Highlights
► Three different ESCRT pathways activate ESCRT-III on distinct target membranes. ► Assembly of ESCRT-III filaments on membranes. ► Vps4 mediated disassembly of ESCRT-III and its role in MVB vesicle formation.
Keywords: ESCRT, Vps4, Multivesicular body (MVB), HIV, Cytokinesis
Abstract
The ESCRT (endosomal sorting complex required for transport) pathway promotes the final membrane scission step at the end of cytokinesis, assists viral budding and generates multivesicular bodies (MVBs). These seemingly unrelated processes require a topologically similar membrane deformation and scission event that buds membranes/vesicles out of the cytoplasm. The topology of this budding reaction is ‘opposite’ to reactions that bud endocytic and secretory vesicles into the cytoplasm. Here we summarize recent findings that help to understand how the ESCRT machinery, in particular the ESCRT-III complex, assembles on its target membranes, executes membrane scission and is disassembled by the AAA-ATPase Vps4.
1. Introduction
The identification of the endosomal sorting complexes required for transport (ESCRT) in yeast provided the first insight into the molecular mechanism that is required to generate multivesicular bodies (MVBs) [1–3]. MVBs are specialized organelles of the endosomal system that are required for the lysosomal degradation of membrane proteins [4]. They consist of a limiting membrane and small intralumenal vesicles (ILVs). Like all vesicle budding reactions, the formation of intralumenal MVB vesicles requires three distinct steps. First: cargo recognition and sorting. Second: membrane budding. Third: vesicle scission from a donor membrane (endosome). While endocytic and secretory vesicles bud from their donor membranes into the cytoplasm, the budding and scission of the cargo-laden MVB vesicles has the opposite topology. MVB vesicles bud away from the cytoplasm into the lumen of endosomes (Fig. 1). Thereby, cargo proteins and lipids are sequestered inside MVBs and thus removed from the cytoplasm. Fusion of MVBs with the lysosome releases ILVs into the lumen of the lysosome where they are degraded together with their contents. In yeast, the MVB pathway requires the vps (vacuolar protein sorting) class E genes that assemble into five functionally distinct ESCRT complexes (-0, -I, -II, -III and Vps4). Loss of any of these genes interrupts the MVB pathway. MVB vesicles are no longer formed; membrane proteins and lipids, which are degraded via the MVB pathway, accumulate in the so-called class E compartment inside cells [5]. Not surprisingly, ESCRT dysfunction contributes to diseases ranging from neurodegeneration to cancer [6].
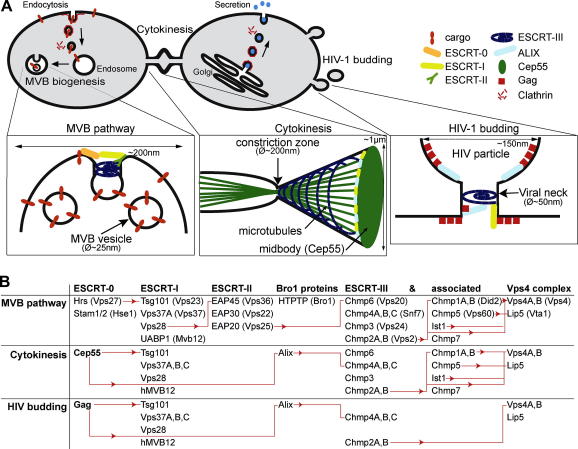
Fig. 1.
The three ESCRT pathways. (A) In the MVB pathway ESCRT-0 initiates the sequential recruitment of ESCRT-I, -II to endosomes. In cytokinesis, Cep55 recruits ESCRT-I and Alix to the midbody. HIV-1 Gag recruits ESCRT-I and Alix to the plasma membrane. All processes require ESCRT-III and Vps4 for membrane remodelling, potentially in different assemblies. (B) ESCRT pathways: The red lines mark direct protein–protein interactions and the arrowheads indicate the direction of the interaction.
Parts of the ESCRT machinery also function during cell abscission at the final stage of cytokinesis and during retroviral budding [7]. These different processes require topologically similar membrane rearrangements that rely on the assembly of the ESCRT-III complex and AAA-ATPase Vps4 complex at the corresponding target membrane (Fig. 1). Somehow, ESCRT-III complexes can pinch off membrane necks and release ‘end-products’ that differ in size: MVB vesicles have a diameter of 25 nm in yeast and 50 nm in mammals. The membrane stalk connecting budding HIV-1 to its host has a diameter of 50–100 nm and mature HIV-1 has an diameter of 120–150 nm [8]. During cytokinesis a 1 μm intracellular bridge is narrowed into a 200 nm constriction zone that is cleaved by ESCRT-III [9,10]. This ‘promiscuity’ sets the ESCRT pathway for membrane scission apart from classical membrane budding events driven by protein coats like clathrin, COP-I, or -II. Their property to form defined ‘protein cages’ that bud membranes, determines a fixed vesicle diameter.
The assembly pathways for ESCRT-III and Vps4 on their respective target membranes appear to be more flexible, allowing the same set of molecules to scission membranes in different biological processes. The following steps are mandatory for ESCRT function:
-
1.
