Abstract
Synaptic transmission is achieved by exocytosis of small, synaptic vesicles containing neurotransmitters across the plasma membrane. Here, we use a DNA-tethered freestanding bilayer as a target architecture that allows observation of content transfer of individual vesicles across the tethered planar bilayer. Tethering and fusion are mediated by hybridization of complementary DNA-lipid conjugates inserted into the two membranes, and content transfer is monitored by the dequenching of an aqueous content dye. By analyzing the diffusion profile of the aqueous dye after vesicle fusion, we are able to distinguish content transfer across the tethered bilayer patch from vesicle leakage above the patch.
SNARE-mediated exocytosis of synaptic vesicles containing neurotransmitters across the pre-synaptic plasma membrane is a critical step for synaptic transmission (1) and has been studied by a variety of techniques in cellular systems (2). Currently, many researchers use simpler in vitro model systems to probe the underlying molecular mechanisms of membrane fusion at the single event level by fluorescence microscopy. Two types of assays are commonly employed: transfer of lipid dye from one membrane to the other during fusion (lipid-mixing), and transfer of an aqueous content dye through the fusion pore (content transfer) (e.g., (3)). Although content transfer has been studied electrochemically in live cells for some time (2,4), content transfer in simple fluorescence-based model systems has generally been difficult to achieve and observe. Consequently, many researchers use only lipid-mixing to study vesicle fusion in their experimental designs. Although lipid-mixing may be an accurate reporter for some fusion intermediates, we (5,6) and others (7) have observed discrepancies between the amount of lipid-mixing and content transfer in model fusion systems, suggesting that complete lipid mixing may occur without content transfer.
Here we present the observation of content transfer from small unilamellar vesicles (SUVs, 50–100 nm in diameter) containing a self-quenched, aqueous dye across a tethered, planar bilayer. At the heart of our system are DNA-lipid conjugates, described by us (5,8) and others (9), which can be inserted into lipid membranes to mediate specific membrane-membrane interactions such as tethering, docking, and fusion by DNA hybridization. Here, these DNA-lipid conjugates are used in two different ways depending on their relative orientations on the interacting surfaces: First, DNA-lipids are used as tethers to construct the target DNA-tethered bilayer patch by hybridization of DNA partners coupled at the 5′ end to a lipid (Fig. 1 A). Second, DNA-lipid partners coupled at opposite ends (one at the 3′ end and the antisense at the 5′ end) are used to specifically mediate SUV-bilayer docking and fusion (Fig. 1 B). We have previously shown that DNA-lipids can promote SUV-to-SUV fusion in bulk (5,6), and here we show this for individual events. We highlight that the geometry of our system is designed to mimic the fusion of synaptic vesicles to the plasma membrane.
Figure 1.
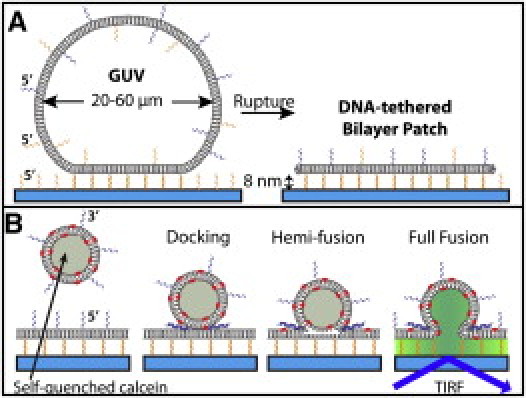
(A) Formation of a DNA-tethered bilayer patch. A GUV displaying 5′-anchored DNA-lipids with two different 24-mer sequences (blue and gold) binds to the gold antisense DNA strand on the substrate by DNA hybridization. Over time, the GUV ruptures to form a DNA-tethered bilayer patch, held at the distance specified by the DNA-duplex (8 nm for a 24-mer). Note that nothing is to scale. (B) Fluorescence assay to detect individual lipid mixing and content transfer events. An SUV displaying the 3′-anchored blue antisense DNA-lipid docks and subsequently fuses to the patch by DNA hybridization, transferring a self-quenched content dye into the 8-nm gap between patch and surface.
