Abstract
The functional implications of transient receptor potential melastatin 3 (TRPM3) activation, the most recently described member of the melastatin subfamily of cation permeable TRP channels, have begun to be elucidated in recent years. The discovery of TRPM3 activation by the steroid pregnenolone sulfate (PregS) has shed new light on the physiological role of this channel. For example, TRPM3 activation enhances insulin secretion from β pancreatic cells, induces contraction of vascular smooth muscle, and is also involved in the detection of noxious heat. Although TRPM3 expression has been detected in several regions of the developing and mature brain, little is known about the roles of TRPM3 in brain physiology. Here, we demonstrate the abundant expression of TRPM3 steroid-sensitive channels in the developing cerebellar cortex. We also show that TRPM3-like channels are expressed at glutamatergic synapses in neonatal Purkinje cells (PCs). We recently showed that PregS potentiates spontaneous glutamate release onto neonatal PCs during a period of active glutamatergic synapse formation; we now show that this effect of PregS is mediated by TRPM3-like channels. Mefenamic acid, a recently discovered TRPM3 antagonist, blocked the effect of PregS on glutamate release. The PregS effect on glutamate release was mimicked by other TRPM3 agonists (nifedipine and epipregnanolone sulfate) but not by a TRMP3-inactive steroid (progesterone). Our findings identify TRPM3 channels as novel modulators of glutamatergic transmission in the developing brain.
Keywords: TRPM3 channels, pregnenolone sulfate, neurosteroid, glutamate release, cerebellum, Purkinje cell
Introduction
The mammalian transient receptor potential melastatin (TRPM) channel subfamily includes 8 members based on their homology to melastatin, a putative tumor suppressor that may be involved in the pathophysiology of melanoma. The members of the TRPM subfamily are characterized by sequence similarities along the 700 amino acid-long N-terminal and transmembrane domains, whereas the C-terminal domains show diverse structures (reviewed in Venkatachalam & Montell 2007, Wu et al. 2011). TRPM proteins assemble into ion conducting channels that respond to a variety of stimuli including temperature, osmolarity, various chemical signals, changes in membrane voltage, oxidative stress, and intracellular Ca2+ (Harteneck 2005). While most of the TRPM channels are activated in a unique manner, TRPM3 behaves as a polymodal regulated ion channel (Harteneck & Schultz 2007, Oberwinkler & Phillipp 2007). Beside the recently described activation by temperature, TRPM3 is activated by the neuroactive steroids pregnenolone sulfate (PregS) and epipregnanolone sulfate, the L-type voltage-gated Ca2+ channel antagonist nifedipine, and also by hypotonicity (Grimm et al. 2003, Lee et al. 2003, Grimm et al. 2005, Wagner et al. 2008, Majeed et al. 2010, Vriens et al. 2011). Electrophysiological experiments in heterologous expression systems demonstrated that TRPM3 forms an ion channel permeable to Ca2+, Na+, Mg2+, and Mn2+ (Grimm et al. 2005, Oberwinkler et al. 2005). The polymodal activation mode of TRPM3 corresponds to the diversity of cells expressing TRPM3; these channels have been linked to vascular smooth muscle contraction, modulation of glucose-induced insulin release from pancreatic islets, detection of noxious heat in dorsal root ganglia, and development of oligodendrocytes (Wagner et al. 2008, Naylor et al. 2010, Hoffmann et al. 2010, Vriens et al. 2011).
Since the first functional description of TRPM3, northern blot analyses argued for the expression of TRPM3 in the brain (Lee et al. 2003, Grimm et al. 2003, Oberwinkler et al. 2005). These studies revealed the presence of several distinct transcription products in the mouse brain. The detection of sequence discrepancies due to alternative splicing and exon usage suggests the expression of multiple TRPM3 mRNA transcripts (Lee et al. 2003, Oberwinkler et al. 2005). In situ hybridization and immunohistochemistry approaches revealed expression of TRPM3 in the epithelial cells of the choriod plexus, oligodendrocytes and neurons (Oberwinkler et al. 2005, Hoffmann et al. 2010). The functional consequences of TRPM3 activation in the brain have just begun to be elucidated and one physiological role for TRPM3 in the brain has been recently suggested. At the onset of myelination and into adulthood, TRPM3 expression was found in oligodendrocytes, where TRPM3 activation induced increases in intracellular Ca2+. These findings suggest that TRPM3 channels are important players in oligodendrocyte differentiation and CNS myelination (Hoffmann et al. 2010). In the immature brain, the presence of TRPM3 was also detected (Hoffmann et al. 2010); however, the physiological role of these channels in the developing brain has not been explored. Studies from our laboratory suggest that the TRPM3 activator, PregS, is an important regulator of the formation and refinement of glutamatergic synapses in the developing brain (Mameli et al. 2005, Valenzuela et al. 2008). In the developing cerebellum, PregS potently and reversibly enhances glutamate release onto Purkinje cells (PCs) of neonatal rats, an effect that is independent of several PregS-sensitive targets; namely, N-methyl-D-aspartate (NMDA), glycine, α7 nicotinic acetylcholine, and σ1 receptors, as well as voltage-gated Ca2+ channels (Zamudio-Bulcock & Valenzuela 2011). PregS increases glutamate release by enhancing Ca2+ entrance into presynaptic terminals via a target that is sensitive to La3+, a non-selective agent that modulates a number of TRP channel subtypes and is known to block TRPM3 (Zamudio-Bulcock & Valenzuela 2011, Zholos 2010). Based on this finding, we hypothesized that TRPM3 channels are expressed at developing glutamatergic synapses on PCs and that they mediate the PregS-induced enhancement of glutamatergic transmission. We tested this hypothesis using immunohistochemistry and slice electrophysiology.
Materials and Methods
Unless specified, all chemicals were from Sigma (St. Louis, MO) or Tocris Bioscience (Ellisville, MO). All animal procedures were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee and conformed to National Institutes of Health guidelines.