Recruitment and activation of the individual ESCRT-III subunits.
-
2.
Ordered assembly of ESCRT-III filaments.
-
3.
Vps4 mediated disassembly of ESCRT-III filaments into its individual subunits.
2. Recruitment and activation of the individual ESCRT-III subunits
2.1. Molecular architecture of the ESCRT-III subunits
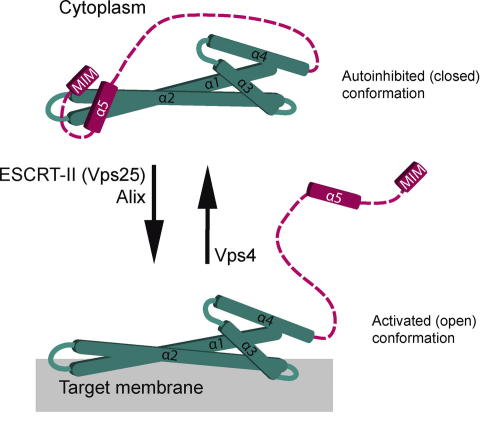
In yeast, the ESCRT-III complex consists of four core subunits, Vps20, Snf7, Vps24 and Vps2 and three accessory components Did2, Vps60 and Ist1 [3,11–13]. They have 12 mammalian homologues: CHMP6 (Vps20), CHMP4A,B,C (Snf7), CHMP3 (Vps24), CHMP2A,B (Vps2) CHMP5 (Vps60), Chmp1A,B (Did2), CHMP7 and Ist1. The homologous ESCRT-III core subunits are relatively small (221–241 amino acids) proteins and probably adopt a common molecular architecture (Fig. 2) [14,15,16]. Yet, their function is not redundant. Their positively charged N-terminal region consists of two helices (α1, α2) that form a 70 Å hairpin structure important for membrane binding and homo- or hetero-dimerization. This hairpin together with α3 and α4 forms an asymmetric anti-parallel four-helix bundle. The C-terminal acidic region (α5 and α6) is negatively charged and constitutes an autoinhibitory region. The intramolecular interaction of helix α5 with the N-terminal hairpin keeps the protein inactive in the cytoplasm [17,18]. The C-terminus also harbors the so-called MIT (microtubule-interacting and transport)-interacting motif (MIM) for interaction with Vps4 [19–21]. Each ESCRT-III subunit has one MIM1 (Vps2, Vps24, Did2), one MIM2 (Vps20, Snf7, Vps60) or both (Ist1). Each ESCRT-III subunit cycles between an inactive cytoplasmic monomer, and its active state as part of a membrane bound ESCRT-III filament (Fig. 2). The activation of a subunit requires a series of intramolecular conformational changes that release autoinhibition, stabilize membrane interactions, enable interaction with other ESCRT-III molecules (homo- or hetero-oligomerization) and expose the MIM domain (Fig. 2). Three different pathways are known to activate ESCRT-III assembly on different target membranes.
Fig. 2.
ESCRT-III subunit cycling. Cartoon of an ESCRT-III subunit based on the crystal structure of human CHMP3. Inactive, autoinhibited ESCRT-III subunits can be activated either by Vps25 or Alix. Vps4 resets the active ESCRT-III subunits to the ‘inactive’ state.
2.2. Recruitment of ESCRT-III to endosomes: the MVB-ESCRT pathway
The MVB pathway is essential for the degradation of ubiquitinated membrane proteins. All five ESCRT complexes are required and follow a sequential recruitment to endosomes that is initiated by the ESCRT-0 complex (Fig. 1) [22,23]. The Y-shaped ESCRT-III complex induces the activation of the first ESCRT-III subunit, Vps20 [1,24,25]. Two copies of Vps25 that form the arms of the ‘Y’, provide certain geometry since each activates one Vps20 subunit. Activated Vps20 then might nucleate the assembly of two ESCRT-III filaments [26,27]. ESCRT-0 and -II are only required for the assembly of ESCRT-III on endosomes.
2.3. Recruitment of ESCRT-III to the Midbody: the cytokinetic ESCRT pathway
A final membrane abscission step at the end of cytokinesis physically separates two daughter cells. Abscission requires the recruitment of the ESCRT-I complex, Alix, all ESCRT-III subunits and Vps4 (Fig. 1). The centrosomal protein CEP55 initiates ESCRT assembly at the midbody [28,29]. It recruits Alix (a Bro1 domain containing factor) and Tsg101 (ESCRT-1) into two cortical rings (diameter of app. 1 μm) to either side of the midbody via its ESCRT- and ALIX-binding region (EBAR) [30]. ESCRT-I and Alix then trigger the assembly of long ESCRT-III filaments that extend form the midbody into the constriction zone [9,10].
Members of the crenarcheal kingdom that lack the homologues of tubulin (FtsZ) and actin (MreB) proteins, use the ESCRT-III - Vps4 system for binary cell division [31,32]. A protein called CdvA localizes first to membranes at the site of cell division and recruits the first ESCRT-III subunit (Saci_1373) to membranes [33]. Subsequently, the remaining three ESCRT-III subunits assemble into cortical filaments at the site of cytokinesis. The mode of Vps4 recruitment to ESCRT-III is conserved and occurs prior to abscission [19]. The conservation of the ESCRT-III-Vps4 system in prokaryotes points to its function as the minimal membrane remodeling machinery.
