arthritis and rheumatism. 2008;58:1823–1832. doi: 10.1002/art.23474. [DOI] [PubMed] [Google Scholar]
- Ghate JV, Jorizzo JL. Behcet’s disease and complex aphthosis. Journal of the American Academy of Dermatology. 1999;40:1–18. doi: 10.1016/s0190-9622(99)70523-2. quiz 19–20. [DOI] [PubMed] [Google Scholar]
- Gorsky M, Epstein J, Rabenstein S, Elishoov H, Yarom N. Topical minocycline and tetracycline rinses in treatment of recurrent aphthous stomatitis: a randomized cross-over study. Dermatology online journal. 2007;13:1. [PubMed] [Google Scholar]
- Gorsky M, Epstein J, Raviv A, Yaniv R, Truelove E. Topical minocycline for managing symptoms of recurrent aphthous stomatitis. Spec Care Dentist. 2008;28:27–31. doi: 10.1111/j.1754-4505.2008.00006.x. [DOI] [PubMed] [Google Scholar]
- Hamazaki K, Itomura M, Hamazaki T, Sawazaki S. Effects of cooking plant oils on recurrent aphthous stomatitis: a randomized, placebo-controlled, double-blind trial. Nutrition (Burbank, Los Angeles County, Calif. 2006;22:534–538. doi: 10.1016/j.nut.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hamdy AA, Ibrahem MA. Management of aphthous ulceration with topical quercetin: a randomized clinical trial. The journal of contemporary dental practice. 2010;11:E009–E016. [PubMed] [Google Scholar]
- Hello M, Barbarot S, Bastuji-Garin S, Revuz J, Chosidow O. Use of thalidomide for severe recurrent aphthous stomatitis: a multicenter cohort analysis. Medicine. 2010;89:176–182. doi: 10.1097/MD.0b013e3181dfca14. [DOI] [PubMed] [Google Scholar]
- Hofer MF. Cured by tonsillectomy: was it really a PFAPA syndrome? The Journal of pediatrics. 2008;153:298. doi: 10.1016/j.jpeds.2008.04.050. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Peters U, Maki M, Miettinen A, Collin P. Celiac sprue in patients with chronic oral mucosal symptoms. Journal of clinical gastroenterology. 1998;26:23–26. doi: 10.1097/00004836-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Jorizzo JL, Taylor RS, Schmalstieg FC, Solomon AR, Jr, Daniels JC, Rudloff HE, Cavallo T. Complex aphthosis: a forme fruste of Behcet’s syndrome? Journal of the American Academy of Dermatology. 1985;13:80–84. doi: 10.1016/s0190-9622(85)70147-8. [DOI] [PubMed] [Google Scholar]
- Jurge S, Kuffer R, Scully C, Porter SR. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral diseases. 2006;12:1–21. doi: 10.1111/j.1601-0825.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- Karaca S, Seyhan M, Senol M, Harputluoglu MM, Ozcan A. The effect of gastric Helicobacter pylori eradication on recurrent aphthous stomatitis. International journal of dermatology. 2008;47:615–617. doi: 10.1111/j.1365-4632.2008.03667.x. [DOI] [PubMed] [Google Scholar]
- Kaufman I, Caspi D, Yeshurun D, Dotan I, Yaron M, Elkayam O. The effect of infliximab on extraintestinal manifestations of Crohn’s disease. Rheumatology international. 2005;25:406–410. doi: 10.1007/s00296-004-0467-8. [DOI] [PubMed] [Google Scholar]
- Kleinman DV, Swango PA, Pindborg JJ. Epidemiology of oral mucosal lesions in United States schoolchildren: 1986–87. Community dentistry and oral epidemiology. 1994;22:243–253. doi: 10.1111/j.1600-0528.1994.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Kolseth I, Herlofson BB, Pedersen A. Norwegian LongoVital and recurrent aphthous ulceration: a randomized, double-blind, placebo-controlled study. Oral diseases. 2005;11:374–378. doi: 10.1111/j.1601-0825.2005.01133.x. [DOI] [PubMed] [Google Scholar]
- Koray M, Ak G, Kurklu E, Tanyeri H, Aydin F, Oguz FS, Temurhan S, Ciltci H, Carin M, Onal AE, Ozdilli K. The effect of beta-glucan on recurrent aphthous stomatitis. J Altern Complement Med. 2009;15:111–112. doi: 10.1089/acm.2008.0118. [DOI] [PubMed] [Google Scholar]
