Abstract
In the new Brassica napus microspore culture system, wherein embryos with suspensors are formed, ab initio mimics zygotic embryogenesis. The system provides a powerful in vitro tool for studying the diverse developmental processes that take place during early stages of plant embryogenesis. Here, we studied in this new culture system both the temporal and spatial distribution of nuclear DNA synthesis places and the organization of the microtubular (MT) cytoskeleton, which were visualized with a refined whole mount immunolocalization technology and 3D confocal laser scanning microscopy. A ‘mild’ heat stress induced microspores to elongate, to rearrange their MT cytoskeleton and to re-enter the cell cycle and perform a predictable sequence of divisions. These events led to the formation of a filamentous suspensor-like structure, of which the distal tip cell gave rise to the embryo proper. Cells of the developing pro-embryo characterized endoplasmic (EMTs) and cortical microtubules (CMTs) in various configurations in the successive stages of the cell cycle. However, the most prominent changes in MT configurations and nuclear DNA replication concerned the first sporophytic division occurring within microspores and the apical cell of the pro-embryo. Microspore embryogenesis was preceded by pre-prophase band formation and DNA synthesis. The apical cell of the pro-embryo exhibited a random organization of CMTs and, in relation to this, isotropic expansion occurred, mimicking the development of the apical cell of the zygotic situation. Moreover, the apical cell entered the S phase shortly before it divided transversally at the stage that the suspensor was 3–8 celled.
Keywords: Androgenesis, Brassica napus, Nuclear DNA synthesis, Microtubular cytoskeleton
Introduction
Embryogenesis in angiosperm plants is a highly regulated process. The zygote divides and a pro-embryo is formed consisting of a suspensor and an apical domain that will give rise to the embryo proper. The embryo proper in turn develops from a globular structure into an organism consisting of an embryonic axis with root and shoot meristems and cotyledons (West and Harada 1993). Embryogenesis can be induced in cells other than the zygote as well. When microspores and pollen of certain plant species are subject to stress, e.g. high and/or low temperature treatment, carbohydrate or nitrogen starvation or colchicine treatment, the normal gametophytic pathway is blocked and sporophytic development occurs, which leads to embryo formation (Maluszynski et al. 2003; Wedzony et al. 2009). Brassica napus ‘Topas’ appeared to be an excellent model system to induce embryos in cultures of isolated anthers (Thomas and Wenzel 1975) and microspore suspensions (Binarova et al. 1993, 1997; Custers 2003; Custers et al. 1994, 2001; Gu et al. 2004; Hause et al. 1992, 1993, 1994; Joosen et al. 2007; Lichter 1982; Pechan and Keller 1988; Smýkal 2000; Supena et al. 2008; Telmer et al. 1995; Zaki and Dickinson 1990, 1991, 1995) subjected to increased shock temperature. A lot of effort was made to analyse the initiation and maintenance of the embryogenic pathway with respect to the deviating nuclear DNA synthesis (Binarova et al. 1993, 1997), expression of heat stress proteins (Cordewener et al. 1995), protein phosphorylation (Cordewener et al. 2000), cytoskeletal aberrations (Hause et al. 1993; Simmonds 1994; Simmonds and Keller 1999) and differential gene expression (Boutilier et al. 2002). Whereas zygotic embryogenesis in B. napus is developmentally characterized by the formation of a pro-embryo consisting of a single file of cells (Tykarska 1976, 1979, 1980, 1987), microspore-derived embryos often deviate in their development and first form a multicellular structure that attains the eventual shape and structure of a zygotic globular embryo. Moreover, in such a system of microspore embryogenesis, a number of divisions that lead to the formation of the pro-embryo stage with a suspensor does not occur first (Binarova et al. 1997; Custers et al. 1994; Hause et al. 1994). Incidentally, it was observed that embryos derived from microspore suspensions were similar to zygotic embryos and had a pro-embryo stage in which a suspensor was formed on top where the embryo proper developed (Hause et al. 1994). Recently, culture procedures were modified and adapted to a laboratory scale, which resulted in the development of a much more refined microspore embryogenesis system that mimics the zygotic pathway (Joosen et al. 2007; Supena et al. 2008). As these embryos have a pattern development highly similar to zygotic embryos, they are of high value when studying polarized, embryogenic development and stage dependent gene expression in vitro under defined conditions. A relatively short and controlled heat treatment of isolated microspores and pollen is essential for obtaining such embryos in B. napus.
The morphogenesis of the embryo and the pattern formation within the embryo is the result of a consorted interplay of the cellular events of division, growth and differentiation. Microtubules (MTs) play a central role in these processes mainly by directing cell expansion and by the establishment of the cell division plane (Sedbrook and Kaloriti 2008; Webb and Gunning 1991; Ye et al. 1997). MT assembly is regulated by the cell cycle machinery (Zheng 2004). However, changes in the organization of MTs mainly concern interphase cortical microtubules (CMTs) and take place at the G2/M transition. It is known that the rearrangements of MTs in the future site and plane of division begin just after DNA replication and are completed just before mitosis (Gunning and Sammut 1990).
Studies of nuclei and MT involvement and behaviour in the zygotic-like pattern of embryo development promises to be helpful in understanding the role of the cytoskeleton during embryo development in angiosperms.
To focus on these aspects, this paper describes the spatial distribution of cell division from the microspore stage onwards by visualizing nuclear DNA synthesis through the application of the thymidine analogue Bromodeoxyuridine (BrdU). The information about the organization of the MT cytoskeleton completes and links the data concerning nuclear DNA synthesis. To analyse this relation during polarized, microspore-derived embryo development, ‘whole mount’ preparations and immunocytochemistry were applied. Perfect 3D visualization elucidates the embryo pattern formation and regulation of cell growth in successive developmental stages under in vitro conditions.
Materials and methods
Donor plants
Plants of B. napus L., cultivar ‘Topas’ line DH 4079 were grown under greenhouse conditions at 20/18°C day/night temperature with additional light (Philips SON-T lamps) at a 16 h/8 h day/night regime until the beginning of bolting. The plants were then transferred to a growth chamber and kept at 10°C with 16 h illumination per day provided by 150 μEm−2 s−1 HPI (Philips) lamps. Plants were watered twice a week with N:P:K = 15:15:18 soluble fertilizer. Inflorescences were harvested after at least 2 weeks of cold treatment, and flower buds of 3.1–3.3 mm in length were used for isolation of microspores.