2.4. Recruitment of ESCRT-III to the plasma membrane: the retroviral ESCRT pathway
HIV and other retroviruses require ESCRT-I, Alix, a specific subset of ESCRT-III subunits (Chmp4 and Chmp2) and Vps4 to bud out of infected cells (Fig. 1) [34]. Two late domains in HIV-Gag hijack ESCRT-I (by mimicking the Hrs–Tsg101 interaction) and Alix to the plasma membrane [35–37]. Alix accumulates together with Gag at budding sites [38]. Only when Gag assembly was complete, ESCRT-I and Alix initiated ESCRT-III assembly [39,40]. Thus, during HIV budding ESCRT-I, Chmp4, Chmp2 and Vps4 function at budding sites prior to virus release.
Taken together three specific adaptors (ESCRT-0, Cep55 and Gag) initiate distinct ESCRT pathways to activate the assembly of ESCRT-III filaments at defined target membranes.
3. Assembly of the ESCRT-III filament
ESCRT-III function requires the polymerization of its subunits into filaments with defined structure and stoichiometry. Individual ESCRT-III subunits can oligomerize into ring-like or helical filaments and tubes in vitro and in vivo, which may indicate structural and functional properties of the membrane scission competent complex. First evidence for the formation of ESCRT-III filaments was provided by over-expression of Snf7 (Chmp4) [42]. Snf7 assembled into flat ring-like filaments at the plasma membrane. The binding of ATPase deficient Vps4 (a substrate traps that blocks ESCRT-III disassembly [43]) to these Snf7 filaments, changed their morphology and generated Snf7 tubes that projected out of the cytoplasm, pushing the plasma membrane outward. In vitro, ESCRT-III subunits polymerize in a concentration dependent manner. Snf7 assembled into rings and sheets. Vps24 assembled into a two intertwined helical filaments [44]. Vps24 and Vps2 formed 1:1 hetero- oligomeric tubes of 50–100 nm in diameter that narrow into a closed dome at one end [45]. Ist1 assembled into large homo-polymeric tubes with a diameter of app. 700 nm. Chmp1 (Did2) polymerized into tubes with a diameter of app. 200 nm [15]. Despite the self-assembly properties of most subunits it is clear that ESCRT-III filaments require a defined order of subunit assembly.
3.1. ESCRT-III assembly during MVB sorting
ESCRT-III assembly on endosomes requires ESCRT-II mediated activation of Vps20 [1]. Two steps result in full activation of Vps20 (Fig. 2). First: the N-terminal myristoyl residue together with the positively charged N-terminus probably serve as the primary targeting signals that direct Vps20 to endosomes. Membrane binding may induce a conformational change that facilitates the interaction with Vps25 (ESCRT-II) [25,46]. Second: binding of helix α1 to Vps25 could displace helices α5/α 6, thereby releasing autoinhibition [26,47]. Activated Vps20 (helix α2) could then nucleate Snf7 filaments in a ratio of approximately 1:10-20 molecules of Vps20 to Snf7 [48]. The Snf7 homo-oligomer is the major ESCRT-III component on endosomes. Snf7 homo-oligomerization is capped by Vps24. Subsequent recruitment of Vps2 completes the ESCRT-III filament [1,3,48]. The same order of ESCRT-III assembly with similar stoichiometry was used to reconstitute MVB vesicle formation in vitro [46,49,50]. When larger ESCRT-III filaments were generated, either by over-expression of Snf7 or by delaying Vps4 mediated disassembly, viewer but larger MVB vesicles were formed [26,13,51,52]. Hence the size of the ESCRT-III filament contributes to MVB vesicle formation. Additionally, ESCRT-III filaments could sequester MVB cargo by forming a ring-like fence [48]. Bro1-Doa4 mediated cargo de-ubiquitination occurs at this stage without the risk of cargo escaping the MVB sorting process. Once inside the ESCRT-III ring, cargo is committed to enter the intralumenal vesicle (Figs. 1 and 3). Consistently, binding of Bro1 to Snf7 delays disassembly of ESCRT-III filaments, thereby coordinating cargo de-ubiquitination and ESCRT-III function [52]. Hence, ESCRT-III filaments may have a dual role during MVB sorting: membrane budding/scission and cargo sequestration.
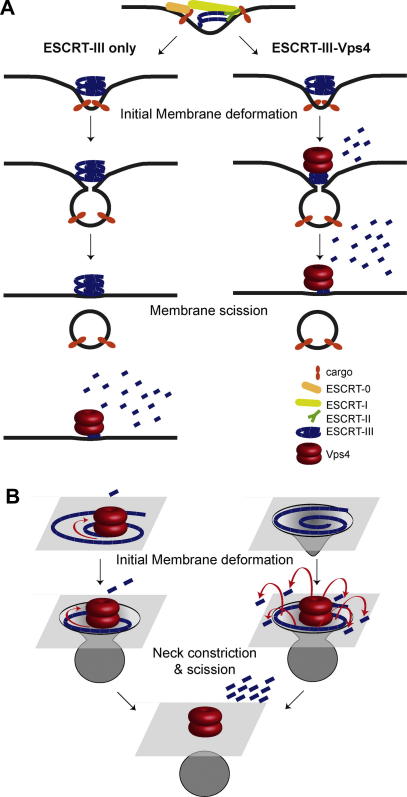
Fig. 3.