Immunohistochemistry
TRPM3 expression in the cerebellar cortex was studied using immunohistochemistry. Parasagittal cerebellar cortex sections were prepared from 5 postnatal day (P) 6–8 male Sprague Dawley rats (Harlan, Indianapolis, IN) from three different litters. Tissue preparation was performed as follows: brains were removed after deep anesthesia with 250 mg/kg ketamine and immersion-fixed in 4% paraformaldehyde overnight. Subsequently, brains were cryo-protected in 30% sucrose in phosphate buffered saline (PBS) at 4°C until they sank to the bottom. Brains were then imbedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA) and flash-frozen by submersion in isopentane cooled in a dry ice/methanol bath. Brains were then cryo-sectioned in the sagittal plane, placed on superfrost plus micro slides (VWR international, West Chester, PA), and stored at −20°C for a period no longer than two weeks. Sections were washed 3 times in PBS and permeabilized with 0.5% Triton X-100. Simultaneously, nonspecific binding was blocked with 10% normal donkey serum, 10% normal goat serum, and 10% bovine serum albumin. Primary antibodies (polyclonal rabbit anti-TRPM3; polyclonal guinea pig anti-VGlut2, AB5907 Millipore, Billerica, MA; and monoclonal mouse anti-calbindin, sc-70478, Santa Cruz Biotechnology, Inc, Santa Cruz, CA), were incubated overnight at 4°C. The anti-TRPM3 antibody was raised in rabbits and affinity purified as previously described (Hoffmann et al. 2010), using the following synthetic peptide NH2-CDMPYMSQAQEIHLQEKEAEEPEKPTKE-CONH2 corresponding to positions 795 to 821 of TRPM3 splice variants A and B. All secondary antibodies were purchased from Millipore. Secondary antibodies (goat anti-rabbit alexa fluor 555 for TRPM3 detection, donkey anti-guinea pig CY5 conjugate for VGlut2 detection and donkey anti-mouse alexa 488 for calbindin detection) were incubated for 1 h at room temperature. Sections were rinsed and mounted with Vectashield (Vector Laboratories, Burlingame, CA) and examined with a Zeiss LSM510 confocal microscope (Carl Zeiss, Germany). All confocal images were taken using a 40x immersion objective. Confocal images used to evaluate TRPM3 expression throughout the cerebellar cortex were taken using an optical section thickness of 2 μm. For colocalization analysis, Z-stack images were taken using an optical section thickness of 1 μm, which was the optimal pinhole diameter for best resolution. Detector gain and laser percent output parameters were optimized for each channel in one section and maintained throughout all the studies. Two z-stack image files from different cerebellar cortical regions were taken per each animal.
Co-localization was determined using Slidebook 5 image analysis software (Intelligent Imaging Innovations, Inc., Denver, CO). After background subtraction for each individual channel, one mask per channel was created. Subsequently, the area over the PC layer region was selected and extrapolated to each image in the z-stack. Then, a colocalization mask across two or three channels within that area was created. Colocalization masks were created using the math function “AND”, which recognizes the voxels that are positive for both proteins. Percent colocalization was calculated using the total number of voxels in an individual channel mask compared to the number of colocalized voxels in the respective colocalization mask. (Vo et al. 2004).
Slice electrophysiology
Cerebellar vermis parasagittal slices (250 μm-thick) were prepared from neonatal (P4–P10) male Sprague–Dawley rats and whole-cell patch-clamp recordings were performed as previously described (Mameli et al. 2008, Zamudio-Bulcock & Valenzuela 2011). Alpha-amino-3-hydroxy-5-methyl-4- isoxazole-propionic acid (AMPA) receptor-mediated miniature excitatory postsynaptic currents (AMPA-mEPSCs) were recorded from PCs at 32°C at a holding potential of −70 mV. The artificial cerebrospinal fluid (ACSF) was equilibrated with 95% O2/5% CO2 and contained the following (in mM): 126 NaCl, 2 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2, 10 glucose, 10 μM 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid (SR 95531), and 0.5 μM tetrodotoxin (TTX; Calbiochem, La Jolla, CA). The internal solution contained (in mM): 150 CsCl, 1.5 MgCl2, 10 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES), 0.1 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) (Calbiochem, La Jolla, CA), 2 Na2-ATP, 0.4 Na-GTP, at pH 7.3. Using these recording conditions, we previously demonstrated that mEPSCs are blocked by 1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepine (GYKI 53655; 50 μM), confirming that these events are AMPA receptor mediated (Zamudio-Bulcock & Valenzuela 2011). If the access resistance changed >30%, the recording was discarded.
Data were acquired with pClamp 10 (Molecular Devices, Sunnyvale, CA) at a filtering frequency of 2 kHz and an acquisition rate of 10 kHz. Miniature events were analyzed using Mini Analysis (Synaptosoft, Decatur, GA). In all cases, changes in AMPA-mEPSC frequency were quantified by measuring changes in inter-event interval. Individual recordings were analyzed using the Kolmogorov–Smirnov (K–S) test using a conservative value for significance of p <0.01. Statistical analyses of pooled data were performed with GraphPad Prizm 4 (GraphPad Software, San Diego, CA). Data were initially analyzed with the D’Agostino and Pearson omnibus normality tests. If data followed a normal distribution, these were analyzed using parametric tests. If this was not the case, then non-parametric tests were used. The level of significance was p <0.05. Data were normalized to baseline and expressed as percent of baseline ± standard error of the mean.
Results
TRPM3 expression in the neonatal cerebellar cortex
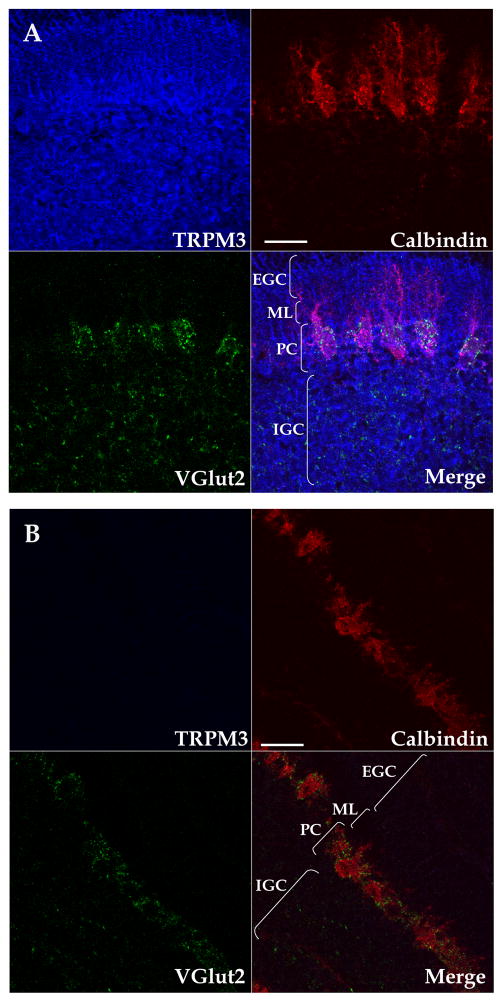
We performed immunohistochemistry experiments to assess the expression of TRPM3 in the neonatal cerebellar cortex using parasagittal sections from P6-8 rats. During the neonatal period, the cerebellar cortex consists of the external granule cell layer, the nascent molecular layer, the PC layer, and the internal granule cell layer (Fig 1A). Glutamatergic axons form temporary synapses on PC bodies, which are arranged in a pseudomonolayer (Eilers et al. 2001, Kawa 2003, Kurihara et al. 1997). During the first 14 postnatal days, all glutamatergic terminals onto PCs express the vesicular glutamate transporter VGlut2. Subsequently, VGlut2 is expressed selectively in climbing fiber (CF) terminals and VGlut1 becomes the main vesicular glutamate transporter in parallel fiber (PF) terminals (Miyazaki et al. 2003). Therefore, we used VGlut2 as a marker for glutamatergic terminals in the neonatal cerebellar cortex. To detect PCs, we used the Ca2+-binding protein calbindin, which is highly expressed in PCs. We found a strong TRPM3 expression in PCs (Fig. 1A). Other cells within the PC layer were labeled by the anti-TRPM3 antibody; based on their localization, these cells likely correspond to developing Bergman glia, which enwrap CF-to-PC synapses during postnatal development (Douyard et al. 2007). TRPM3 was also found in the inner granule cell layer, the nascent molecular layer, and the external granule cell layer (Fig. 1A). Preincubation of the anti-TRPM3 antisera with the synthetic peptide used for immunization, completely blunted the staining reaction, demonstrating the specificity of the immunohistochemical assay (Fig. 1B).