Microspore isolation and culture
Microspores were isolated following the protocol of Custers (2003) with modifications by Joosen et al. (2007).
Three types of cultures were conducted in NLN-13 modified Lichter (1982) medium with 13% sucrose (w/v) without potato extract and growth regulators in 6 cm Petri dishes: (1) culture at 18°C, where the microspores continued normal development resulting in pollen maturation, (2) culture at 32 ± 0.2°C for 5 days and thereafter 25°C, which is actually a conventional microspore culture, and (3) culture at 32 ± 0.2°C for 24 h and thereafter 25°C, which is a mild heat stress induced culture resulting in microspore-derived embryos with suspensors. Progress of development in cultures was monitored with light microscopy. Regularly samples were taken for DAPI staining (4′,6-diamidino-2-phenylindole 1 μg/ml + 1% Triton-X100) and observation under an epifluorescence microscope.
BrdU labelling
To analyse DNA synthesis and cell proliferation under embryogenic conditions, a pulse labelling with the thymidine substitute, 5-bromo 2′-deoxy-uridine (BrdU) was applied after 7 days of culture. The BrdU labelling solution (RPN201, Amersham Biosciences) was added to the cultures at a final dilution of 1:500. Cells cultured between 8 and 9 days were labelled for 24 h with BrdU. They were then cultured in medium without BrdU for another 24 h and later fixed. Morphological development of induced microspores was monitored in cultures without BrdU.
All experiments were repeated at least four times.
Immunolocalization of microtubules and places of DNA synthesis
The protocol for whole mount immunolocalization was adapted from Friml et al. (2003) and Szechyńska-Hebda et al. (2006) with substantial modification that improved preservation of cell structures and penetration of antibodies according to Dubas et al. (2011).
Samples were collected with special hand-made sieves prepared from ‘blue’ pipette tips (5 mm in diameter) with nylon mesh (11 μm, Millipore). Sieves were immediately placed in Eppendorf tubes in a freshly prepared prefixation mixture containing 1% paraformaldehyde (PFA, Sigma 76240) and 0.025% glutaraldehyde (GA, Sigma 49626) in a microtubule stabilizing buffer [MTSB: 50 mM 1.4-piperazinediethane sulfonic acid (PIPES, Sigma P-1851), 5 mM EGTA (Sigma O-3778), 5 mM MgSO4, pH 7.0, adjusted with 5 M KOH]. The fixative was removed from Eppendorf tubes after 10 min, and the samples were re-dipped in MTSB with 3% PFA and 0.025% GA for 30 min at RT. Next, the samples were washed with MTSB with 0.025% Triton X-100 for 10 min then treated with 0.05 M NH4Cl and 0.05 M NaBH4 for 5 min and washed again. To make microspore and pollen walls permeable to antibodies, they were partly digested in a mixture of 1% cellulase (“Ozonuka R-10” from Trichoderma viride, SERVA 16419), 0.8% pectinase (from Rhizopus, SIGMA P-2401), 0.02% pectolyase (from Aspergillus japonicus, SIGMA P-3026) and 0.3% macerozyme (R-10 from Rhizopus lyophil, SERVA 28302) in MTSB for 1 h at 37°C. The cells were then washed 5× 10 min each with MTSB/0.025% Triton X-100. From that point onward, the protocols described by Friml et al. (2003) and Szechyńska-Hebda et al. (2006) were applied with minor variations. To enhance cell wall and cell membrane permeability, the material was incubated in MTSB with 10% DMSO and 3% Nonidet P-40 for 50 min at RT. After rinsing, a blocking step was performed with 2% BSA in MTSB at 30°C. Primary monoclonal antibodies [anti-α-tubulin clone DM1A raised in mouse, dilution 1:1,000, Sigma T-9026 or anti- BrdU (Bromodeoxyuridine) monoclonal antibody, dilution1:1,000, Amersham Bioscience RPN202] were applied overnight in MTSB with 3% BSA at 4°C. Cells were washed 6× 10 min with MTSB/0.025% Triton (washing buffer), after which the secondary antibody GaM/IgG/Alexa 488 (Molecular Probes A-11001, dilution 1:100) was applied in blocking buffer for 3 h at 37°C in darkness. Thereafter, material was washed 6× 10 min with MTSB/0.025% Triton/0.02% NaN3 and 6× 10 min with MQ water. Samples were stored in PBS containing 0.02% NaN3. Control experiments were performed by omitting the first antibody. For DNA counterstaining, the samples were incubated in 0.1% propidium iodide (PI, Sigma-Aldrich, P-4170) for 15 min, washed in PBS and embedded on slides in Citifluor-glycerol (Citifluor Ltd. in glycerol, AF2, Enfield Cloisters).
Microscopic observation
Confocal laser-scanning microscopy (CLSM) and fluorescence microscopy were performed with a CELL MAP IC Bio-Rad (Microscience Division, Hemel Hempstead, UK) CLSM mounted on a Nikon Eclipse TE 2000-S inverted microscope. Fluorescence was examined under filters EX 470-490/DM 510 BA/515 EF (Alexa 488) and EX 510-560/DM 580 BA/590 EF (PI). CLSM images were collected by a Kalman averaging of four full scans. 3D images and z-projections of the cells were obtained by collecting a series of approximately 30–60 optical sections in the Z-axis, each section 0.5–1 μm thick. Images were acquired and processed using software programs, including Lasersharp 2000 software (Microscience Division, Hemel Hempstead, UK), Confocal Assistant 4.02 (written by Todd Clark Brelje), CorelDRAW® ESSENTIALS 9.0 and Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA, USA).
Cell tracking experiments were performed at 3 min intervals over 96 h with non-immobilized cultures that were left on an inverted microscope equipped with Hoffman Modulation Contrast and a Sony CCD camera.