Models of ESCRT mediated membrane scission. (A) The ‘ESCRT-III only’ model (left panel): ESCRT-III triggers membrane budding and scission. Vps4 activity is limited to ESCRT-III recycling. The ‘ESCRT-III Vps4’ (right panel) model presumes an active role for Vps4. Vps4 mediated disassembly of ESCRT-III results in membrane scission. (B) Left panel: The ‘Purse String model’: Vps4 removes one ESCRT-III subunit after another. The shrinking filament constricts and scission the membrane. Right panel: ESCRT-III assembly has deformed the membrane. Binding of Vps4 and rapid ESCRT-III disassembly trigger membrane scission.
3.2. ESCRT-III assembly during cytokinesis
All 12 ESCRT-III subunits appear to contribute to cytokinesis, with Chmp6 (Vps20) being least important [53]. Mechanistically, it is not well understood how ESCRT-III assembly is activated at the midbody. ESCRT-II is not required for cytokinesis. Instead, Alix could activate Chmp4 (Snf7) directly. The interaction of the Bro1 domain with the autoinhibitory C-terminus of Chmp4 (Snf7) may facilitate the release of autoinhibition and activate ESCRT-III assembly [54]. Alix dimers could even activate the assembly of two Snf7 filaments, in analogy to the Y-shaped ESCRT-II complex. Alternatively, Alix could ’cross-link’ and thereby stabilize two Snf7 filaments [55]. Ultra-structural analysis revealed that the ESCRT-III subunits organize into long helical filaments of 17 nm in diameter that span a distance over 1 μm [9] (Fig. 1). Once ESCRT-III filaments reach the constriction site, GFP-Vps4 accumulates at the site of membrane scission. Ten minutes later, membrane abscission takes place [10]. ‘Cytokinetic’ ESCRT-III filaments constrict and scission membranes of large dimensions (0.2–1 μm), maybe therefore they are longer and more stable when compared to those for MVB sorting or HIV budding [10,9].
3.3. ESCRT-III assembly during HIV budding
Only Chmp4 and Chmp2, but not Chmp3 (Vps24) are absolutely required for HIV budding [56], suggesting that distinct ESCRT-III filaments execute membrane scission during MVB sorting and cytokinesis. Like in cytokinesis the mechanism underlying Chmp4 activation is unclear, but may also involve Alix mediated activation of Chmp4. GFP-Chmp4 and GFP-Vps4 were recruited for 35–120 s to budding sites. Two to five Vps4 complexes assembled at each budding site prior to viral release [41]. Dominate-negative Vps4 or depletion of Chmp2 (Vps2), which is required for the recruitment of Vps4, stabilizes ring like striations in the necks of the arrested viral buds (Fig. 1) [40,56]. Upon depletion of Chmp4 (Snf7), these rings were no longer detectable, indicating that they correspond to ESCRT-III filaments that need to be disassembled prior to HIV release.
Overall, it seems possible that three activation pathways result in the assembly of specific ESCRT-III filaments, each with different subunit requirements, length and dynamics. It is currently unknown how the assembly, the stoichiometry and the dynamics of these ESCRT-III filaments are controlled.
4. ESCRT-III disassembly
4.1. The Vps4 complex
From archeae to humans, Vps4 releases ESCRT-III subunits from endosomes into the cytoplasm. Vps4 is a mechano-enzyme of the type I AAA (ATPase associated with a variety of cellular activities) ATPases family. In the cytoplasm Vps4 is an inactive protomer (mono/dimer) [43]. Vps4 protomers assemble on ESCRT-III filaments into the active dodecameric complex consisting of two stacked hexameric rings with a central pore [57]. The N-terminal MIT (microtubule interacting and transport) domain is required for ESCRT-III interaction. MITs are asymmetric three-helical bundles that bind directly to MIMs of each ESCRT-III subunits. Sterically, one MIT domain could bind two ESCRT-III subunits simultaneously, one with MIM1, the other with MIM2 [19,21,58]. A flexible linker region connects the MIT domain to the single AAA-domain. Like all AAA-ATPase Vps4 converts the energy from ATP hydrolysis into mechanical power to disassemble the ESCRT-III filament.
