Abstract
Objective
Type 1 diabetes and obesity has increased in childhood. We therefore tested the hypothesis that type 1 diabetes HLA-DQ risk genotypes may be associated with an increased body mass index (BMI).
Design
The type 1 diabetes high risk HLA-DQ A1*05:01-B1*02:01/A1*03:01-B1*03:02 genotype along with lower risk DQ genotypes were determined at the time of clinical onset by PCR and hybridization with allele-specific probes. Body mass index was determined after diabetes was stabilized.
Subjects
A total of 2403 incident type 1 diabetes children below 18 years of age were ascertained in the Swedish national Better Diabetes Diagnosis (BDD) studybetween May 2005 to September 2009. All children classified with type 1 diabetes including positivity for at least one islet autoantibody were investigated.
Results
Overall, type 1 diabetes HLA-DQ risk was negatively associated with BMI (p<0.0008). The proportion of the highest risk A1*05:01-B1*02:01/A1*03:01-B1*03:02 genotype decreased with increasing BMI (p<0.0004). However, lower risk type 1 diabetes DQ genotypes were associated with an increased proportion of patients who were overweight or obese (p<0.0001). Indeed, the proportion of patients with the low risk A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype increased with increasing body mass index (p<0.003). The magnitude of association on the multiplicative scale between the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype and increased body mass index was significant (p<0.006). The odds ratio in patients with this genotype of being obese was 1.80 (95% CI 1.21–2.61; p<0.006). The increased proportion of overweight type 1 diabetes children with the A1*05:01-B1*02:01 haplotype was most pronounced in children diagnosed between 5 and 9 years of age.
Conclusions
Susceptibility for childhood type 1 diabetes was unexpectedly found to be associated with the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype and an increased BMI. These results support the hypothesis that overweight may contribute to the risk of type 1 diabetes in children positive for HLA-DQ A1*05:01-B1*02:01.
Keywords: islet autoantibodies, body mass index, GAD65 autoantibodies, IA-2 autoantibodies, insulin autoantibodies, HLA genotype
Introduction
The incidence of childhood type 1 diabetes is increasing worldwide (1). A recent report from Finland, which has the highest incidence in the world (1), suggested that the incidence is also increasing faster than before (2). In Sweden, which is second to Finland in type 1 diabetes incidence worldwide, the incidence has nearly doubled from 23 in 1983 to 40/100.000/year in 2000 (3, 4). While the etiology of type 1 diabetes remains to be determined, the pathogenesis of the disease is strongly associated with islet autoimmunity. More than 90% of children with newly diagnosed type 1 diabetes have at least one autoantibody against either glutamic acid decarboxylase (GAD65), insulin, insulinoma antigen-2 (IA-2) or the three variants of the Zn transporter-8 (ZnT8) (5–7). The human leukocyte antigen (HLA) class II region on the short arm of chromosome 6 confers approximately half of the genetic risk of type 1 diabetes (5, 8). The simultaneous presence of both the HLA-DQ A1*05:01-B1*02:01 and the A1*03:01-B1*03:02 haplotype represents the highest risk genotype (5). Several recent studies show that there has been a decrease over time in the proportion of the high risk genotype, with an increase in low to moderate HLA risk genotypes among type 1 diabetes patients in the UK (9), Finland (10), Colorado (11), Australia (12) and in Sweden (13). These observations suggest that a possible increase in environmental exposures may have contributed to an increase in type 1 diabetes among lower risk HLA genotype children, who in the past would not have developed type 1 diabetes.
Several environmental factors have been implicated in the etiology and pathogenesis of islet autoimmunity and type 1 diabetes (7). Secular trends in increased body mass index (BMI) and sedentary life style have been thought to contribute (10, 14). Rapid gain in weight has been associated with an increased type 1 diabetes incidence (15, 16) and others have suggested that obesity (17), insulin resistance, or both may be risk factors for type 1 diabetes (16, 18, 19).
The national population-based Better Diabetes Diagnosis (BDD) study prospectively ascertain nearly all newly diagnosed diabetes patients from Sweden younger than 18 years of age (20). We tested if there was any association between HLA-DQ genotype and an increase in BMI, which could contribute to the increase in type 1 diabetes risk. Our hypothesis was that type 1 diabetes HLA-DQ risk genotypes may be associated with an increased body BMI.
Methods
Subjects
All children in Sweden below the age of 18 years with newly diagnosed diabetes are referred to and followed in a hospital clinic by a pediatric diabetes team.
In 2005, a prospective national initiative, the Better Diabetes Diagnosis (BDD) study, was initiated to carefully monitor all children and adolescents with newly diagnosed diabetes for genetic risk and clinical phenotypes, including HLA genotypes, islet autoantibodies and BMI (20). A total of 95% (40/42) pediatric clinics in Sweden participate in BDD.
