Abstract
Aim
Hypertension is related to abnormalities in autonomic nervous system (ANS) function, with increased sympathetic output and decreased parasympathetic tone. Lifestyle interventions are the first line of treatment in hypertension, and decreased blood pressure (BP) effects may be related to changes in ANS function. Using heart rate recovery (HRR) from exercise as an index of parasympathetic tone and plasma norepinephrine as an index of sympathetic tone, we investigated the effects of lifestyle interventions on ANS function in patients with elevated BP.
Methods
Sedentary participants with elevated BP were randomly assigned to either an Exercise only (N=25), Exercise plus Dietary Approaches to Stop Hypertension (DASH) diet (N=12), or Waitlist control (N=15) 12-week intervention. Plasma norepinephrine was measured at rest and participants performed a peak exercise test before and after the intervention. HRR was calculated as peak heart rate (HR) minus HR at 1-minute post exercise.
Results
HRR showed a significant group by time interaction; both intervention groups showed increases in HRR from pre- to post-intervention, while waitlist showed no change. Similarly, both exercise plus diet and exercise groups, but not waitlist, showed significant reductions in BP from pre to post-intervention. Linear regression revealed that BP post-intervention was significantly predicted by change in HRR when controlling for pre BP, age, gender and BMI.
Conclusions
Lifestyle interventions induced training-reduced BP and altered autonomic tone, indexed by HRR. This study indicates the importance of behavioural modification in hypertension and that increased parasympathetic function is associated with success in reduction of BP.
Keywords: Autonomic nervous system, Heart rate recovery, Hypertension, Parasympathetic nervous system
Introduction
Elevated blood pressure (BP) is an independent risk factor for cardiovascular disease (CVD), and despite advances in our understanding of this relationship, the associated morbidity and mortality underscore the remaining importance of further establishing associated mechanisms of BP control (Chobanian, 2009). The etiology of abnormality in autonomic nervous system (ANS) control of BP in hypertension has been studied for many years, and has led to the ‘neurogenic hypothesis of hypertension’ which describes the imbalance of elevated sympathetic nervous system (SNS) activation and parasympathetic nervous system (PNS) impairment in essential hypertension (Madhi et al., 2007, Julius and Majahalme, 2000).
Evidence for increased sympathetic activation underlying elevated BP includes several different measures of resting SNS activity including plasma norepinephrine, muscle sympathetic nerve activity and norepinephrine radiolabelling (Esler, 2010). Such data firmly establish elevated SNS activity as a feature of hypertension and have revealed multifactorial causes such as alterations in respiratory pattern, leading to intermittent hypoxia, increased plasma osmolality, vascular inflammation and angiotensin II mediated sympathoexcitation (Grassi et al., 2010). The resulting effects of elevated SNS activity are a similarly long list, including insulin resistance, elevated coagulation, arterial stiffening and vasoconstriction (Mancia et al., 1999). The hypofunction of the parasympathetic arm of the ANS in hypertension has received less attention, partly for reasons of measurement accessibility; however, heart rate variability (HRV) and heart rate recovery (HRR) measures have been successful in demonstrating reduced vagal tone in hypertension (Singh et al., 1998, Polonia et al., 2006).