The planar, free-standing DNA-tethered bilayer patch is constructed by exposing giant unilamellar vesicles (GUVs, 20–60 μm in diameter) that display two different DNA sequences (blue and gold in Fig. 1; and see Table S1 in the Supporting Material for sequences) to a glass coverslip displaying DNA complementary to the gold strand on the GUV. As DNA hybrids form between the GUV and the substrate, the GUV flattens and eventually ruptures, forming a stable tethered membrane patch held ∼8 nm above the substrate surface for a 24-mer DNA duplex tether (10). The lateral dimension of the membrane patch depends on the size of the GUV, and can be up to 100 μm in diameter. A range of GUVs compositions can be used (11), and a small (0.01 mol %) amount of lipid dye is included to locate the tethered patch.
Once the tethered patch is located by total internal reflectance fluorescence microscopy, SUVs containing a self-quenched content dye (100 mM calcein) and displaying 3′-anchored DNA complementary to the 5′-anchored blue strand on the patch are manually pipetted into the solution above the patch surface. The SUVs are observed to dock and subsequently fuse to the tethered patches. Docking is determined by the appearance of the SUV on the patch, detected by the faint fluorescence of the self-quenched content dye. Fusion is detected by content transfer of the dye across the patch membrane, resulting in the rapid dequenching of the dye as it becomes diluted in the gap between the bottom surface of the tethered patch and the substrate. This is observed as a radially expanding fluorescent explosion monitored by total internal reflectance fluorescence microscopy at 5.5-ms time resolution. Movie S1 in the Supporting Material shows a typical fluorescent burst, and frame snapshots are shown in Fig. 2. Consistent with previous bulk studies (5,6), SUVs displaying either noncomplementary DNA-lipids or no DNA-lipids neither dock nor fuse to the target tethered patch. SUVs displaying a complementary DNA-lipid, but with the lipid on its 5′ end, are observed to tether but not fuse with the target membrane.
Figure 2.
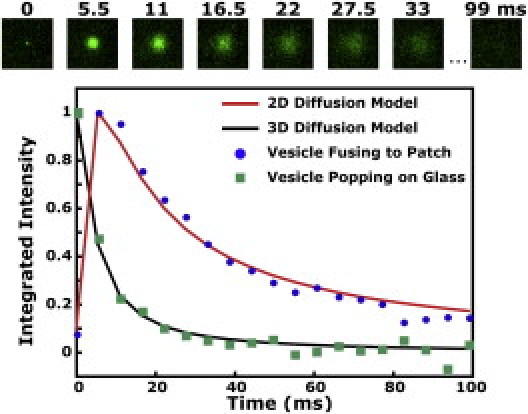
Fluorescence time decay analysis to distinguish a content transfer event from content leakage or bursting. The time trace of the integrated fluorescence intensity is fit by a two- or three-dimensional mathematical diffusion model (see text and the Supporting Material). The trace from an SUV fusing to the tethered bilayer patch (blue circles) is better fit by the two-dimensional diffusion model (red), consistent with genuine fusion, whereas the trace from an SUV popping on a glass surface (green squares) is better fit by the three-dimensional model (black). Fluorescence images of an SUV fusing to a tethered patch are displayed.
The time evolution of the fluorescent bursts was quantified by calculating the normalized integrated intensity within a 4-μm radius circle, and a mathematical diffusion model was used to determine whether the bursts are due to content transfer across the tethered bilayer (true fusion) or merely result from SUVs bursting (or leaking) content dye above the bilayer. If true fusion occurs, then the dye molecules should exhibit roughly two-dimensional diffusion in the ∼8-nm space between the membrane patch and the surface as they spread radially outward. Conversely, if bursting/leaking occurs, then the dye molecules should escape much more quickly, exhibiting three-dimensional diffusion in the open half-space above the patch.