All children in Sweden who develop diabetes are diagnosed according to the recommendations by the American Diabetes Association (21) and are invited to participate in BDD.
Blood samples for HLA genotypes and islet autoantibodies were obtained at clinical onset of diabetes from a total of 2742 patients, recruited between May 2005 and September 2009. Patients classified with type 2 diabetes (n=43), Maturity Onset Diabetes of the Young (MODY)(n=28), secondary diabetes (n=5) or yet unclassifiable diabetes (n=60) were excluded. In this study a total of 2403 type 1 diabetes patients who also had at least one islet cell autoantibody (GAD65, IA-2 or insulin autoantibody) were included in the comparison of HLA genotypes with the general population. The male: female ratio was 1.22:1 and the mean age at onset was 9.8 (1st quartile was 6.3 and 3rd quartile 13.4) years of age.
In the BMI analysis, we included a total of 2294 islet autoantibody positive type 1 diabetes patients with BMI measurements between 5 and 26 weeks after the clinical onset of diabetes. Children diagnosed with diabetes below 2 years of age (1.9%) were excluded from the analysis between HLA genotypes and BMI-class as comparison data for BMI is lacking in this age group and a total of 2242 children were analyzed. For BMI standard deviation score (BMI SDS) correlation, a total of 2278 children were analyzed as 16 individuals were lacking values as they were older than 18 years at follow-up and the reference group did not contain individuals older than 18 years.
The Regional Ethics Board at the Karolinska Institute, Stockholm, Sweden approved the BDD study and informed consent was obtained from the parents to all children in the BDD study.
Healthy control population
The frequency of HLA genotypes in healthy control children in this study was obtained in the Diabetes Prediction Study in Skåne (DiPiS), a prospective population-based study in Skåne, the most southern province of Sweden with 1.2 million inhabitants (15, 22, 23). Children were included in the DiPiS study between September 2000 and August 2004. At a total of 35,683 cord blood samples were obtained for HLA-typing in the maternity clinics in Malmö, Lund, Helsingborg, Kristianstad and Ystad from 48,058 children born during this period.
HLA typing
HLA-DQ types were determined as previously described (15, 20, 22, 23). Briefly, blood was dropped onto Whatman filters to generate dried blood spots (DBS). The DBS was used to obtain 3-mm punches, which were used directly for PCR amplification of DQA1 and DQB1 alleles as described (24). Single-stranded DNA was hybridized with two sets of probes, the first containing Eu- DQB1*06:02/3, Sm-DQB1*06:03/4 and Tb-Control and the second containing Eu-DQB1*03:02, Sm-DQB1*03:01 and Tb-DQB1*02. Samples positive for DQB1*02 were further analyzed for DQA1*02:01 and 05 alleles to separate subjects with DR3 from DR7. HLA-DQA1 typing was performed with the same technique as for DQB1 typing with some modifications as described (24) with use of Sm-DQA1*05 and Tb-DQA1*02:01 probes.
Autoantibodies
Autoantibodies (Ab) to GAD65, IA-2 and insulin were determined by first incubating labeled auto antigen in serum and then separating antibody-bound labeled auto antigen from free by Sepharose-Protein A (Amersham Biosciences, Uppsala, Sweden) and the radioactivity counted in a Beta Plate Reader (Perkin Elmer Life Sciences) as described (20, 22, 25) using the World Health Organization (WHO) reference sample as standard (26). The GAD65Ab and IA-2Ab assays showed mean inter-assay coefficients of variation of 14% and intra-assay of 8%. In the insulin autoantibody competing assay (15, 20, 22), the inter-assay and intra-assay coefficient of variations were 7.2 % and 6.8%, respectively. Samples above the 99th percentile were considered to have high levels. Our laboratory participates in the Diabetes Autoantibody Standardization Program and showed in 2009 the best IA-2Ab assay performance of all participating laboratories.
BMI
We used ISO-BMI (defined in reference 27) to classify the patients into normal weight, overweight or obese. BMI was determined when the diabetes had been stabilized and not earlier than 5 week after onset; mean disease duration was 3 months (range 1 to 6 months). We excluded very young children below 2 years of age, as ISO-BMI classes were not available. The proportion of overweight or obese patients was compared to that in the general population (20%) in Sweden (28) using a two tailed one sample test for proportion. Therefore we used sex and age BMI SDS expressed as standard deviations from the reference mean as described (28). BMI SDS was not available for 16 individuals who were older than 18 years at follow-up.
Statistical evaluation
Differences in frequencies between patients and controls were tested with standard Pearsons Chi square test. Chi square test for trends in proportion of HLA genotypes between groups with increasing BMI was carried out using the proportion trend.test command in R (The R Foundation for Statistical Computing, http://www.r-project.org/foundation). Two-tailed one sample test for proportion was used to compare proportions of overweight and obese patients in different HLA groups with that in the general population (29).