Lifestyle interventions are the first line of treatment for hypertension. Cross-sectional data has shown that exercise training and physical activity are protective against CVD, but interventional studies often find that training does not alter traditional risk factors, including BP, blood lipids, diabetes etc. to the extent predicted by epidemiology (Mora et al., 2007, Edwards et al., 2007). This ‘risk-factor gap’ has led to the suggestion that exercise must provide protection beyond these traditional indices (Joyner and Green, 2009). The effects of exercise on ANS function and balance are fairly well established, with association between exercise training and reduced heart rate variability (Routledge et al., 2010) and protection against age-related baroreflex function (Monahan et al., 2000) reduction documented. Thus, it is not unreasonable to suggest that the benefits of exercise training on CVD outcomes may well include effects on ANS balance. Indeed, Fraga et al (Fraga et al., 2007) have shown that SNS activity, indexed by muscle sympathetic nerve activity, is reduced by exercise training in patients with congestive heart failure. Recently the effects of diet alterations in hypertension control have gained attention with inclusion in the recommendations of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (Chobanian et al., 2003). A randomized, controlled trial recently demonstrated that the DASH (Dietary Approaches to Stop Hypertension) diet alone reduced BP and improved ANS function in patients with elevated BP, and that DASH diet combined with exercise and weight-management showed even greater improvements than DASH diet alone (Blumenthal et al., 2010). Given the literature linking elevated BP to reduced vagal tone and increased SNS activity, the examination of ANS balance as a mechanism of blood pressure reduction through diet and exercise interventions is well supported.
While there are numerous established indices of assessing SNS and PNS activity, many measures involve invasive procedures which limit clinical application. The generalizability of autonomic outflow to specific organs or vascular beds has been questioned, but sympathetic outflow has been found correlate well with more specific markers such as muscle sympathetic nerve activity (Wallin et al., 1996). In the current study we chose to use resting plasma norepinephrine as a marker of SNS activity, which has fairly consistently been shown to be elevated in patients with hypertension (Grassi, 2009, Palatini and Julius, 2009) and has the advantage of being relatively non-invasive. To index PNS activity we used heart rate recovery (HRR) after maximal exercise, an index which has been shown through blockade studies to be predominantly determined by parasympathetic reactivation (Lahiri et al., 2008), and has been shown to be reduced in hypertension and prehypertension (Erdogan et al., 2010, Polonia et al., 2006).
Using indices of ANS function, the purpose of the present study was to examine the effects of a 12 week lifestyle intervention in previously sedentary patients with elevated BP. Our groups included an exercise only intervention, a combined exercise plus DASH diet intervention, and a waitlist control group. Given that both DASH diet and exercise interventions alone have been shown to reduce BP we hypothesised that the combined diet plus exercise intervention would result in great BP reductions than exercise alone, and that changes in autonomic function would be predictive of BP improvements.
Materials and Methods
Participants
Participants were recruited via advertisements, word of mouth referral and referral from medical practices in the San Diego area. Fifty-two participants with elevated BP (>120/80) were included; age range was 25–60 years. Potential participants were excluded if they had a history of a major medical illness (with the exception of hypertension), current psychiatric diagnoses (including alcohol or drug abuse), or if they were receiving psychotropic medications. Four patients who were taking hypertensive medication were slowly tapered off their medications for 3 weeks, prior to participation. In all cases, BP remained within the inclusion range (<170/105 mmHg), and thus the patients were retained in the sample. The project was approved by the University of California, San Diego (UCSD) Human Subjects Committee.
Procedure
Written informed consent was obtained from all subjects before participation in the study. Participants received a medical examination. Participants completed testing prior to and following the 12-week intervention. Subjects were instructed to refrain from caffeine, vigorous exercise, alcohol, and smoking for 24 h prior to the testing day. Exercise testing comprised a maximal exercise test BP was recorded seated at rest 3 consecutive times prior to exercise, the mean of these 3 measures was defined as resting BP pre- and post-intervention. Blood was drawn via an i.v. catheter following a 30 min resting period, with no food 2 hr and no caffeine 12 hr prior, between the hours of 11.30am – 1pm. The UCSD Institutional Review Board approved the protocol, and written consent was obtained from all participants.