Figure 1. Pseudocolored confocal images of TRPM3 expression profile in the neonatal cerebellar cortex.
(A) TRPM3 (blue), calbindin (red) and VGlut2 (green) expression in a 2 μm-thick sagittal cerebellar confocal section at P8. Channels are shown separately and a merged image is shown in the bottom right division. Similar results were obtained in sections from 4 additional rats. (B) Competition with the immunizing peptide eliminates TRPM3 protein staining. Similar results were obtained in sections from another rat. External granule cell layer (EGC); Molecular layer (ML ), Purkinje cell layer (PC), internal granule cell layer (IGC). Scale bars: 50 μm.
TRPM3 is expressed at glutamatergic synapses in neonatal PCs
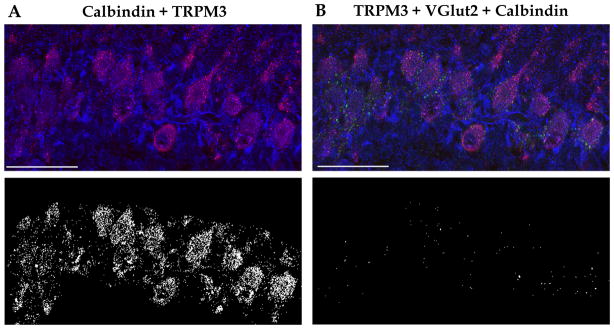
TRPM3 expression in the PC layer and its localization with glutamatergic synapses in the PC layer at P6-8 was further analyzed by quantifying signals from double- and triple-stained sections. We found TRPM3 abundantly expressed in PC cell bodies; the quantification revealed that 37.32 ± 11.1 % of calbindin colocalized with TRPM3 and 22.64 ± 3.56 % of TRPM3 staining overlapped with calbindin staining (Fig. 2A; n = 5). In addition, 43.37 ± 4.84 % of glutamatergic terminals on PCs, visualized by VGlut2 and calbindin staining, were also positive for TRPM3 (Fig. 2B, n = 5). Consistent with the presence of immature CF terminals not associated with PCs at this age (Hashimoto et al. 2009), not all VGlut2 staining colocalized with calbindin (32.06 ± 3.13 %, not shown; n = 5).
Figure 2. Confocal images and colocalization masks for TRPM3, VGlut2 and calbindin in the PC layer.
(A) Top, merge of calbindin (red) and TRPM3 (blue). Bottom, colocalization of calbindin and TRPM3 is illustrated by masking of regions with no overlap between these proteins. Confocal images correspond to a 1 μm-thick sagittal cerebellar confocal section at P8. (B) Top, merged image of TRPM3 (blue), calbindin (red) and VGlut2 (green). Bottom, colocalization of calbindin, TRPM3, and VGlut2 is illustrated by masking of regions with no overlap between these proteins. Scale bars: 50 μm. See text for quantification of results from 5 separate experiments.
Characterization of increases in glutamate release induced by TRPM3-like channels
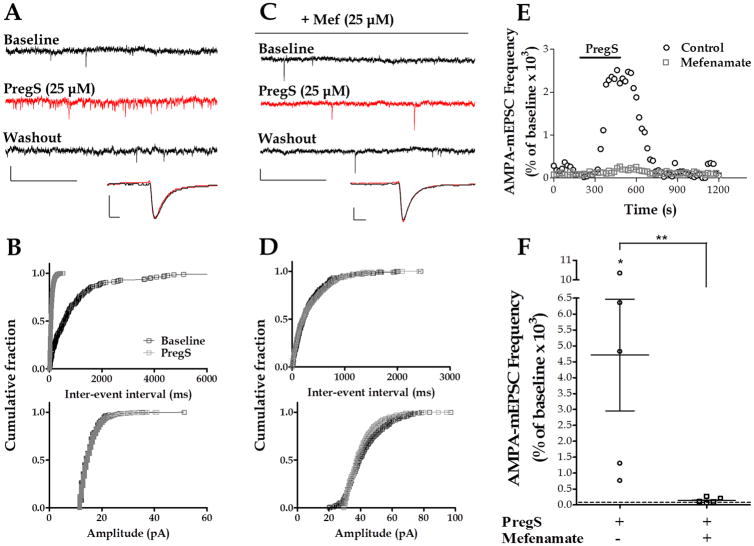
Our previous finding that PregS increases glutamate release at PCs, taken together with our present results indicating that TRPM3 channels are expressed at glutamatergic synapses in these cells, prompted us to assess the role of these channels in the mechanism of action of PregS. To this end, we used recently characterized TRPM3 pharmacological tools. Within the group of fenamates, a class of small compounds derived from N-phenyl-substituted anthranilic acid, mefenamic acid has been described to selectively block TRPM3 with a half-maximal inhibitory concentration of 6.6 ± 1.8 μM (IC50 of > 300 μM on other TRP channels) (Klose et al. 2011). Therefore, we tested whether the PregS-induced increase of quantal glutamate release was affected by this potent TRPM3 channel blocker. To measure quantal glutamate release, we recorded AMPA-mEPSCs from PCs using whole-cell patch-clamp techniques. Using this approach, we previously demonstrated that PregS reversibly increases AMPA-mEPSC frequency but not amplitude, indicating that this neuroactive steroid acts by increasing glutamate release rather than via modulation of postsynaptic AMPA receptors (Zamudio-Bulcock & Valenzuela 2011). In agreement with that report, we found that PregS (25 μM) strongly increases AMPA-mEPSC frequency (4,716 ± 1,755 % of baseline, with a raw change of 13.16 ± 5.68 Hz from baseline, n = 5) but not amplitude (110.6 ± 6.9 % of baseline, with a raw change of 1.79 ± 1.19 pA from baseline, n = 5) (Fig. 3A, B, E and F). The K-S test showed that, in 5 out of 5 cells, PregS significantly decreased the inter-event interval of AMPA-mEPSCs. In 3 of these cells, the K-S test showed that PregS did not significantly change the amplitude of the events and in 2 cells the amplitude increased. In the presence of mefenamic acid, the PregS-induced increase of AMPA-mEPSC frequency, was significantly reduced (138.7 ± 39.58 % of baseline, with a raw change of 0.076 ± 0.3 Hz from baseline, n = 5) (Fig. 3C, D, E and F). The K-S test showed that, in the presence of mefenamic acid, PregS did not significantly change the inter-event interval in 2 out of 5 cells, significantly increased it in 1 cell and significantly decreased it in 2 cells.
