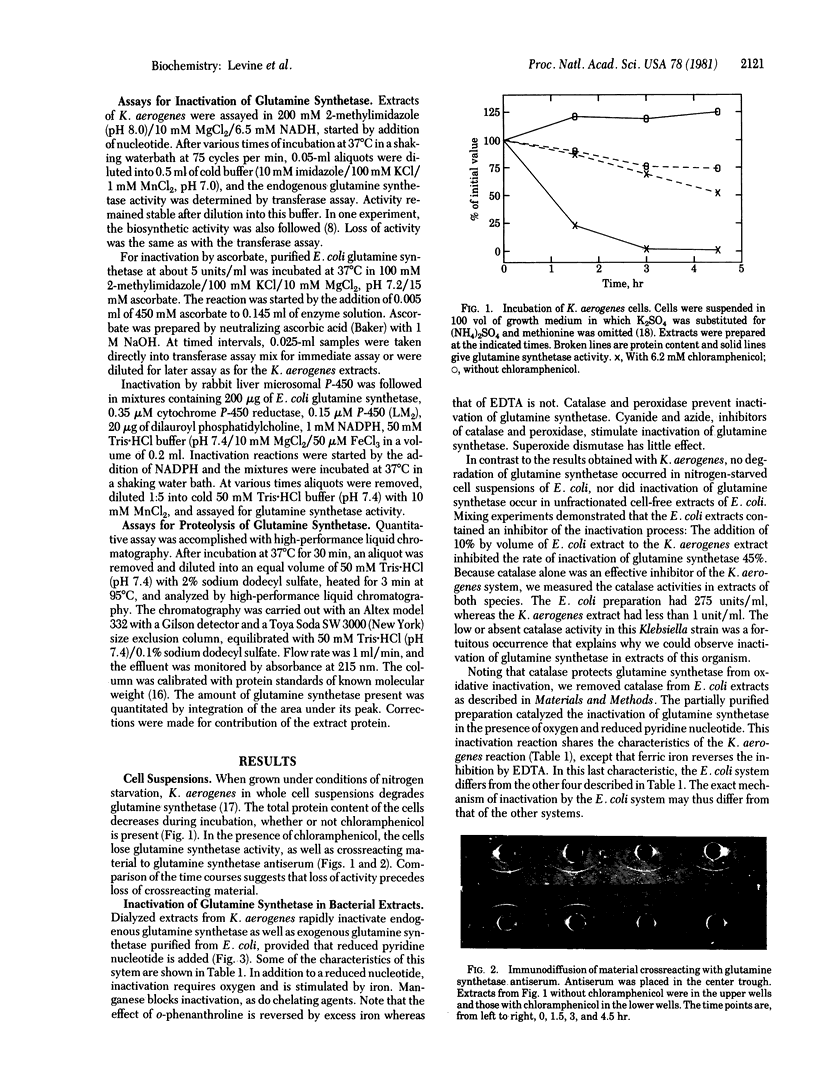
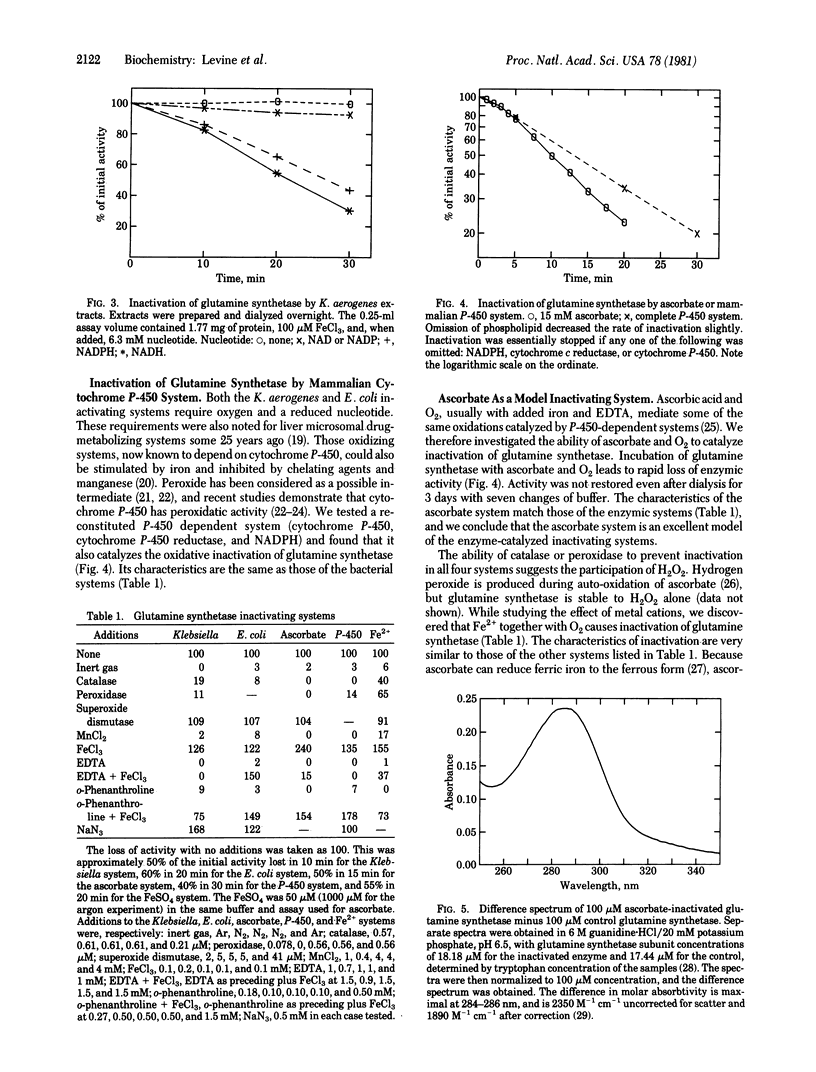
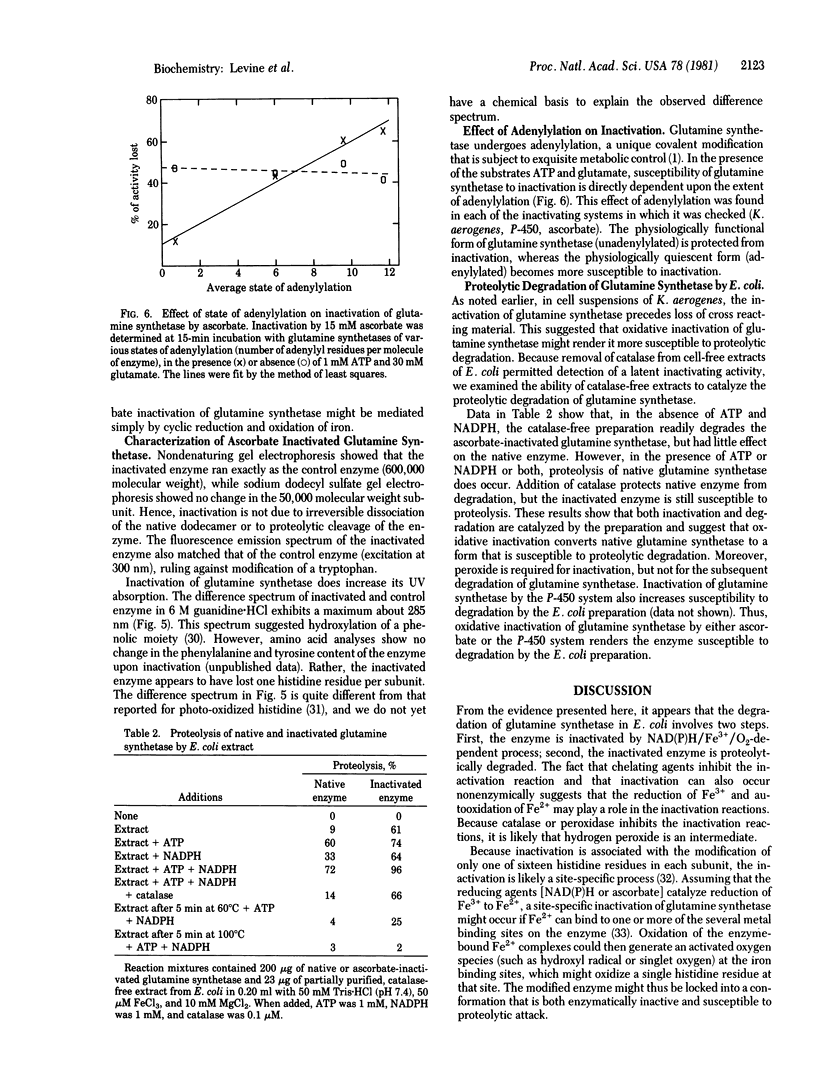
Abstract
We partially purified a preparation from Escherichia coli that proteolytically degrades the enzyme glutamine synthetase [L-glutamate:ammonia ligase (ADP-forming), EC 6.3.1.2]. The degradation is at least a two-step process. First, the glutamine synthetase undergoes an oxidative modification. This modification leads to loss of catalytic activity and also renders the protein susceptible to proteolytic attack in the second step. The oxidative step displays characteristics of a mixed-function oxidation, requiring both molecular oxygen and a reduced nucleotide. This step can also be catalyzed by a purified, mammalian cytochrome P-450 system, as well as by a model system consisting of ascorbic acid and oxygen. Catalase blocks this oxidative modification step. Thus, the overall process of proteolytic degradation can be observed only if care is taken to remove catalase activity from the extracts. The inactivation reaction is dependent on the state of adenylylation of the glutamine synthetase, suggesting that this a physiologically important reaction. If so, then mixed-function oxidases are now implicated in the process of intracellular protein turnover.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V., Kon H. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci U S A. 1979 Feb;76(2):954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R. C., 2nd, Coon M. J. On the mechanism of action of cytochrome P-450. Spectral intermediates in the reaction of P-450LM2 with peroxy compounds. J Biol Chem. 1980 May 10;255(9):4100–4111. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- GILLETTE J. R., BRODIE B. B., LA DU B. N. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957 Apr;119(4):532–540. [PubMed] [Google Scholar]