The synergy index (SIM) was used to estimate the magnitude of association between environmental exposure (increased BMI) and HLA genotype. A deviation from unity was taken to imply deviation from a multiplicative model (30).
Results
HLA association to antibody positive type 1 diabetes
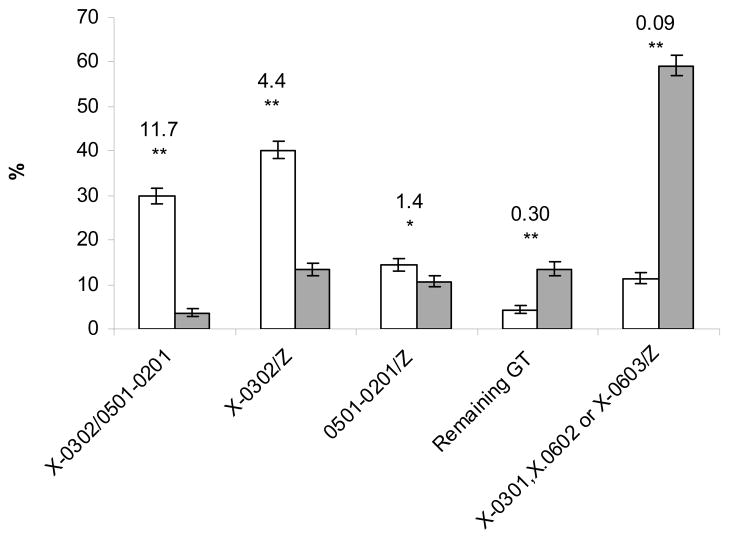
Comparison of genotype frequencies between control children (DiPiS) and the BDD type 1 diabetes children revealed that six genotypes, all either containing the A1*X-B1*03:02 haplotype or A1*05:01-B1*02:01 haplotype or both were positively associated with type 1 diabetes (Supplementary Table 1). Nineteen different genotypes were significantly negatively associated with type 1 diabetes, all but one (A1*02:01-B1*02:01/A1*X-B1*05:01) carrying at least one of the A1*X-B1*06:02, A1*X-B1*06:03 or A1*X-B1*03:01 haplotypes (SupplementaryTable 1). Hence most of the association to antibody positive type 1 diabetes in the Swedish population can be summarized as a positive association to A1*X-B1*03:02 and A1*05:01-B1*02:01 and negative association to A1*X-B1*06:02, A1*X-B1*06:03 and A1X-B1*03:01 haplotypes. A1*X-B1*06:02 acts dominantly over both A1*X-B1*03:02 and A1*05:01-B1*02:01, while A1*X-B1*06:03 and A1*X-B1*03:01 acts dominantly over A1*05:01-B1*02:01. This lead us to grouping the genotypes into five groups shown in figure 1 which captures most of the HLA association to type 1 diabetes.
Figure 1. Frequency of HLA genotypes among antibody positive type 1 diabetes patients and controls.
The HLA-DQ genotype frequencies among 2403 islet autoantibody positive type 1 diabetes patients (open bars) diagnosed compared to the frequencies among 2000 controls (grey bars). Numbers given in the figures are OR and the 95th confidence interval.
* p <0.05, ** p<1 10−8 X = DQA alleles that have not been genotyped, Z = any DQA1-DQB1 haplotype, for further details see table 1. GT is genotype.