Exercise testing
Exercise tests were performed by a certified cardiopulmonary technician under the supervision of an investigator and a physician. Subjects underwent a VO2peak test on a treadmill using the standard Bruce protocol. Given that all study participants were healthy with the exception of elevated BP, the Bruce protocol was selected in order to impose the least additional time burden to this already time-intensive study. Speed and grade of the treadmill increased gradually from 1.7 mph and 10% every 3 min until exhaustion. Following cessation of the test treadmill speed was reduced to 1.7 mph and 0% incline, and subjects continued to walk slowly for 2 min as a cool down period. Subject’s expired gas was analyzed by Sensormedics metabolic cart equipped with Vmax software (version 6–2A), and ECG was recorded using Marquette CardioSoft V.3 (GE medical systems, Milwaukee, WI). Oxyhemoglobin saturation (SpO2) was monitored using pulse oximetry (Ohmeda, Datex, Louisville, CO), and perceived effort during the exercise was recorded using Borg’s 6–20 scale ratings of perceived exertion (RPE) (Borg, 1971). Heart rate, BP and perceived exertion were recorded during the final minute of each stage, at peak exercise and after 1, 2 and 5 min of recovery. Total exercise time was also recorded. Heart rate recovery (HRR) was defined as the difference between maximal heart rate achieved and heart rate at 1 min after cessation of peak exercise.
Lifestyle intervention
At the completion of pre-intervention testing participants were randomized to one of three groups: exercise (Ex), diet plus exercise (DiEx), or control (Con) intervention. Randomization was performed according to pre allocation by random order by computer to equal distribution, however, an imbalance in subject attrition resulted in a larger final group size for Ex. In both Ex and the DiEx intervention arms, the exercise goal was to achieve 5 or more days per week of moderate-intensity cardiovascular physical activity for at least 30–60 min. Subjects met with a personal trainer twice a week at the local YMCA (Young Men’s Christian Association, Mission Valley or La Jolla, San Diego, CA) and were encouraged to exercise an additional 3 times a week. Minimum requirements for exercise were the 2 supervised sessions per week, which participants were instructed to make up is missed, if participants missed two consecutive weeks of training with the personal trainer, they were dropped from the study. Participants were asked to record all of their exercise activities including the type of exercise, the pace, the duration, their HR, their perceived exertion (1–10 scale), and whether or not they were with the trainer. HR was monitored (Polar HR monitor FS1) throughout exercise tasks; subjects were advised to exercise within 60–75% of their previously measured maximum HR using a similar heart rate monitor which was provided for the duration of the study.
The DiEx intervention included all aspects of the exercise intervention with the addition of diet regimen provided by a registered dietician. The goal of the diet intervention was to promote a reduction in energy intake relative to expenditure of 500–1000 kcal/day. This was achieved through a lower energy density diet based on the “Dietary Approaches to Stop Hypertension” (DASH) clinical study (Somers et al., 1991). Dieticians (GCRC Nutrition Services Core, UCSD Medical Center, San Diego, CA) communicated with participants at least once a week via telephone or email to set goals and ensure successful implementation of the dietary plan. Adherence to the dietary intervention was monitored by completion of a daily diary of the types of food participants were eating, the method of cooking, and whether food was home-cooked or from a restaurant. In addition, all study participants completed three 24-hr dietary recalls at baseline, 6-weeks, and after the 12-week intervention using the Nutrition Data System (NDS University of Minnesota, Minneapolis, MN) and software for collection and analysis.
Subjects in the Con group continued their usual lifestyle for 12 weeks, and then completed post-intervention testing.
Plasma catecholamine analysis
Plasma was stored at −80ºCuntil assay. Catecholamines were determined using a COMT-based radioenzymatic assay (Kennedy and Ziegler, 1990). Intra-assay and inter-assay coefficients of variation were <7%and <11%, respectively.
Statistical analyses
A series of one-way ANOVAs were used to assess baseline differences among the groups. Effects of intervention were investigated using a series of 3 Group (Ex, DiEx, Con) × 2 Time (pre-intervention, post-intervention) repeated measures multivariate analyses of variance (MANOVAs) were performed on cardiovascular and fitness variables. Associations between predictor variables (markers of SNS and PNS activity) and BP were assessed using hierarchical linear regressions. A 5% significance level was adopted throughout and eta-squared (η2), a measure of effect size was determined. Occasional missing data are reflected in the reported degrees of freedom. All analyses were performed using PASW Statistics 17.0 (SPSS Inc. Chicago IL).