- Kovacs L, Hlavata A, Baldovic M, Paulovicova E, Dallos T, Fehervizyova Z, Kadasi L. Elevated immunoglobulin D levels in children with PFAPA syndrome. Neuro endocrinology letters. 2010;31:743–746. [PubMed] [Google Scholar]
- Koybasi S, Parlak AH, Serin E, Yilmaz F, Serin D. Recurrent aphthous stomatitis: investigation of possible etiologic factors. American journal of otolaryngology. 2006;27:229–232. doi: 10.1016/j.amjoto.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Kozlak ST, Walsh SJ, Lalla RV. Reduced dietary intake of vitamin B12 and folate in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2010;39:420–423. doi: 10.1111/j.1600-0714.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahteenoja H, Toivanen A, Viander M, Maki M, Irjala K, Raiha I, Syrjanen S. Oral mucosal changes in coeliac patients on a gluten-free diet. European journal of oral sciences. 1998;106:899–906. doi: 10.1046/j.0909-8836.1998.eos106501.x. [DOI] [PubMed] [Google Scholar]
- Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, Moulin B, Frouget T, Le Meur Y, Glotz D, Heng AE, Onno C, Buchler M, Girardot-Seguin S, Hurault de Ligny B. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant. 2009;9:1115–1123. doi: 10.1111/j.1600-6143.2009.02615.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jung JY, Bang D. The efficacy of topical 0.2% hyaluronic acid gel on recurrent oral ulcers: comparison between recurrent aphthous ulcers and the oral ulcers of Behcet’s disease. J Eur Acad Dermatol Venereol. 2008;22:590–595. doi: 10.1111/j.1468-3083.2007.02564.x. [DOI] [PubMed] [Google Scholar]
- Leong SC, Karkos PD, Apostolidou MT. Is there a role for the otolaryngologist in PFAPA syndrome? A systematic review. International journal of pediatric otorhinolaryngology. 2006;70:1841–1845. doi: 10.1016/j.ijporl.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Lierl M. Periodic fever syndromes: a diagnostic challenge for the allergist. Allergy. 2007;62:1349–1358. doi: 10.1111/j.1398-9995.2007.01534.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Zeng X, Chen Q, Cai Y, Chen F, Wang Y, Zhou H, Lin M, Shi J, Wang Z, Zhang Y. An evaluation on the efficacy and safety of amlexanox oral adhesive tablets in the treatment of recurrent minor aphthous ulceration in a Chinese cohort: a randomized, double-blind, vehicle-controlled, unparallel multicenter clinical trial. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2006;102:475–481. doi: 10.1016/j.tripleo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Long SS. Syndrome of Periodic Fever, Aphthous stomatitis, Pharyngitis, and Adenitis (PFAPA)--what it isn’t. What is it? The Journal of pediatrics. 1999;135:1–5. doi: 10.1016/s0022-3476(99)70316-1. [DOI] [PubMed] [Google Scholar]
- Lynde CB, Bruce AJ, Rogers RS., 3rd Successful treatment of complex aphthosis with colchicine and dapsone. Archives of dermatology. 2009;145:273–276. doi: 10.1001/archdermatol.2008.591. [DOI] [PubMed] [Google Scholar]
- Majorana A, Sapelli PL, Malagoli A, Meini A, Pillan MN, Duse M, Ugazio AG. [Celiac disease and recurrent aphthous stomatitis. The clinical and immunogenetic aspects] Minerva stomatologica. 1992;41:33–40. [PubMed] [Google Scholar]
- Marshall GS, Edwards KM, Butler J, Lawton AR. Syndrome of periodic fever, pharyngitis, and aphthous stomatitis. The Journal of pediatrics. 1987;110:43–46. doi: 10.1016/s0022-3476(87)80285-8. [DOI] [PubMed] [Google Scholar]
- Marshall GS, Edwards KM, Lawton AR. PFAPA syndrome. The Pediatric infectious disease journal. 1989;8:658–659. doi: 10.1097/00006454-198909000-00026. [DOI] [PubMed] [Google Scholar]