Figure 3. Mefenamic acid inhibits the PregS-induced increase of AMPA-mEPSC frequency.
(A) Sample traces of AMPA-mEPSC recordings at P7 in a control PC (calibration: 20 pA, 0.5 s) and corresponding average mEPSC traces (calibration: 8 pA, 3 ms). (B) Corresponding cumulative probability plots; PregS significantly decreased the inter-event interval (p < 0.01 by K–S test) but not the amplitude. (C) Sample traces of AMPA-mEPSC recordings in the presence of mefenamic acid (Mef) at P6 (calibration: 20 pA, 0.5 s) and corresponding average mEPSC traces (calibration: 8 pA, 3 ms). (D) Corresponding cumulative probability plots. (E) Time courses of AMPA-mEPSC frequency corresponding to the control and mefenamic acid recordings shown in A and C. (F) Summary of the effect of PregS on AMPA-mEPSC frequency (* p < 0.05 vs. zero by Wilcoxon Signed Rank Test). In the presence of mefenamic acid the effect of PregS was significantly reduced (Mann-Whitney test, ** p <0.05; n = 5).
PregS has been shown to produce some activation of recombinant TRPM3 channels at concentrations in the low μM range (Wagner et al. 2008). We therefore tested the effects of lower concentrations of PregS on AMPA-mEPSCs. We found that 2.5 μM PregS enhanced quantal glutamate release in the majority of neonatal PCs tested; the K-S test showed that in 4 out of 5 cells, 2.5 μM PregS significantly decreased the inter-event interval of AMPA-mEPSCs (329 ± 115.2 % of baseline, with a raw change of 0.56 ± 0.28 Hz, data not shown, n=5). The amplitude was not affected by 2.5 μM PregS; the K-S test showed that, in 3 out of 5, cells PregS did not significantly change the amplitude of the events, in 1 cell it increased it and in 1 cell it decreased it (98.92 ± 5.41 % of baseline, with a raw change of 0.71 ± 1.11 Hz, data not shown, n=5). At a lower concentration (0.5 μM), PregS did not significantly affect the inter-event interval in 4 out of 7 cells, significantly decreased it in 2 out of 7 cells and increased it in 1 cell (124.2 ± 33.29 % of baseline, with a raw change of −0.28 ± 0.13 pA, data not shown, n=7). The amplitude was not affected by 0.5 μM PregS; the K-S test showed that, in 5 out of 7 cells, PregS did not significantly changed the amplitude of the events and in 2 cells it decreased it (96.51 ± 3.41 % of baseline, with a raw change of −1.14 ± 0.82 pA, data not shown, n=5).
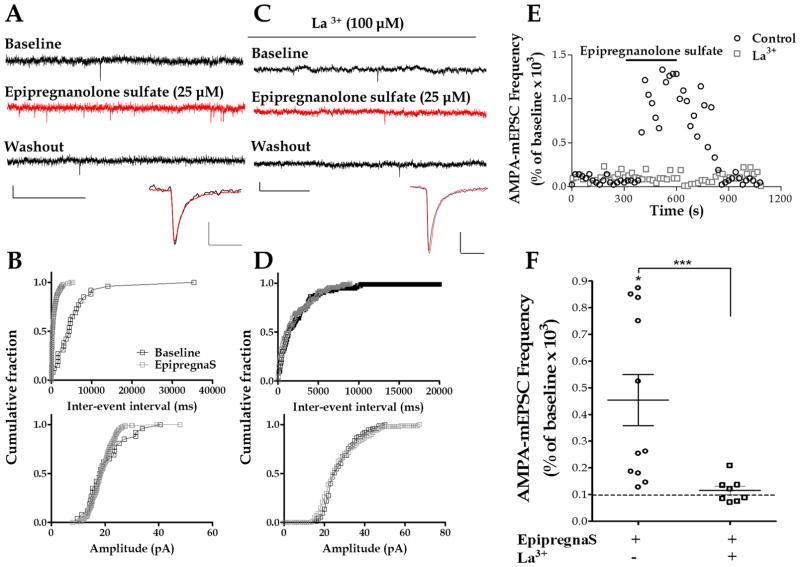
Epipregnanolone sulfate, a pregnanolone isomer derived from progesterone, has been shown to activate recombinant TRPM3 channels expressed in HEK cells (Majeed et al. 2010). Therefore, we tested whether bath application of epipregnanolone sulfate affected glutamatergic transmission onto neonatal cerebellar PCs. Bath application of epipregnanolone sulfate (25 μM) significantly increased AMPA-mEPSC frequency (454.3 ± 95.3 % of baseline, with a raw change of 0.67 ± 0.14 Hz from baseline, n = 11) (Fig. 4A, B, E, and F). The K-S test showed that, 9 out of 11 cells, had a significant decrease in the inter-event interval, with no change in the other 2 cells. The amplitude of these events was not significantly affected by epipregnanolone sulfate (106.2 ± 9.95 % of baseline, with a raw change of −0.69 ± 1.71 pA from baseline). The K-S test showed that, in 8 out of 11 cells, epipregnanolone sulfate did not significantly affect AMPA-mEPSC amplitude; in 3 cells, it induced a significant decrease in the amplitude of these events. We have previously reported that La3+, a non-selective agent that blocks TRPM3 channels, significantly reduces the PregS-induced increase of AMPA-mEPSC frequency (Zholos 2010, Zamudio-Bulcock & Valenzuela 2011). In the presence of La3+ (100 μM), epipregnanolone sulfate did not significantly increase AMPA-mEPSC frequency (114.9 ± 16.22 % of baseline, with a raw change of 0.08 ± 0.13 Hz from baseline, n = 8) (Fig. 4C, D, E and F). The K-S test showed that in the presence of La3+, epipregnanolone sulfate did not significantly affect the inter-event interval in 5 out of 8 cells and that there was a significant decrease in 2 cells, and a significant increase in 1 cell.
Figure 4. Epipregnanolone sulfate increases AMPA-mEPSC frequency.