Results
Morphogenesis after heat shock treatment
In the process of microspore- and pollen-derived embryogenesis, two developmental pathways were distinguished:
When microspore suspension was cultured under strong heat stress for 5 days at 32 ± 0.2°C and then transferred to 25°C, multicellular structures were formed within the microspore wall. These structures emerged from the exine by day 5 and at about day 7 they showed features of globular embryos, which developed into heart stage embryos without suspensors in 9–12-day old cultures. This is the typical embryogenic pathway known in conventional microspore cultures (data not presented).
When microspore suspension was subjected to a short heat treatment during the first day at 32°C and then cultured at 25°C, it did not form multicellular structures, but gave rise to suspensor-like structures (Figs. 1, 2, 3, 4 5). From the sixth day of culture, some microscopically noticeable changes in microspores occurred. At the apical pole, close to the intensively stretched exine, intensive nuclear movement was noticed. Microspore volume enlargement and elongation preceded the first, slightly asymmetric mitotic division inside the original wall of the microspore on the eighth day of culture (Figs. 1a–i, 2c). When two-celled structures divided, the new cell plate was in such a position that a file of three cells was formed (Fig. 2d). During the following 3 days, repeated cell divisions lead to the formation of linear files of three up to eight cells (Fig. 2e–g). The tip cell of the suspensor-like structure was delineated to become the embryo proper (Fig. 2e, f).
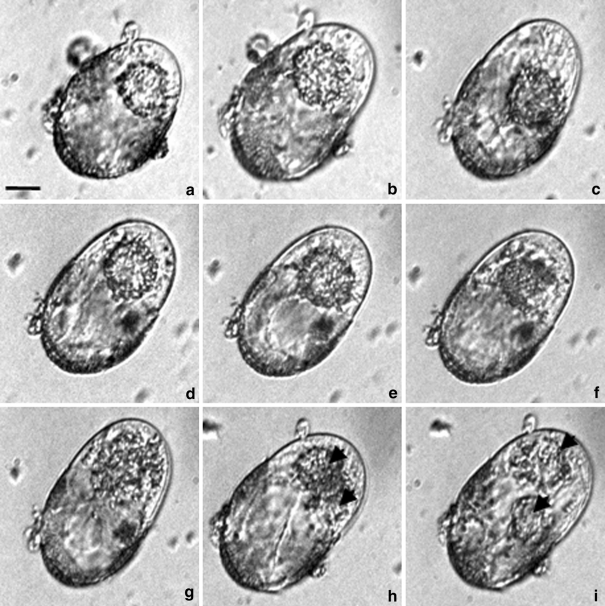
Fig. 1.
Time-lapse photographs showing the earliest stages of embryogenesis in the microspore suspension culture of Brassica napus. a–f The microspore volume enlarged and resulted in the cell emerging from the microspore parental wall at one pole. The nucleus moved from and towards the apical pole and came close to the intensively stretched sporoderm wall. g–i Chromatin condensation and slightly asymmetric mitotic division. h–i Structure with two nuclei (arrowheads). Images a–f were taken from day 7 to day 8 of culture. Cultures were continuously kept in a NLN-13 medium. Bar 20 μm
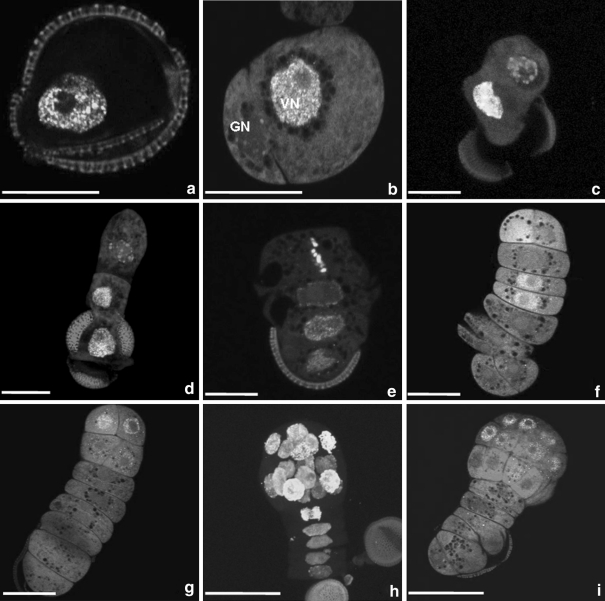
Fig. 2.
Visualization of DNA synthesis by BrdU incorporation and immunocytochemical labelling with FITC in microspores and pollen of Brassica napus induced to sporophytic development by the mild heat stress treatment. a Microspore with labelled nucleus. b Bi-cellular pollen grain shows labelling only in the nucleus (VN) of the vegetative cell and not in the generative cell nucleus (GN). Filamentous structures emerging from microspores. c Two-celled pro-embryo exhibits labelling in the nucleus of the basal cell, while the nucleus of the apical cell is not labelled. d Linear file of three cells. Two nuclei below the apical cell incorporated BrdU. Labelling in both cells might indicate a synchronous S-phase. e The tip cells of the suspensor-like structure is delineated to become the embryo proper. Labelled, mitotic chromosomes in the tip cell lie in the equatorial plane along the longitudinal division plane. Embryo proper stages. f Two-celled embryo proper with a long, suspensor-like filament. Labelling shows two labelled nuclei in the suspensor-like structure and only one labelled nucleus in the embryo proper. g Embryo proper in the octant stage with four labelled nuclei. h Globular embryo stage with labelled nuclei in the embryo proper. Note the cell with labelled telophase chromosomes in the hypophysis region. i Globular embryo with labelled nuclei in the protoderm and inner cells of the embryo proper. Green fluorescence (FITC) demonstrates the temporal and spatial distribution of BrdU incorporation and thus DNA synthesis during the S-phase. Red fluorescence is caused by PI staining. Bar 20 μm
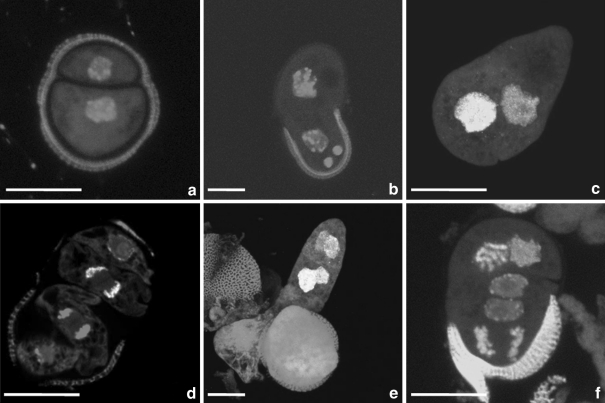
Fig. 3.