4.2. Vps4 recruitment to ESCRT-III filaments
A recruitment complex regulates the assembly of Vps4 on ESCRT-III. Its central component is Vps2, which functions together with Did2 and Ist1. Vps2 is the last core subunit added to the ESCRT-III filament and thereby provides a timer for filament disassembly [3,48]. Vps4 assembly may include 12 different, partially redundant, protein–protein interactions with this recruitment complex [59]. At first, the MIT domain of Vps4 contacts the MIM of Vps2 which is supported by Did2 and Ist1 [11–13,59]. Vps4 complex assembly proceeds by binding to the cofactor Vta1 [60]. Dimers of Vta1 stabilize the Vps4 complex and enhance its ATPase activity, finally resulting in a dodecameric Vps4 complex bound to three [61] or six Vta1 dimers [62]. Vta1 contains two MIT domains of which only MIT2 binds to Vps60. Thus in theory, the Vps4-Vta1 complex could interact simultaneously with 24 ESCRT-III subunits [62]. Alternatively, Vta1 may crosslink different Vps4 complexes to generate Vps4 arrays on ESCRT-III [63]. The interaction of Vps4 with ESCRT-III subunits increases ATP hydrolysis, suggesting that the Vps4 complex could function like a substrate-activated enzyme [64,65].
4.3. Vps4 mediated disassembly of the ESCRT-III complex
In addition to the interaction of MIT with MIMs which guide Vps4 recruitment, the pore of Vps4 dodecamer together with the acidic regions surrounding the MIMs of ESCRT-III contribute to the disassembly reaction [65]. Once assembled, the Vps4-Vta1 complex appears to remain stable for some time, suggesting that Vps4 could progressively disassemble ESCRT-III filaments [66].
Mechanistically, ATP hydrolysis driven motions of Vps4 could translocate ESCRT-III subunits through the central pore [67]. Alternatively, Vps4 might use ATP to act as a molecular crowbar and change ESCRT-III subunit conformation just enough for release from the filament. During each process, Vps4 ATPase activity is required to reset ESCRT-III subunits to the inactive, autoinhibited state to ready them for a new round of ESCRT-III filament formation (Fig. 2). The exact molecular mechanism of Vps4 mediated ESCRT-III disassembly and its role in membrane remodeling and scission is unclear.
5. Contribution of Vps4 to ESCRT-III mediated membrane scission
Several models on how ESCRT-III could execute membrane scission have been postulated recently [68,69]. It is common to all models that ESCRT-III subunits are not consumed in the budding reaction, since they are not localized inside the buds but remain at bud necks. Two major paradigms exist for ESCRT-III mediated membrane scission: The ‘ESCRT-III only’ and the ‘ESCRT-III-Vps4’ models (Fig. 3A).
5.1. The ‘ESCRT-III-only’ model for membrane scission
The model predicts that the assembly of ESCRT-III is sufficient for membrane scission (Fig. 3A, left panel). In vitro reconstitution experiments suggest that Vps20, Snf7 and Vps24 are scission competent ESCRT-III subunits. Vps2 and Vps4 are only required for repeated rounds of membrane scission [49,50]. All ESCRT-III subunits interact with membranes via their positively charged N- termini [14], thus their membrane binding could couple filament assembly to membrane deformation. Circular ESCRT-III filaments could form ever-narrowing spirals in the neck of a growing vesicle. Due to the interactions of the filament with the membrane, the necks could be constricted to a size below a critical threshold (<2 nm) that would favor scission [70]. Alternatively, the ‘dome model’ proposes, that membrane scission involves the assembly of Vps24 and Vps2 into a dome-shaped hetero-polymer, which functions as a scaffold that forces its surrounding membrane into extreme curvature for scission [71,45]. Yet, Vps2 is not required for membrane scission in vitro [50] and Vps24 is dispensable for HIV budding [56], suggesting that either other ESCRT-III subunits could form a similar ’dome’ or a dome may not be required at this stage of the budding process. Vps4 would function after membrane scission. The energy of ATP hydrolysis would be invested to reset the autoinhibition of ESCRT-III subunits. The autoinhibited ESCRT-III proteins could store this energy in the cytoplasm and release it upon membrane binding and incorporation into filaments (Fig. 2). The released energy could be transformed into a mechanical force to remodel and scission membranes. In this model, Vps4 would act similar to the AAA-ATPase NSF that disassembles (and reloads) SNARE complexes after vesicle fusion.
5.2. The ‘ESCRT-III Vps4’ model
In vivo experiments suggest that Vps4 mediated disassembly of ESCRT-III filaments actively participates in membrane remodeling and scission (Fig. 3A, right panel). Vps4 is recruited to viral bud sites and to the midbody prior to membrane scission. Loss of the ESCRT-III accessory proteins, Ist1 and Did2, which regulate Vps4 function, results in an increase in MVB vesicle diameter [13,51]. Modulating Snf7 oligomer stability by the over-expression of the Bro1-domain, delayed Vps4 mediated ESCRT-III disassembly and caused in the accumulation of budding intermediates that did no yet detach from the limiting membrane of the endosome [52]. Blocking of Vps4 activity resulted in the formation of ring like ESCRT-III filaments within the necks of arrested viral buds. The mechanism of Vps4 activity on ESCRT-III remains unclear. Maybe Vps4 acts like a winch, pulling in and successively removing one ESCRT-III subunit at the time, thereby gradually shrinking the diameter of the ESCRT-III filament and constricting the membrane (‘purse string’ model) (Fig. 3B, left panel) [46]. Alternatively, ESCRT-III filaments/domes may have already narrowed the neck to a critical diameter. The recruitment of Vps4 into the dome would allow for multiple simultaneous interactions between Vps4 and ESCRT-III subunits. By binding as many ESCRT-III subunits as possible, the substrate dependent ATPase activity of Vps4 would become maximal. The sudden disassembly of the ESCRT-III dome would relax the strain on the bent membrane and thereby trigger scission (Fig. 3B, right panel).