(A) Sample traces of AMPA-mEPSC recordings at P6 in a control PC (calibration: 20 pA, 0.5 s) and corresponding average traces (calibration: 6 pA, 8 ms). (B) Corresponding cumulative probability plots; epipregnanolone sulfate significantly decreased the inter-event interval (p < 0.01 by K–S test) but not the amplitude of these events. (C) Sample traces of AMPA-mEPSC recordings in a La3+ treated cell at P7 (calibration: 20 pA, 0.5 s) and corresponding average traces (calibration: 8 pA, 12 ms). (D) Corresponding cumulative probability plots; in the presence of La3+. (E) Time courses of AMPA-mEPSC frequency corresponding to the control and the La3+ recordings shown in A and C. (F) Summary of the effect of epipregnanolone sulfate (EpipregnanS) on AMPA-mEPSC frequency (* p < 0.05 vs. zero by Wilcoxon Signed Rank Test). In the presence of La3+ the effect of epipregnanolone sulfate was significantly reduced (Mann-Whitney test, *** p <0.05; n = 8).
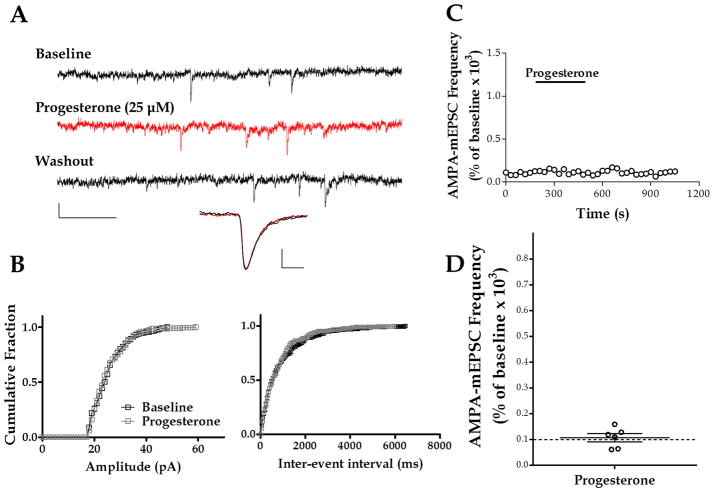
As a control, we tested progesterone, a steroid that has been shown to lack stimulating actions on TRPM3 (Majeed et al. 2010, Wagner et al. 2008). The application of progesterone (25 μM) affected neither the frequency (107.1 ± 15.57 % of baseline, with a raw change of 0.25 ± 0.13 Hz from baseline, n = 6) nor the amplitude (108 ± 10.15 % of baseline, with a raw change of 1.27 ± 2.70 pA from baseline) of AMPA-mEPSCs (Fig. 5). In 6 out of 6 cells, the K-S test showed no significant change in the inter-event interval of these events. The K-S test showed no significant change in AMPA-mEPSC amplitude in 4 out of 6 cells; there was a significant increase in 1 cell and a significant decrease in 1 cell.
Figure 5. Progesterone changes neither the frequency nor the amplitude of AMPA-mEPSCs.
(A) Sample traces of AMPA-mEPSC recordings at P9; calibration: 20 pA, 0.2 sec. Average traces calibration: 4 pA, 3 ms. (B) Corresponding cumulative probability plots for amplitude (left panel) and inter-event interval (right panel). (C) Time course of AMPA-mEPSC frequency for the recording shown in A. (D) Summary of the effect of progesterone on AMPA- mEPSC frequency (n = 6).
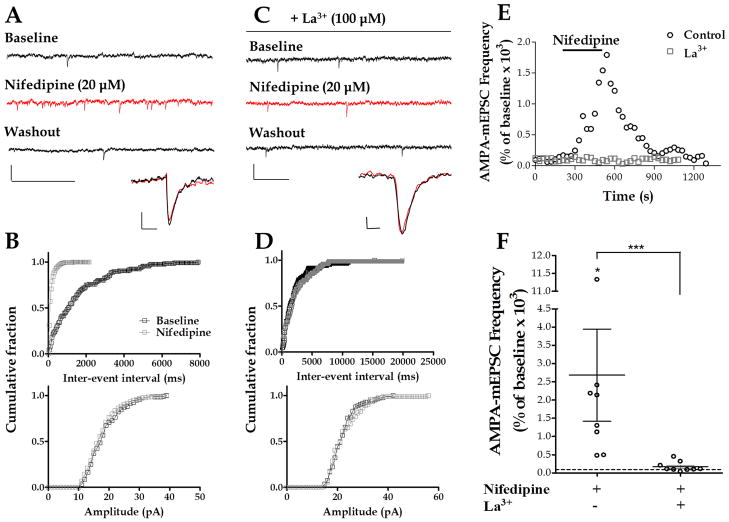
The increase in AMPA-mEPSC frequency induced by steroid sulfates and the blockade of this effect by both mefenamic acid and La3+ provide strong evidence for the participation of TRPM3-like channels in this process. To exclude effects based on the steroid structure of the activating compounds, we chose nifedipine, a non-steroideal L-type Ca2+ channel blocker with TRPM3-stimulating properties (Wagner et al. 2008). Bath application of nifedipine (20 μM) significantly increased AMPA-mEPSC frequency (2,680 ± 1,265 % of baseline, with a raw change of 9.13 ± 3.07 Hz from baseline, n = 8) (Fig. 6A,B,E and F). The K-S test showed that, in 8 out of 8 cells, there was a significant decrease in the inter-event interval. The amplitude of AMPA-mEPSCs was not significantly changed by nifedipine (90.92 ± 2.25% of baseline, with a raw change of −1.96 ± 0.47 pA from baseline) (Fig. 6A and B). The K-S test showed that, in 5 out of 8 cells, nifedipine did not change the amplitude of these events. In the other 3 cells, nifedipine significantly decreased the amplitude of these events. La3+ (100 μM) significantly reduced the nifedipine-induced increase of AMPA-mEPSC frequency (169.1 ± 44.67 % of baseline, with a raw change of 0.62 ± 0.37 Hz from baseline, n = 9) (Fig. 6C, D, E and F). In the presence of La3+, the K-S test showed that nifedipine did not change the inter-event interval of these events in 5 out of 9 cells, and that it significantly decreased it in 3 cells and increased it in 1 cell.
Figure 6. Nifedipine increases AMPA-mEPSC frequency.
(A) Sample traces of AMPA-mEPSC recordings at P6 in a control PC (calibration: 50 pA, 0.5 s) and corresponding average traces (calibration: 4 pA, 10 ms). (B) Corresponding cumulative probability plots; nifedipine significantly decreased the inter-event interval (p < 0.01 by K–S test) without affecting the amplitude. (C) Sample traces of AMPA-mEPSC recordings in La3+ at P8 (calibration: 50 pA, 0.2 s) and corresponding average traces (calibration: 4 pA, 4 ms). (D) Corresponding cumulative probability plots illustrating the lack of a significant effect of nifedipine in presence of La3+. (E) Time courses of AMPA-mEPSC frequency corresponding to the control and the La3+ recordings shown in A and C. (F) Summary of the effect of nifedipine on AMPA-mEPSC frequency (* p < 0.05 vs. zero by Wilcoxon Signed Rank Test). In the presence of La3+, the effect of nifedipine was significantly reduced (Mann-Whitney test, *** p <0.0001; n = 9).