- Martin MD, Sherman J, van der Ven P, Burgess J. A controlled trial of a dissolving oral patch concerning glycyrrhiza (licorice) herbal extract for the treatment of aphthous ulcers. General dentistry. 2008;56:206–210. quiz 211-2, 224. [PubMed] [Google Scholar]
- Mc Carty MA, Jorizzo JL. Complex aphthosis: evaluation for Behcet’s disease? Advances in experimental medicine and biology. 2003;528:303–310. doi: 10.1007/0-306-48382-3_60. [DOI] [PubMed] [Google Scholar]
- Meini A, Pillan MN, Plebani A, Ugazio AG, Majorana A, Sapelli PL. High prevalence of DRW10 and DQW1 antigens in celiac disease associated with recurrent aphthous stomatitis. The American journal of gastroenterology. 1993;88:972. [PubMed] [Google Scholar]
- Meng W, Dong Y, Liu J, Wang Z, Zhong X, Chen R, Zhou H, Lin M, Jiang L, Gao F, Xu T, Chen Q, Zeng X. A clinical evaluation of amlexanox oral adhesive pellicles in the treatment of recurrent aphthous stomatitis and comparison with amlexanox oral tablets: a randomized, placebo controlled, blinded, multicenter clinical trial. Trials. 2009;10:30. doi: 10.1186/1745-6215-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura MA, Hirota SK, Sugaya NN, Sanches JA, Jr, Migliari DA. Systemic treatment in severe cases of recurrent aphthous stomatitis: an open trial. Clinics (Sao Paulo, Brazil) 2009;64:193–198. doi: 10.1590/S1807-59322009000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moezzi M, Moezzi A, Lengyel ZS, Csete B, Szepes E. Sympathetic blockade in treating recurrent aphthous ulcers (RAU) European Academy of Dermatology and Venereology JEADV. 2005;19:13. [Google Scholar]
- Moghadamnia AA, Motallebnejad M, Khanian M. The efficacy of the bioadhesive patches containing licorice extract in the management of recurrent aphthous stomatitis. Phytother Res. 2009;23:246–250. doi: 10.1002/ptr.2601. [DOI] [PubMed] [Google Scholar]
- Motallebnejad M, Moghadamnia A, Talei M. The Efficacy of Hypericum perforatum Extract on Recurrent Aphthous Ulcers. J Med Sci. 2008;8:39–43. [Google Scholar]
- Mousavi F, Mojaver YN, Asadzadeh M, Mirzazadeh M. Homeopathic treatment of minor aphthous ulcer: a randomized, placebo-controlled clinical trial. Homeopathy. 2009;98:137–141. doi: 10.1016/j.homp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Murray B, Biagioni PA, Lamey PJ. The efficacy of amlexanox OraDisc on the prevention of recurrent minor aphthous ulceration. J Oral Pathol Med. 2006;35:117–122. doi: 10.1111/j.1600-0714.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- Murray B, McGuinness N, Biagioni P, Hyland P, Lamey PJ. A comparative study of the efficacy of Aphtheal in the management of recurrent minor aphthous ulceration. J Oral Pathol Med. 2005;34:413–419. doi: 10.1111/j.1600-0714.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- Nally FF, Blake GC. Letter: Recurrent aphthae: treatment with vitamin B12, folic acid, and iron. British medical journal. 1975;3:308. doi: 10.1136/bmj.3.5978.308-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanke Y, Kamatani N, Okamoto T, Ogiuchi H, Kotake S. Irsogladine is effective for recurrent oral ulcers in patients with Behcet’s disease : an open-label, single-centre study. Drugs in R&D. 2008;9:455–459. doi: 10.2165/0126839-200809060-00008. [DOI] [PubMed] [Google Scholar]
- Nolan A, Baillie C, Badminton J, Rudralingham M, Seymour RA. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J Oral Pathol Med. 2006;35:461–465. doi: 10.1111/j.1600-0714.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- Nowak M, Dziechciarz P, Dwilewicz-Trojaczek J. [The frequency of coeliac disease occurrence in patients with recurrent aphthous stomatitis (RAS)-preliminary report] Wiad Lek. 2002;55:542–546. [PubMed] [Google Scholar]
