Abstract
Background
The prognosis for patients with esophageal cancer is poor, even among those who undergo potentially curative esophagectomy. The neutrophil:lymphocyte ratio (NLR) is hypothesized to reflect the systemic inflammatory response created by a tumor and is possibly predictive of tumor aggressiveness and propensity for metastasis.
Methods
We performed a single-center retrospective analysis of esophageal cancer patients who underwent attempted curative esophagectomy at Weill Cornell Medical Center between 1996 and 2009. We collected data on patient demographics, clinical characteristics, and receipt of neoadjuvant treatment. Preoperative blood tests were used to calculate NLR. Elevated NLR was defined a priori as ≥5.0. Logistic regression modeling was performed to analyze characteristics associated with elevated NLR. We conducted Kaplan-Meier analyses and Cox regression modeling to determine estimates and predictors of disease-free and overall survival.
Results
We identified a total of 295 patients who underwent esophagectomy. The median duration of follow-up was 31 months (interquartile range [IQR] 13–61). There were 56 patients (18.9%) who had elevated NLR preoperatively. Receipt of neoadjuvant therapy was independently associated with high NLR (odds ratio [OR] 2.14, 95% confidence interval [95% CI] 1.02–4.51). In multivariable analyses, elevated NLR was associated with significantly worse disease-free (hazard ratio [HR] 2.26, 95% CI 1.43–3.55) and overall survival (HR 2.31, 95% CI 1.53–3.50).
Conclusions
Preoperative NLR is a potential prognostic marker for recurrence and death after esophagectomy. It is unclear whether NLR reflects the degree of inflammatory response to the primary tumor or other patient-specific or tumor characteristics that predispose to recurrence. Further investigation is warranted to clarify the mechanisms explaining the observed associations between elevated NLR and poor outcomes in esophageal cancer.
Esophageal cancer is the 6th leading cause of cancer death worldwide.1 The prognosis of this disease is extremely poor, with a 16% 5-year survival in the United States.2 The primary curative treatment for esophageal cancer is esophagectomy, with or without neoadjuvant therapy. Unfortunately, even after attempts at curative therapy, the majority of patients eventually develop local or distant recurrent disease.
Tumors interact directly and indirectly with host inflammatory cells.3 This tumor-generated inflammatory response may result in an increased propensity for metastasis via upregulation of cytokines and inflammatory mediators, inhibition of apoptosis, promotion of angiogenesis, and damage of DNA.4 Prior studies have shown correlations between the degree of systemic inflammatory response and outcomes in various malignancies.5 The neutrophil:lymphocyte ratio (NLR) is one particular nonspecific marker of systemic inflammation. Published data suggest that an elevated preoperative NLR (≥5) may correlate with an increased risk of recurrence and death in patients who undergo hepatic resection for colorectal liver metastases and for primary hepatocellular carcinoma.6,7
Given the high rate of local recurrence and distant metastasis in esophageal cancer, we decided to investigate preoperative NLR as a predictor of recurrence after attempted curative esophagectomy. We performed a retrospective analysis of prospectively collected data on patients with esophageal cancer, with the primary hypothesis that elevated preoperative NLR is associated with an increased risk of disease recurrence after esophagectomy.
MATERIALS AND METHODS
The study was conducted using a database of patients with histologically confirmed esophageal cancer who had undergone esophagectomy at Weill Cornell Medical Center (New York, NY) between January 1, 1996 and June 30, 2009. Patient demographic and clinical information as well as tumor characteristics and patient follow-up were entered in a prospective fashion. The following data were extracted from the database: age (at time of esophagectomy), gender, race/ethnicity, smoking history (ever or never), comorbidities, aspirin or NSAID use, tumor stage and subsite (dichotomized as GE junction/lower or mid/upper esophagus), cell type, receipt of neoadjuvant therapy, and year of surgery. Specifically, the following comorbid conditions were recorded in the database: chronic obstructive pulmonary disease, congestive heart failure, coronary artery disease, and diabetes mellitus. All subjects had minimum 6 months follow-up time.