Discussion
This study provides evidence supporting the hypothesis that TRPM3 channels mediate the effect of PregS on glutamate release in the neonatal cerebellar cortex and constitutes the first demonstration of a physiological effect of TRPM3 channels in neurotransmission. The findings can be summarized as follows. Immunohistochemistry experiments indicate that TRPM3 splice variants A and/or B are abundantly expressed in the developing cerebellar cortex and that these colocalize with glutamatergic synapses in PCs. Furthermore, the previously reported effect of PregS on the frequency of AMPA-mEPSCs is inhibited by the TRPM3 blocker, mefenamic acid. In addition, the TRPM3 agonists, epipregnanolone sulfate and nifedipine, mimicked the PregS-induced increase of AMPA-mEPSC frequency; however, the TRPM3 inactive agent progesterone was not able to mimic the PregS effect.
TRPM3 channels are expressed in the developing cerebellar cortex
The expression of TRPM3 channels in the neonatal cerebellar cortex was found to be abundant in all 4 layers of the developing cerebellar cortex. This expression profile suggests that these channels may play a role in the maturation of cerebellar cortex circuitry. During the neonatal period there are ongoing cell maturation and migration processes that depend on Ca2+ signaling and, therefore, might be influenced by TRPM3 channel activation. Certainly, future studies should focus on investigating the role of these channels on the overall development of the cerebellar cortex.
Importantly, our results suggest that a considerable number of VGlut2-containing glutamatergic synapses in PCs express TRPM3 channels, although electron microscopic studies will be needed to elucidate the precise synaptic compartment containing these channels. TRPM3 and calbindin showed colocalization, but most of the TRPM3 staining in the PC layer was not localized on PCs. Collectively, these results indicate that in the PC layer, TRPM3 is expressed in three locations. First, TRPM3 is localized in VGlut2 positive glutamatergic synapses onto PCs, which, at least in part, correspond to CF-to-PC synapses, as suggested by our finding that PregS alters paired-pulse plasticity of CF-evoked EPSCs (Zamudio-Bulcock & Valenzuela 2011). Second, TRPM3 is present in PC somata in locations other than glutamatergic synapses, perhaps in developing GABAergic pinceaux synapses and/or axonal collaterals from neighboring PCs (Watt et al. 2009); supporting this possibility are previous findings that PregS, although less strongly, also increases GABAergic transmission onto neonatal PCs (Zamudio-Bulcock & Valenzuela 2011). Third, TRPM3 is expressed in other cell types present in the developing PC layer, with the most likely candidates being Bergman glia and migrating granule cells. In the neonatal cerebellar cortex, Bergmann glial fibers associate with granule cells migrating from the external granule cell layer to their final destination in the internal granule cell layer. Therefore, it will be important to assess whether these channels are located on Bergmann glia and if they play a role granule cell migration (Yamada et al. 2000).
Steroid-sensitive TRPM3 channels increase glutamate release
Studies with recombinant TRPM3 channels showed that PregS activates TRPM3 channels with an apparent EC50 value of 23 μM (at 80 mV), with concentrations below 10 μM producing responses that are less than 25% of maximum (Wagner et al. 2008). In agreement with these studies, we found that lower contentrations of PregS (2.5 μM in this study and 5 μM in Zamudio-Bulcock & Valenzuela 2011) also potentiate glutamatergic transmission in the neonatal cerebellum. The magnitude of the effect of these PregS concentrations is about 7–10% of that produced by 25 μM PregS, in agreement with the results of (Wagner et al. 2008). It should be noted that it is currently a matter of controversy whether significant concentrations of PregS per se are endogenously produced in the rodent brain (for details, see Schumacher et al. 2008, Valenzuela et al. 2008). However, previous work from our laboratory suggests that developing pyramidal neurons in the rat hippocampus release a PregS-like neurosteroid in a retrograde fashion, reaching synaptic concentrations near 17 μM (Mameli et al. 2005). Therefore, it is possible that TRPM3 channels are activated by PregS-like neurosteroids present in developing synapses under physiological conditions. Moreover, PregS-like neurosteroid levels could be increased under pathophysiological conditions (i.e. fetal alcohol spectrum disorders), leading to enhanced activation of TRPM3 channels and alterations in neuronal circuit development (Valenzuela et al. 2008, Caldeira et al. 2004).
We have previously shown that the effect of PregS on glutamatergic transmission in the neonatal cerebellar cortex is blocked by La3+, a non-specific agent that antagonizes some but not all TRP channels subtypes (TRPC4 and TRPC5 are potentiated; Jung et al. 2003), includingTRPM3 (Zholos 2010). Mefenamic acid, a clinically used non-steroidal anti-inflammatory agent, was recently shown to be a selective and potent TRPM3 blocker (Klose et al. 2011). This finding enabled us to more selectively test the involvement of TRPM3 channels in the mechanism of action of PregS. Mefenamic acid blockade of the PregS-induced increase of AMPA-mEPSC frequency provided the first line of evidence of the involvement of this channel in the PregS effect.
PregS and epipregnanolone sulfate are structurally related compounds. Not surprisingly, similar to PregS, epipregnanolone sulfate has been shown to inhibit GABAA receptors (Park-Chung et al. 1999). PregS is a positive modulator of NMDA receptors whereas epipregnanolone sulfate inhibits these receptors by acting at a distinct site from that of PregS (Park-Chung et al. 1997). In the case of TRPM3 stimulation, both PregS and epipregnanolone sulfate act at the same site; however, these agents stimulate TRPM3 with a different efficacy. PregS-mediated stimulation of TRPM3 requires the sulfate group positioned at ring A and the cis (β) configuration of the side group, both of which epipregnanolone sulfate possess. In fact, the structure of epipregnanolone sulfate only differs from that of PregS in that it lacks the double bond in ring B and this structural difference renders epipregnanolone sulfate less effective in stimulating TRPM3 channels. In HEK cells expressing TRPM3 channels, epipregnanolone sulfate was shown to be less potent than PregS at increasing intracellular Ca2+ (Majeed et al. 2010). Consistent with these findings, we observed that epipregnanolone sulfate significantly increases the frequency of AMPA-mEPSCs in neonatal PCs (Fig. 4), but this effect was of lower magnitude than that induced by the same concentration of PregS (Fig. 3). Importantly, similar to the PregS-induced increase of AMPA-mEPSC frequency, this effect was blocked by La3+(Zamudio-Bulcock & Valenzuela 2011).