Variation in patterns of DNA synthesis as revealed by BrdU pulse labelling in microspores and pollen of Brassica napus induced to sporophytic development by mild heat stress treatment. a Embryogenic microspore without labelling. b Bi-cellular structure derived from a pollen grain without labelling. c Two-celled pro-embryo with labelled nuclei. d Four-celled pro-embryo with deviations in orientation of division plane in suspensor cells. Labelling of chromosomes in telophase in one of two synchronously divided suspensor-like structure cells. e Embryo in which both the apical and the sub-apical cell exhibited a longitudinal division. f Embryo in which the apical and the basal cell divided longitudinally. This lead to twin embryo formation. Green fluorescence (FITC) demonstrates the temporal and spatial distribution of BrdU incorporation and thus DNA synthesis during the S-phase. Red fluorescence of nuclei is caused by PI staining of DNA. Bar 20 μm
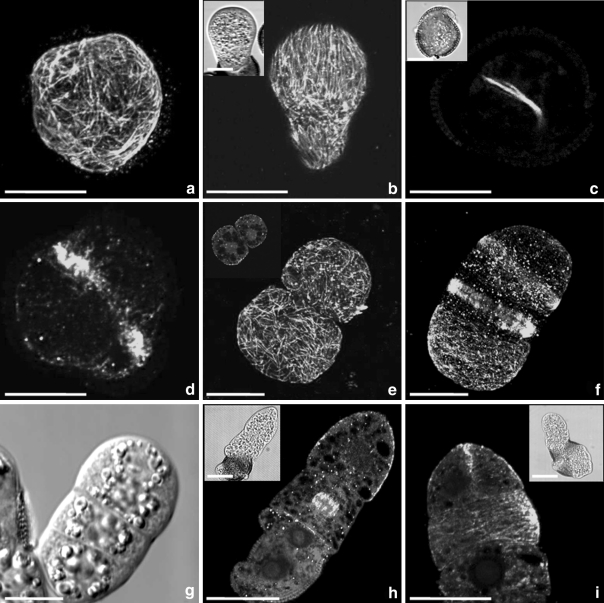
Fig. 4.
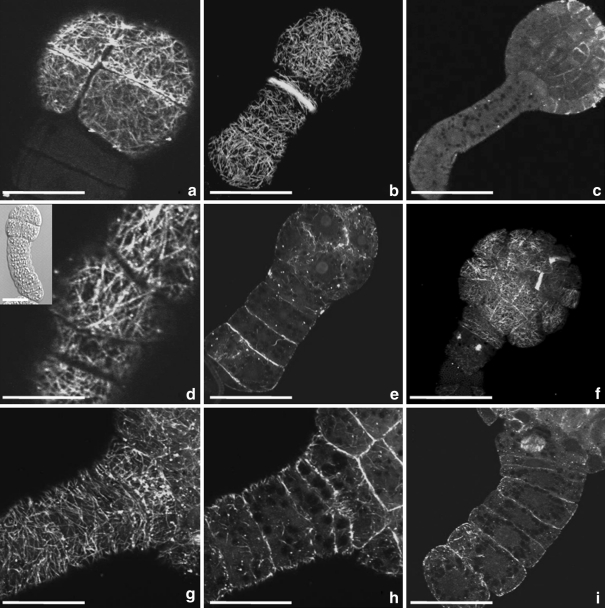
Microtubular configurations in cultured microspores and pollen of Brassica napus after mild heat stress treatment. a Random CMTs in microspore within the exine. b The structure, released from the exine, exhibited CMTs in parallel arrays, either parallel or oblique to the long axis. c. Pre-prophase band (PPB) in microspore marking the near symmetric position of the future cell wall. The small picture in the upper left corner depicts the same structure observed with DIC. d Phragmoplast at the first mitotic division, separating the microspore in two approx. equally sized cells. e Reticulate arrays of short and densely packed cortical and endoplasmic MTs in the smaller apical cell and the larger basal cell of a two-celled pro-embryo derived from microspore. The small picture in the upper left corner depicts endoplasmic MTs and the position of the nuclei in the same structure. f Elongated suspensor-like structure with random CMTs in interphase cells and phragmoplast MTs in transverse position to the long axis in dividing cells. g Four-celled linear pro-embryo depicted with differential interference contrast (DIC). h MTs and chromosomes at metaphase in the division plane transversal to the long axis of the suspensor-like structure. The small picture in the upper left corner depicts the same structure observed with DIC. i Emerged embryo-like structure. Note the MTs close to the plasma membrane of the newly formed longitudinal cell wall between the apical cell derivatives and the parallel arrays of MTs in the sub apical cell. The small picture in the upper right corner depicts the same structure in DIC. Green fluorescence (FITC) shows α-tubuline. Red fluorescence is caused by PI staining. Bar 20 μm
Fig. 5.
Microtubular configurations in the developing embryo proper in suspension cultures of microspores and pollen of Brassica napus after mild heat stress treatment. a CMTs at the octant stage of the embryo proper. b CMTs in the globular embryo proper as well as in suspensor cells. A PPB close to the hypophysis marks the transversal position of the future cell wall. c Fluorescence coming from MTs close to the plasma membranes in protoderm and sub-epidermal cells of the globular embryo proper. d MTs without preferential orientation in the ‘suspensor’ region. The small picture in the upper left corner depicts the same structure in DIC. e Endoplasmic MTs in cells of globular embryo proper and MTs close to the plasma membrane in the cell walls of embryo proper and ‘suspensor’. f–i Cortical MTs, PPBs, mitotic spindles and phragmoplasts in the globular embryo proper. The cells of the globular embryo divided more frequently than those of the suspensor-like structure. Note the mitotic divisions of the hypophyseal cell. Green fluorescence (FITC) shows α-tubuline. Red fluorescence is caused by PI staining. Bar 20 μm
Both late uni-nucleate microspores (Fig. 2a) and bi-cellular pollen (Fig. 2b) in suspensions were able to form suspensor-like structures. The embryos proper attained a globular shape at the quadrant and octant stages (Fig. 2g). Anticlinal and periclinal cell divisions of the outer cells of the globular embryo proper resulted in the development of the protoderm, sub-epidermal cells and the enlargement of the embryo proper (Fig. 2h, i).