Results
Subject Characteristics
Fifty-two participants completed the intervention and returned for post-intervention testing. Ex (N = 25, men = 12), DiEx (N = 12, men = 6) and Con (N = 15, men = 7), groups were not different in age, BMI, resting SBP, resting DBP, resting HR, VO2peak, peak HR and Total maximal exercise test time at baseline (p > .05) (see Table 1).
Table 1.
Mean (SD) participant characteristics and effects of intervention. * Indicates significantly different from pre-intervention.
| Exercise (N=25) | Exercise + Diet (N=12) | Waitlist Control (N=15) | Intervention by Group Interaction | ||||
|---|---|---|---|---|---|---|---|
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | ||
| Age (years) | 46.5 (10.2) | -- | 48.0 (7.7) | -- | 44.9 (10.7) | -- | -- |
| BMI (kg/m2) | 30.1 (3.6) | 29.9 (3.4) | 31.2 (3.8) | 30.4 (4.3) | 30.9 (4.5) | 31.0 (4.4) | n.s. |
| Resting SBP (mmHg) | 140.6 (9.8) | 133.9 (10.9)* | 139.9 (10.5) | 127.8 (11.6)* | 137.6 (11.5) | 135.6 (9.9) | p = .053 |
| Resting DBP (mmHg) | 89.8 (11.2) | 85.6 (8.7) | 85.1 (10.0) | 78.0 (10.7) | 88.2 (9.2) | 84.7 (8.5) | n.s. |
| Resting HR (bpm) | 77.2 (14.4) | 73.3 (12.2) | 77.3 (9.1) | 73.6 (10.4)* | 75.4 (7.6) | 78.5 (9.1) | p = .039 |
| VO2peak (ml/kg/min) | 28.0 (7.2) | 30.0 (6.7)* | 24.5 (5.5) | 29.2 (7.7)* | 28.4 (8.8) | 27.0 (8.0) | p < .001 |
| Peak HR (bpm) | 165.4 (16.1) | 165.4 (12.7) | 154.6 (17.4) | 161.5 (14.8) | 162.9 (17.1) | 165.3 (19.3) | n.s. |
| Total Maximal Exercise Time (min) | 9.58 (2.8) | 11.27 (3.2)* | 9.24 (2.0) | 10.17 (2.1) | 9.78 (3.1) | 9.48 (2.8) | p = .024 |
Diet and Exercise Adherence
Subjects in the Ex group completed a mean (SD) total of 3.2 (0.8) days of exercise per week and a mean (SD) of 173 (69) minutes of per week exercise. Similarly, subjects in the DiEx group completed 3.3 (1.1) days of exercise per week and a mean (SD) of 161 (64) minutes per week of exercise. Participants in the DiEx group significantly reduced average 3 day calorie intake from pre to post-intervention (p = .019, mean (SD) pre- calorie intake = 1865 (775) kcal/day, post-intervention calorie intake = 1362 (590) kcal/day) while average 3 day calorie intake was not different pre- to post-intervention in the Ex or Con groups (p < .05).
Effects of intervention on BP and fitness
Repeated Measures ANOVA (2 Time × 3 Group) were conducted to determine the effects of intervention on BMI, resting SBP, resting DBP, resting HR, VO2peak, peak HR and Total maximal exercise test time. As shown in Table 1, significant Group by Time interactions were found for resting SBP (F(2,49) = 3.12, p = .053, η2 = .113), resting HR (F(2,49) = 3.47, p = .039, η2 = .124), VO2peak (F(2,49) = 9.01, p < .001, η2 = .269), and total maximal exercise time (F(2,49) = 4.03, p = .024, η2 = .141). Post-hoc tests found that the Ex group showed decreased resting SBP and a trend towards decreased resting DBP and resting HR, and significant increases in VO2peak and total max exercise time. The DiEx group similarly showed significant decreases in resting SBP and resting HR and a trend toward decreased resting DBP, and increased VO2peak. The Con group as expected did not show significant changes in any of the variables. In comparison of Ex and DiEx groups, the change in SBP, DBP, HR and VO2peak, was not statistically different between groups, although a trend toward significantly greater changes in the DiEx group were seen for SBP (p = .08) and VO2peak (p = .06).