6. Perspective
It is clear that ESCRT-III and Vps4 constitute a ’minimal’ membrane scission machinery. Yet major questions regarding the underlying molecular mechanism remain unclear: How are membranes scissioned? How is ESCRT-III filament stoichiometry regulated? How does Vps4 disassemble ESCRT-III filaments? Answers to these questions are required to understand this fundamental membrane scission process.
Acknowledgments
We would like to apologize to our colleagues whose work could not be cited due to space restrictions. We would like to thank Marietta Brunner and Martin Muller for critically reading the Manuscript. The Teis lab is funded by the FWF-START (Y444) and the SFB021, Cell Proliferation and cell death in tumors’ and the HFSP CDA00001/2010-C.
References
- 1.Babst M., Katzmann D.J., Snyder W.B., Wendland B., Emr S.D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 2.Katzmann D.J., Babst M., Emr S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 3.Babst M., Katzmann D.J., Estepa-Sabal E.J., Meerloo T., Emr S.D. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 4.Gruenberg J., Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 5.Raymond C.K., Howald-Stevenson I., Vater C.A., Stevens T.H. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuffers S., Brech A., Stenmark H. ESCRT proteins in physiology and disease. Exp. Cell Res. 2009;315:1619–1626. doi: 10.1016/j.yexcr.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Henne W.M., Buchkovich N.J., Emr S.D. The ESCRT Pathway. Dev. Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Carlson L.A. Three-dimensional analysis of budding sites and released virus suggests a revised model for HIV-1 morphogenesis. Cell Host Microbe. 2008;4:592–599. doi: 10.1016/j.chom.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guizetti J., Schermelleh L., Mantler J., Maar S., Poser I., Leonhardt H., Muller- Reichert T., Gerlich D.W. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331:1616–1620. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- 10.Elia N., Sougrat R., Spurlin T.A., Hurley J.H., Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc. Natl. Acad. Sci. USA. 2011;108:4846–4851. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimaano, C., Jones, C.B., Hanono, A., Curtiss, M. and Babst, M. (2007). Ist1 Regulates Vps4 Localization and Assembly. Mol. Biol. Cell. [DOI] [PMC free article] [PubMed]
- 12.Rue S.M., Mattei S., Saksena S., Emr S.D. Novel Ist1-Did2 complex functions at a late step in multivesicular body sorting. Mol. Biol. Cell. 2008;19:475–484. doi: 10.1091/mbc.E07-07-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nickerson D.P., West M., Odorizzi G. Did2 coordinates Vps4- mediated dissociation of ESCRT-III from endosomes. J. Cell Biol. 2006;175:715–720. doi: 10.1083/jcb.200606113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muziol T., Pineda-Molina E., Ravelli R.B., Zamborlini A., Usami Y., Gottlinger H., Weissenhorn W. Structural basis for budding by the ESCRT-III factor CHMP3. Dev. Cell. 2006;10:821–830. doi: 10.1016/j.devcel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Bajorek M. Structural basis for ESCRT-III protein autoinhibition. Nat. Struct. Mol. Biol. 2009 doi: 10.1038/nsmb.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao J., Chen X.W., Davies B.A., Saltiel A.R., Katzmann D.J., Xu Z. Structural basis of Ist1 function and Ist1-Did2 interaction in the multivesicular body pathway and cytokinesis. Mol. Biol. Cell. 2009;20:3514–3524. doi: 10.1091/mbc.E09-05-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamborlini A., Usami Y., Radoshitzky S.R., Popova E., Palu G., Gottlinger H. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc. Natl. Acad. Sci. USA. 2006;103:19140–19145. doi: 10.1073/pnas.0603788103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim S., Kimpler L.A., Hanson P.I. Structure/function analysis of four core ESCRT-III proteins reveals common regulatory role for extreme C-terminal domain. Traffic. 2007;8:1068–1079. doi: 10.1111/j.1600-0854.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 19.Obita T., Saksena S., Ghazi-Tabatabai S., Gill D.J., Perisic O., Emr S.D., Williams R.L. Structural basis for selective recognition of ESCRT-III by the AAA ATPase Vps4. Nature. 2007;449:735–739. doi: 10.1038/nature06171. [DOI] [PubMed] [Google Scholar]
- 20.Scott A., Gaspar J., Stuchell-Brereton M.D., Alam S.L., Skalicky J.J., Sundquist W.I. Structure and ESCRT-III protein interactions of the MIT domain of human VPS4A. Proc. Natl. Acad. Sci. USA. 2005;102:13813–13818. doi: 10.1073/pnas.0502165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuchell-Brereton M.D., Skalicky J.J., Kieffer C., Karren M.A., Ghaffarian S., Sundquist W.I. ESCRT-III recognition by VPS4 ATPases. Nature. 2007;449:740–744. doi: 10.1038/nature06172. [DOI] [PubMed] [Google Scholar]
- 22.Bilodeau P.S., Winistorfer S.C., Kearney W.R., Robertson A.D., Piper R.C. Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. J. Cell Biol. 2003;163:237–243. doi: 10.1083/jcb.200305007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzmann D.J., Stefan C.J., Babst M., Emr S.D. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J. Cell Biol. 2003;162:413–423. doi: 10.1083/jcb.200302136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hierro A., Sun J., Rusnak A.S., Kim J., Prag G., Emr S.D., Hurley J.H. Structure of the ESCRT-II endosomal trafficking complex. Nature. 2004;431:221–225. doi: 10.1038/nature02914. [DOI] [PubMed] [Google Scholar]