Using analytical expressions reported by Wang et al. (12) and described in the Supporting Material, we performed a least-squares fit of the time traces from many fluorescent bursts (n = 125) to a two-dimensional or three-dimensional diffusion model. The majority of the bursts (n = 111) were better fit by the two-dimensional diffusion model, with an average fitted diffusion coefficient of 333 ± 117 μm2/s (see Fig. S2 in the Supporting Material), consistent with the conclusion that true fusion had occurred for those events. Five of the events were better fit by the three-dimensional diffusion model, suggesting that in those cases, the content had burst above the bilayer surface (see Fig. S1 B). The remaining events could not be assigned conclusively to either model. Fig. 2 shows the time trace for an event identified as fusion (blue data) overlaid with the discretized fit of the two-dimensional model (red trace). Consistent with studies in bulk (5,6), the efficiency of our DNA-lipid system to promote content transfer was low—only ∼6% of SUVs were observed to undergo content transfer during the timescale of the experiment. The average docking to fusion time for these SUVs was 1.8 s (see Fig. S3). We note, however, that the docking-to-fusion times can depend strongly on the light level used to collect the data (see cautionary note about light intensity in the Supporting Material).
To further verify the mathematical diffusion analysis, we applied the same models to time traces of SUVs filled with self-quenched calcein bursting into three dimensions after being deposited on an unmodified glass coverslip under buffer. Bursting was induced by flowing in blank SUVs to initiate formation of a supported bilayer (13). A typical bursting time trace is shown in Fig. 2 (green data) and is more closely fit by the three-dimensional diffusion curve (black trace) with a diffusion coefficient of 455 μm2/s.
Two interesting observations were made during the analysis of vesicle fusion events. First, we observed content transfer events in two types of enclosed lipid structures where the sidedness of the transfer across the membrane is unambiguous but quantitative analysis is difficult. The first structure is a DNA-tethered GUV (stable at low densities of DNA tethers on the substrate, see Fig. S5 and Movie S2 for an example content transfer event). The second structure is lipid tubules, which are sometimes created during tethered patch formation (see Movie S3 for example of a fusion event). Second, ∼12% of SUV-to-patch fusion events appeared to be only partial content transfer events, leaving behind some content dye in the SUV (see Fig. S4 and Movie S4). Presumably, this is transient pore formation.
To verify that lipid-mixing and content transfer occurred simultaneously during fusion events, two-color SUV-to-tethered patch fusion experiments were performed, with 2% Texas Red-DHPE (TR) in the SUV as the lipid dye and self-quenched calcein as the content dye. Fig. 3 displays a representative two-color time trace showing that lipid mixing and content transfer do occur simultaneously. The outward radial diffusion of TR in the tethered bilayer after a lipid-mixing event can also be fit to a two-dimensional diffusion model (see Fig. S8). The average diffusion coefficient from many events (n = 168) was 4.1 ± 1.4 μm2/s, consistent with a previous measurement of lipid diffusion in DNA-tethered bilayer patches (10). The efficiency of lipid-mixing (mostly hemifusion events) is ∼60–80%.
Figure 3.
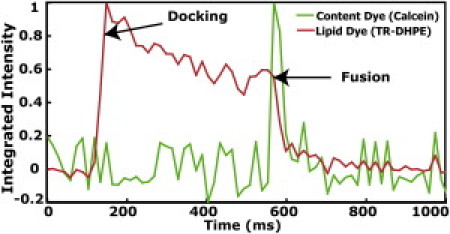
Time trace of a two-color fusion experiment, demonstrating that content transfer and lipid-mixing occur simultaneously. Docking occurs at t ≈ 100 ms, indicated by the appearance of the TR signal (red trace). The self-quenched content dye in the SUV (green trace) is not visible over the noise. After a delay of ∼480 ms during which the TR undergoes some photobleaching, full fusion is observed at t ≈ 580 ms, by content dye dequenching (compare to Fig. 2) followed by a rapid drop in the intensity of both dyes as they diffuse away.
Finally, we emphasize that using a DNA-tethered patch as a mimic of the presynaptic membrane is a strategy that avoids the pitfalls common to glass-supported lipid bilayers (SLBs). Because the tethered patches are flat and separated from the underlying substrate by the DNA duplexes, their adhesion to the surface is greatly diminished (10). It is, perhaps, for this reason that our system permits content transfer during fusion events, whereas groups studying vesicle fusion to SLBs have had difficulty demonstrating content transfer (12). Additionally, integral membrane proteins are often immobile and/or nonfunctional when incorporated into SLBs, making it difficult to use such systems to study SNARE-mediated fusion (14), although some groups have obtained SLBs with mobile SNARE complexes and studied fusion by lipid mixing (15). Because of these considerations, we expect that our DNA-tethered bilayer patches will be readily transferable to other model membrane fusion systems, including systems using reconstituted SNARE proteins.