Nuclear DNA synthesis in heat induced microspores
Fluorescence in nuclei, caused by the incorporation of BrdU, demonstrated the temporal and spatial distribution of S-phases in microspores and pollen grains induced to embryogenesis as well as in the successive stages of zygotic-like microspore-derived embryos (Fig. 2). Because BrdU was administered at a pulse of 24 h, not all nuclei became labelled. Examples without labelling are given for structures derived from a microspore (Fig. 3a) and pollen grain (Fig. 3b). In the majority, microspores induced to embryogenesis had fluorescent nuclei (Fig. 2a). When pollen grains were induced to form embryos, it was found that only the nucleus of the vegetative cell (VC) incorporated BrdU (Fig. 2b). When the suspensor-like structure was three or four cells long, nuclear DNA synthesis was mostly seen in the cells below the apical cell (Fig. 3c, d). In suspensor cells, division occurred in a regulated pattern, resulting in a uniseriate cell file. Regulation of the suspensor-like structure formation relied on the controlled and successive transversal divisions of the basal cell and its derivatives. However, deviations in the orientation of the division plane in suspensor cells occurred occasionally (Fig. 3d). Suspensor-like structures could consist of three up to eight cells when the apical cell entered the S-phase and divided longitudinally (Fig. 2e). This was the onset of embryo proper formation. Not only did the apical cell regularly exhibit a longitudinal division, but the sub-apical cell and even the following cell did as well (Fig. 3e). Next to that, both the apical and the basal cell could divide longitudinally (Fig. 3f). Such structures could become twin embryos. When the embryo proper was at the globular stage, the number of embryo cells with DNA replication increased, but the number of suspensor cells with DNA replication decreased (Fig. 2g–i), except in the region of the hypophysis.
Microtubular configurations in microspores and pollen cultured after mild heat stress
After mild heat stress treatment (32°C for 24 h and then culture at 25°C), cultured microspores enlarged and released from the microspore wall (Fig. 2b, c). They remained spherical or became elongated. At interphase, CMTs and EMTs were abundant. When still within the exine, most cells had CMTs without a clear preferential orientation (Fig. 4a), but when they released from the exine, often parallel arrays of CMTs were observed, either parallel or oblique to the long axis (Fig. 4b). Before the first mitotic division of the microspores, a preprophase band (PPB) was observed (Fig. 4c), marking the almost symmetric position of the future cell wall. After telophase, a phragmoplast developed in the middle of the microspore, separating the microspore into two cells. The apical cell slightly differed in size from the basal cell (Fig. 4d). In two-celled structures derived from microspores, reticulate arrays of CMTs and EMTs were observed in the smaller apical cell and the larger basal cell (Fig. 4e). Both cells had numerous short and densely packed CMTs without preferential orientation. At about 9 days of culture, the basal cell divided again, and three-celled linear structures were formed. After that, four-celled suspensor-like structures developed (Fig. 4f). Again, CMTs in the apical cell did not show a preferential orientation. Cells of the suspensor had random CMTs at interphase. The orientation of the metaphase plate was transverse to the long axis of the suspensor. MTs of the mitotic spindle gave rise to the phragmoplast MTs (Fig. 4f). By repeated transversal divisions, the number of suspensor cells increased (Fig. 4g–h). At about 10 days of culture, the apical, up to now ‘dormant’ cell, divided longitudinally and gave rise to the embryo proper (Fig. 4i).
Microtubule configurations in the developing embryo proper
In contrast to cell divisions in the sub-apical and lower cells of the suspensor-like structure, the apical cell never exhibited a transversal division plane, but only divided longitudinally. The two resulting cells of the embryo proper had a dense network of CMTs at interphase, but a prevalent orientation was not observed (Fig. 4i). Again, there was no overall net orientation of the CMTs during embryo proper development in the four-celled stage, the octant stage and the globular stage (Fig. 5). Although PPBs were found in all embryo proper cells that prepared for mitosis, they were not observed in the initial apical cell that underwent longitudinal division.
The frequent occurrence of PPBs, mitotic spindles and phragmoplasts in many globular embryos indicated that the cells of the globular embryo divided more frequently than those of the suspensor-like structure. However, mitotic divisions of the hypophyseal cell generated the quiescent centre/root cap cell lineage (Fig. 5f, i).
Discussion
Comparison zygotic and microspore-derived embryogenesis
Zygotic embryogenesis of B. napus L. was described from the structural point of view by Tykarska (1976). The zygote divides transversally, resulting in a small apical cell that will form the embryo proper and an elongated basal cell that will give rise to the suspensor and hypophysis. The second transversal division occurs in the elongated basal cell and is again unequal. The apical cell divides in vertical direction when the suspensor is three-celled, and the quadrant and octant stages of the embryo proper occur when the suspensor is in the four- to five-celled stage. Regarding the pathway of embryogenesis in microspores that were subject to mild heat stress treatment, it was observed that the first and the second divisions were also transversal to the long axis of the pro-embryo, and here also the sub-apical cell appeared to be the one to divide transversally. The apical cell only divided either longitudinally, as in zygotic embryos, or in oblique fashion. Sometimes this happened at the moment that the suspensor was in the two- to three-celled stage, sometimes when it was in four- to five-celled stage. This data shows that the early embryogenic pathway of microspore-derived embryos mimics zygotic development, although more variation is seen under in vitro culture conditions. In the octant stage of the zygotic embryo proper, cells divide parallel to the surface of the embryo proper and form the dermatogen (Tykarska 1976). Such development is also observed in the microspore cultures, although some variation did occur with respect to the positioning of the cell walls.
Embryos developed from both microspores and pollen grains. Incidentally, embryogenic structures were seen that contained two cells that were similar to sperm cells (Fig. 3b). This shows that the developmental window for pollen-derived embryogenesis is less limited than previously observed (Hause et al. 1993) and also discussed by Custers et al. (1994) and Supena et al. (2008). Either tri-cellular pollen can be induced to become embryogenic, or the GC of pollen still divided upon the onset of culture, which then means that pollen development continued first before embryogenic development started.