Change in body mass index with HLA genotype among antibody positive type 1 diabetes patients
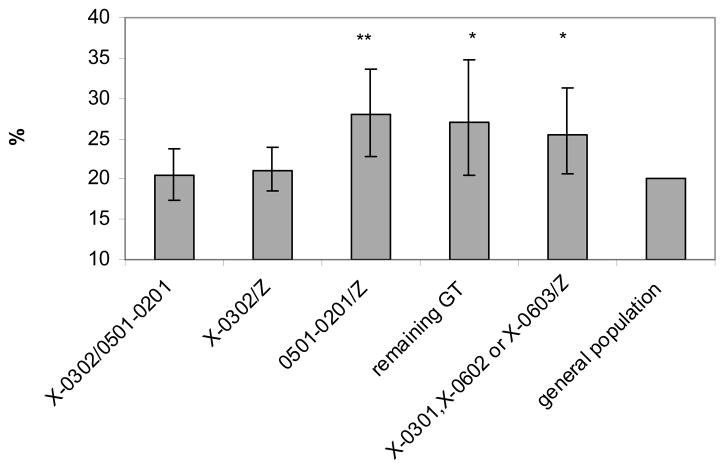
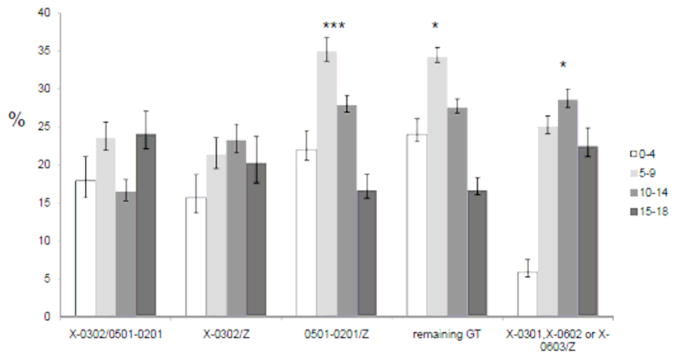
ISO-BMI was used to classify the patients into normal weight, over-weight or obese. Three genotype (A1*05:01-B1*02:01/Z, remaining genotypes and A1*X-B1*03:01, A1*X-B1*06:02 or A1*X-B1*06:03/Z) groups, all associated with moderate or low risk for type 1 diabetes, had increased proportion of antibody positive type 1 diabetes patients who were overweight or obese compared to the general population (p<0.002, 0.04 and 0.03 respectively, Figure 2). Patients carrying the HLA genotypes associated with strongest risk for type 1 diabetes (A1*X-B1*03:02/A1*05:01-B1*02:01; and A1*X-B1*03:02/Z) did not have increased proportion of overweight (p>0.05) or obesity (p>0.05). Interestingly, the increased proportion of overweight and obese type 1 diabetes children among A1:05:01-B1*02:01/Z carriers was most pronounced among 5-9 year olds (p<0.0005, Figure 3). In contrast, among patients belonging to the A1*X-B1*03:01, A1*X-B1*06:02 or A1*X-B1*06:03/Z HLA group, the increased proportion of overweight (p<0.05) was mainly observed among young teenagers (10–14 year olds, Figure 3). In this genotype group there is also a gradual increase in BMI with increasing age at onset as evaluated in a test for trend (p<0.04).
Figure 2. Proportion of overweight or obese islet autoantibody positive type 1 diabetes patients by HLA genotype.
A total of 2183 patients were available to be classified with normal weight, overweight and obese using ISO-BMI determined after stabilization of diabetes.
X = DQA alleles that have not been genotyped, Z = any DQA1-DQB1 haplotype, for further details see table 1. GT is genotype.
* p<0.05, ** p<0.002
Figure 3. The proportion of overweight and obese islet autoantibody positive type 1 diabetes children vary with age at onset and HLA genotype.
A total of 2183 patients were classified with normal weight, overweight and obese using ISO-BMI determined after stabilization of diabetes. X = DQA alleles that have not been genotyped, Z = any DQA1-DQB1 haplotype. Details are shown in Supplementary table 1.
* p<0.05, ***p<0.0005
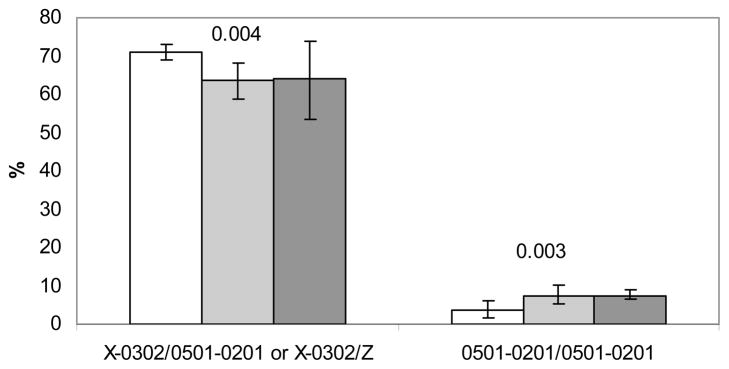
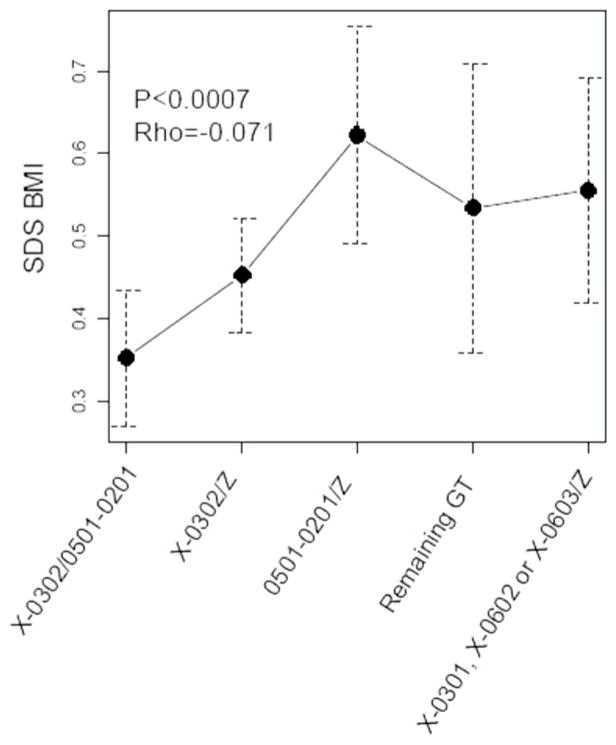
There was an increase in the frequency of the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype with increasing BMI among the antibody positive type 1 diabetes positive patients (p<0.003, Figure 4) while a slight decrease in the frequency of the A1*X-B1*03:02/A1*05:01-B1*02:01 or A1*X-B1*03:02/Z genotypes was observed with increasing BMI (p<0.004, Figure 4). The other genotype groups did not show any significant change in frequency with increasing BMI (data not shown). Further there was a correlation between BMI SDS and HLA genotype when they were ordered by the observed odds ratio (OR) for type 1 diabetes (Figure 5).