The 41 patients who did not have resections for curative intent were excluded from the analyses. Tumor stage was based on the American Joint Committee on Cancer (AJCC) 6th edition staging manual.8 The clinical stage was used if the patient received neoadjuvant therapy, and the pathologic stage was used if the patient did not receive neoadjuvant therapy. In patients who received neoadjuvant therapy, response to treatment was determined by comparing the clinical stage (pretreatment) to the pathological stage (from the surgical resection). Response was defined as an improvement in pathological stage compared with clinical stage. Tumor differentiation was determined by review of pathology reports from the medical records. In a specimen where two grades were reported (e.g., well to moderately differentiated), the more advanced grade was used.
Data on preoperative complete blood cell counts (CBC) were extracted in a retrospective fashion from the medical records. Only subjects with available preoperative CBC with differential were included in the study. All white blood cell and differential counts were taken within 1 week prior to surgery. The neutrophil:lymphocyte ratio (NLR) was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. “High” NLR was defined a priori as a ratio ≥ 5. A cutoff of five has been used in prior studies that evaluated preoperative NLR and outcomes in other malignancies.6,9–14 Three patients demonstrating signs of preoperative sepsis were excluded.
Statistical Analysis
Categorical variables were analyzed using Fisher exact tests and chi-square tests, as appropriate. Continuous variables were analyzed using 2-sided t tests. Multivariable logistic regression was performed to assess for patient and tumor characteristics associated with high preoperative NLR.
For assessment of disease-free survival (DFS), recurrence was defined as development of local recurrence, distant metastasis, or death from esophageal cancer (whichever occurred first). Patients who died within 30 days of the date of surgery (n = 5) were excluded from survival analyses. Kaplan-Meier curves were generated for disease-free and overall survival (OS) to compare patients with high and low NLR. Curves were compared using the log-rank test. Multivariable survival analyses were performed using Cox regression modeling. NLR was treated first as a categorical variable (≥5 or <5) and then as a continuous variable. In light of the unknown effects of neoadjuvant chemotherapy and/or radiation therapy on preoperative NLR, stratified analyses were also performed based on receipt of neoadjuvant therapy. Response to treatment was also included in the stratified models for those patients who received neoadjuvant therapy.
Statistical significance was defined as P < 0.05 or a 95% confidence interval (95% CI) that did not cross 1.00. All analyses were conducted using Stata 10.0 (StataCorp, College Station, TX). The study was approved by the Cornell University and Columbia University Institutional Review Boards.
RESULTS
There were a total of 339 patients with histologically confirmed esophageal cancer who underwent attempted curative esophagectomy between 1996 and 2009 at Weill Cornell Medical Center. Of these, 295 patients had available preoperative white blood cell counts with differentials (to calculate NLR), and this group comprised the study population. The characteristics of these patients are shown in Table 1. The majority of patients were male (80.3%) with a mean age of 62.8 years. The median duration of follow-up was 31 months (IQR 13–61). Adenocarcinomas accounted for 68% of the tumors. There was no difference between the included subjects and 43 subjects excluded because of lack of NLR with regard to age, sex, or tumor histology. A lower proportion of excluded subjects received neoadjuvant therapy (20.9 vs. 41.2% included subjects; P = 0.01).
TABLE 1.