Effects of intervention on indicators of SNS and PNS
HRR and plasma norepinephrine changes after intervention were examined by repeated measures ANOVA. Figure 1 shows the significant Group by Time interaction for HRR (F(2,47) = 3.73, p = .031, η2 = .137), such that DiEx and Ex groups increased HRR from pre to post intervention while Con group did not change, indicating an increase in parasympathetic tone after lifestyle intervention. In contrast, plasma norepinephrine, an indicator of sympathetic activation did not show any significant effects of intervention.
Figure 1.
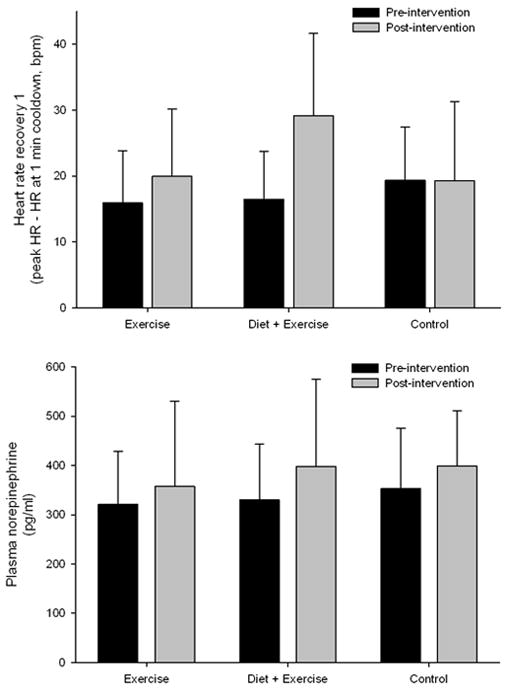
Mean (SD) HRR and plasma norepinephrine pre- and post-intervention for exercise, exercise plus diet and waitlist control groups. * Indicates significant difference pre-post intervention.
Predictors of BP effects of lifestyle intervention
To explore the potential importance of PNS and SNS markers in BP changes elicited by lifestyle intervention, we conducted a series of hierarchical linear regressions. Using the outcome variable of post-intervention SBP or DBP, step 1 included the pre-intervention SBP or DBP respectively, step 2 included gender and age, step 3 included the pre to post intervention changes in VO2peak and BMI, and in step 4 potential predictor variables were entered. Changes in HRR and plasma norepinephrine were calculated as post-intervention minus pre-intervention values and were used as potential predictors. Change in HRR pre to post intervention emerged as a significant predictor in post-intervention DBP (B = −0.31, β = −.41, t = −2.879, p = .006, ΔR2 = .140) and near-significant predictor of post-intervention SBP (B = −0.21, β = −.26, t = −1.909, p = .063, ΔR2 = .055). Norepinephrine change was not associated with SBP or DBP.
Discussion
In the current study, we report the effects of lifestyle interventions in patients with elevated BP, with a focus on the autonomic nervous system. Patients who completed a 12-week exercise or exercise plus DASH diet intervention showed expected improvements in resting BP and fitness (VO2peak) compared to the waitlist control group. Indices of SNS and PNS activity indicated that PNS activity showed increased activity while SNS activity was not altered. Importantly the change in PNS activity was predictive of BP change, even when accounting for changes in fitness and body composition (BMI). Blumenthal et al. (2010) recently reported greater effects of a combined DASH diet plus exercise and weight management program verses DASH diet alone. Although our results do not show significant differences between groups, in both VO2peak and HRR our exercise plus DASH diet group showed a trend (p’s = .06 and 0.8) towards greater increases.