Figure 4. Change in HLA genotype frequency with increasing BMI.
Patients were classified with normal weight (n=1680, white bars), overweight (n=413, grey bars) and obese (n=81, black bars) using ISO-BMI determined after stabilization of diabetes. Proportion of islet autoantibody positive type 1 diabetes patients carrying A1*X-B1*03:02/A1*05:01-B1*02:01 or A1*X-B1*03:02/Z and A1*05:01-B1*02:01/Z genotypes is plotted by BMI class. A test for trend was carried out for each of the genotype groups. X = DQA alleles that have not been genotyped, Z = any DQA1-DQB1 haplotype. Details are shown in Supplementary table 1.
Figure 5. Correlation between HLA genotype and BMI among antibody positive type 1 diabetes patients.
Correlation between BMI SDS and HLA genotypes using two-sided Spearman’s rank correlation among 2278 antibody positive type 1 diabetes patients with BMI SDS value. Error bars represents 95% confidence intervals of BMI SDS.
X = DQA alleles that have not been genotyped, Z = any DQA1-DQB1 haplotype. Details are shown in table 1.
We further assess the magnitude of the association between ISO-BMI class and HLA genotypes in the risk for type 1 diabetes. This analysis was carried out in a case-only setting as described (30) as we did not have BMI data from controls matched for age and gender. The DiPiS controls were newborns. On the multiplicative scale, the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype was associated with ISO-BMI class overweight or obese (SIM=1.83; P<0.006, Table 1). The magnitude of the association on the multiplicative scale between normal weight and the presence of the A1*03:01-B1*03:02 haplotype was also significant (SIM=1.37; p<0.005, Table 1).
Table 1.
Synergy index between HLA-DQ A1*-B1* genotypes and BMI.
| BMI | 05:01-02:01/05:01-02:01 neg |
05:01-02:01/0501-0201 pos |
Synergy index (SIM) (95%CI) | p |
|---|---|---|---|---|
| Normal weight | 1667 | 70 | 1.83 (1.21–2.76) | 0.006 |
| Overweight or obese | 469 | 36 | ||
|
| ||||
| BMI | 0301-0302 neg | 0301-0302 pos | ||
|
| ||||
| Overweight or obese | 477 | 1203 | 1.37 (1.10-1.70) | 0.005 |
| Normal weight | 174 | 320 | ||
Synergy index (SIM)= ORge/(ORe × ORg) when no correlation between exposure and genetic factor was present among controls. The 95% confidence intervals (CI) are shown.
Discussion
The first unexpected and novel finding in the present nation-wide study of a large cohort of newly diagnosed type 1 diabetes children was an increased proportion of overweight or obese patients among carriers of HLA genotypes associated with lower risk for type 1 diabetes. The increased frequency of the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype with increasing body mass index is of particular interest as this genotype did not confer risk for type 1 diabetes in our nationwide investigation carried out more than 20 years ago (5, 32, 33). The magnitude of the association on the multiplicative scale between the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype and increased body mass index also support the notion that this particular HLA type may be sensitive to over weight and obesity to increase the risk for type 1 diabetes. We therefore suggest that overweight individuals with the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype may be more prone to develop type 1 diabetes than individuals with the same genotype who are not overweight. The second unexpected and novel finding was that we did not observe an increase in BMI in the subjects with high-risk HLA genotypes including A1*05:01-B1*02:01/A1*03:01-B1*03:02 and A1*03:01-B1*03:02/Z. In fact, our data suggest that the frequency of these genotypes decrease with increasing BMI. The presence of the A1*03:01-B1*03:02 haplotype also showed an association with BMI on the multiplicative scale to increase type 1 diabetes susceptibility. We therefore speculate that children carrying the A1*03:01-B1*03:02 haplotype have a more aggressive disease process than carriers of the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype. The more aggressive disease process may lead to a more rapid loss of beta cells and a decrease in insulin production, which would rather be consistent with the classic weight loss that is associated with the clinical onset of type 1 diabetes. Children with the type 1 diabetes high-risk A1*05:01-B1*02:01/A1*03:01-B1*03:02 genotype are more often diagnosed at a younger age (5, 8, 34).