Progesterone is a major neurosteroid shown to be inactive at TRPM3 channels (Majeed et al. 2010, Wagner et al. 2008). In-vitro and in-vivo studies have shown that progesterone promotes dendritic growth and spine formation in PCs via modulation of its nuclear receptor, although a role of progesterone membrane receptors in this trophic effect has not been ruled out (reviewed in Tsutsui et al. 2003). Progesterone is known to modulate the release of norepinephrine, serotonin, dopamine, and glutamate. In the prefrontal cortex, progesterone inhibits the dopamine-evoked release of glutamate while it does not affect spontaneous release (reviewed in Zheng 2009). Here, we report that progesterone neither affected the frequency nor the amplitude of AMPA-mEPSCs. These results are consistent with the lack of activity of this neurosteroid on TRPM3. Additionally, these results argue against acute modulation of spontaneous glutamatergic transmission as a mechanism responsible for some of the trophic effects of progesterone on neonatal PCs.
Nifedipine mimics the effect of PregS
Nifedipine is an L-type calcium channel blocker clinically used for the treatment of conditions such as cardiac arrhythmias, angina, hypertension, and preterm labor (Hirasawa & Pittman 2003, Conde-Agudelo et al. 2011). Recently, nifedipine was shown to paradoxically activate TRPM3 channels in recombinant experiments and in pancreatic islets cells with a potency similar to that of PregS (Wagner et al. 2008). It is noteworthy that activation of voltage-gated Ca2+ channels is not involved in the effect of PregS on neonatal PCs, as bath application of Cd2+ does not prevent the PregS-induced increase of AMPA-mEPSC frequency (Zamudio-Bulcock & Valenzuela 2011). In this study, we show that nifedipine mimics the PregS-induced increase of glutamate release in neonatal PCs and that, like the effect of PregS, this effect is sensitive to La3+. Ca2+ channel blockers, including nifedipine, are used in obstetrics and gynecology for the management of hypertensive disorders of pregnancy and preterm labor. The teratogenicity of these agents has been reported in animals; however, human data are inconclusive (Tranquilli & Giannubilo 2009). Nevertheless, a recent systematic review linked the use of nifedipine and other Ca2+ channel blockers with significant higher risks for a wide range of feto-maternal adverse events, including cardiac, respiratory, and renal alterations (Khan et al. 2011). Given that nifedipine can readily cross the blood brain barrier (Janicki et al. 1988), and that human TRPM3 channels are also strongly modulated by PregS and other TRPM3 channel activators (Majeed et al. 2010), our results suggest that using nifedipine during the third-trimester of pregnancy should be avoided, as it may cause alterations in cerebellar neuronal circuit formation. Importantly, this effect may not be limited to cerebellar neurons, as another study showed that nifedipine increases glutamate release in magnocellular neurons of the supraoptic nucleus via a Ca2+ channel-independent mechanism (Hirasawa & Pittman 2003).
In conclusion, the present study provides evidence indicating that TRPM3 channels are novel modulators of glutamatergic transmission. A mature PC receives glutamatergic input from numerous granule cell projections, the PFs, and from a single axonal projection of an inferior olivary neuron, the CF. The one to one CF-to-PC association is preceded during development by multinumerary CF-to-PC synapses and it is achieved at the end of the second postnatal week in rodents (approximately equivalent to end of the third trimester of human pregnancy) via synaptic competition among CFs and between CF and PFs. In addition, CF synapse formation starts during embryonic development, while PF-to-PC synapse formation starts at the end of the first postnatal week (Bosman & Konnerth 2009, Cesa & Strata 2009). During the developmental period at which the TRPM3-mediated enhancement of glutamate release occurs, the multinumerary CF-to-PC synapses are functionally differentiated and the process of early synapse elimination starts (Kano & Hashimoto 2009). While the mechanisms controlling the late stages of CF synapse elimination are rather well understood (Kano & Hashimoto 2009, Bosman & Konnerth 2009), the early stages of CF synapse elimination have not been fully characterized. CF plasticity mechanisms may play a central role in CF functional differentiation and elimination. It has been shown that, in response to the same stimuli, strong CF inputs undergo long-term potentiation while weak ones undergo long-term depression (Bosman et al. 2008, Ohtsuki & Hirano 2008, Valenzuela et al. 2010). The mechanisms underlying the differential synaptic properties among CFs are not well understood. We previously showed that the PregS-induced enhancement of glutamate release occurs at developing CF-to-PC synapses (Zamudio-Bulcock & Valenzuela 2011). Therefore, the interesting possibility that TRPM3 channel activation by PregS-like neurosteroids released from PCs is involved in the differential strengthening of CF inputs needs to be further assessed.
Acknowledgments
This work was supported by NIH Grants MH70386 and AA14973, and the Deutsche Forschungsgemeinschaft. The confocal microscopy facility at the UNM Cancer Center is supported by grant NCI P30 CA118100. The authors report no conflict of interest. We would like to acknowledge Dr. Donald Partridge for critically reading the manuscript and providing helpful advice. We would also like to acknowledge Genevieve Phillips and Rebecca Lee for their assistance with the acquisition and analysis of confocal images.