- O’Farrelly C, O’Mahony C, Graeme-Cook F, Feighery C, McCartan BE, Weir DG. Gliadin antibodies identify gluten-sensitive oral ulceration in the absence of villous atrophy. J Oral Pathol Med. 1991;20:476–478. doi: 10.1111/j.1600-0714.1991.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Olson JA, Feinberg I, Silverman S, Jr, Abrams D, Greenspan JS. Serum vitamin B12, folate, and iron levels in recurrent aphthous ulceration. Oral surgery, oral medicine, and oral pathology. 1982;54:517–520. doi: 10.1016/0030-4220(82)90189-x. [DOI] [PubMed] [Google Scholar]
- Olszewska M, Sulej J, Kotowski B. Frequency and prognostic value of IgA and IgG endomysial antibodies in recurrent aphthous stomatitis. Acta dermato-venereologica. 2006;86:332–334. doi: 10.2340/00015555-0087. [DOI] [PubMed] [Google Scholar]
- Padeh S, Brezniak N, Zemer D, Pras E, Livneh A, Langevitz P, Migdal A, Pras M, Passwell JH. Periodic fever, aphthous stomatitis, pharyngitis, and adenopathy syndrome: clinical characteristics and outcome. The Journal of pediatrics. 1999;135:98–101. doi: 10.1016/s0022-3476(99)70335-5. [DOI] [PubMed] [Google Scholar]
- Padeh S, Stoffman N, Berkun Y. Periodic fever accompanied by aphthous stomatitis, pharyngitis and cervical adenitis syndrome (PFAPA syndrome) in adults. Isr Med Assoc J. 2008;10:358–360. [PubMed] [Google Scholar]
- Pakfetrat A, Mansourian A, Momen-Heravi F, Delavarian Z, Momen-Beitollahi J, Khalilzadeh O, Basir-Shabestari S. Comparison of colchicine versus prednisolone in recurrent aphthous stomatitis: A double-blind randomized clinical trial. Clinical and investigative medicine. 2010;33:E189–E195. doi: 10.25011/cim.v33i3.13725. [DOI] [PubMed] [Google Scholar]
- Palopoli J, Waxman J. Recurrent aphthous stomatitis and vitamin B12 deficiency. Southern medical journal. 1990;83:475–477. doi: 10.1097/00007611-199004000-00029. [DOI] [PubMed] [Google Scholar]
- Passarini B, Infusino SD, Barbieri E, Varotti E, Gionchetti P, Rizzello F, Morselli C, Tambasco R, Campieri M. Cutaneous manifestations in inflammatory bowel diseases: eight cases of psoriasis induced by anti-tumor-necrosis-factor antibody therapy. Dermatology. 2007;215:295–300. doi: 10.1159/000107622. [DOI] [PubMed] [Google Scholar]
- Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, Ondei P, Rubis N, Diadei O, Gherardi G, Prandini S, Panozo A, Bravo RF, Carminati S, De Leon FR, Gaspari F, Cortinovis M, Motterlini N, Ene-Iordache B, Remuzzi A, Remuzzi G. Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol. 2010;21:1031–1040. doi: 10.1681/ASN.2009121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro L, Torretta S, Pietrogrande MC, Dellepiane RM, Pavesi P, Bossi A, Drago L, Capaccio P. Outcome of tonsillectomy in selected patients with PFAPA syndrome. Archives of otolaryngology--head & neck surgery. 2009;135:548–553. doi: 10.1001/archoto.2009.56. [DOI] [PubMed] [Google Scholar]
- Pignatello R, Basile L, Puglisi G. Chitosan glutamate hydrogels with local anesthetic activity for buccal application. Drug delivery. 2009;16:176–181. doi: 10.1080/10717540902861267. [DOI] [PubMed] [Google Scholar]
- Piskin S, Sayan C, Durukan N, Senol M. Serum iron, ferritin, folic acid, and vitamin B12 levels in recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol. 2002;16:66–67. doi: 10.1046/j.1468-3083.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- Porter SR, Al-Johani K, Fedele S, Moles DR. Randomised controlled trial of the efficacy of HybenX in the symptomatic treatment of recurrent aphthous stomatitis. Oral diseases. 2009;15:155–161. doi: 10.1111/j.1601-0825.2008.01503.x. [DOI] [PubMed] [Google Scholar]