Patient and tumor characteristics, based on preoperative NLR, of patients who underwent esophagectomy for esophageal cancer, 1996–2009
| Low NLR (n = 239) |
High NLR (n = 56) |
P value | |
|---|---|---|---|
| Mean age, years (± SD) | 62.8 (±11.1) | 62.8 (±12.6) | 0.50 |
| Sex, male | 191 (79.9%) | 46 (82.1%) | 0.85 |
| Race/ethnicity | 0.44 | ||
| White | 212 (88.7%) | 49 (96.1%) | |
| Black | 5 (2.1%) | 1 (1.9%) | |
| Asian | 18 (7.5%) | 1 (1.9%) | |
| Hispanic | 4 (1.7%) | 0 | |
| Stage | 0.90 | ||
| 1 | 41 (17.5%) | 9 (16.7%) | |
| 2 | 73 (30.5%) | 19 (33.9%) | |
| 3 | 105 (43.9 %) | 24 (42.9%) | |
| 4 | 20 (8.4%) | 4 (7.4%) | |
| Histology | 0.67 | ||
| Adenocarcinoma | 171 (71.9%) | 43 (76.7%) | |
| Squamous | 63 (26.3%) | 12 (21.4%) | |
| Othera | 3 (1.3%) | 1 (1.79%) | |
| Tumor subsite | 0.19 | ||
| GE junction, lower | 190 (79.6%) | 49 (87.5) | |
| Mid, upper | 49 (20.5) | 7 (12.5) | |
| Neoadjuvant treatment | 95 (39.7%) | 32 (57.1%) | 0.02 |
| Tumor differentiation | 0.57 | ||
| Well | 22 (9.2%) | 6 (10.7%) | |
| Moderate | 124 (51.2%) | 23 (41.1%) | |
| Poor | 93 (38.9%) | 24 (42.8%) | |
| Aspirin/NSAID use | 58 (23.1%) | 15 (26.3%) | 0.36 |
| Smoking history, ever | 175 (73.2%) | 43 (76.7%) | 0.74 |
| Comorbidities | 0.75 | ||
| 0 | 196 (82.0%) | 46 (82.1%) | |
| 1 | 38 (15.9%) | 10 (17.8%) | |
| >1 | 5 (2.1%) | 0 |
Other (small cell = 2, GIST = 1, undifferentiated = 1)
The mean NLR for the study population was 3.68 (SD 3.66); 56 (18.9%) patients had a high preoperative NLR (≥5). The associations between patient and tumor characteristics and NLR are shown in Table 1. In multivariable analysis, only receipt of neoadjuvant therapy was associated with elevated preoperative NLR (odds ratio [OR] 2.14, 95% CI 1.02–4.51).
Of the 127 patients who received neoadjuvant therapy, 69 patients had a pretreatment NLR available. Of these patients, 15 (21.7%) had an elevated NLR. There was no association between response to therapy and elevated pretreatment NLR (P = 0.95) or preoperative NLR (P = 0.53). There was also no significant change between pretreatment NLR and preoperative NLR (paired t test; P = 0.38).
Disease-Free and Overall Survival
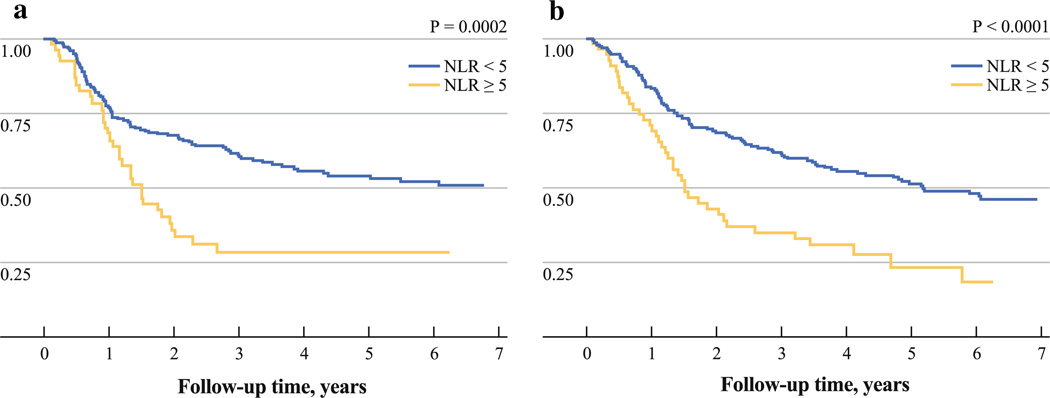
The median disease-free survival was 23.6 months, and the median overall survival was 31.4 months. Patients with high preoperative NLR had significantly worse disease-free and overall survival compared with those with low NLR (DFS, P = 0.0002; OS, P < 0.0001) (Fig. 1a, b). There were 5 patients (1.6%) who died within 30 days of the date of surgery, and all of these patients had low NLR preoperatively.