As the incidence of type 1 diabetes has doubled the last 20 years it is interesting to note that the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype was not a risk gene in our national study in 1986-1987 of children with new onset of diabetes, thus the frequency of this genotype was not reported in our publication (32). The observed frequency among patients in the 1986–1987 study was 2.5% whereas it has now increased to 4.8%, and is significantly associated with type 1 diabetes with an OR of 2.8 (Supplementary Table 1). Our results in the present large Swedish cohort of type 1 diabetes children (20) thereby support prior investigations in several countries that there has been a temporal change in the proportion of the different HLA types in the children who develop type 1 diabetes (9-13). The temporal trend in HLA genotype risk in combination with the increase in the absolute incidence could be explained by an increasing environmental exposure affecting both children with high risk HLA genotypes and even more so the children with low risk HLA genotypes, who in the past would not have developed type 1 diabetes.
In Sweden, childhood obesity has increased compared to 20 years ago and about 20% children are now overweight (31). Our observation that the high risk HLA-genotype A1*05:01-B1*02:01/A1*03:01-B1*03:02 was not associated with an increased risk for elevated BMI is most likely due to the fact that children with this genotype tend to develop type 1 diabetes at a younger age (34). Several investigators have reported that the increase in type 1 diabetes incidence rate may be correlated with an increase of overweight and obese children in the population (14, 16, 18, 19). Our observation that a higher mean age standardized BMI was a significant risk factor for individuals with lower risk HLA genotypes suggests a hitherto unrecognized relationship between HLA, BMI and an increased risk for type 1 diabetes in the Swedish childhood population. Indeed, as shown in figure 2 the proportion of overweight patients were higher for HLA genotypes associate with lower risk of developing type 1 diabetes than for HLA genotypes associated with increased risk of developing type 1 diabetes.
There are several possible mechanisms to explain the increased risk of type 1 diabetes with increasing BMI in children with lower risk HLA genotypes. Fat cells secrete adipokines and cytokines, which may affect not only beta cell function (35)but also either increase the risk for or accelerate islet autoimmunity by a direct effect of e.g. IL-6 on the function of autoimmunity suppressing T regulatory cells (36). Furthermore, overweight increase insulin resistance and the combination between islet autoimmunity and a demand for more insulin with increasing BMI may lead to an exhaustion of beta cells particularly in this group of children with lower risk HLA genotypes.
A potential limitation in studies such as this is inclusion of overweight or obese children who may have type 2 diabetes. In this study from Sweden, 97% (n=2608) of the newly diagnosed children and adolescents with diabetes were clinically classified as type 1 diabetes and 92% (n=2403) of the type 1 diabetes patients have islet autoantibodies. We have only included clinically classified type 1 diabetes patients positive at the time of diagnosis for one or several islet autoantibodies. Patients classified with type 2 diabetes (n=43) were excluded. The present results are therefore not likely to be explained by an admixture of patients with type 2 diabetes since type 2 diabetes is by definition not associated with autoimmunity (21).
The test the magnitude of association between BMI and HLA genotypes was carried out in a case only setting since we did not have BMI measurements for the control group. In order to be able to draw the conclusion that there is an interaction in such an analysis one has to assume that among controls there is no correlation between BMI and HLA genotype. This seems a fair assumption. In an independent investigation where both BMI and HLA DRB1 genotypes were available from 894 Swedish controls at the age of 20 years (37), BMI did not differ between DR3 positive individuals (mean 20.8) and the DR3 negative individuals (mean 21.2) suggesting that DR3 and BMI do not correlate among controls. Another limitation with a case only study design is that it is only possible to test for interaction on the multiplicative scale and not on the additive scale. There is intense debate currently regarding on which scale is best to measure interaction in order to detect biological interaction, i.e. genetics and environment (38). The conclusion seems to be that interaction on both scales may indicate a biological interaction.
We conclude that the A1*05:01-B1*02:01/A1*05:01-B1*02:01 genotype is associated with an increased BMI in children with newly diagnosed type 1 diabetes. Individuals with this genotype have an increased risk for type 1 diabetes today compared to about twenty years ago. Our data suggest that overweight needs to be considered as a possible risk factor for type 1 diabetes in children who have HLA-DQ A1*05:01-B1*02:01 haplotype.
Supplementary Material
Acknowledgments
We thank Ali Shalouie, Barbro Gustavsson, Ida Hansson, Rasmus Håkansson, Britt Buveris, and Qefsere Bramini for expert technical assistance. We also thank the Epidemiologic Investigation of Multiple Sclerosis (EIMS) study group for kindly sharing their data on BMI and HLA in the general Swedish population.