- Haugen D. A., van der Hoeven T. A., Coon M. J. Purified liver microsomal cytochrome P-450. Separation and characterization of multiple forms. J Biol Chem. 1975 May 10;250(9):3567–3570. [PubMed] [Google Scholar]
- Imamura T., Konishi K., Yokoyama M., Konishi K. High-speed gel filtration of polypeptides in sodium dodecyl sulfate. J Biochem. 1979 Sep;86(3):639–642. doi: 10.1093/oxfordjournals.jbchem.a132567. [DOI] [PubMed] [Google Scholar]
- MITOMA C., POSNER H. S., REITZ H. C., UDENFRIEND S. Enzymatic hydroxylation of aromatic compounds. Arch Biochem Biophys. 1956 Apr;61(2):431–441. doi: 10.1016/0003-9861(56)90366-6. [DOI] [PubMed] [Google Scholar]
- McLEAN D. J., GIESE A. C. Absorption spectra of proteins and amino acids after ultraviolet irradiation. J Biol Chem. 1950 Dec;187(2):537–542. [PubMed] [Google Scholar]
- Miller R. E., Shelton E., Stadtman E. R. Zinc-induced paracrystalline aggregation of glutamine synthetase. Arch Biochem Biophys. 1974 Jul;163(1):155–171. doi: 10.1016/0003-9861(74)90465-2. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- Niles E. G., Westhead E. W. The variable subunit structure of lysine-sensitive aspartylkinase from Escherichia coli TIR-8. Biochemistry. 1973 Apr 24;12(9):1715–1722. doi: 10.1021/bi00733a009. [DOI] [PubMed] [Google Scholar]
- Nordblom G. D., White R. E., Coon M. J. Studies on hydroperoxide-dependent substrate hydroxylation by purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1976 Aug;175(2):524–533. doi: 10.1016/0003-9861(76)90541-5. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- REDDI K. K. Ultraviolet absorption studies on tobacco mosaic virus. Biochim Biophys Acta. 1957 May;24(2):238–241. doi: 10.1016/0006-3002(57)90188-9. [DOI] [PubMed] [Google Scholar]