FIG. 1.
Kaplan–Meier a disease-free survival and b overall survival curves after esophagectomy for esophageal cancer, comparing patients with high preoperative NLR (≥5) to low NLR (<5)
In multivariable analyses, preoperative NLR ≥ 5 was associated with worse DFS (HR 2.26, 95% CI 1.44–3.56) (Table 2) and OS (HR 2.32, 95% CI 1.53–3.50) (Table 3). When treated as a continuous variable, increased NLR remained a significant predictor of worse DFS (HR 1.04, 95% CI 1.00–1.08) and OS (HR 1.06, 95% CI 1.02–1.10). Older age, male sex, greater number of comorbidities, and more advanced tumor stage were associated with worse overall survival. Greater tumor differentiation was associated with improved DFS and OS. More advanced stage and surgery after 2002 were associated with increased risk of disease recurrence. In a post hoc analysis, patients who underwent surgery after 2002 were found to have higher comorbidity scores compared with the earlier time period (P < 0.0001). The analyses were also repeated excluding the 10 R1 cases, and no qualitative differences in the results were seen.
TABLE 2.
Univariate and multivariable Cox regression analyses for disease-free survival among patients who underwent attempted curative surgery for esophageal cancer, 1996–2009
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| NLR | ||||||
| <5 | 1.00 | Referent | 1.00 | Referent | ||
| ≥5 | 2.08 | (1.40–3.10) | <0.001 | 2.26 | (1.44–3.56) | <0.0001 |
| Neutrophils | 1.01 | (1.00–1.02) | 0.04 | 1.00 | (0.99–1.02) | 0.69 |
| Lymphocytes | 0.98 | (0.97–1.00) | 0.112 | 0.99 | (0.96–1.02) | 0.37 |
| Age, per year | 0.99 | (0.97–1.01) | 0.39 | 1.00 | (0.98–1.02) | 0.79 |
| Sex | ||||||
| Female | 1.00 | Referent | 1.00 | Referent | ||
| Male | 1.10 | (0.68–1.76) | 0.69 | 1.56 | (0.88–2.77) | 0.13 |
| Race/ethnicity | ||||||
| White | 1.00 | Referent | 1.00 | Referent | ||
| Black | 0.31 | (0.04–2.25) | 0.25 | 0.49 | (0.06–3.81) | 0.49 |
| Asian | 0.64 | (0.28–1.45) | 0.29 | 0.56 | (0.23–1.41) | 0.22 |
| Hispanic | 0.65 | (0.09–4.65) | 0.67 | 0.57 | (0.07–4.55) | 0.60 |
| Stage | ||||||
| 1 | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 4.39 | (1.85–10.44) | 0.001 | 2.39 | (0.96–5.71) | 0.06 |
| 3 | 9.46 | (4.09–21.85) | <0.0001 | 4.93 | (2.02–12.05) | <0.0001 |
| 4 | 8.05 | (3.02–21.48) | <0.0001 | 4.48 | (1.58–12.55) | 0.004 |
| Histology | ||||||
| Adenocarcinoma | 1.00 | Referent | 1.00 | Referent | ||
| Squamous | 1.06 | (0.71–1.57) | 0.79 | 1.33 | (0.79–2.24) | 0.28 |
| Tumor differentiation | ||||||
| Well | 0.24 | (0.11–0.56) | 0.001 | 0.28 | (0.11–0.68) | 0.005 |
| Moderately | 0.53 | (0.36–0.75) | 0.001 | 0.56 | (0.37–0.85) | 0.006 |
| Poorly | 1.00 | Referent | 1.00 | Referent | ||
| Neoadjuvant Rx | ||||||
| No | 1.00 | Referent | 1.00 | Referent | ||
| Yes | 2.58 | (1.81–3.69) | <0.001 | 1.37 | (0.91–2.12) | 0.14 |
| Aspirin/NSAIDs | ||||||
| No | 1.00 | Referent | 1.00 | Referent | ||
| Yes | 1.31 | (0.88–1.92) | 0.18 | 1.06 | (0.68–1.67) | 0.79 |