The study was supported in part by the Swedish Child Diabetes Foundation, the National Institutes of Health (DK63861, DK26190), the Swedish Research Council, the Swedish Diabetes Association Research Fund, Knut & Alice Wallenberg Foundation, Lion Club International District 101-S and the Skåne County Council for Research and Development.
Footnotes
Conflict of interest
The authors have no competing financial interests in relation to the work described.
References
- 1.DIAMOND PG. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 2.Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 3.Pundziute-Lycka A, Dahlquist G, Urbonaite B, Zalinkevicius R. Time trend of childhood type 1 diabetes incidence in Lithuania and Sweden, 1983–2000. Acta Paediatr. 2004;93:1519–1524. doi: 10.1080/08035250410026680. [DOI] [PubMed] [Google Scholar]
- 4.Pundziute-Lycka A, Dahlquist G, Nystrom L, Arnqvist H, Bjork E, Blohme G, et al. The incidence of Type I diabetes has not increased but shifted to a younger age at diagnosis in the 0–34 years group in Sweden 1983–1998. Diabetologia. 2002;45:783–791. doi: 10.1007/s00125-002-0845-2. [DOI] [PubMed] [Google Scholar]
- 5.Graham J, Hagopian WA, Kockum I, Li LS, Sanjeevi CB, Lowe RM, et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–1355. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 6.Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, et al. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes. 2008;57:2693–2697. doi: 10.2337/db08-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54:S52–61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 8.Redondo MJ, Fain PR, Eisenbarth GS. Genetics of type 1A diabetes. Recent Prog Horm Res. 2001;56:69–89. doi: 10.1210/rp.56.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364:1699–1700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 10.Hermann R, Knip M, Veijola R, Simell O, Laine AP, Akerblom HK, et al. Temporal changes in the frequencies of HLA genotypes in patients with Type 1 diabetes--indication of an increased environmental pressure? Diabetologia. 2003;46:420–425. doi: 10.1007/s00125-003-1045-4. [DOI] [PubMed] [Google Scholar]
- 11.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith GJ, Rewers M, et al. Trends in high-risk HLA susceptibility genes among Colorado youth with type 1 diabetes. Diabetes Care. 2008;31:1392–1396. doi: 10.2337/dc07-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fourlanos S, Varney MD, Tait BD, Morahan G, Honeyman MC, Colman PG, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31:1546–1549. doi: 10.2337/dc08-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resic-Lindehammer S, Larsson K, Ortqvist E, Carlsson A, Cederwall E, Cilio CM, et al. Temporal trends of HLA genotype frequencies of type 1 diabetes patients in Sweden from 1986 to 2005 suggest altered risk. Acta Diabetol. 2008;45:231–235. doi: 10.1007/s00592-008-0048-5. [DOI] [PubMed] [Google Scholar]
- 14.Knip M, Reunanen A, Virtanen SM, Nuutinen M, Viikari J, Akerblom HK. Does the secular increase in body mass in children contribute to the increasing incidence of type 1 diabetes? Pediatr Diabetes. 2008;9:46–49. doi: 10.1111/j.1399-5448.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 15.Larsson HE, Hansson G, Carlsson A, Cederwall E, Jonsson B, Larsson K, et al. Children developing type 1 diabetes before 6 years of age have increased linear growth independent of HLA genotypes. Diabetologia. 2008;51:1623–1630. doi: 10.1007/s00125-008-1074-0. [DOI] [PubMed] [Google Scholar]
- 16.Waldhor T, Schober E, Rami B. Regional distribution of risk for childhood diabetes in Austria and possible association with body mass index. Eur J Pediatr. 2003;162:380–384. doi: 10.1007/s00431-003-1184-0. [DOI] [PubMed] [Google Scholar]
- 17.Hypponen E, Virtanen SM, Kenward MG, Knip M, Akerblom HK. Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care. 2000;23:1755–1760. doi: 10.2337/diacare.23.12.1755. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbloom AL. Obesity, Insulin Resistance, beta-Cell Autoimmunity, and the Changing Clinical Epidemiology of Childhood Diabetes. Diabetes Care. 2003;26:2954–2956. doi: 10.2337/diacare.26.10.2954. [DOI] [PubMed] [Google Scholar]