Cell cycle events
BrdU incorporation pulse label experiments revealed the spatial and temporal distribution of the S-phase in the various cells of the pro-embryo and embryo proper. First, it was found that when pollen became embryogenic, only the VC nucleus became fluorescently labelled. This finding corresponds with observations of Binarova et al. (1993). Some pro-embryos in the two-celled stage did not exhibit BrdU labelling at all (Fig. 3a, b), which indicates that the S-phase and possibly cell division had happened before the pulse labelling period. Other times, only one nucleus was labelled in two-celled structures, which pointed to the absence of synchrony as expected in apical and sub-apical cells. When the lower two cells in a three-celled embryo were labelled (Fig. 2d), this could be caused in two different ways, either the S-phase proceeded in the two cells synchronously, or the S-phase occurred in the basal cell of the two-celled stage and the nucleus divided after the S-phase, by which the daughter cells retained the label. Longitudinal cell divisions in zygotic pro-embryos are exclusively found in the apical cell, by which the quadrant embryo proper develops. In microspore cultures, not only does the apical cell exhibit longitudinal division, but sometimes the opposite cell in the cell file, the basal cell, does as well (Fig. 3f). In addition, the sub-apical cell had divided longitudinally in a number of embryos, too (Fig. 3d, e). These events clearly show that although there is polarity within the developing microspore-derived pro-embryo, it is less profound than observed in the zygotic pathway. This is already expressed during the first division, which is far less unequal in microspores than in zygotes.
Regarding the cell cycle in the apical cell of the microspore-derived pro-embryo, it appeared that most interphase nuclei were not labelled with BrdU, whereas the apical cells at mitosis had fluorescent chromosomes. Because BrdU incorporation was followed by a 24-h lag phase without BrdU, the span between S-phase and mitosis is limit. In the globular embryo stage, most BrdU incorporation was found in the cells of the embryo proper, and indeed, cell division in the suspensor occurred less frequently. Again, cell division was not as strictly regulated as in zygotic embryogenesis. Bi-polar pro-embryos developed as well, and formed twin embryos eventually.
Microtubular arrangements
The MT cytoskeleton is of undoubted importance during plant embryogenesis. Because of the zygote’s and pro-embryo’s inaccessibility inside the maternal tissue and the consequent technical difficulties in cytoskeleton visualization, studies which refer to the role of MTs are scarce (Huang et al. 1990; Webb and Gunning 1991). An in vitro culture, with numerous embryos produced over a short time, offers a novel approach to the study of experimental plant embryogenesis. Ovules cultured in vitro (Sauer and Friml 2004) and non-embryogenic and embryogenic microspore suspensions cultures (Supena et al. 2008; Dubas et al. 2011) can be effectively used in many staining protocols. The significance of such staining opportunity increases when it is hard to obtain transformants.
The MT cytoskeleton was visualized in order to relate its 3D configuration and dynamic changes with the morphogenesis of the embryo. The application of fixation, proper enzymatic digestion of cell walls and DMSO treatment, in combination with CLSM observation, resulted in excellent preservation and visualization of the MT cytoskeleton in successive phases of the cell cycle in cells from the microspore stage until the globular embryo stage.
When isolated microspores and pollen were cultured at 32°C for 24 h and then at 25°C, programmed sporophytic development proceeded and embryogenic structures resembling zygotic embryos with suspensors were formed. The first sign of sporophytic development upon heat shock treatment of microspores was the change in the orientation of the mitotic spindle by 90° (Hause et al. 1993). Hereafter, cell division lead to two approx. equally sized cells. From this stage onward, suspensor-like structures were formed when short heat shock treatment was performed (Joosen et al. 2007; Supena et al. 2008). Here, we show that the first mitotic division was markedly postponed until 7–8 days after heat treatment, and the division was preceded by the appearance of a PPB in the prophase. The appearance of PPB in heat-induced microspores marks sporophytic development and is important for embryogenesis (Simmonds and Keller 1999; Dubas et al. 2010).
At the moment that single celled structures or bi-cellular structures were released from the wall of the microspore, they did not contain large vacuoles, and MTs radiated to the periphery. Patterns of MTs in the cortical cytoplasm varied from criss-cross textures to parallel arrays. When criss-cross textures dominated, isotropic expansion was observed. Parallel arrays of MTs were found in regions where elongation occurred. The transformation of an unordered into directional pattern of MT organization could be crucial for setting up the main axis characteristic for early embryogenesis.
From the MT configurations observed in bi- and multicellular embryos, CMTs and EMTs were abundant in interphase cells, and PPB and spindle MTs clearly exhibited the orientation of cell division. EMTs ran from the periphery of the nucleus towards the cell cortex in radiating patterns, whereas the CMTs were organized in various patterns ranging from parallel arrangements to random patterning. During interphase, random patterns were observed in cells that expanded isotropically, while elongating cells contained a cortical pattern of aligned MTs perpendicular to the direction of growth (Cyr and Palevitz 1995; Wasteneys 2002).
The orientation of interphase MTs in the apical cell of the pro-embryo and in the first cells of the embryo proper was typical for cells exhibiting isotropic growth, which was in agreement with the observations of Webb and Gunning (1991). Contrary to this, cells that have anisotropic growth often exhibit parallel arrays of CMTs (Cyr 1994; Wasteneys 2002). This holds true for the cells in the older elongating heart-shaped and torpedo-shaped embryos and sometimes for cells of the suspensor. The orientation of MTs in suspensor cells is described as perpendicular to the direction of cell elongation and is believed to play a role in cell-shaping (Gunning and Hardham 1982; Paredez et al. 2006). Although there is a debate about the function of MTs in determining or influencing the deposition of cellulose microfibrils, they might function as such in the older long and elongating cells. The cells of the suspensor, however, are disk shaped, i.e. much wider than higher, and in these cells, CMTs and EMTs appeared in various orientations. It is suggested that in these cells, where space is limited, the deposition of cellulose microfibrils is probably not regulated by alignment of synthase complexes along or mediated by MTs (Emons and Mulder 2000) rather than by the limited space available (Fig. 5g). In the present investigations, PPBs were seen in microspores and many suspensor cells, but not in the apical cells of proembryos. Either the phenomenon does not occur in these cells or the stage is short termed.