| Smoking Hx | ||||||
| Never | 1.00 | Referent | 1.00 | Referent | ||
| Ever | 1.02 | (0.69–1.51) | 0.94 | 1.39 | (0.85–2.07) | 0.15 |
| Tumor subsite | ||||||
| GEJ/lower | 1.00 | Referent | 1.00 | Referent | ||
| Mid/upper | 1.36 | (0.87–2.08) | 0.16 | 1.53 | (0.91–2.56) | 0.11 |
| Comorbidities | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | ||
| 1 | 0.97 | (0.59–1.56) | 0.89 | 0.77 | (0.45–1.32) | 0.35 |
| >1 | 10.26 | (2.44–43.07) | 0.001 | 8.07 | (1.81–35.97) | 0.006 |
| Year of surgery | ||||||
| 2002 or before | 1.00 | Referent | 1.00 | Referent | ||
| After 2002 | 1.22 | (0.85–1.77) | 0.28 | 1.59 | (1.01–2.43) | 0.04 |
TABLE 3.
Univariate and multivariable Cox regression analyses for overall survival among patients who underwent attempted curative surgery for esophageal cancer, 1996–2009
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| NLR | ||||||
| <5 | 1.00 | Referent | 1.00 | Referent | ||
| ≥5 | 2.09 | (1.46–3.01) | <0.001 | 2.32 | (1.53–3.50) | <0.0001 |
| Neutrophils | 1.02 | (1.01–1.03) | <0.001 | 1.01 | (0.99–1.03) | 0.56 |
| Lymphocytes | 0.98 | (0.96–0.99) | 0.005 | 0.99 | (0.97–1.02) | 0.16 |
| Age, per year | 1.01 | (0.99–1.02) | 0.23 | 1.02 | (1.00–1.04) | 0.01 |
| Sex | ||||||
| Female | 1.00 | Referent | 1.00 | Referent | ||
| Male | 1.38 | (0.88–2.14) | 0.16 | 1.97 | (1.11–3.48) | 0.02 |
| Race/ethnicity | ||||||
| White | 1.00 | Referent | 1.00 | Referent | ||
| Black | 0.26 | (0.03–1.83) | 0.18 | 0.57 | (0.08–4.40) | 0.59 |
| Asian | 0.76 | (0.37–1.56) | 0.46 | 0.78 | (0.35–1.78) | 0.57 |
| Hispanic | 0.60 | (0.08–4.30) | 0.61 | 0.57 | (0.07–4.44) | 0.59 |
| Stage | ||||||
| 1 | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 2.94 | (1.52–5.66) | 0.001 | 2.01 | (0.92–4.41) | 0.08 |
| 3 | 4.94 | (2.62–9.32) | <0.001 | 3.79 | (1.78–8.07) | 0.001 |
| 4 | 5.47 | (2.55–11.70) | <0.001 | 4.44 | (1.86–10.56) | 0.001 |
| Histology | ||||||
| Adenocarcinoma | 1.00 | Referent | 1.00 | Referent | ||
| Squamous | 0.95 | (0.67–1.37) | 0.82 | 0.97 | (0.58–1.62) | 0.93 |
| Tumor differentiation | ||||||
| Well | 0.36 | (0.17–0.71) | 0.002 | 0.57 | (0.39–0.84) | 0.004 |
| Moderate | 0.58 | (0.41–0.82) | 0.003 | 0.38 | (0.17–0.83) | 0.02 |
| Poor | 1.00 | Referent | 1.00 | Referent | ||
| Neoadjuvant Rx | ||||||
| No | 1.00 | Referent | 1.00 | Referent | ||
| Yes | 1.71 | (1.24–2.35) | <0.001 | 1.09 | (0.75–1.55) | 0.65 |
| Aspirin/NSAIDs | ||||||
| No | 1.00 | Referent | 1.00 | Referent | ||
| Yes | 1.22 | (0.86–1.75) | 0.26 | 0.99 | (0.66–1.51) | 0.99 |
| Smoking Hx | ||||||
| Never | 1.00 | Referent | 1.00 | Referent | ||
| Ever | 1.31 | (0.89–1.91) | 0.17 | 1.05 | 0.67–1.62 | 0.84 |
| Tumor subsite | ||||||
| GEJ/lower | 1.00 | Referent | 1.00 | Referent | ||
| Mid/upper | 1.09 | (0.72–1.64) | 0.67 | 1.36 | (0.81–2.26) | 0.24 |
| Comorbidities | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | ||
| 1 | 0.99 | (0.63–1.54) | 0.95 | 0.71 | (0.43–1.20) | 0.18 |