References
- Bosman LW, Konnerth A. Activity-dependent plasticity of developing climbing fiber-Purkinje cell synapses. Neuroscience. 2009;162:612–623. doi: 10.1016/j.neuroscience.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Bosman LW, Takechi H, Hartmann J, Eilers J, Konnerth A. Homosynaptic long-term synaptic potentiation of the “winner” climbing fiber synapse in developing Purkinje cells. J Neurosci. 2008;28:798–807. doi: 10.1523/JNEUROSCI.4074-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira JC, Wu Y, Mameli M, Purdy RH, Li PK, Akwa Y, Savage DD, Engen JR, Valenzuela CF. Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J Neurochem. 2004;90:1530–1539. doi: 10.1111/j.1471-4159.2004.02686.x. [DOI] [PubMed] [Google Scholar]
- Cesa R, Strata P. Axonal competition in the synaptic wiring of the cerebellar cortex during development and in the mature cerebellum. Neuroscience. 2009;162:624–632. doi: 10.1016/j.neuroscience.2009.02.061. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Romero R, Kusanovic JP. Nifedipine in the management of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204:134 e131–120. doi: 10.1016/j.ajog.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douyard J, Shen L, Huganir RL, Rubio ME. Differential neuronal and glial expression of GluR1 AMPA receptor subunit and the scaffolding proteins SAP97 and 4.1N during rat cerebellar development. J Comp Neurol. 2007;502:141–156. doi: 10.1002/cne.21294. [DOI] [PubMed] [Google Scholar]
- Eilers J, Plant TD, Marandi N, Konnerth A. GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. J Physiol. 2001;536:429–437. doi: 10.1111/j.1469-7793.2001.0429c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem. 2003;278:21493–21501. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- Grimm C, Kraft R, Schultz G, Harteneck C. Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine. Mol Pharmacol. 2005;67:798–805. doi: 10.1124/mol.104.006734. [DOI] [PubMed] [Google Scholar]
- Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:307–314. doi: 10.1007/s00210-005-1034-x. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Schultz G. TRPV4 and TRPM3 as Volume-Regulated Cation Channels. 2007 [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron. 2009;63:106–118. doi: 10.1016/j.neuron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Pittman QJ. Nifedipine facilitates neurotransmitter release independently of calcium channels. Proc Natl Acad Sci U S A. 2003;100:6139–6144. doi: 10.1073/pnas.0936131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Grimm C, Kraft R, et al. TRPM3 is expressed in sphingosine-responsive myelinating oligodendrocytes. J Neurochem. 2010;114:654–665. doi: 10.1111/j.1471-4159.2010.06644.x. [DOI] [PubMed] [Google Scholar]
- Janicki PK, Siembab D, Paulo EA, Krzascik P. Single-dose kinetics of nifedipine in rat plasma and brain. Pharmacology. 1988;36:183–187. doi: 10.1159/000138382. [DOI] [PubMed] [Google Scholar]
- Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. The Journal of biological chemistry. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19:154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kawa K. Glycine facilitates transmitter release at developing synapses: a patch clamp study from Purkinje neurons of the newborn rat. Brain Res Dev Brain Res. 2003;144:57–71. doi: 10.1016/s0165-3806(03)00159-7. [DOI] [PubMed] [Google Scholar]
- Khan K, Zamora J, Lamont RF, et al. Safety concerns for the use of calcium channel blockers in pregnancy for the treatment of spontaneous preterm labour and hypertension: a systematic review and meta-regression analysis. J Matern Fetal Neonatal Med. 2011;23:1030–1038. doi: 10.3109/14767050903572182. [DOI] [PubMed] [Google Scholar]
- Klose C, Straub I, Riehle M, Ranta F, Krautwurst D, Ullrich S, Meyerhof W, Harteneck C. Fenamates as TRP channel blockers: mefenamic acid selectively blocks TRPM3. Br J Pharmacol. 2011;162:1757–1769. doi: 10.1111/j.1476-5381.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, Inoue Y, Watanabe M. Impaired parallel fiber-->Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor delta2 subunit. J Neurosci. 1997;17:9613–9623. doi: 10.1523/JNEUROSCI.17-24-09613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Chen J, Sun L, et al. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3) J Biol Chem. 2003;278:20890–20897. doi: 10.1074/jbc.M211232200. [DOI] [PubMed] [Google Scholar]
- Majeed Y, Agarwal AK, Naylor J, Seymour VA, Jiang S, Muraki K, Fishwick CW, Beech DJ. Cis-isomerism and other chemical requirements of steroidal agonists and partial agonists acting at TRPM3 channels. Br J Pharmacol. 2010;161:430–441. doi: 10.1111/j.1476-5381.2010.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther. 2008;327:910–917. doi: 10.1124/jpet.108.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Carta M, Partridge LD, Valenzuela CF. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J Neurosci. 2005;25:2285–2294. doi: 10.1523/JNEUROSCI.3877-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Fukaya M, Shimizu H, Watanabe M. Subtype switching of vesicular glutamate transporters at parallel fibre-Purkinje cell synapses in developing mouse cerebellum. Eur J Neurosci. 2003;17:2563–2572. doi: 10.1046/j.1460-9568.2003.02698.x. [DOI] [PubMed] [Google Scholar]
- Naylor J, Li J, Milligan CJ, et al. Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ Res. 2010;106:1507–1515. doi: 10.1161/CIRCRESAHA.110.219329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwinkler J, Lis A, Giehl KM, Flockerzi V, Philipp SE. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J Biol Chem. 2005;280:22540–22548. doi: 10.1074/jbc.M503092200. [DOI] [PubMed] [Google Scholar]
- Oberwinkler J, Phillipp SE. Trpm3. Handb Exp Pharmacol. 2007:253–267. doi: 10.1007/978-3-540-34891-7_15. [DOI] [PubMed] [Google Scholar]
- Ohtsuki G, Hirano T. Bidirectional plasticity at developing climbing fiber-Purkinje neuron synapses. Eur J Neurosci. 2008;28:2393–2400. doi: 10.1111/j.1460-9568.2008.06539.x. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol Pharmacol. 1997;52:1113–1123. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Liere P, Akwa Y, Rajkowski K, Griffiths W, Bodin K, Sjovall J, Baulieu EE. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int. 2008;52:522–540. doi: 10.1016/j.neuint.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Tranquilli AL, Giannubilo SR. Use and safety of calcium channel blockers in obstetrics. Curr Med Chem. 2009;16:3330–3340. doi: 10.2174/092986709789057699. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Sakamoto H, Ukena K. Biosynthesis and action of neurosteroids in the cerebellar Purkinje neuron. J Steroid Biochem Mol Biol. 2003;85:311–321. doi: 10.1016/s0960-0760(03)00229-2. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Lindquist B, Zamudio-Bulcock PA. A review of synaptic plasticity at Purkinje neurons with a focus on ethanol-induced cerebellar dysfunction. Int Rev Neurobiol. 2010;91:339–372. doi: 10.1016/S0074-7742(10)91011-8. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Partridge LD, Mameli M, Meyer DA. Modulation of glutamatergic transmission by sulfated steroids: role in fetal alcohol spectrum disorder. Brain Res Rev. 2008;57:506–519. doi: 10.1016/j.brainresrev.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo YP, Hutton JC, Angleson JK. Recycling of the dense-core vesicle membrane protein phogrin in Min6 beta-cells. Biochem Biophys Res Commun. 2004;324:1004–1010. doi: 10.1016/j.bbrc.2004.09.147. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, et al. TRPM3 Is a Nociceptor Channel Involved in the Detection of Noxious Heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Wagner TF, Loch S, Lambert S, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Cuntz H, Mori M, Nusser Z, Sjostrom PJ, Hausser M. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci. 2009;12:463–473. doi: 10.1038/nn.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2011;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;418:106–120. [PubMed] [Google Scholar]
- Zamudio-Bulcock PA, Valenzuela CF. Pregnenolone sulfate increases glutamate release at neonatal climbing fiber-to-Purkinje cell synapses. Neuroscience. 2011;175:24–36. doi: 10.1016/j.neuroscience.2010.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Zholos A. Pharmacology of transient receptor potential melastatin channels in the vasculature. British journal of pharmacology. 2010;159:1559–1571. doi: 10.1111/j.1476-5381.2010.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]