Lifestyle changes including increased physical activity and diet adjustment are considered a key treatments for elevated BP, and intervention studies show that BP is significantly reduced after exercise training (Bacon et al., 2004, Lesniak and Dubbert, 2001, Tsai et al., 2002) and after DASH diet (Blumenthal et al., 2010). The mechanisms of this effect are less well established, and are suggested to include reduction in peripheral vascular resistance mediated by neurohormonal and structural adaptations (Pescatello et al., 2004, Edwards et al., 2007). The effects of exercise training on the function of the autonomic nervous system are acknowledged, with the reduction of sympathetic activation by endurance training shown in normotensive patients (Grassi et al., 1994) and found to be related to BP reduction in hypertensive patients (Brown et al., 2002). In the current study we found no change in resting plasma norepinephrine, but given that this global measure provides no indication of regional SNS outflow, it is possible that further measures might have reflected alterations in the system. However, we did detect changes in PNS function, which importantly were predictive of BP reduction. This finding is in line with previous reports of exercise training modulation of ANS function. To our knowledge, this is the first report that change in HRR, is predictive of successful reduction of BP through lifestyle intervention in patients with elevated BP. Exercise training effects on increasing PNS activity (HRV) have been shown in healthy, athlete and patient populations (Buch et al., 2002), but effects in patients with elevated blood pressure are limited and somewhat mixed (Somers et al., 1991, Loimaala et al., 2000, Collier et al., 2009). Thus, our data adds support for a role in BP control through PNS activity in exercise interventions. The effects of the DASH diet on BP are gaining support, however, a recent study of DASH diet alone effects found that although BP was reduced after 30 days, parasympathetic function (HRV at rest) was not altered (Hodson et al., 2010). This difference is likely due to the exercise component in our intervention, and indicates attention is due to potentially differing mechanisms of BP reduction by dietary changes and exercise. It is perhaps surprising to note that the change in PNS activity was a significant predictor of resting BP reduction above those changes in fitness (VO2peak) and body composition (BMI). This finding highlights the potential importance of assessing the changes in PNS function even when interventions include weight loss and fitness increases.
The mechanism through which exercise training might alter PNS activity and thus influence BP is not well understood, although roles for the renin-angiotensin-aldosterone system, oxidative stress and inflammation have been suggested (Buch et al., 2002, Edwards et al., 2007). Increases in oxidative stress alongside reduced nitric oxide availability are a feature of hypertension (Hamilton et al., 2001), which contribute to reduced PNS function through nitric oxide facilitation of vagal neurotransmission (Herring and Paterson, 2001). Recent animal evidence suggests that exercise training in the spontaneously hypertensive rat reduces oxidative stress through increase of superoxide dismutase (Kimura et al., 2010), and therefore may provide a link to greater PNS function. The parasympathetic nervous system has been shown to interact with immune function with increased PNS function having anti-inflammatory effects. Indeed, lower pro-inflammatory cytokine production is associated with greater heart rate variability (PNS activity) (Marsland et al., 2007). Cardiovascular disease has a large inflammatory aspect, and chronic low-grade inflammation is identified as a hallmark of hypertension. Given that physical activity is associated with reduced inflammation (Edwards et al., 2007, Wegge et al., 2004, Adamopoulos et al., 2002) and increased PNS activity it is possible that the cholinergic anti-inflammatory effect (Tracey, 2002) may explain a potential pathway through which exercise acts to reduce inflammation as well as BP. Future studies with multiple testing as training progresses might well discover the kinetics of changes in ANS activity and inflammatory markers, and thereby uncover the directionality of pathways of change.