| >1 | 8.97 | (2.75–29.17) | <0.001 | 6.81 | (1.95–23.75) | 0.003 |
| Year of surgery | ||||||
| 2002 or before | 1.00 | Referent | 1.00 | Referent | ||
| After 2002 | 1.22 | (0.87–1.70) | 0.25 | 1.36 | (0.91–2.02) | 0.13 |
Analyses were subsequently performed evaluating the individual components of NLR, specifically neutrophil and lymphocyte counts (Table 3). On their own, lymphocytes were significant predictors of overall survival and disease-free survival. Neutrophils were significant for overall survival only. However, when both were included in the model, neither was significantly associated with DFS or OS, even when the NLR was not analyzed.
In multivariable analyses stratified by receipt of neoadjuvant therapy, high NLR was associated with significantly increased risk of disease recurrence among patients who received neoadjuvant treatment (HR 2.83, 95% CI 1.52–5.25), but not among those who did not receive neoadjuvant therapy (HR 1.44, 95% CI 0.65– 3.16). Elevated NLR was independently associated with increased risk of death among both patients who received neoadjuvant treatment (HR 2.72, 95% CI 1.49–4.98) and those who did not (HR 1.93, 95% CI 1.01–3.66).
Subanalyses by tumor types were performed, and NLR remained an independent predictor of both OS and DFS. For adenocarcinoma, high NLR was associated with significantly increased risk of disease recurrence (HR 2.47, 95% CI 1.44–4.28; P = 0.001) and overall survival (HR 2.29, 95% CI 1.41–3.73; P = 0.001). For squamous cell carcinoma, NLR was independently associated with increased risk of overall survival (HR 3.66, 95% CI 1.32–10.10; P = 0.01), and a nonsignificant increased risk of DFS (HR 2.35, 95% CI 0.83–6.64; P = 0.12).
DISCUSSION
The prognosis for patients with esophageal cancer is poor, even among those who undergo potentially curative esophagectomy. In the present study, we analyzed data from a cohort of patients with esophageal cancer to evaluate neutrophil:lymphocyte ratio (NLR) as a predictor of disease recurrence and overall mortality. We found that an elevated preoperative NLR was associated with a nearly 2-fold increased risk of recurrence and death, independent of other patient and tumor characteristics associated with poor outcomes. Consistent with prior studies of esophageal cancer, the following factors were also associated with worse OS: older age, male sex, comorbidities, more advanced tumor stage, and worse tumor differentiation.
It is interesting to note that, in stratified analyses, elevated preoperative NLR was associated with worse DFS only in patients who received neoadjuvant therapy. Elevated NLR prior to receipt of neoadjuvant therapy was not associated with response to treatment, and the neutrophil:lymphocyte ratio did not change significantly after receipt of neoadjuvant therapy. Perhaps preoperative NLR reflects the propensity for postoperative cancer metastasis and may reflect some underlying biological phenomenon in the tumor itself.