- Renneberg R., Scheller F., Ruckpaul K., Pirrwitz J., Mohr P. NADPH and H2O2-dependent reactions of cytochrome P-450LM compared with peroxidase catalysis. FEBS Lett. 1978 Dec 15;96(2):349–353. doi: 10.1016/0014-5793(78)80434-7. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Smyrniotis P. Z., Davis J. N., Wittenberger M. E. Enzymic procedures for determining the average state of adenylylation of Escherichia coli glutamine synthetase. Anal Biochem. 1979 May;95(1):275–285. doi: 10.1016/0003-2697(79)90217-3. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Ciardi J. E., Stadtman E. R. Comparative biochemical and immunological studies of bacterial glutamine synthetases. J Bacteriol. 1973 Sep;115(3):858–868. doi: 10.1128/jb.115.3.858-868.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UDENFRIEND S., CLARK C. T., AXELROD J., BRODIE B. B. Ascorbic acid in aromatic hydroxylation. I. A model system for aromatic hydroxylation. J Biol Chem. 1954 Jun;208(2):731–739. [PubMed] [Google Scholar]
- Weil L., Seibles T. S., Herskovits T. T. Photooxidation of bovine insulin sensitized by methylene blue. Arch Biochem Biophys. 1965 Aug;111(2):308–320. doi: 10.1016/0003-9861(65)90191-8. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. The role of superoxide in the destruction of erythrocyte targets by human neutrophils. J Biol Chem. 1980 Oct 25;255(20):9912–9917. [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. The role of non-haem iron. Biochem J. 1969 Jun;113(2):325–332. doi: 10.1042/bj1130325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J Biol Chem. 1965 Nov;240(11):4509–4514. [PubMed] [Google Scholar]