Our observations on the dynamics in DNA replication and MT cytoskeleton organization contribute new insight into the microspore embryogenic process under mild heat in vitro inductive conditions. In the present study, we have found there is a clear similarity to zygotic embryo development, which makes this system a valuable tool to investigate the successive stages of embryo development.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Binarova P, Straatman K, Hause B, Hause G, van Lammeren AAM. Nuclear DNA synthesis during the induction of embryogenesis in cultured microspores and pollen of Brassica napus L. Theor Appl Genet. 1993;87:9–16. doi: 10.1007/BF00223736. [DOI] [PubMed] [Google Scholar]
- Binarova P, Hause G, Cenklová V, Cordewener JHG, van Lookeren Campagne MM. A short severe heat shock is required to induce embryogenesis in late bicellular pollen of Brassica napus L. Sex Plant Reprod. 1997;10:200–208. doi: 10.1007/s004970050088. [DOI] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AA, Miki BL, Custers JB, van Lookeren Campagne MM. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14(8):1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordewener JHG, Hause G, Görgen E, Busink R, Hause B, Dons HJM, van Lammeren AAM, van Lookeren Campagne MM, Pechan P. Changes in synthesis and localization of members of the 70-kDa class of heat shock proteins accompany the induction of embryogenesis in Brassica napus L. microspores. Planta. 1995;196:747–755. doi: 10.1007/BF01106770. [DOI] [Google Scholar]
- Cordewener JHG, Bergervoet J, Liu CM. Changes in protein synthesis and phosphorylation during microspore embryogenesis in Brassica napus. J Plant Physiol. 2000;156:156–163. [Google Scholar]
- Custers JBM. Microspore culture in rapeseed (Brassica napus L.) In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I, editors. Doubled haploid production in crop plant: a manual. Dordrecht: Kluwer; 2003. pp. 185–194. [Google Scholar]
- Custers JBM, Cordewener JHG, Nöllen Y, Dons HJM, van Lookeren Campagne MM. Temperature controls both gametophytic and sporophytic development in microspore culture of Brassica napus. Plant Cell Rep. 1994;13:267–271. doi: 10.1007/BF00233317. [DOI] [PubMed] [Google Scholar]
- Custers JBM, Cordewener JHG, Fiers MA, Massen BTH, van Lookeren Campagne MM, Liu CM. Androgenesis in Brassica. A model system to study the initiation of plant embryogenesis. In: Bhojwani SS, Soh WY, editors. Current trends in the embryology of angiosperms. The Netherlands: Kluwer; 2001. pp. 451–470. [Google Scholar]
- Cyr RJ. Microtubules in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol. 1994;10:153–180. doi: 10.1146/annurev.cb.10.110194.001101. [DOI] [PubMed] [Google Scholar]
- Cyr RJ, Palevitz BA. Organization of cortical microtubules in plant cells. Curr Opin Cell Biol. 1995;7:65–71. doi: 10.1016/0955-0674(95)80046-8. [DOI] [PubMed] [Google Scholar]
- Dubas E, Wędzony M, Petrovska B, Salaj J, Żur I. Cell structural reorganization during induction of androgenesis in isolated microspore cultures of triticale (×Triticosecale Wittm.) Acta Biol Crac Ser Bot. 2010;52(1):73–86. [Google Scholar]
- Dubas E, Wędzony M, Custers J, Kieft H, van Lammeren AAM (2011) Gametophytic development of Brassica napus pollen in vitro enables examination of cytoskeleton and nuclear movements. Protoplasma (in press). doi:10.1007/s00709-011-0287-0 [DOI] [PMC free article] [PubMed]
- Emons AMC, Mulder BM. How the deposition of cellulose microfibrils builds cell wall architecture. Trends Plant Sci. 2000;5:35–40. doi: 10.1016/S1360-1385(99)01507-1. [DOI] [PubMed] [Google Scholar]
- Friml J, Benkova E, Mayer U, Palme K, Muster G. Automated whole mount localisation techniques for plant seedlings. Plant J. 2003;34(1):115–124. doi: 10.1046/j.1365-313X.2003.01705.x. [DOI] [PubMed] [Google Scholar]
- Gu HH, Hagberg P, Zhou WJ. Cold pretreatment enhances microspore embryogenesis in oilseed rape (Brassica napus L.) Plan Growth Regul. 2004;42:137–143. doi: 10.1023/B:GROW.0000017488.29181.fa. [DOI] [Google Scholar]
- Gunning BES, Hardham AR. Microtubules. Annu Rev Plant Physiol. 1982;33:651–698. doi: 10.1146/annurev.pp.33.060182.003251. [DOI] [Google Scholar]
- Gunning BES, Sammut M. Rearrangements of microtubules involved in establishing cell division planes start immediately after DNA synthesis and are completed just before mitosis. Plant Cell. 1990;2:1273–1282. doi: 10.1105/tpc.2.12.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause G, Hause B, van Lammeren AAM. Microtubular and actin filament configurations during microspore and pollen development in Brassica napus cv. Topas. Can J Bot. 1992;70:1369–1376. doi: 10.1139/b92-172. [DOI] [Google Scholar]
- Hause B, Hause G, Pechan P, van Lammeren AAM. Cytoskeletal changes and induction of embryogenesis in microspore and pollen cultures of Brassica napus L. Cell Biol Int. 1993;17(2):153–168. doi: 10.1006/cbir.1993.1052. [DOI] [Google Scholar]
- Hause B, van Veenendaal WLH, Hause G, van Lammeren AAM. Expression of polarity during early development of microspore-derived and zygotic embryos of Brassica napus L. cv. Topas. Bot Acta. 1994;107(6):407–415. [Google Scholar]
- Huang BQ, Russell SD, Strout GW, Mao LJ. Organization of isolated embryo sacs and eggs of Plumbago zeylanica (Plumbaginaceae) before and after fertilization. Am J Bot. 1990;77:1401–1410. doi: 10.2307/2444750. [DOI] [Google Scholar]