Our study is not the first to evaluate NLR and cancer outcomes. Previous studies have shown this measure to be an independent predictor of survival in resectable primary colorectal cancer, colorectal cancer liver metastases, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, non-small cell lung cancer, and ovarian cancer.6,9,11,13–18 Rashid et al. recently evaluated NLR and its association with survival after resection for esophageal cancer.19 In multivariable analysis, NLR as a continuous variable was not significantly associated with risk of death. However, it is unclear which potential confounders were included in the final model. In a secondary unadjusted analysis, there was no significant difference in overall survival with categorization of preoperative NLR ≥ 5 or <5. Disease-free survival analyses were not reported. Reasons for the discrepant findings with regard to overall survival compared with the present study are not clear. One reason may be that the authors used pathological staging only, whereas we took into account whether the patient received neoadjuvant therapy, and thus included clinical stage as well as pathological stage, potentially more accurately reflecting disease burden.
Why have studies consistently found associations between nonspecific markers of systemic inflammation and risk of postoperative disease recurrence? Host inflammatory cells can both suppress and stimulate tumor growth, and inflammation plays a major role in the development and progression of various solid tumors.11,20,21 Specifically, inflammatory cells interact directly and indirectly with the primary tumor to promote angiogenesis, extracellular matrix remodeling, and preparation of the metastatic niche.3 NLR reflects systemic inflammation, and other similar measures, including the Glasgow Prognostic Score, C-reactive protein levels, hypoalbuminemia, and thrombocytosis, have also been associated with outcomes after surgery for gastroesophageal cancer and other various tumor types.5,22–25 These measures of systemic inflammation may serve as a composite “score” that reflects the degree of host inflammatory cell activity that promotes tumor growth and progression.
Elevated NLR may also provide clues with regard to the tumor microenvironment. Significant literature exists that describes the roles of macrophages and lymphocytes in various tumor types.4 In a recent study by Fridlender et al., tumor-associated neutrophils (TANs) were also implicated as a potentially important factor in tumor growth.26 In mouse models of non-small cell lung cancer and mesothelioma, TGF-β expression within the tumor microenvironment was associated with expression of neutrophil-attracting chemokines and infiltration by TANs, which in turn resulted in an increase in tumor growth. Conceivably, an elevated circulating NLR could reflect high numbers of TANs, which may be associated with a more aggressive tumor phenotype.
The current study has several strengths. Data on the cohort was recorded prospectively, including follow-up data related to local recurrence, distant metastasis, and death. The follow-up period was adequate to capture recurrence and death in the majority of the patients. In a subset of patients who received neoadjuvant therapy, both pretreatment and preoperative NLR were available to assess for an association with response to therapy. In the survival analyses, we excluded patients who died within the first 30 days so as to eliminate the effects of operative mortality on the outcomes. Patients who had macroscopic residual disease after surgery were also excluded. Additionally, we were able to adjust for the majority of known prognostic factors for esophageal cancer, including age, sex, smoking history, comorbidities, and tumor stage. We also adjusted for tumor grade and subsite, both of which are prognostic measures now included in the most recent edition of the American Joint Committee on Cancer (AJCC) staging for esophageal cancer.27
Our study also has certain limitations. Preoperative neutrophil:lymphocyte ratio was evaluated retrospectively and was not available for the entire cohort. We did not record the number of positive lymph nodes, a measure that is included in the updated AJCC staging system. We chose to dichotomize NLR as ≥5 or <5 in order to be consistent with prior studies that have used the same cutoff value.6,9–14 However, it is unclear whether a different cutoff value would serve as a better predictor of disease recurrence in esophageal cancer, or whether NLR would be better categorized as “high,” “intermediate,” and “low” instead of dichotomized.