- 25.Teo H., Perisic O., Gonzalez B., Williams R.L. ESCRT-II, an endosome- associated complex required for protein sorting: crystal structure and interactions with ESCRT-III and membranes. Dev. Cell. 2004;7:559–569. doi: 10.1016/j.devcel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Teis D., Saksena S., Judson B.L., Emr S.D. ESCRT-II coordinates the assembly of ESCRT-III filaments for cargo sorting and multivesicular body vesicle formation. EMBO J. 2010;29:871–883. doi: 10.1038/emboj.2009.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fyfe I., Schuh A.L., Edwardson J.M., Audhya A. Association of ESCRT-II with VPS20 generates a curvature sensitive protein complex capable of nucleating filaments of ESCRT-III. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.266411. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita E., Sandrin V., Chung H.Y., Morham S.G., Gygi S.P., Rodesch C.K., Sundquist W.I. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. Embo J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlton J.G., Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 30.Lee H.H., Elia N., Ghirlando R., Lippincott-Schwartz J., Hurley J.H. Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55. Science. 2008;322:576–580. doi: 10.1126/science.1162042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindas A.C., Karlsson E.A., Lindgren M.T., Ettema T.J., Bernander R. A unique cell division machinery in the Archaea. Proc. Natl. Acad. Sci. USA. 2008;105:18942–18946. doi: 10.1073/pnas.0809467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samson R.Y., Obita T., Freund S.M., Williams R.L., Bell S.D. A role for the ESCRT system in cell division in archaea. Science. 2008;322:1710–1713. doi: 10.1126/science.1165322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samson R.Y., Obita T., Hodgson B., Shaw M.K., Chong P.L., Williams R.L., Bell S.D. Molecular and structural basis of ESCRT-III recruitment to membranes during archaeal cell division. Mol. Cell. 2011;41:186–196. doi: 10.1016/j.molcel.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Serrano J., Neil S.J. Host factors involved in retroviral budding and release. Nat. Rev. Microbiol. 2011;9:519–531. doi: 10.1038/nrmicro2596. [DOI] [PubMed] [Google Scholar]
- 35.Pornillos O. HIV Gag mimics the Tsg101-recruiting activity of the human Hrs protein. J. Cell Biol. 2003;162:425–434. doi: 10.1083/jcb.200302138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrus J.E. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Serrano J., Zang T., Bieniasz P.D. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 2001;7:1313–1319. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- 38.Jouvenet N., Zhadina M., Bieniasz P.D., Simon S.M. Dynamics of ESCRT protein recruitment during retroviral assembly. Nat. Cell Biol. 2011;13:394–401. doi: 10.1038/ncb2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strack B., Calistri A., Craig S., Popova E., Gottlinger H.G. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 40.von Schwedler U.K. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 41.Baumgartel V. Live-cell visualization of dynamics of HIV budding site interactions with an ESCRT component. Nat. Cell Biol. 2011;13:469–474. doi: 10.1038/ncb2215. [DOI] [PubMed] [Google Scholar]
- 42.Hanson P.I., Roth R., Lin Y., Heuser J.E. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J. Cell Biol. 2008;180:389–402. doi: 10.1083/jcb.200707031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babst M., Wendland B., Estepa E.J., Emr S.D. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. Embo J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghazi-Tabatabai S. Structure and disassembly of filaments formed by the ESCRT-III subunit Vps24. Structure. 2008;16:1345–1356. doi: 10.1016/j.str.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Lata S., Schoehn G., Jain A., Pires R., Piehler J., Gottlinger H.G., Weissenhorn W. Helical structures of ESCRT-III are disassembled by VPS4. Science. 2008;321:1354–1357. doi: 10.1126/science.1161070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saksena S., Wahlman J., Teis D., Johnson A.E., Emr S.D. Functional reconstitution of ESCRT-III assembly and disassembly. Cell. 2009;136:97–109. doi: 10.1016/j.cell.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Im Y.J., Wollert T., Boura E., Hurley J.H. Structure and function of the ESCRT-II-III interface in multivesicular body biogenesis. Dev. Cell. 2009;17:234–243. doi: 10.1016/j.devcel.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teis D., Saksena S., Emr S.D. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev. Cell. 2008;15:578–589. doi: 10.1016/j.devcel.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Wollert T., Hurley J.H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wollert T., Wunder C., Lippincott-Schwartz J., Hurley J.H. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nickerson D.P., West M., Henry R., Odorizzi G. Regulators of Vps4 ATPase activity at endosomes differentially influence the size and rate of formation of intralumenal vesicles. Mol. Biol. Cell. 2010;21:1023–1032. doi: 10.1091/mbc.E09-09-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wemmer M., Azmi I., West M., Davies B., Katzmann D., Odorizzi G. Bro1 binding to Snf7 regulates ESCRT-III membrane scission activity in yeast. J. Cell Biol. 2011;192:295–306. doi: 10.1083/jcb.201007018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morita E., Colf L.A., Karren M.A., Sandrin V., Rodesch C.K., Sundquist W.I. Human ESCRT-III and VPS4 proteins are required for centrosome and spindle maintenance. Proc. Natl. Acad. Sci. USA. 2010;107:12889–12894. doi: 10.1073/pnas.1005938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fisher R.D., Chung H.Y., Zhai Q., Robinson H., Sundquist W.I., Hill C.P. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell. 2007;128:841–852. doi: 10.1016/j.cell.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 55.Pires R. A crescent-shaped ALIX dimer targets ESCRT-III CHMP4 filaments. Structure. 2009;17:843–856. doi: 10.1016/j.str.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morita E., Sandrin V., McCullough J., Katsuyama A., Baci Hamilton I., Sundquist W.I. ESCRT-III protein requirements for HIV-1 budding. Cell Host Microbe. 2011;9:235–242. doi: 10.1016/j.chom.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landsberg M.J., Vajjhala P.R., Rothnagel R., Munn A.L., Hankamer B. Three-dimensional structure of AAA ATPase Vps4: advancing structural insights into the mechanisms of endosomal sorting and enveloped virus budding. Structure. 2009;17:427–437. doi: 10.1016/j.str.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 58.Kieffer C., Skalicky J.J., Morita E., De Domenico I., Ward D.M., Kaplan J., Sundquist W.I. Two distinct modes of ESCRT-III recognition are required for VPS4 functions in lysosomal protein targeting and HIV-1 budding. Dev. Cell. 2008;15:62–73. doi: 10.1016/j.devcel.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 59.Shestakova A., Hanono A., Drosner S., Curtiss M., Davies B.A., Katzmann D.J., Babst M. Assembly of the AAA ATPase Vps4 on ESCRT-III. Mol. Biol. Cell. 2010;21:1059–1071. doi: 10.1091/mbc.E09-07-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azmi I., Davies B., Dimaano C., Payne J., Eckert D., Babst M., Katzmann D.J. Recycling of ESCRTs by the AAA-ATPase Vps4 is regulated by a conserved VSL region in Vta1. Cell Biol. 2006;172:705–717. doi: 10.1083/jcb.200508166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu Z., Gonciarz M.D., Sundquist W.I., Hill C.P., Jensen G.J. Cryo-EM structure of dodecameric Vps4p and its 2:1 complex with Vta1p. J. Mol. Biol. 2008;377:364–377. doi: 10.1016/j.jmb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao J., Xia H., Zhou J., Azmi I.F., Davies B.A., Katzmann D.J., Xu Z. Structural basis of Vta1 function in the multivesicular body sorting pathway. Dev. Cell. 2008;14:37–49. doi: 10.1016/j.devcel.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang D., Hurley J.H. Structural role of the Vps4-Vta1 interface in ESCRT-III recycling. Structure. 2010;18:976–984. doi: 10.1016/j.str.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azmi I.F., Davies B.A., Xiao J., Babst M., Xu Z., Katzmann D.J. ESCRT-III family members stimulate Vps4 ATPase activity directly or via Vta1. Dev. Cell. 2008;14:50–61. doi: 10.1016/j.devcel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 65.Merrill S.A., Hanson P.I. Activation of human VPS4A by ESCRT-III proteins reveals ability of substrates to relieve enzyme autoinhibition. J. Biol. Chem. 2010;285:35428–35438. doi: 10.1074/jbc.M110.126318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies B.A., Azmi I.F., Payne J., Shestakova A., Horazdovsky B.F., Babst M., Katzmann D.J. Coordination of substrate binding and ATP hydrolysis in Vps4- mediated ESCRT-III disassembly. Mol. Biol. Cell. 2010;21:3396–4408. doi: 10.1091/mbc.E10-06-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott A. Structural and mechanistic studies of VPS4 proteins. Embo J. 2005;24:3658–3669. doi: 10.1038/sj.emboj.7600818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peel S., Macheboeuf P., Martinelli N., Weissenhorn W. Divergent pathways lead to ESCRT-III-catalyzed membrane fission. Trends Biochem. Sci. 2011;36:199–210. doi: 10.1016/j.tibs.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Hurley J.H., Hanson P.I. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat. Rev. Mol. Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bashkirov P.V., Akimov S.A., Evseev A.I., Schmid S.L., Zimmerberg J., Frolov V.A. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell. 2008;135:1276–1286. doi: 10.1016/j.cell.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fabrikant G., Lata S., Riches J.D., Briggs J.A., Weissenhorn W., Kozlov M.M. Computational model of membrane fission catalyzed by ESCRT-III. PLoS Comput. Biol. 2009;5:e1000575. doi: 10.1371/journal.pcbi.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]