- Porter SR, Scully C, Flint S. Hematologic status in recurrent aphthous stomatitis compared with other oral disease. Oral surgery, oral medicine, and oral pathology. 1988;66:41–44. doi: 10.1016/0030-4220(88)90064-3. [DOI] [PubMed] [Google Scholar]
- Pourahmad M, Rahiminejad M, Fadaei S, Kashafi H. Effects of camel thorn distillate on recurrent oral aphthous lesions. J Dtsch Dermatol Ges. 2010;8:348–352. doi: 10.1111/j.1610-0387.2010.07316.x. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Grainger P, Bradshaw MH, Mohammad AR, Powala CV, Nolan A. Subantimicrobial dose doxycycline in the treatment of recurrent oral aphthous ulceration: a pilot study. J Oral Pathol Med. 2007;36:236–240. doi: 10.1111/j.1600-0714.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Procaccini M, Campisi G, Bufo P, Compilato D, Massaccesi C, Catassi C, Lo Muzio L. Lack of association between celiac disease and dental enamel hypoplasia in a case-control study from an Italian central region. Head & face medicine. 2007;3:25. doi: 10.1186/1746-160X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renko M, Salo E, Putto-Laurila A, Saxen H, Mattila PS, Luotonen J, Ruuskanen O, Uhari M. A randomized, controlled trial of tonsillectomy in periodic fever, aphthous stomatitis, pharyngitis, and adenitis syndrome. The Journal of pediatrics. 2007;151:289–292. doi: 10.1016/j.jpeds.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Richards D. GRADING--levels of evidence. Evid Based Dent. 2009;10:24–25. doi: 10.1038/sj.ebd.6400636. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Rubio JA, Sanchez R. Effectiveness of two oral pastes for the treatment of recurrent aphthous stomatitis. Oral diseases. 2007;13:490–494. doi: 10.1111/j.1601-0825.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- Rogers RS., 3rd Complex aphthosis. Advances in experimental medicine and biology. 2003;528:311–316. doi: 10.1007/0-306-48382-3_61. [DOI] [PubMed] [Google Scholar]
- Rogers RS, 3rd, Hutton KP. Screening for haematinic deficiencies in patients with recurrent aphthous stomatitis. The Australasian journal of dermatology. 1986;27:98–103. doi: 10.1111/j.1440-0960.1986.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Rose JD, Smith DM, Allan FG, Sircus W. Recurrent aphthous ulceration and jejunal biopsy. British medical journal. 1978;1:1145. doi: 10.1136/bmj.1.6120.1145-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet N, Laurent C, Susarla SM, Samet-Rubinsteen N. The effect of bee propolis on recurrent aphthous stomatitis: a pilot study. Clinical oral investigations. 2007;11:143–147. doi: 10.1007/s00784-006-0090-z. [DOI] [PubMed] [Google Scholar]
- Scully C, Hodgson T, Lachmann H. Auto-inflammatory syndromes and oral health. Oral diseases. 2008;14:690–699. doi: 10.1111/j.1601-0825.2008.01484.x. [DOI] [PubMed] [Google Scholar]
- Sedghizadeh PP, Shuler CF, Allen CM, Beck FM, Kalmar JR. Celiac disease and recurrent aphthous stomatitis: a report and review of the literature. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2002;94:474–478. doi: 10.1067/moe.2002.127581. [DOI] [PubMed] [Google Scholar]
- Sharma NL, Sharma VC, Mahajan VK, Shanker V, Ranjan N, Gupta M. Thalidomide: an experience in therapeutic outcome and adverse reactions. The Journal of dermatological treatment. 2007;18:335–340. doi: 10.1080/09546630701386993. [DOI] [PubMed] [Google Scholar]
- Sharquie KE, Al-Tammimy SM, Al-Mashhadani S, Hayani RK, Al-Nuaimy AA. Lactic acid 5 percent mouthwash is an effective mode of therapy in treatment of recurrent aphthous ulcerations. Dermatology online journal. 2006;12:2. [PubMed] [Google Scholar]
- Sharquie KE, Hayani RK. BCG as a new therapeutic and prophylactic agent in patients with severe oral aphthosis. Clinical and experimental rheumatology. 2005;23:914. [PubMed] [Google Scholar]