- Joosen R, Cordewener J, Supena EDJ, Vorst O, Lammers M, Maliepaard Ch, Zeilmaker T, Miki B, America T, Custers J, Boutilier K. Combined transcriptome and proteome analysis identifies pathways and robust markers associated with the establishment of Brassica napus microspore-derived embryo development. Plant Physiol. 2007;144:155–172. doi: 10.1104/pp.107.098723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter R. Induction of haploid plants from isolated pollen of Brassica napus. Z Pflanzenphysiol. 1982;105:427–434. [Google Scholar]
- Maluszynski M, Kasha KJ, Forster BP, Szarejko I (2003) Double haploid production in crop plants. A manual. Kluwer, Dordrecht, p 428
- Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312:1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- Pechan PM, Keller WA. Identification of potentially embryogenic microspores in Brassica napus. Physiol Plant. 1988;74:377–384. doi: 10.1111/j.1399-3054.1988.tb00646.x. [DOI] [Google Scholar]
- Sauer M, Friml J. In vitro culture of Arabidopsis embryos within their ovules. Plant J. 2004;40(5):835–843. doi: 10.1111/j.1365-313X.2004.02248.x. [DOI] [PubMed] [Google Scholar]
- Sedbrook JC, Kaloriti D. Microtubules, MAPs and plant directional cell expansion. Trends Plant Sci. 2008;13:303–310. doi: 10.1016/j.tplants.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Simmonds DH. Mechanism of induction of microspore embryogenesis in Brassica napus; significance of the PPBs of microtubules in the first sporophytic division. In: Akkas N, editor. Biomechanics of active movement and division of cells. NATO ASI series, H 84. Berlin: Springer; 1994. pp. 569–574. [Google Scholar]
- Simmonds DH, Keller WA. Significance of preprophase bands in induction of embryogenesis from microspores of Brassica napus. Planta. 1999;208:383–391. doi: 10.1007/s004250050573. [DOI] [Google Scholar]
- Smýkal P. Pollen embryogenesis—the stress mediated switch from gametophytic to sporophytic development. Current status and future prospects. Biol Plantarum. 2000;43(4):481–489. doi: 10.1023/A:1002835330799. [DOI] [Google Scholar]
- Supena EDJ, Winarto B, Riksen T, Dubas E, van Lammeren A, Offringa R, Boutilier K, Custers J. Regeneration of zygotic-like microspore-derived embryos suggests an important role for the suspensor in early embryo patterning. J Exp Bot. 2008;59(4):803–814. doi: 10.1093/jxb/erm358. [DOI] [PubMed] [Google Scholar]
- Szechyńska-Hebda M, Wędzony M, Dubas E, Kieft H, van Lammeren A. Visualisation of microtubules and actin filaments in fixed BY-2 suspension cells using an optimized whole mount immunolabeling protocol. Plant Cell Rep. 2006;25(8):758–766. doi: 10.1007/s00299-005-0089-y. [DOI] [PubMed] [Google Scholar]
- Telmer CA, Newcomb W, Simmonds DH. Cellular changes during heat shock induction and embryo development of cultured microspores of Brassica napus cv. Topas. Protoplasma. 1995;185:106–112. doi: 10.1007/BF01272758. [DOI] [Google Scholar]
- Thomas E, Wenzel G. Embryogenesis from microspores of Brassica napus. Z Pflanzenzuecht. 1975;74:77–81. [Google Scholar]
- Tykarska T (1976) Rape embryogenesis I: the proembryo development. Acta Soc Bot Pol XLV:3–16
- Tykarska T (1979) Rape embryogenesis II: development of embryo proper. Acta Soc Bot Pol XLVIII:391–421
- Tykarska T. Rape embryogenesis. 111. Embryo development in time. Acta Soc Bot Pol. 1980;49:369–385. [Google Scholar]
- Tykarska T. Rape embryogenesis. V. Accumulation of lipid bodies. Acta Acta Soc Bot Pol. 1987;56:573–584. [Google Scholar]
- Wasteneys GO. Microtubule organization in the green kingdom: chaos or self-order? J Cell Sci. 2002;115:1345–1354. doi: 10.1242/jcs.115.7.1345. [DOI] [PubMed] [Google Scholar]
- Webb MC, Gunning BES. The microtubular cytoskeleton during development of the zygote, proembryo and free-nuclear endosperm in Arabidopsis thaliana (L.) Heynh. Planta. 1991;184:187–195. doi: 10.1007/BF01102418. [DOI] [PubMed] [Google Scholar]
- Wedzony M, Forster BP, Zur I, Golemiec E, Szechyńska-Hebda M, Dubas E, Gołębiowska G (2009) Progress in doubled haploid technology in higher plants. In: Touraev A et al. (eds) Advances in haploid production in higher plants. Springer Science+Business Media B.V., pp 1–33
- West MAL, Harada JJ. Embryogenesis in higher plants: an overview. Plant Cell. 1993;5:1361–1369. doi: 10.1105/tpc.5.10.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XL, Zee SY, Yeung EC. Suspensor development in Nun orchid Phaius tanker.illiae. Int J Plant Sci. 1997;158:704–712. doi: 10.1086/297482. [DOI] [Google Scholar]
- Zaki MAM, Dickinson HG. Structural changes during the first divisions of embryos resulting, from anther and free microspore culture in Brassica napus. Protoplasma. 1990;156:149–162. doi: 10.1007/BF01560653. [DOI] [Google Scholar]
- Zaki MAM, Dickinson HG. Microspore-derived embryos in Brassica: the significance of division symmetry in pollen mitosis I to embryogenic development. Sex Plant Reprod. 1991;4:48–55. doi: 10.1007/BF00194572. [DOI] [Google Scholar]
- Zaki MAM, Dickinson HG. Modification of cell development in vitro: the effect of colchicine on anther and isolated microspore culture in Brassica napus. Plant Cell Tissue Org Cult. 1995;40:255–270. doi: 10.1007/BF00048132. [DOI] [Google Scholar]
- Zheng Y. G protein control of microtubule assembly. Annu Rev Cell Dev Biol. 2004;20:867–894. doi: 10.1146/annurev.cellbio.20.012103.094648. [DOI] [PubMed] [Google Scholar]