It is important to note some potential limitations to our study, in particular the population chosen. The current study included a group of sedentary patients with a wide age-range who had untreated elevated BP. Given that we studied mildly hypertensive patients, it is unclear whether our findings would generalize to more severe hypertension, and thus future studies should be extended into a range of higher BPs. Although our large variation in age provides a population with good generalizability, given that age is associated with ANS function even within hypertensive populations (Kornet et al., 2005) it is a limitation that we cannot separate the role of age. Finally, the measure of SNS activity we used, plasma norepinephrine, although previously shown to be elevated in hypertension, does have limitations, in particular its non-specificity. Thus we are cautious in interpreting our findings that PNS activity was altered but SNS activity unaffected by lifestyle intervention, as different methods of assessment may have revealed changes.
In the current report we show that 12-week lifestyle interventions which reduced BP also reduced HRR after maximal exercise. This non-invasive and easily obtained measure of parasympathetic function was further found to be a significant predictor of the interventions reducing BP, even when accounting for the intervention effects on body composition and fitness. Given the interaction between PNS activity and inflammation (Tracey, 2002), and the importance of inflammation in hypertension and CVD development, understanding of the role of lifestyle factors deserves increasing attention.
Acknowledgments
This work was supported in part by NIH grants HL57265-08, HL073355, and MO1RR-00827.
Footnotes
Conflict of Interest
Authors have no potential conflicts of interest
References
- Adamopoulos S, Parissis J, Karatzas D, Kroupis C, Georgiadis M, Karavolias G, Paraskevaidis J, Koniavitou K, Coats AJ, Kremastinos DT. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:653–663. doi: 10.1016/s0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- Bacon SL, Sherwood A, Hinderliter A, Blumenthal JA. Effects of exercise, diet and weight loss on high blood pressure. Sports Med. 2004;34:307–316. doi: 10.2165/00007256-200434050-00003. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170:126–35. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. The perception of physical performance. In: SHEPHARD RJ, editor. Frontiers of Fitness. Springfield, Il: Charles C. Thomas; 1971. [Google Scholar]
- Brown MD, Dengel DR, Hogikyan RV, Supiano MA. Sympathetic activity and the heterogenous blood pressure response to exercise training in hypertensives. J Appl Physiol. 2002;92:1434–42. doi: 10.1152/japplphysiol.00477.2001. [DOI] [PubMed] [Google Scholar]
- Buch AN, Coote JH, Townend JN. Mortality, cardiac vagal control and physical training--what’s the link? Exp Physiol. 2002;87:423–35. doi: 10.1111/j.1469-445x.2002.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Chobanian AV. Shattuck Lecture. The hypertension paradox--more uncontrolled disease despite improved therapy. N Engl J Med. 2009;361:878–87. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Collier SR, Kanaley JA, Carhart R, Jr, Frechette V, Tobin MM, Bennett N, Luckenbaugh AN, Fernhall B. Cardiac autonomic function and baroreflex changes following 4 weeks of resistance versus aerobic training in individuals with pre-hypertension. Acta Physiol (Oxf) 2009;195:339–48. doi: 10.1111/j.1748-1716.2008.01897.x. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Ziegler MG, Mills PJ. The potential anti-inflammatory benefits of improving physical fitness in hypertension. J Hypertens. 2007;25:1533–42. doi: 10.1097/HJH.0b013e328165ca67. [DOI] [PubMed] [Google Scholar]
- Erdogan D, Gonul E, Icli A, Yucel H, Arslan A, Akcay S, Ozaydin M. Effects of normal blood pressure, prehypertension, and hypertension on autonomic nervous system function. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.04.079. [DOI] [PubMed] [Google Scholar]
- Esler M. The 2009 Carl Ludwig Lecture: Pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol. 2010;108:227–37. doi: 10.1152/japplphysiol.00832.2009. [DOI] [PubMed] [Google Scholar]
- Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail. 2007;9:630–6. doi: 10.1016/j.ejheart.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–7. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Calhoun DA, Mancia G. Physical training and baroreceptor control of sympathetic nerve activity in humans. Hypertension. 1994;23:294–301. doi: 10.1161/01.hyp.23.3.294. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Quarti-Trevano F. The ‘neuroadrenergic hypothesis’ in hypertension: current evidence. Exp Physiol. 2010;95:581–6. doi: 10.1113/expphysiol.2009.047381. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–34. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Nitric oxide-cGMP pathway facilitates acetylcholine release and bradycardia during vagal nerve stimulation in the guinea-pig in vitro. J Physiol. 2001;535:507–18. doi: 10.1111/j.1469-7793.2001.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson L, Harnden KE, Roberts R, Dennis AL, Frayn KN. Does the DASH diet lower blood pressure by altering peripheral vascular function? J Hum Hypertens. 2010;24:312–9. doi: 10.1038/jhh.2009.65. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009;587:5551–8. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius S, Majahalme S. The changing face of sympathetic overactivity in hypertension. Ann Med. 2000;32:365–70. doi: 10.3109/07853890008995939. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–53. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- Kimura H, Kon N, Furukawa S, Mukaida M, Yamakura F, Matsumoto K, Sone H, Murakami-Murofushi K. Effect of endurance exercise training on oxidative stress in spontaneously hypertensive rats (SHR) after emergence of hypertension. Clin Exp Hypertens. 2010;32:407–15. doi: 10.3109/10641961003667930. [DOI] [PubMed] [Google Scholar]
- Kornet L, Hoeks AP, Janssen BJ, Houben AJ, De Leeuw PW, Reneman RS. Neural activity of the cardiac baroreflex decreases with age in normotensive and hypertensive subjects. J Hypertens. 2005;23:815–23. doi: 10.1097/01.hjh.0000163151.50825.e2. [DOI] [PubMed] [Google Scholar]
- Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–33. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Lesniak KT, Dubbert PM. Exercise and hypertension. Curr Opin Cardiol. 2001;16:356–359. doi: 10.1097/00001573-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Loimaala A, Huikuri H, Oja P, Pasanen M, Vuori I. Controlled 5-mo aerobic training improves heart rate but not heart rate variability or baroreflex sensitivity. J Appl Physiol. 2000;89:1825–9. doi: 10.1152/jappl.2000.89.5.1825. [DOI] [PubMed] [Google Scholar]
- Madhi SA, Adrian P, Kuwanda L, Jassat W, Jones S, Little T, Soininen A, Cutland C, Klugman KP. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine. 2007;25:2451–7. doi: 10.1016/j.vaccine.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–8. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom Med. 2007;69:709–16. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol. 2000;529(Pt 1):263–71. doi: 10.1111/j.1469-7793.2000.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–8. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep. 2009;11:199–205. doi: 10.1007/s11906-009-0035-4. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- Polonia J, Amaral C, Bertoquini S, Martins L. Attenuation of heart rate recovery after exercise in hypertensive patients with blunting of the nighttime blood pressure fall. Int J Cardiol. 2006;106:238–43. doi: 10.1016/j.ijcard.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26:303–12. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–7. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- Somers VK, Conway J, Johnston J, Sleight P. Effects of endurance training on baroreflex sensitivity and blood pressure in borderline hypertension. Lancet. 1991;337:1363–8. doi: 10.1016/0140-6736(91)93056-f. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Chang WY, Kao CC, Lu MS, Chen YJ, Chan P. Beneficial effect on blood pressure and lipid profile by programmed exercise training in Taiwanese patients with mild hypertension. Clin Exp Hypertens. 2002;24:315–324. doi: 10.1081/ceh-120004234. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491 ( Pt 3):881–7. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism. 2004;53:377–381. doi: 10.1016/j.metabol.2003.10.016. [DOI] [PubMed] [Google Scholar]