The results of our analyses of a prospective cohort of esophageal cancer patients demonstrate that preoperative NLR is an independent predictor of both disease recurrence and death after esophagectomy. Several studies have now shown NLR to be predictive of outcomes in various epithelial malignancies. However, various “cutoffs” for elevated NLR have been used in these studies. If NLR is to be incorporated into clinical practice as a prognostic marker, it is unclear how patients should be categorized and whether the same categorizations can be used for different tumor types. NLR is a nonspecific measure with unclear biologic relevance. Future studies should be aimed at the identification of circulating markers of inflammatory cell subtypes that better reflect the interaction between the primary tumor and the host immune system.
ACKNOWLEDGMENT
Dr. Abrams is supported in part by a career development award from the National Cancer Institute (K07 CA132892). Dr. Sharaiha is supported in part by a training grant from the National Cancer Institute (T32-CA 09529).
REFERENCES
- 1.American Cancer Society. Global cancer facts and figures. [Accessed 30 Mar 2010];2007 http://www.cancer.org/downloads/STT/Global_Facts_and_Figures_2007_rev2.pdf.
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 6.Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Malik HZ, Gomez D, Wong V, Al-Mukthar A, Toogood GJ, Lodge JP, et al. Predictors of early disease recurrence following hepatic resection for colorectal cancer metastasis. Eur J Surg Oncol. 2007;33:1003–1009. doi: 10.1016/j.ejso.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 8.AJCC cancer staging manual. 6th edn. New York: Springer-Verlag; 2002. [Google Scholar]
- 9.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 10.Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–814. doi: 10.1097/SLA.0b013e318142d964. [DOI] [PubMed] [Google Scholar]
- 11.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 12.Halazun KJ, Hardy MA, Rana AA, Woodland DC, IV, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 13.Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 14.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97:513–518. doi: 10.1002/jso.21001. [DOI] [PubMed] [Google Scholar]
- 15.Nakahara K, Monden Y, Ohno K, Fujii Y, Hashimoto J, Kitagawa Y, et al. Importance of biologic status to the postoperative prognosis of patients with stage III nonsmall cell lung cancer. J Surg Oncol. 1987;36:155–160. doi: 10.1002/jso.2930360302. [DOI] [PubMed] [Google Scholar]
- 16.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 17.Gomez D, Morris-Stiff G, Wyatt J, Toogood GJ, Lodge JP, Prasad KR. Surgical technique and systemic inflammation influences long-term disease-free survival following hepatic resection for colorectal metastasis. J Surg Oncol. 2008;98:371–376. doi: 10.1002/jso.21103. [DOI] [PubMed] [Google Scholar]
- 18.Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pretreatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashid F, Waraich N, Bhatti I, Saha S, Khan RN, Ahmed J, et al. A pre-operative elevated neutrophil: lymphocyte ratio does not predict survival from oesophageal cancer resection. World J Surg Oncol. 2010;8:1. doi: 10.1186/1477-7819-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aliustaoglu M, Bilici A, Ustaalioglu BB, Konya V, Gucun M, Seker M, et al. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol. 2010;27:1060–1065. doi: 10.1007/s12032-009-9335-4. [DOI] [PubMed] [Google Scholar]
- 21.Liao Q, Zhao YP, Yang YC, Li LJ, Long X, Han SM. Combined detection of serum tumor markers for differential diagnosis of solid lesions located at the pancreatic head. Hepatobiliary Pancreat Dis Int. 2007;6:641–645. [PubMed] [Google Scholar]
- 22.Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197–201. doi: 10.1016/s0002-9610(01)00684-5. [DOI] [PubMed] [Google Scholar]
- 23.Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Aoki T, et al. Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2003;83:248–252. doi: 10.1002/jso.10275. [DOI] [PubMed] [Google Scholar]
- 24.Guillem P, Triboulet JP. Elevated serum levels of C-reactive protein are indicative of a poor prognosis in patients with esophageal cancer. Dis Esophagus. 2005;18:146–150. doi: 10.1111/j.1442-2050.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Teruya M, Kishiki T, Kaneko S, Endo D, Takenaka Y, et al. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008;144:729–735. doi: 10.1016/j.surg.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2010. [Google Scholar]