Acknowledgments
This work was supported in part by grants from the National Science Foundation Biophysics Program, grant No. GM069630 from the National Institutes of Health, and award No. DMR-0213618 (CPIMA) by The Materials Research and Engineering Center Program of the National Science Foundation. R.J.R. is supported by a National Science Foundation Graduate Fellowship and an Althouse Family Stanford Graduate Fellowship, B.v.L. by a Gabilan Stanford Graduate Fellowship, and P.M.B. by the Danish Council for Independent Research—Natural Sciences.
Footnotes
Poul Martin Bendix's current address is Niels Bohr Institute, Copenhagen, Denmark.
Supporting Material
References and Footnotes
- 1.Brunger A.T., Weninger K., Chu S. Single-molecule studies of the neuronal SNARE fusion machinery. Annu. Rev. Biochem. 2009;78:903–928. doi: 10.1146/annurev.biochem.77.070306.103621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge S., Koseoglu S., Haynes C.L. Bioanalytical tools for single-cell study of exocytosis. Anal. Bioanal. Chem. 2010;397:3281–3304. doi: 10.1007/s00216-010-3843-0. [DOI] [PubMed] [Google Scholar]
- 3.Chernomordik L., Chanturiya A., Zimmerberg J. The hemifusion intermediate and its conversion to complete fusion: regulation by membrane composition. Biophys. J. 1995;69:922–929. doi: 10.1016/S0006-3495(95)79966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dias A.F., Dernick G., Lindau M. An electrochemical detector array to study cell biology on the nanoscale. Nanotechnology. 2002;13:285–289. [Google Scholar]
- 5.Chan Y.-H. M., van Lengerich B., Boxer S.G. Lipid-anchored DNA mediates vesicle fusion as observed by lipid and content mixing. Biointerphases. 2008;3:FA17–FA21. doi: 10.1116/1.2889062. [DOI] [PubMed] [Google Scholar]
- 6.Chan Y.-H. M., van Lengerich B., Boxer S.G. Effects of linker sequences on vesicle fusion mediated by lipid-anchored DNA oligonucleotides. Proc. Natl. Acad. Sci. USA. 2009;106:979–984. doi: 10.1073/pnas.0812356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyoung M., Srivastava A., Brunger A.T. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc. Natl. Acad. Sci. USA. 2011;108:E304–E313. doi: 10.1073/pnas.1107900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshina-Ishii C., Boxer S.G. Arrays of mobile tethered vesicles on supported lipid bilayers. J. Am. Chem. Soc. 2003;125:3696–3697. doi: 10.1021/ja029783+. [DOI] [PubMed] [Google Scholar]
- 9.Stengel G., Zahn R., Höök F. DNA-induced programmable fusion of phospholipid vesicles. J. Am. Chem. Soc. 2007;129:9584–9585. doi: 10.1021/ja073200k. [DOI] [PubMed] [Google Scholar]
- 10.Chung M., Lowe R.D., Boxer S.G. DNA-tethered membranes formed by giant vesicle rupture. J. Struct. Biol. 2009;168:190–199. doi: 10.1016/j.jsb.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung M., Boxer S.G. Stability of DNA-tethered lipid membranes with mobile tethers. Langmuir. 2011;27:5492–5497. doi: 10.1021/la200234h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T., Smith E.A., Weisshaar J.C. Lipid mixing and content release in single-vesicle, SNARE-driven fusion assay with 1–5 ms resolution. Biophys. J. 2009;96:4122–4131. doi: 10.1016/j.bpj.2009.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson J.M., Ha T., Boxer S.G. Early steps of supported bilayer formation probed by single vesicle fluorescence assays. Biophys. J. 2002;83:3371–3379. doi: 10.1016/S0006-3495(02)75337-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowen M.E., Weninger K., Chu S. Single molecule observation of liposome-bilayer fusion thermally induced by soluble n-ethyl maleimide sensitive-factor attachment protein receptors (SNAREs) Biophys. J. 2004;87:3569–3584. doi: 10.1529/biophysj.104.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domanska M.K., Kiessling V., Tamm L.K. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J. Biol. Chem. 2009;284:32158–32166. doi: 10.1074/jbc.M109.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