- Sharquie KE, Najim RA, Al-Hayani RK, Al-Nuaimy AA, Maroof DM. The therapeutic and prophylactic role of oral zinc sulfate in management of recurrent aphthous stomatitis (ras) in comparison with dapsone. Saudi medical journal. 2008;29:734–738. [PubMed] [Google Scholar]
- Shemer A, Amichai B, Trau H, Nathansohn N, Mizrahi B, Domb AJ. Efficacy of a mucoadhesive patch compared with an oral solution for treatment of aphthous stomatitis. Drugs in R&D. 2008;9:29–35. doi: 10.2165/00126839-200809010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulason S, Holbrook WP, Kristmundsdottir T. Clinical assessment of the effect of a matrix metalloproteinase inhibitor on aphthous ulcers. Acta odontologica Scandinavica. 2009;67:25–29. doi: 10.1080/00016350802526559. [DOI] [PubMed] [Google Scholar]
- Sood A, Midha V, Sood N, Malhotra V. Adult celiac disease in northern India. Indian J Gastroenterol. 2003;22:124–126. [PubMed] [Google Scholar]
- Srinivasan U, Weir DG, Feighery C, O’Farrelly C. Emergence of classic enteropathy after longstanding gluten sensitive oral ulceration. BMJ (Clinical research ed. 1998;316:206–207. doi: 10.1136/bmj.316.7126.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens FM. Celiac Disease Clinical Manifestations. Practical Gastroenterology. 1980;4:10–14. [Google Scholar]
- Sunitha M, Shanmugam S. Evaluation of salivary nitric oxide levels in oral mucosal diseases: A controlled clinical trial. Indian J Dent Res. 2006;17:117–120. doi: 10.4103/0970-9290.29878. [DOI] [PubMed] [Google Scholar]
- Tasher D, Somekh E, Dalal I. PFAPA syndrome: new clinical aspects disclosed. Archives of disease in childhood. 2006;91:981–984. doi: 10.1136/adc.2005.084731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel A, Kara C, Balkaya V, Orbak R. An evaluation of different treatments for recurrent aphthous stomatitis and patient perceptions: Nd:YAG laser versus medication. Photomedicine and laser surgery. 2009;27:101–106. doi: 10.1089/pho.2008.2274. [DOI] [PubMed] [Google Scholar]
- Thomas KT, Feder HM, Jr, Lawton AR, Edwards KM. Periodic fever syndrome in children. The Journal of pediatrics. 1999;135:15–21. doi: 10.1016/s0022-3476(99)70321-5. [DOI] [PubMed] [Google Scholar]
- Thongprasom K, Youngnak P, Aneksuk V. Hematologic abnormalities in recurrent oral ulceration. The Southeast Asian journal of tropical medicine and public health. 2002;33:872–877. [PubMed] [Google Scholar]
- Thornhill MH, Baccaglini L, Theaker E, Pemberton MN. A randomized, double-blind, placebo-controlled trial of pentoxifylline for the treatment of recurrent aphthous stomatitis. Archives of dermatology. 2007;143:463–470. doi: 10.1001/archderm.143.4.463. [DOI] [PubMed] [Google Scholar]
- Tyldesley WR. Stomatitis and recurrent oral ulceration: is a full blood screen necessary? The British journal of oral surgery. 1983;21:27–30. doi: 10.1016/0007-117x(83)90027-6. [DOI] [PubMed] [Google Scholar]
- van der Hilst JC, Bodar EJ, Barron KS, Frenkel J, Drenth JP, van der Meer JW, Simon A. Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine. 2008;87:301–310. doi: 10.1097/MD.0b013e318190cfb7. [DOI] [PubMed] [Google Scholar]
- Veloso FT, Saleiro JV. Small-bowel changes in recurrent ulceration of the mouth. Hepato-gastroenterology. 1987;34:36–37. [PubMed] [Google Scholar]
- Volkov I, Rudoy I, Abu-Rabia U, Masalha T, Masalha R. Case report: Recurrent aphthous stomatitis responds to vitamin B12 treatment. Canadian family physician Medecin de famille canadien. 2005;51:844–845. [PMC free article] [PubMed] [Google Scholar]
- Volkov I, Rudoy I, Freud T, Sardal G, Naimer S, Peleg R, Press Y. Effectiveness of vitamin B12 in treating recurrent aphthous stomatitis: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Med. 2009;22:9–16. doi: 10.3122/jabfm.2009.01.080113. [DOI] [PubMed] [Google Scholar]