- 19.Wilkin TJ. Diabetes: 1 and 2, or one and the same? Progress with the accelerator hypothesis Pediatr Diabetes. 2008;9:23–32. doi: 10.1111/j.1399-5448.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 20.Delli AJ, Lindblad B, Carlsson A, Forsander G, Ivarsson SA, Ludvigsson J, et al. Type 1 diabetes patients born to immigrants to Sweden increase their native diabetes risk and differ from Swedish patients in HLA types and islet autoantibodies. Pediatr Diabetes. 2010;11:513–20. doi: 10.1111/j.1399-5448.2010.00637.x. [DOI] [PubMed] [Google Scholar]
- 21.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch KF, Lernmark B, Merlo J, Cilio CM, Ivarsson SA, Lernmark A. Cord blood islet autoantibodies and seasonal association with the type 1 diabetes high-risk genotype. J Perinatol. 2008;28:211–217. doi: 10.1038/sj.jp.7211912. [DOI] [PubMed] [Google Scholar]
- 23.Larsson HE, Lynch K, Lernmark B, Hansson G, Lernmark A, Ivarsson SA. Relationship between increased relative birthweight and infections during pregnancy in children with a high-risk diabetes HLA genotype. Diabetologia. 2007;50:1161–1169. doi: 10.1007/s00125-007-0648-6. [DOI] [PubMed] [Google Scholar]
- 24.Kiviniemi M, Hermann R, Nurmi J, Ziegler AG, Knip M, Simell O, et al. A high-throughput population screening system for the estimation of genetic risk for type 1 diabetes: an application for the TEDDY (the Environmental Determinants of Diabetes in the Young) study. Diabetes Technol Ther. 2007;9:460–472. doi: 10.1089/dia.2007.0229. [DOI] [PubMed] [Google Scholar]
- 25.Grubin CE, Daniels T, Toivola B, Landin-Olsson M, Hagopian WA, Li L, et al. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia. 1994;37:344–350. doi: 10.1007/BF00408469. [DOI] [PubMed] [Google Scholar]
- 26.Mire-Sluis AR, Gaines Das R, Lernmark A. The World Health Organization International Collaborative Study for islet cell antibodies. Diabetologia. 2000;43:1282–1292. doi: 10.1007/s001250051524. [DOI] [PubMed] [Google Scholar]
- 27.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Q, Albertsson-Wikland K, Karlberg J. Population-based body mass index reference values from Goteborg, Sweden: birth to 18 years of age. Acta Paediatr. 2000;89:582–592. [PubMed] [Google Scholar]
- 29.Blair RC, Taylor RA. Biostatisctics for the health sciences. Prentice Hall; Upper Saddle River, New Jersey: 2008. p. 552. [Google Scholar]
- 30.Khoury MJ, Flanders WD. Nontraditional epidemiologic approaches in the analysis of gene-environment interaction: case-control studies with no controls! Am J Epidemiol. 1996;144:207–213. doi: 10.1093/oxfordjournals.aje.a008915. [DOI] [PubMed] [Google Scholar]
- 31.Sjoberg A, Lissner L, Albertsson-Wikland K, Marild S. Recent anthropometric trends among Swedish school children: evidence for decreasing prevalence of overweight in girls. Acta Paediatr. 2008;97:118–123. doi: 10.1111/j.1651-2227.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 32.Kockum I, Sanjeevi CB, Eastman S, Landin-Olsson M, Dahlquist G, Lernmark A. Complex interaction between HLA DR and DQ in conferring risk for childhood type 1 diabetes. Eur J Immunogenet. 1999;26:361–372. doi: 10.1046/j.1365-2370.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 33.Kockum I, Sanjeevi CB, Eastman S, Landin-Olsson M, Dahlquist G, Lernmark A. Population analysis of protection by HLA-DR and DQ genes from insulin-dependent diabetes mellitus in Swedish children with insulin-dependent diabetes and controls. Eur J Immunogenet. 1995;22:443–465. doi: 10.1111/j.1744-313x.1995.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanjeevi CB, Sedimbi SK, Landin-Olsson M, Kockum I, Lernmark A. Risk conferred by HLA-DR and DQ for type 1 diabetes in 0-35-year age group in Sweden. Ann N Y Acad Sci. 2008;1150:106–111. doi: 10.1196/annals.1447.061. [DOI] [PubMed] [Google Scholar]
- 35.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 36.Guzik TJ, Mangalat D, Korbut R. Adipocytokines - novel link between inflammation and vascular function? J Physiol Pharmacol. 2006;57:505–528. [PubMed] [Google Scholar]
- 37.Hedstrom AK, Baarnhielm M, Olsson T, Alfredsson L. Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology. 2009;73:696–701. doi: 10.1212/WNL.0b013e3181b59c40. [DOI] [PubMed] [Google Scholar]
- 38.Ahlbom A, Alfredsson L. Interaction: A word with two meanings creates confusion. Eur J Epidemiol. 2005;20:563–564. doi: 10.1007/s10654-005-4410-4. [DOI] [PubMed] [Google Scholar]
- 39.Haldane S. The estimation and significance of the logarithm of a ratio of frequencies. Ann Hum Genet. 1956;20:309–311. doi: 10.1111/j.1469-1809.1955.tb01285.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.