- Walker JE. Aphthous ulceration and vitamin B12 deficiency. The British journal of oral surgery. 1973;11:165–170. doi: 10.1016/0007-117x(73)90036-x. [DOI] [PubMed] [Google Scholar]
- Weckx LL, Hirata CH, Abreu MA, Fillizolla VC, Silva OM. [Levamisole does not prevent lesions of recurrent aphthous stomatitis: a double-blind placebo-controlled clinical trial] Revista da Associacao Medica Brasileira (1992) 2009;55:132–138. doi: 10.1590/s0104-42302009000200014. [DOI] [PubMed] [Google Scholar]
- Weusten BL, van de Wiel A. Aphthous ulcers and vitamin B12 deficiency. The Netherlands journal of medicine. 1998;53:172–175. doi: 10.1016/s0300-2977(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Wong KK, Finlay JC, Moxham JP. Role of Tonsillectomy in PFAPA Syndrome. Archives of otolaryngology--head & neck surgery. 2008;134:16–19. doi: 10.1001/archoto.2007.15. [DOI] [PubMed] [Google Scholar]
- Wray D, Ferguson MM, Hutcheon WA, Dagg JH. Nutritional deficiencies in recurrent aphthae. Journal of oral pathology. 1978;7:418–423. doi: 10.1111/j.1600-0714.1978.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Wray D, Ferguson MM, Mason DK, Hutcheon AW, Dagg JH. Recurrent aphthae: treatment with vitamin B12, folic acid, and iron. British medical journal. 1975;2:490–493. doi: 10.1136/bmj.2.5969.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TY, Jang TY. The value of local botulinum toxin A injection in the treatment of the pain of aphthous ulcer. Eur Arch Otorhinolaryngol. 2009;266:445–448. doi: 10.1007/s00405-008-0756-z. [DOI] [PubMed] [Google Scholar]
- Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A, Meric-Bernstam F. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K, Kurata T, Yashiro M, Tsuge M, Ohtsuki S, Morishima T. The effect of ascorbate on minor recurrent aphthous stomatitis. Acta Paediatr. 2010;99:442–445. doi: 10.1111/j.1651-2227.2009.01628.x. [DOI] [PubMed] [Google Scholar]
- Yazdanpanah MJ, Mokhtari MB, Mostofi K, Soleimani M, Ebrahimirad M, Esmaili H, Ahmadi SN. Oral poliovirus vaccine in management of recurrent aphthous stomatitis. Acta microbiologica et immunologica Hungarica. 2008;55:343–350. doi: 10.1556/AMicr.55.2008.3.5. [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Imamura T, Yokoi K, Shibata M, Kano G, Osone S, Yagi K, Todo S, Murakami Y, Yamada Y, Yamada H, Satomura S, Ishida H. Potential use of procalcitonin concentrations as a diagnostic marker of the PFAPA syndrome. European journal of pediatrics. 2007;166:621–622. doi: 10.1007/s00431-006-0281-2. [DOI] [PubMed] [Google Scholar]
- Zand N, Ataie-Fashtami L, Djavid GE, Fateh M, Alinaghizadeh MR, Fatemi SM, Arbabi-Kalati F. Relieving pain in minor aphthous stomatitis by a single session of non-thermal carbon dioxide laser irradiation. Lasers in medical science. 2009;24:515–520. doi: 10.1007/s10103-008-0555-1. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Chen Q, Meng W, Jiang L, Wang Z, Liu J, Lin M, Zhou H, Chen X, Zhao M, Zeng X. Evaluation of penicillin G potassium troches in the treatment of minor recurrent aphthous ulceration in a Chinese cohort: a randomized, double-blinded, placebo and no-treatment-controlled, multicenter clinical trial. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2010;109:561–566. doi: 10.1016/j.tripleo.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC. Epidemiology of Adamantiades-Behcet’s disease. In: Bang D, Lee ES, Lee S, editors. Proceedings of the 8th and 9th International Conference on Behcet’s Disease; Seoul: Design Mecca Publishing; 2001. pp. 43–47. [Google Scholar]


